Improvement Effect and Mechanism of Hydroxytyrosol on Skin Aging Induced Advanced Glycation End Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
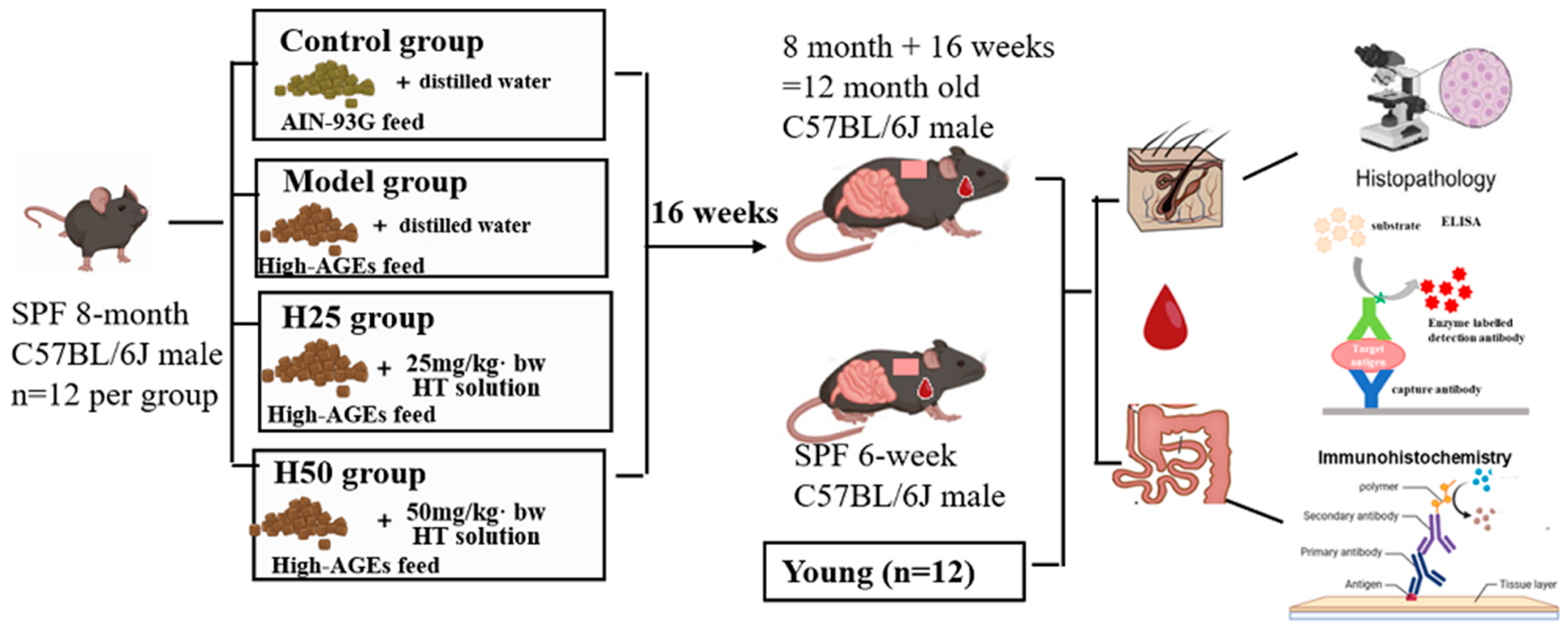
2.2. Experimental Animals and Treatment
2.3. High-AGEs Feed Preparation
2.4. General Situation of Animals
2.5. Determination of Skin Moisture Content
2.6. Histopathological Evaluation of Skin Tissue
2.7. Measurement of Skin Hydroxyproline (HYP) Levels
- (1)
- Coating: 96-well plates were coated with 100 μL/well antigen/antibody solution in carbonate-bicarbonate buffer (pH 9.6), incubated overnight at 4 °C.
- (2)
- Washing: Wells were washed 3–5 times with phosphate-buffered saline containing 0.05% Tween-20 (PBST), with residual liquid removed by aspiration followed by plate inversion on absorbent paper.
- (3)
- Blocking: 200 μL blocking buffer (5% bovine serum albumin in PBST) was added per well, incubated for 1 h at room temperature (RT, 22 ± 2 °C).
