Unlocking Polyphenol Efficacy: The Role of Gut Microbiota in Modulating Bioavailability and Health Effects
Abstract
1. Introduction
2. Methodology
3. Understanding the Efficacy of Dietary Polyphenols
4. Sources, Bioavailability, and Metabolism of Dietary Phenolic Classes
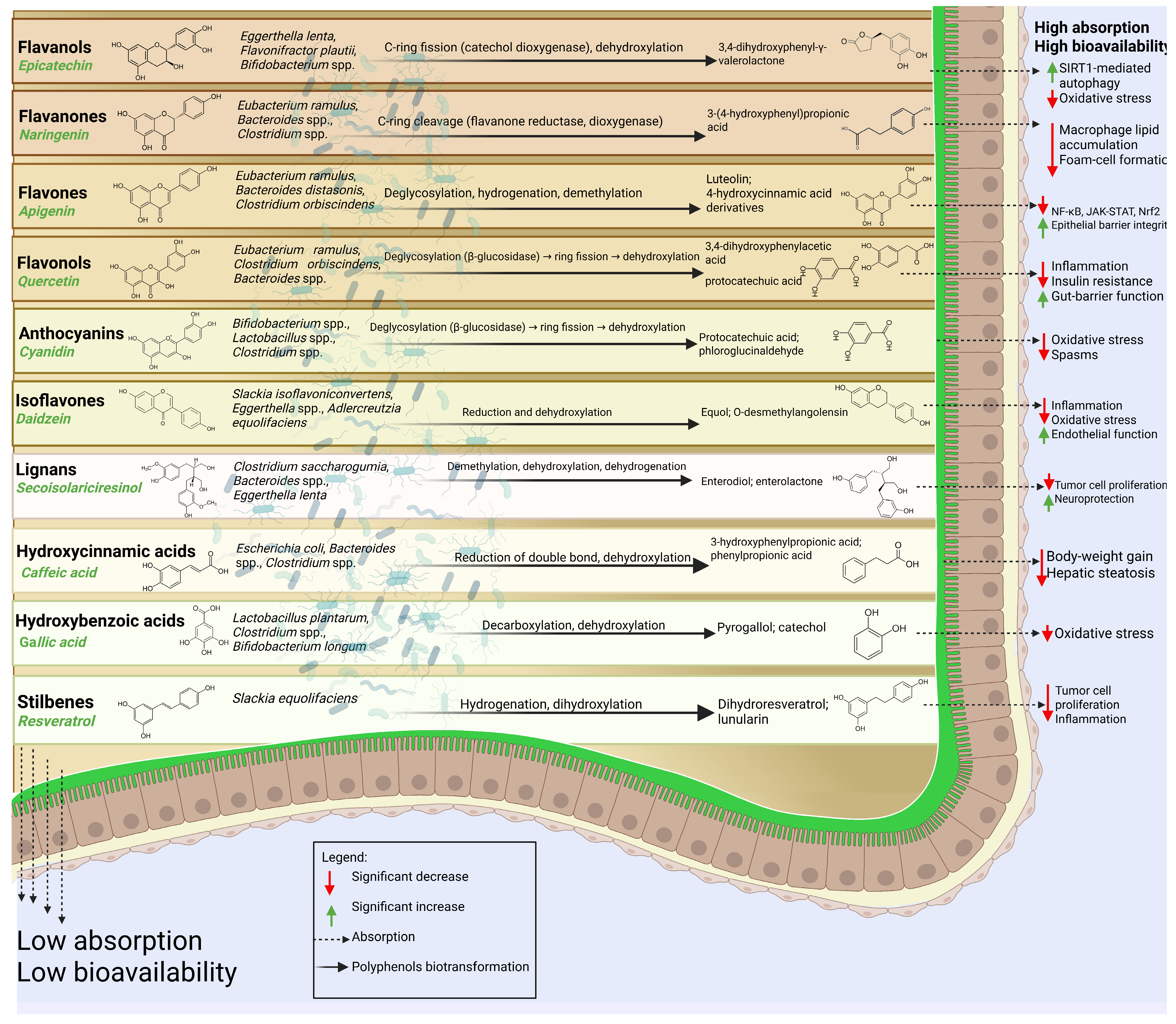
4.1. Flavonoids
4.1.1. Flavonols
4.1.2. Flavones
4.1.3. Flavanones
4.1.4. Flavanols
4.1.5. Anthocyanins
4.1.6. Isoflavones
4.2. Phenolic Acids
4.2.1. Hydroxybenzoic Acids
4.2.2. Hydroxycinnamic Acids
4.3. Stilbenes
Resveratrol
4.4. Lignans
4.4.1. Secoisolariciresinol
4.4.2. Matairesinol
4.5. Other Polyphenols
4.5.1. Curcuminoids
4.5.2. Tannins
4.5.3. Coumarins
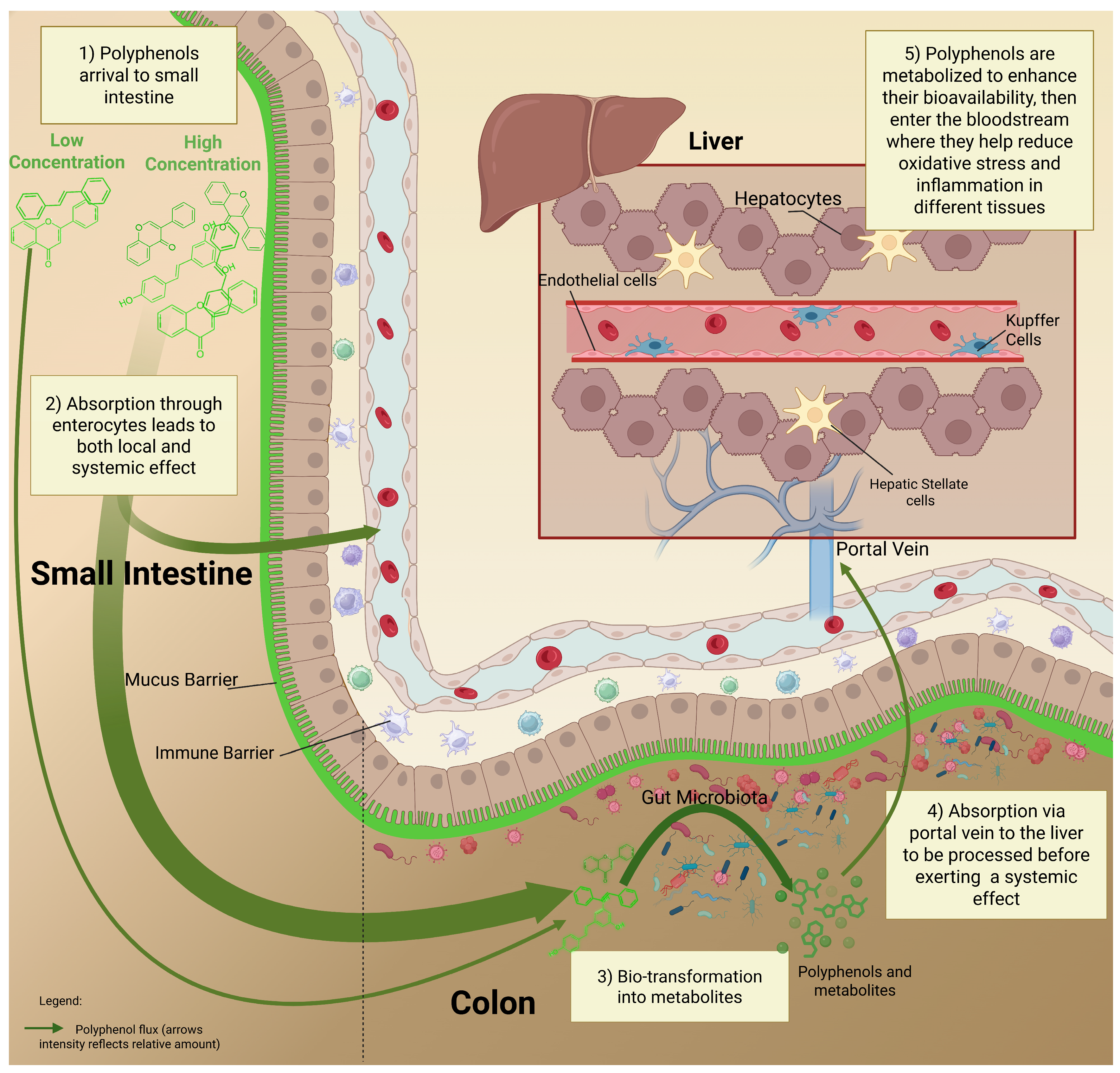
5. The Fate of Polyphenols in the GI Tract: From Dose to Metabolism
6. Possible Adverse Effects of High Amounts of Polyphenol Intake
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP Binding Cassette |
| AhR | Aryl Hydrocarbon Receptor |
| Akt-eNOS | Protein Kinase B-endothelial Nitric Oxide Synthase Pathway |
| BDMC | Bisdemethoxycurcumin |
| EFSA | European Food Safety Authority |
| EPIC | European Prospective Investigation into Cancer and Nutrition |
| GI | Gastrointestinal |
| HBA | Hydroxybenzoic acid |
| HCAs | Hydroxycinnamic Acid |
| HDL | High-Density Lipoprotein |
| HIV | Human Immunodeficiency Virus |
| IL-22 | Interleukin-22 |
| IL-6 | Interleukin-6 |
| LDL | Low-Density Lipoprotein |
| LPH | Lactase-Phlorizin Hydrolase |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MCT1 | Monocarboxylate Transporter 1 |
| MEAL | Mediterranean Healthy Eating |
| NF-kB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| P38/MAPK/ERK | p38 Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase |
| PI3k/Akt | Phosphoinositide 3-Kinase/Protein Kinase B Pathway |
| PREDIMED | Prevención con Dieta Mediterránea (Prevention with Mediterranean Diet Study) |
| PSA | Prostate-Specific Antigen |
| ROS | Reactive Oxygen Species |
| SCFAs | Short Chain Fatty Acids |
| SDG | Secoisolariciresinol Diglucoside |
| SECO | Secoisolariciresinol |
| SGLT1 | Sodium Glucose Cotransporter 1 |
| SUN | Seguimiento Universidad de Navarra (Spanish cohort study) |
| TDAC | Total Dietary Antioxidant Capacity |
| TDI | Tolerable Daily Intake |
| TLR4/MyD88 | Toll-like Receptor 4/Myeloid Differentiation Primary Response 88 |
| TNF-α | Tumor Necrosis Factor-alpha |
| UK | United Kingdom |
References
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the Role of the Human Gut Microbiome in Health and Diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Graziani, A.; Frühbeck, G.; Baffy, G.; Garruti, G.; Di Ciaula, A.; Bonfrate, L. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations? Eur. J. Intern. Med. 2024, 119, 13–30. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S4), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Durdevic, M.; Leber, B.; di Vora, K.; Rainer, F.; Krones, E.; Douschan, P.; Spindelboeck, W.; Durchschein, F.; Zollner, G.; et al. Changes in the Intestinal Microbiome during a Multispecies Probiotic Intervention in Compensated Cirrhosis. Nutrients 2020, 12, 1874. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression—A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Moser, A.M.; Spindelboeck, W.; Halwachs, B.; Strohmaier, H.; Kump, P.; Gorkiewicz, G.; Högenauer, C. Effects of an oral synbiotic on the gastrointestinal immune system and microbiota in patients with diarrhea-predominant irritable bowel syndrome. Eur. J. Nutr. 2019, 58, 2767–2778. [Google Scholar] [CrossRef]