- (4)
- Standards/Samples: Serial dilution standards and tissue homogenates (prepared in RIPA buffer) were added to wells and incubated for 2 h at RT.
- (5)
- Detection Antibody: Wells received 100 μL horseradish peroxidase-conjugated detection antibody (1:2000 in blocking buffer), incubated for 1 h at RT.
- (6)
- Substrate Reaction: 100 μL tetramethylbenzidine (TMB) substrate was added per well and incubated for exactly 30 min at RT in darkness. Reactions were terminated with 50 μL 2 M H2SO4.
- (7)
- Absorbance Measurement: Optical density at 450 nm (reference 630 nm) was measured within 30 min using a microplate reader (BioTek Synergy H1). HYP concentrations of seven mice in each group were calculated using four-parameter logistic regression against the standard curve.
2.8. Evaluation of Oxidative Stress Markers
2.9. Measurement of Serum Inflammatory Cytokines
2.10. Intestinal Morphology and Barrier Function Evaluation
2.11. Statistical Analysis
3. Results
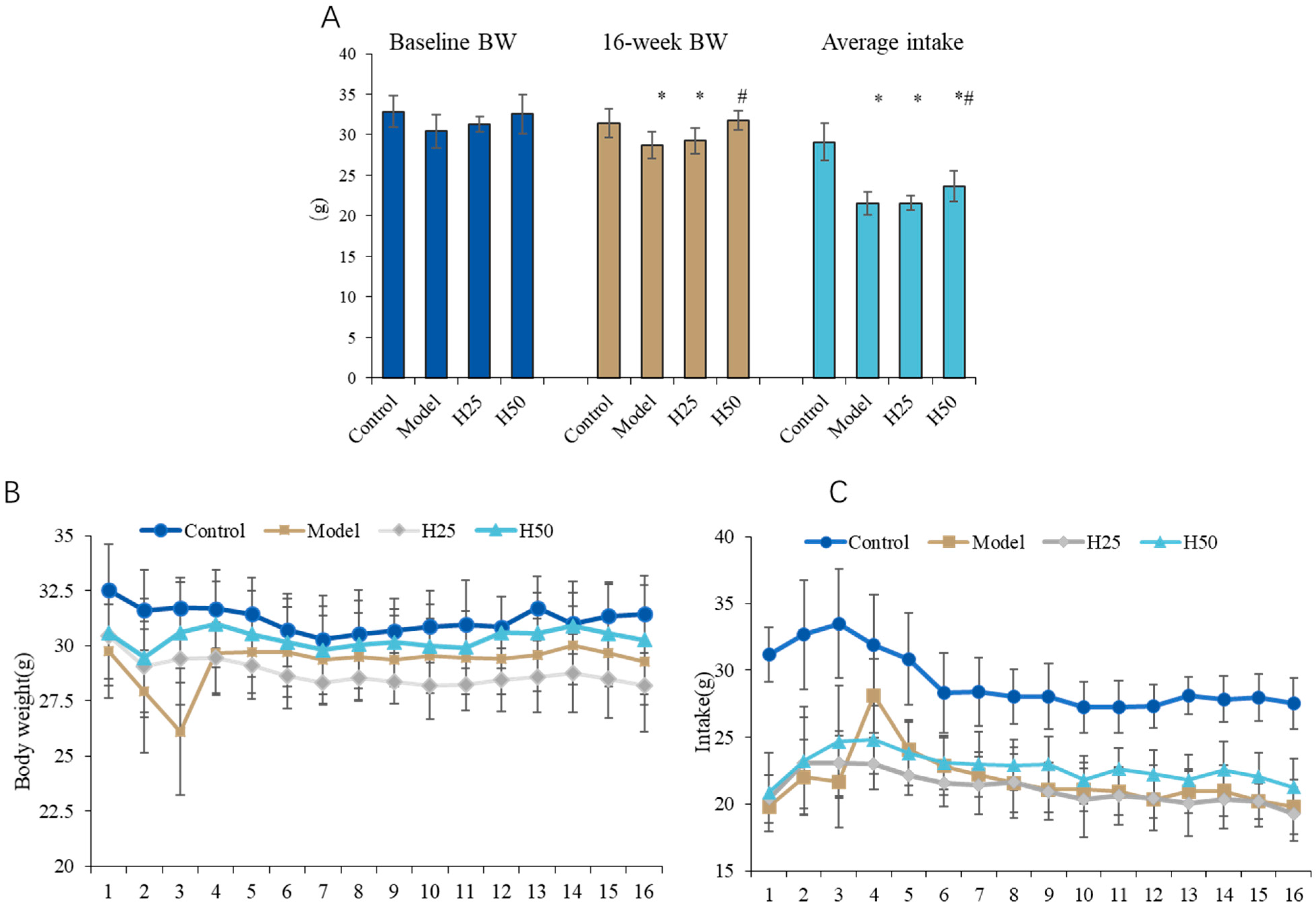
3.1. General Situation of Animals
Changes in the Body Weight and Food Intake of Mice
3.2. The Improvement Effect of Hydroxytyrosol on Skin Aging in Mice
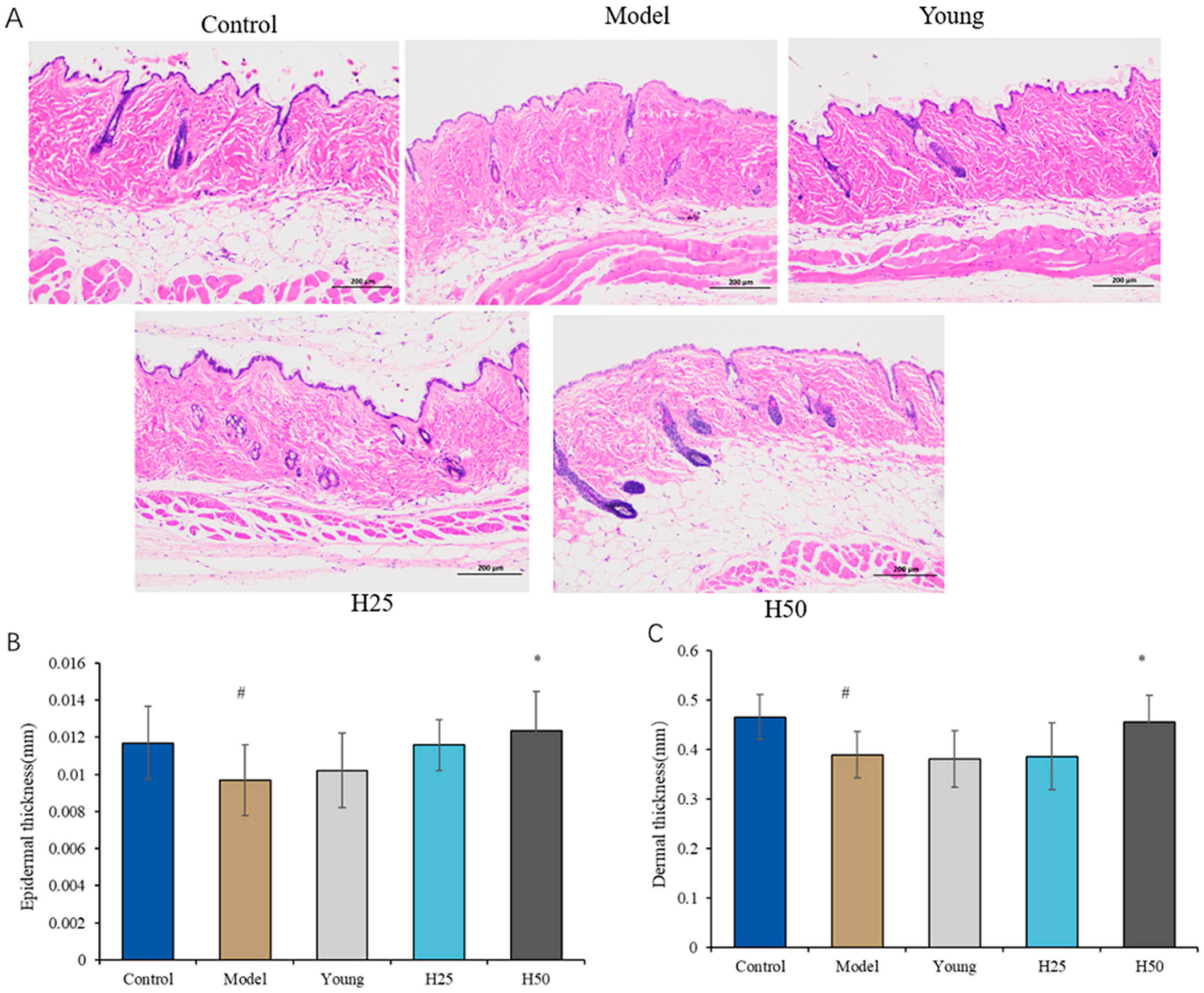
3.2.1. Histopathological Evaluation of Skin Tissue
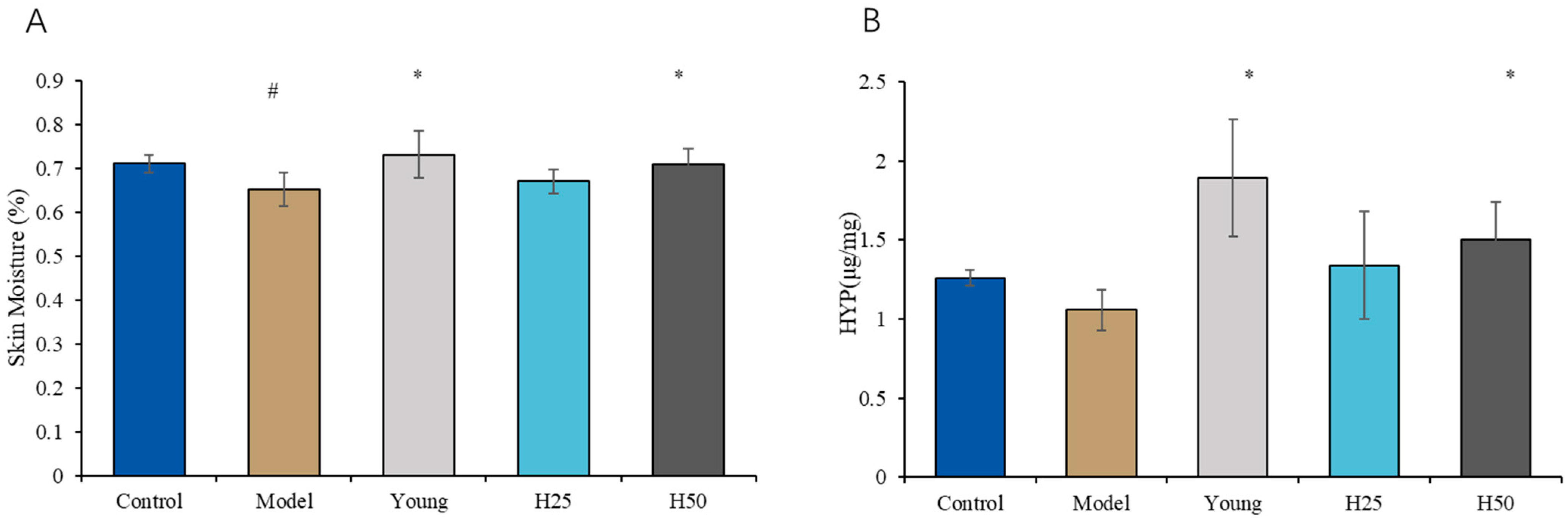
3.2.2. Determination of Skin Moisture Content and Hydroxyproline (HYP) Levels
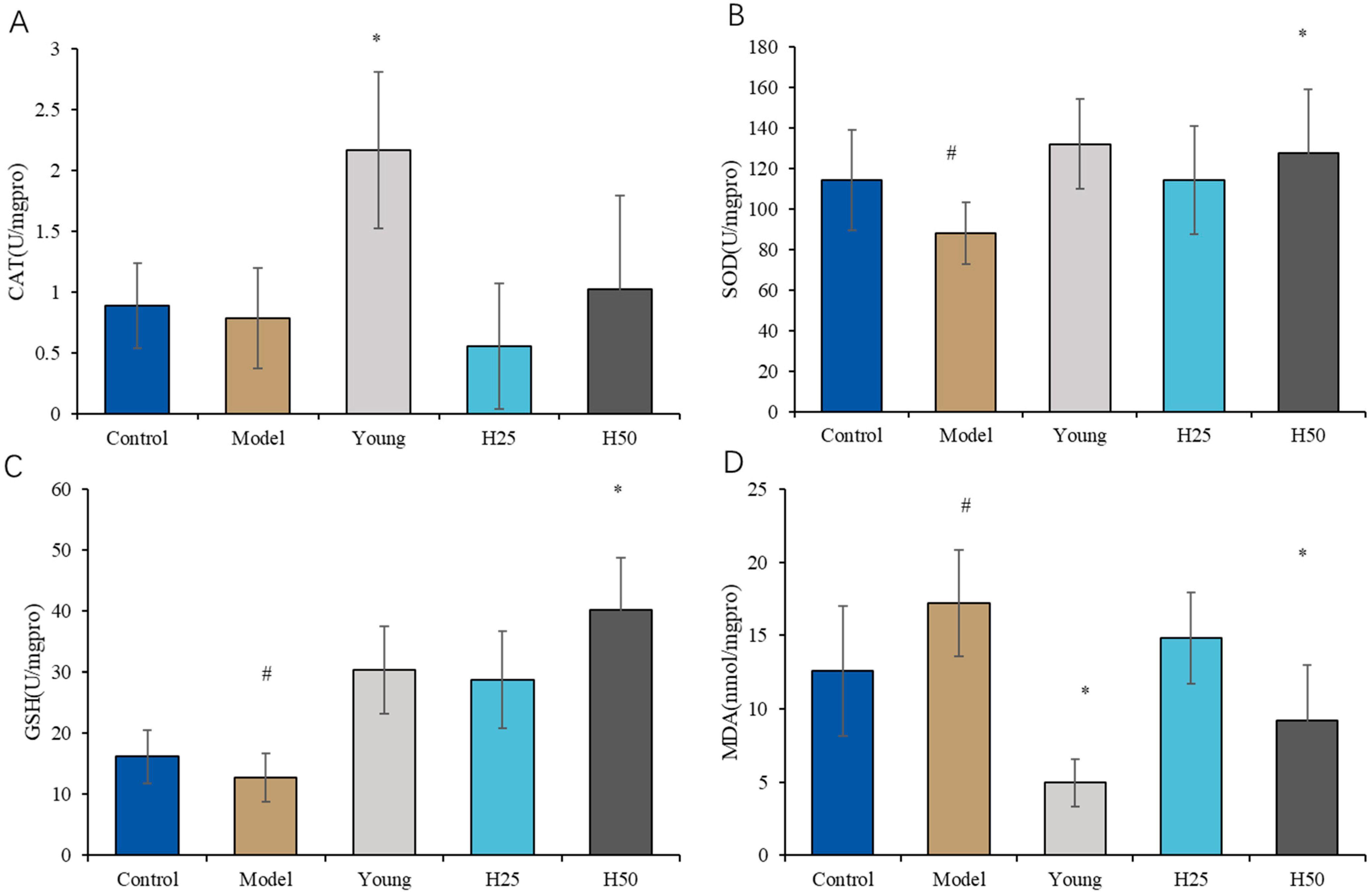
3.2.3. Evaluation of Oxidative Stress Markers
3.3. Measurement of Serum Inflammatory Cytokines
3.4. Cytokines Intestinal Morphology and Barrier Function Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGES | Advanced Glycation End Products |
| β-actin | Beta-action |
| CAT | Catalase |
| CML | Carboxy Methyl Lysine |
| ECM | Extracellular Matrix |
| GSH-Px | Glutathione Peroxidase |
| HYP | Hydroxyproline |
| HT | Hydroxytyrosol |
| IL-1α | Interleukin-1 Alpha |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin 6 |
| LDL | Low Density Lipoprotein |
| MMP | Matrix Metalloproteinase |
| PC | Positive Cell Ratio |
| RAGE | Receptor for Advanced Glycation End Products |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| TNF-α | Tumor Necrosis Factor-Alpha |
| ZO-1 | Zonula Occludens 1 |
References
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.; Hay, R.J.; Griffiths, C.E. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, 230–242. [Google Scholar] [CrossRef]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- Gunn, D.A.; de Craen, A.J.; Dick, J.L.; Tomlin, C.C.; van Heemst, D.; Catt, S.D.; Griffiths, T.; Ogden, S.; Maier, A.B.; Murray, P.G.; et al. Facial appearance reflects human familial longevity and cardiovascular disease risk in healthy individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.C.; Hossain, S.; Shobo, A.; Zhong, Y.; Jourdain, R.; Hancock, M.A.; George, K.; Breton, L.; Multhaup, G. Neurodegenerative Disease-Related Proteins within the Epidermal Layer of the Human Skin. J. Alzheimers Dis. 2019, 69, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Haiminen, N.; Carrieri, A.P.; Hu, R.; Jiang, L.; Parida, L.; Russell, B.; Allaband, C.; Zarrinpar, A.; Vázquez-Baeza, Y.; et al. Human Skin, Oral, and Gut Microbiomes Predict Chronological Age. mSystems 2020, 5, e00630-19. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.L.; Pitcher, L.E.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Development of New Approaches for Skin Care. Plast. Reconstr. Surg. 2022, 150, 12S–19S. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transpl. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Wautier, J.L.; Stern, D. Activation of receptor for advanced glycation end products: A mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ. Res. 1999, 84, 489–497. [Google Scholar] [CrossRef]