- Kim, H.K.; Rutten, N.B.M.M.; Besseling-van der Vaart, I.; Niers, L.E.M.; Choi, Y.H.; Rijkers, G.T.; van Hemert, S. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef. Microbes 2015, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Vivarelli, S.; Costa, C.; Teodoro, M.; Giambò, F.; Tsatsakis, A.M.; Fenga, C. Polyphenols: A route from bioavailability to bioactivity addressing potential health benefits to tackle human chronic diseases. Arch. Toxicol. 2023, 97, 3–38. [Google Scholar] [CrossRef]
- Yang, M.; Abdullah; Ahmad, N.; Hussain, M.; Lu, X.; Xu, J.; Zhong, H.; Guan, R. A review of recent advances on cyanidin-3-glucoside: The biotransformation, absorption, bioactivity and applications of nano-encapsulation. Food Funct. 2023, 14, 6320–6345. [Google Scholar] [CrossRef]
- Wang, M.; Lu, Y.; Wu, Q.; Chen, G.; Zhao, H.; Ho, C.T.; Li, S. Biotransformation and Gut Microbiota-Mediated Bioactivity of Flavonols. J. Agric. Food Chem. 2023, 71, 8317–8331. [Google Scholar] [CrossRef]
- Cuciniello, R.; Di Meo, F.; Filosa, S.; Crispi, S.; Bergamo, P. The Antioxidant Effect of Dietary Bioactives Arises from the Interplay between the Physiology of the Host and the Gut Microbiota: Involvement of Short-Chain Fatty Acids. Antioxidants 2023, 12, 1073. [Google Scholar] [CrossRef]
- Gade, A.; Kumar, M.S. Gut microbial metabolites of dietary polyphenols and their potential role in human health and diseases. J. Physiol. Biochem. 2023, 79, 695–718. [Google Scholar] [CrossRef]
- Oumeddour, D.Z.; Al-Dalali, S.; Zhao, L.; Zhao, L.; Wang, C. Recent advances on cyanidin-3-O-glucoside in preventing obesity-related metabolic disorders: A comprehensive review. Biochem. Biophys. Res. Commun. 2024, 729, 150344. [Google Scholar] [CrossRef]
- Láng, L.; McArthur, S.; Lazar, A.S.; Pourtau, L.; Gaudout, D.; Pontifex, M.G.; Müller, M.; Vauzour, D. Dietary (Poly)phenols and the Gut-Brain Axis in Ageing. Nutrients 2024, 16, 1500. [Google Scholar] [CrossRef]
- Gao, F.; Yang, P.; Wang, W.; Wang, K.; Zhao, L.; Wang, Y.; Liao, X. Unveiling the multifaceted roles of anthocyanins: A review of their bioavailability, impacts on gut and system health, and industrial implications. Curr. Res. Food Sci. 2025, 11, 101137. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z. Cyanidin-3-glucoside: Targeting atherosclerosis through gut microbiota and anti-inflammation. Front. Nutr. 2025, 12, 1627868. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Xiao, J. 2-Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 45–67. [Google Scholar]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Alqudah, S.; Claesen, J. Mechanisms of gut bacterial metabolism of dietary polyphenols into bioactive compounds. Gut Microbes 2024, 16, 2426614. [Google Scholar] [CrossRef]
- Quesada-Vázquez, S.; Eseberri, I.; Les, F.; Pérez-Matute, P.; Herranz-López, M.; Atgié, C.; Lopez-Yus, M.; Aranaz, P.; Oteo, J.A.; Escoté, X.; et al. Polyphenols and metabolism: From present knowledge to future challenges. J. Physiol. Biochem. 2024, 80, 603–625. [Google Scholar] [CrossRef]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols Rich Diets and Risk of Type 2 Diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Zhang, H. Dietary polyphenols for tumor therapy: Bioactivities, nano-therapeutic systems and delivery strategies. Food Funct. 2025, 16, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Serale, N.; Parodi, A.; Voci, A.; Vergani, L.; Daher, A. Antitumor Activity of Ethanolic Extract from Thymbra spicata L. aerial Parts: Effects on Cell Viability and Proliferation, Apoptosis Induction, STAT3, and NF-kB Signaling. Nutr. Cancer 2021, 73, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; John Britto, J.S.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Thompson, A.S.; Bondonno, N.P.; Jennings, A.; Murray, K.; Perez-Cornago, A.; Hodgson, J.M.; Tresserra-Rimbau, A.; Kühn, T.; Cassidy, A. High diversity of dietary flavonoid intake is associated with a lower risk of all-cause mortality and major chronic diseases. Nat. Food 2025, 6, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Diab, F.; Zbeeb, H.; Baldini, F.; Portincasa, P.; Khalil, M.; Vergani, L. The Potential of Lamiaceae Herbs for Mitigation of Overweight, Obesity, and Fatty Liver: Studies and Perspectives. Molecules 2022, 27, 5043. [Google Scholar] [CrossRef]
- Hedayati, N.; Yaghoobi, A.; Salami, M.; Gholinezhad, Y.; Aghadavood, F.; Eshraghi, R.; Aarabi, M.-H.; Homayoonfal, M.; Asemi, Z.; Mirzaei, H.; et al. Impact of polyphenols on heart failure and cardiac hypertrophy: Clinical effects and molecular mechanisms. Front. Cardiovasc. Med. 2023, 10, 1174816. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; D’Angelo, S. Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview. Antioxidants 2021, 10, 507. [Google Scholar] [CrossRef]
- Khalil, M.; Rita Caponio, G.; Diab, F.; Shanmugam, H.; Di Ciaula, A.; Khalifeh, H.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Unraveling the beneficial effects of herbal Lebanese mixture “Za’atar”. History, studies, and properties of a potential healthy food ingredient. J. Funct. Foods 2022, 90, 104993. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Annunziata, G.; Verde, L.; Fascì-Spurio, F.; Reytor-González, C.; Muscogiuri, G.; Frias-Toral, E.; Barrea, L. Nutritional Strategies for Battling Obesity-Linked Liver Disease: The Role of Medical Nutritional Therapy in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Management. Curr. Obes. Rep. 2025, 14, 7. [Google Scholar] [CrossRef]
- Williamson, G. Bioavailability of Food Polyphenols: Current State of Knowledge. Annu. Rev. Food Sci. Technol. 2025, 16, 315–332. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Morzel, M.; Canon, F.; Guyot, S. Interactions between Salivary Proteins and Dietary Polyphenols: Potential Consequences on Gastrointestinal Digestive Events. J. Agric. Food Chem. 2022, 70, 6317–6327. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2016, 52, 291–305. [Google Scholar] [CrossRef]