- Scheijen, J.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Bastos, D.H.M.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Katta, R. Sugar Sag: Glycation and the Role of Diet in Aging Skin. Ski. Ther. Lett. 2015, 20, 1–5. [Google Scholar]
- Chen, C.Y.; Zhang, J.Q.; Li, L.; Guo, M.-M.; He, Y.-F.; Dong, Y.-M.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, T.L. Dietary advanced glycation end-products elicit toxicological effects by disrupting gut microbiome and immune homeostasis. J. Immunotoxicol. 2021, 18, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mishra, M. AGE-RAGE Stress, Stressors, and Antistressors in Health and Disease. Int. J. Angiol. 2018, 27, 1–12. [Google Scholar]
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in Dermatology. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Rietjens, S.J.; Bast, A.; Haenen, G.R.M.M. New Insights into Controversies on the Antioxidant Potential of the Olive Oil Antioxidant Hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Maio, M.C.; Minniti, G.; de Góes Corrêa, N.; Barbalho, S.M.; Quesada, K.; Guiguer, E.L.; Sloan, K.P.; Detregiachi, C.R.P.; Araújo, A.C.; et al. Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review. Metabolites 2023, 13, 243. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Kihara, T.; Ibi, K.; Senshu, M.; Nejishima, H.; Takeda, Y.; Imai, K.; Ogawa, H. Olive-Derived Hydroxytyrosol Shows Anti-inflammatory Effect without Gastric Damage in Rats. Biol. Pharm. Bull. 2019, 42, 1120–1127. [Google Scholar] [CrossRef]
- Shan, C.; Xiong, Y.; Miao, F.; Liu, T.; Akhtar, R.W.; Shah, S.A.H.; Gao, H.; Zhu, E.; Cheng, Z. Hydroxytyrosol mitigates Mycoplasma gallisepticum-induced pulmonary injury through downregulation of the NF-κB/NLRP3/IL-1β signaling pathway in chicken. Poult. Sci. 2023, 102, 102582. [Google Scholar] [CrossRef]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef]