- Khalil, M.; Piccapane, F.; Vacca, M.; Celano, G.; Mahdi, L.; Perniola, V.; Apa, C.A.; Annunziato, A.; Iacobellis, I.; Procino, G.; et al. Nutritional and Physiological Properties of Thymbra spicata: In Vitro Study Using Fecal Fermentation and Intestinal Integrity Models. Nutrients 2024, 16, 588. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Khalil, M.; Abdallah, H.; Razuka-Ebela, D.; Calasso, M.; De Angelis, M.; Portincasa, P. The Impact of Za’atar Antioxidant Compounds on the Gut Microbiota and Gastrointestinal Disorders: Insights for Future Clinical Applications. Antioxidants 2023, 12, 426. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Zhao, T.; Yang, X.; Zhang, J.; Yang, H. Bioavailability and mechanisms of dietary polyphenols affected by non-thermal processing technology in fruits and vegetables. Curr. Res. Food Sci. 2024, 8, 100715. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef]
- Jia, H.; Ren, F.; Liu, H. Evaluation of bioaccessibility and bioavailability of dietary bioactives and their application in food systems. Food Biosci. 2024, 62, 105428. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, R.; Rawat, H.; Kumar, V.; Jagtap, C.; Jain, A. The influence of food matrix on the stability and bioavailability of phytochemicals: A comprehensive review. Food Humanit. 2024, 2, 100202. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Sebastian, R.S.; Goldman, J.D.; Enns, C.W.; Moshfegh, A.J. Usual Intakes of Flavonoids: Estimates from What We Eat in America, NHANES 2007–2010. FASEB J. 2017, 31, 647.643. [Google Scholar] [CrossRef]
- Qayum, J.; Bibi, A.; Preet, G.; Farid, A. Flavonoid Associated Preclinical and Clinical Trials Involved in Insulin Resistance/Hyperglycemia, Obesity, Liver Intoxication, Aging, and Cardiovascular Diseases. In Role of Flavonoids in Chronic Metabolic Diseases: From Bench to Clinic; Scrivener Publishing LLC: Beverly, MA, USA, 2024; pp. 571–589. [Google Scholar]
- Akhlaghi, M.; Ghobadi, S.; Mohammad Hosseini, M.; Gholami, Z.; Mohammadian, F. Flavanols are potential anti-obesity agents, a systematic review and meta-analysis of controlled clinical trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 675–690. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids–food sources, health benefits, and mechanisms involved. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2018; pp. 1–27. [Google Scholar]
- Zhang, Y.; Ying, L.; Can, C.; Jie, C.; Wei, C.; Yu, Z.; Cheng, W.; Jia, W.; Xin, Z.; Zhao, X. Dietary Flavonol and Flavone Intakes and Their Major Food Sources in Chinese Adults. Nutr. Cancer 2010, 62, 1120–1127. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated Dietary Flavonoid Intake and Major Food Sources of U.S. Adults1,2. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.N.; Spencer, J.P.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G.G. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef]
- Demirel, S.; Yilmaz, D.A. Effects of flavonoids on vascular activity. Glob. Transl. Med. 2024, 3, 2458. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Fedirko, V.; De Magistris, M.S.; Ericson, U.; Amiano, P.; Trichopoulou, A. Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24 h dietary recall cohort. Br. J. Nutr. 2011, 106, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, S.; Harnly, J.; Bhagwat, S.; Beecher, G.; Doherty, R.; Holden, J.; Haytowitz, D.; Eldridge, A.; Peterson, J.; Dwyer, J. USDA’s flavonoid database: Flavonoids in fruit. Agric. Res. Serv. 2002, 103, 54. [Google Scholar] [CrossRef]