- Luo, L.; Li, R.; Wang, G.; Chen, J.; Chen, L.; Qin, L.Q.; Yu, Z.; Wan, Z. Age-dependent effects of a high-fat diet combined with dietary advanced glycation end products on cognitive function and protection with voluntary exercise. Food Funct. 2022, 13, 4445–4458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.X.; He, L.Z.; Li, Y.B.; Yu, Y.; Lu, Q.; Liu, R. Diet with high content of advanced glycation end products induces oxidative stress damage and systemic inflammation in experimental mice: Protective effect of peanut skin procyanidins. Food Sci. Hum. Wellness 2024, 13, 3570–3581. [Google Scholar] [CrossRef]
- Peng, H.; Gao, Y.Q.; Zeng, C.Y.; Hua, R.; Guo, Y.N.; Wang, Y.D.; Wang, Z. Effects of Maillard reaction and its product AGEs on aging and age-related diseases. Food Sci. Hum. Wellness 2024, 13, 1118–1134. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.M.; Park, J.; Cho, J.Y. Skin Protective Effect of Epigallocatechin Gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef]
- Quan, T. Molecular insights of human skin epidermal and dermal aging. J. Dermatol. Sci. 2023, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Mu, T.; Sun, H.; Blecker, C.; Richel, A. Photoprotective effects of sweet potato leaf polyphenols and caffeic acid against UV-induced skin-damage in BALB/C nude mice. Food Funct. 2022, 13, 7075–7087. [Google Scholar] [CrossRef]
- Lee, S.G.; Nguyen, N.H.; Lee, Y.I.; Jung, I.; Kim, I.A.; Jang, H.; Shin, H.; Lee, J.H. The Role of Cacao Powder in Enhancing Skin Moisture and Reducing Wrinkles: A 12-Week Clinical Trial and In Vitro Study. Curr. Issues Mol. Biol. 2024, 46, 12574–12587. [Google Scholar] [CrossRef] [PubMed]
- Kazanci, A.; Kurus, M.; Atasever, A. Analyses of changes on skin by aging. Skin Res. Technol. 2016, 23, 48–60. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Zhang, X.; Li, Z. Optical features for chronological aging and photoaging skin by optical coherence tomography. Lasers Med. Sci. 2013, 28, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Fisher, G.J.; Voorhees, J.J.; Quan, T.J.; Jo, C.; Medicine, M. Actin cytoskeleton assembly regulates collagen production via TGF-β type II receptor in human skin fibroblasts. J. Cell Mol. Med. 2018, 22, 4085–4096. [Google Scholar] [CrossRef]
- Yoo, J.H.; Lee, J.S.; Jang, J.H.; Jung, J.I.; Kim, E.J.; Choi, S.Y. AGEs Blocker™ (Goji Berry, Fig, and Korean Mint Mixed Extract) Inhibits Skin Aging Caused by Streptozotocin-Induced Glycation in Hairless Mice. Prev. Nutr. Food Sci. 2023, 28, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Qu, Y. A study on the delayed effect of tilapia skin collagen on skin aging for mice and its possible mechanism. J. Cosmet. Dermatol. 2023, 22, 3436–3444. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.; Cai, X.; Sun, Y.; Zhao, C.; Liu, F.; Liu, D. Effects of dimethylaminoethanol and compound amino acid on D-galactose induced skin aging model of rat. Sci. World J. 2014, 2014, 507351. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, G.Q.; Ma, Z.; Qin, L.Q.; Zhai, Y.J.; Yu, Z.L.; Xue, M.; Zhang, Y.H.; Wan, Z. Dietary AdvancedGlycationEnd Products–InducedCognitive Impairment in Aged ICR Mice: Protective Role of Quercetin. Mol. Nutr. Food Res. 2020, 64, 1901019. [Google Scholar] [CrossRef]
- Sloseris, D.; Forde, N.R. AGEing of collagen: The effects of glycation on collagen’s stability, mechanics and assembly. Matrix Biol. 2025, 35, 153–160. [Google Scholar] [CrossRef]