- Lan, H.; Wang, H.; Chen, C.; Hu, W.; Ai, C.; Chen, L.; Teng, H. Flavonoids and gastrointestinal health: Single molecule for multiple roles. Crit. Rev. Food Sci. Nutr. 2024, 64, 10987–11005. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Mulligan, A.A.; Luben, R.N.; Lentjes, M.A.; Heiss, C.; Kelm, M.; Merx, M.W.; Spencer, J.P.; Schroeter, H.; Kuhnle, G.G. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European Union. Br. J. Nutr. 2014, 111, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Knaze, V.; Zamora-Ros, R.; Lujan-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Riboli, E.; Van Rossum, C.T.; Bueno-de-Mesquita, H.B.; Trichopoulou, A. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2012, 108, 1095–1108. [Google Scholar] [CrossRef]
- Raman, G.; Shams-White, M.; Avendano, E.E.; Chen, F.; Novotny, J.A.; Cassidy, A. Dietary intakes of flavan-3-ols and cardiovascular health: A field synopsis using evidence mapping of randomized trials and prospective cohort studies. Syst. Rev. 2018, 7, 100. [Google Scholar] [CrossRef]
- Spencer, J.P. Metabolism of tea flavonoids in the gastrointestinal tract. J. Nutr. 2003, 133, 3255S–3261S. [Google Scholar] [CrossRef]
- Saini, R.K.; Khan, M.I.; Shang, X.; Kumar, V.; Kumari, V.; Kesarwani, A.; Ko, E.-Y. Dietary Sources, Stabilization, Health Benefits, and Industrial Application of Anthocyanins—A Review. Foods 2024, 13, 1227. [Google Scholar] [CrossRef]
- Khalil, M.; Hayek, S.; Khalil, N.; Serale, N.; Vergani, L.; Calasso, M.; De Angelis, M.; Portincasa, P. Role of Sumac (Rhus coriaria L.) in the management of metabolic syndrome and related disorders: Focus on NAFLD-atherosclerosis interplay. J. Funct. Foods 2021, 87, 104811. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S.; et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2011, 106, 1090–1099. [Google Scholar] [CrossRef]
- Festa, J.; Hussain, A.; Al-Hareth, Z.; Singh, H.; Da Boit, M. Anthocyanins and Vascular Health: A Matter of Metabolites. Foods 2023, 12, 1796. [Google Scholar] [CrossRef]
- Tiwari, V.; Saloni, S.; Apoorv, T.; Bhawna, S.; Satveer, K.; Anjali, S.; Mona, Y.; Archana, B.; Garg, M. Effect of dietary anthocyanins on biomarkers of type 2 diabetes and related obesity: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2024, 64, 7517–7534. [Google Scholar] [CrossRef]
- Popa, D.-S.; Rusu, M.E. Isoflavones: Vegetable Sources, Biological Activity, and Analytical Methods for Their Assessment. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; Shiomi, N., Waisundara, V.Y., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Chun, O.K.; Chung, S.J.; Song, W.O. Urinary Isoflavones and Their Metabolites Validate the Dietary Isoflavone Intakes in US Adults. J. Am. Diet. Assoc. 2009, 109, 245–254. [Google Scholar] [CrossRef]
- Ivashkevich, A. The role of isoflavones in augmenting the effects of radiotherapy. Front. Oncol. 2022, 12, 800562. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Rothwell, J.A.; Scalbert, A.; Knaze, V.; Romieu, I.; Slimani, N.; Fagherazzi, G.; Perquier, F.; Touillaud, M.; Molina-Montes, E.; et al. Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2013, 110, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, B.H.J.; Azouz, H.J.; Aldalati, A.M.Z.; AlTinawi, B.M.H.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-Derived and Dietary Hydroxybenzoic Acids—A Comprehensive Study of Structural, Anti-/Pro-Oxidant, Lipophilic, Antimicrobial, and Cytotoxic Activity in MDA-MB-231 and MCF-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Nao, J.; Dong, X. The Therapeutic Potential of Hydroxycinnamic Acid Derivatives in Parkinson’s Disease: Focus on In Vivo Research Advancements. J. Agric. Food Chem. 2023, 71, 10932–10951. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, M.; Li, X.; Wang, D.; Yu, L.; Ma, F.; Jiang, J.; Zhang, L.; Li, P. Contributions of Common Foods to Resveratrol Intake in the Chinese Diet. Foods 2024, 13, 1267. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Chowdhury, R.; Akter, M.A.; Ali, M.A.; Afroz, M.; Akbor, M.S.; Sonia, F.A.; Mubarak, M.S.; Islam, M.T. A mechanistic insight into the anticancer potentials of resveratrol: Current perspectives. Phytother. Res. 2024, 38, 3877–3898. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Deng, X.; Xiao, X.; Zeng, J. Integrative evidence construction for resveratrol treatment of nonalcoholic fatty liver disease: Preclinical and clinical meta-analyses. Front. Pharmacol. 2023, 14, 1230783. [Google Scholar] [CrossRef] [PubMed]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol Diglucoside of Flaxseed and Its Metabolites: Biosynthesis and Potential for Nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef]
- Plaha, N.S.; Awasthi, S.; Sharma, A.; Kaushik, N. Distribution, biosynthesis and therapeutic potential of lignans. 3 Biotech 2022, 12, 255. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Song, G.; Bae, H. Matairesinol Induces Mitochondrial Dysfunction and Exerts Synergistic Anticancer Effects with 5-Fluorouracil in Pancreatic Cancer Cells. Mar. Drugs 2022, 20, 473. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Augustin, M.A.; Sanguansri, L.; Shen, Z.; Ng, K.; Ajlouni, S. Enhanced Bioaccessibility of Curcuminoids in Buttermilk Yogurt in Comparison to Curcuminoids in Aqueous Dispersions. J. Food Sci. 2016, 81, H769–H776. [Google Scholar] [CrossRef]
- Joshi, P.; Bisht, A.; Paliwal, A.; Dwivedi, J.; Sharma, S. Recent updates on clinical developments of curcumin and its derivatives. Phytother. Res. 2023, 37, 5109–5158. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Kleszcz, R.; Majchrzak-Celińska, A.; Baer-Dubowska, W. Tannins in cancer prevention and therapy. Br. J. Pharmacol. 2025, 182, 2075–2093. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Rajasekar, N.; Sivanantham, A. Therapeutic potential of plant-derived tannins in non-malignant respiratory diseases. J. Nutr. Biochem. 2021, 94, 108632. [Google Scholar] [CrossRef]
- Fotland, T.; Paulsen, J.E.; Sanner, T.; Alexander, J.; Husøy, T. Risk assessment of coumarin using the bench mark dose (BMD) approach: Children in Norway which regularly eat oatmeal porridge with cinnamon may exceed the TDI for coumarin with several folds. Food Chem. Toxicol. 2012, 50, 903–912. [Google Scholar] [CrossRef]
- Pal, D.; Bareth, K.; Rani, P.; Kandar, C.C.; Mishra, A. Coumarins as Emerging Anti-Viral Compounds from Natural Origins: Ethnopharmacology, Chemistry, Mechanism of Action, Clinical and Preclinical Studies, and Future Perspectives. In Anti-Viral Metabolites from Medicinal Plants; Pal, D., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1039–1065. [Google Scholar]