- Ali, S.M. In vivo confocal Raman spectroscopic imaging of the human skin extracellular matrix degradation due to accumulated intrinsic and extrinsic aging. Photodermatol. Photoimmunol. Photomed. 2021, 37, 140–152. [Google Scholar] [CrossRef]
- Ye, X.; Tong, Z.; Dang, Y.; Tu, Q.; Weng, Y.; Liu, J.; Zhang, Z. Effects of blood glucose fluctuation on skin biophysical properties, structure and antioxidant status in an animal model. Clin. Exp. Dermatol. 2010, 35, 78–82. [Google Scholar] [CrossRef]
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in D-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar] [CrossRef]
- Serin, Y.; Akbulut, G.; Uğur, H.; Yaman, M. Recent Developments in In-Vitro Assessment of Advanced Glycation End Products. Curr. Opin. Food Sci. 2021, 40, 136–143. [Google Scholar] [CrossRef]
- Iwamura, M.; Yamamoto, Y.; Kitayama, Y.; Higuchi, K.; Fujimura, T.; Hase, T.; Yamamoto, H. Epidermal expression of receptor for advanced glycation end products (RAGE) is related to inflammation and apoptosis in human skin. Exp. Dermatol. 2016, 25, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. [Google Scholar] [CrossRef] [PubMed]
- Batarfi, W.A.; Yunus, M.H.M.; Hamid, A.A.; Lee, Y.T.; Maarof, M. Hydroxytyrosol: A Promising Therapeutic Agent for Mitigating Inflammation and Apoptosis. Pharmaceutics 2024, 16, 1504. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Hernandez, M.M.A. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Shan, C.; Miao, F. Immunomodulatory and antioxidant effects of hydroxytyrosol in cyclophosphamide-induced immunosuppressed broilers. Poult. Sci. 2022, 101, 101516. [Google Scholar] [CrossRef]
- Terracina, S.; Petrella, C.; Francati, S.; Lucarelli, M.; Barbato, C.; Minni, A.; Ralli, M.; Greco, A.; Tarani, L.; Fiore, M.; et al. Antioxidant Intervention to Improve Cognition in the Aging Brain: The Example of Hydroxytyrosol and Resveratrol. Int. J. Mol. Sci. 2022, 23, 15674. [Google Scholar] [CrossRef]