- Cao, H.; Högger, P.; Arroo, R.; Xiao, J. Flavonols with a catechol or pyrogallol substitution pattern on ring B readily form stable dimers in phosphate buffered saline at four degrees celsius. Food Chem. 2020, 311, 125902. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. The first tract of alimentary canal as an extractor. Release of phytochemicals from solid food matrices during simulated digestion. J. Food Biochem. 2012, 36, 555–568. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, C.G.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Wang, M.D.; Fang, J.G. Gastrointestinal stability of dihydromyricetin, myricetin, and myricitrin: An in vitro investigation. Int. J. Food Sci. Nutr. 2017, 68, 704–711. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Tutel’ian, V.A.; Lashneva, N.V. Biologically active substances of plant origin. Flavonols and flavones: Prevalence, dietary sourses and consumption. Vopr. Pitan. 2013, 82, 4–22. [Google Scholar] [PubMed]
- Cattivelli, A.; Zannini, M.; De Angeli, M.; D’Arca, D.; Minischetti, V.; Conte, A.; Tagliazucchi, D. Bioaccessibility of Flavones, Flavanones, and Flavonols from Vegetable Foods and Beverages. Biology 2024, 13, 1081. [Google Scholar] [CrossRef]
- Montaña, M.P.; Massad, W.A.; Criado, S.; Biasutti, A.; García, N.A. Stability of flavonoids in the presence of riboflavin-photogenerated reactive oxygen species: A kinetic and mechanistic study on quercetin, morin and rutin. Photochem. Photobiol. 2010, 86, 827–834. [Google Scholar] [CrossRef]
- Bradwell, J.; Hurd, M.; Pangloli, P.; McClure, A.; Dia, V.P. Storage stability of sorghum phenolic extracts’ flavones luteolin and apigenin. LWT 2018, 97, 787–793. [Google Scholar] [CrossRef]
- Laib, I.; Kehal, F.; Arris, M.; Maameri, M.I.; Lachlah, H.; Bensouici, C.; Mosbah, R.; Houasnia, M.; Barkat, M. Effect of in vitro gastrointestinal digestion on phenolic compounds and the antioxidant activity of Camellia sinensis L. green tea from organic farming. Nutr. Clin. Métabolisme 2021, 35, 212–221. [Google Scholar] [CrossRef]
- Murota, K. Absorption pathway of dietary flavonoids: The potential roles of the lymphatic transport in the intestine. Funct. Foods Health Dis. 2020, 10, 274–289. [Google Scholar] [CrossRef]
- Han, J. Chemical Aspects of Gut Metabolism of Flavonoids. Metabolites 2019, 9, 136. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Sun, D.-L.; Chen, Z.-J.; Chen, T.; Li, L.-P.; Xu, Z.-H.; Jiang, H.-D.; Zeng, S. Relative contribution of small and large intestine to deglycosylation and absorption of flavonoids from Chrysanthemun morifolium extract. J. Agric. Food Chem. 2010, 58, 10661–10667. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Calani, L.; Cordero, C.; Salvatore, S.; Pellegrini, N.; Brighenti, F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition 2010, 26, 1110–1116. [Google Scholar] [CrossRef]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef]
- Mikell, J.R.; Herath, W.; Khan, I.A. Eleven microbial metabolites of 6-hydroxyflavanone. Chem. Pharm. Bull. 2015, 63, 579–583. [Google Scholar] [CrossRef][Green Version]
- Brett, G.M.; Hollands, W.; Needs, P.W.; Teucher, B.; Dainty, J.R.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br. J. Nutr. 2008, 101, 664–675. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, Y.J.; Yang, Y.K.; Kim, J.Y.; Kwon, O. Dietary flavan-3-ols intake and metabolic syndrome risk in Korean adults. Nutr. Res. Pract. 2012, 6, 68–77. [Google Scholar] [CrossRef]