- Nakamura, M.; Saito, H.; Kasanuki, J.; Tamura, Y.; Yoshida, S. Cytokine production in patients with inflammatory bowel disease. Gut 1992, 33, 933–937. [Google Scholar] [CrossRef]
- Vitale, S.; Strisciuglio, C.; Pisapia, L. Cytokine production profile in intestinal mucosa of paediatric inflammatory bowel disease. PLoS ONE 2017, 12, e0182313. [Google Scholar] [CrossRef]


| Ingredients | Before Baking | After Baking | F | p |
|---|---|---|---|---|
| Energy (kJ) | 1576 ± 145 | 1760 ± 189 | 1.790 | 0.252 |
| Crude protein (%) | 17.82 ± 2.23 | 18.68 ± 1.87 | 0.262 | 0.636 |
| Crude fat (%) | 7.00 ± 1.11 | 7.73 ± 1.23 | 0.582 | 0.488 |
| Carbohydrates (%) | 64.31 ± 5.98 | 68.12 ± 6.75 | 0.535 | 0.505 |
| CML (mg) | 0.02 ± 0.01 | 0.14 ± 0.05 * | 16.615 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Ma, Y.; Sun, M.; Zhang, H.; Han, Y.; Wang, J.; Zhu, W.; Zhang, Z. Improvement Effect and Mechanism of Hydroxytyrosol on Skin Aging Induced Advanced Glycation End Products. Nutrients 2025, 17, 2810. https://doi.org/10.3390/nu17172810
Fan R, Ma Y, Sun M, Zhang H, Han Y, Wang J, Zhu W, Zhang Z. Improvement Effect and Mechanism of Hydroxytyrosol on Skin Aging Induced Advanced Glycation End Products. Nutrients. 2025; 17(17):2810. https://doi.org/10.3390/nu17172810
Chicago/Turabian StyleFan, Rui, Yuxin Ma, Meng Sun, Haohao Zhang, Yaxin Han, Junbo Wang, Wenli Zhu, and Zhaofeng Zhang. 2025. "Improvement Effect and Mechanism of Hydroxytyrosol on Skin Aging Induced Advanced Glycation End Products" Nutrients 17, no. 17: 2810. https://doi.org/10.3390/nu17172810
APA StyleFan, R., Ma, Y., Sun, M., Zhang, H., Han, Y., Wang, J., Zhu, W., & Zhang, Z. (2025). Improvement Effect and Mechanism of Hydroxytyrosol on Skin Aging Induced Advanced Glycation End Products. Nutrients, 17(17), 2810. https://doi.org/10.3390/nu17172810