- Elegbede, J.L.; Li, M.; Jones, O.G.; Campanella, O.H.; Ferruzzi, M.G. Interactions Between Flavonoid-Rich Extracts and Sodium Caseinate Modulate Protein Functionality and Flavonoid Bioaccessibility in Model Food Systems. J. Food Sci. 2018, 83, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Freidl, R.; Thumann, T.; Sadjak, S.; Kunert, O.; Bauer, R.; Pferschy-Wenzig, E. Ring B substitution pattern and glycosylation strongly affect the stability of flavonols towards in vitro digestion. Planta Medica 2022, 88, 1450–1451. [Google Scholar] [CrossRef]
- Xiao, J. Recent advances on the stability of dietary polyphenols. Efood 2022, 3, e21. [Google Scholar] [CrossRef]
- Hackman, R.M.; Polagruto, J.A.; Zhu, Q.Y.; Sun, B.; Fujii, H.; Keen, C.L. Flavanols: Digestion, absorption and bioactivity. Phytochem. Rev. 2008, 7, 195–208. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Stalmach, A. Chapter 42-Bioavailability of Dietary Anthocyanins and Hydroxycinnamic Acids. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 561–576. [Google Scholar]
- Teixeira, M.; De Luca, L.; Faria, A.; Bordiga, M.; de Freitas, V.; Mateus, N.; Oliveira, H. First Insights on the Bioaccessibility and Absorption of Anthocyanins from Edible Flowers: Wild Pansy, Cosmos, and Cornflower. Pharmaceuticals 2024, 17, 191. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Giusti, M.M. The Stability and Absorption of Anthocyanins in the Mouth. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health; Brooks, M.S.-L., Celli, G.B., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 186–215. [Google Scholar]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Ursa, H.; Natasa Poklar, U. The Metabolism of Anthocyanins. Curr. Drug Metab. 2014, 15, 3–13. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Thomson, B.M.; Shaw, I.C. Bioactive Isoflavones in Functional Foods: The Importance of Gut Microflora on Bioavailability. Nutr. Rev. 2003, 61, 204–213. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Chen, J.; Lin, H.; Hu, M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother. Pharmacol. 2005, 55, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.B.; Pow-Sang, J.; Spiess, P.; Dickinson, S.; Schell, M.J. A phase II randomized clinical trial using aglycone isoflavones to treat patients with localized prostate cancer in the pre-surgical period prior to radical prostatectomy. Oncotarget 2020, 11, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiao, W.; Wenqi, C.; Qin, L.; Wang, L. Encapsulation of phenolic acids within food-grade carriers systems: A systematic review. Crit. Rev. Food Sci. Nutr. 2025, 65, 2765–2784. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A review. Rocz. Panstw. Zakl. Hig. 2013, 64, 79–84. [Google Scholar] [PubMed]
- Cong, D.; Fong, A.K.; Lee, R.; Pang, K.S. Absorption of benzoic acid in segmental regions of the vascularly perfused rat small intestine preparation. Drug Metab. Dispos. 2001, 29, 1539–1547. [Google Scholar]
- Yadav, M.; Lomash, A.; Kapoor, S.; Pandey, R.; Chauhan, N.S. Mapping of the benzoate metabolism by human gut microbiome indicates food-derived metagenome evolution. Sci. Rep. 2021, 11, 5561. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hydroxycinnamic acids on gut microbiota and health. Compr. Rev. Food Sci. Food Saf. 2021, 20, 710–737. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cha, K.H.; Kim, C.Y.; Nho, C.W.; Pan, C.-H. Bioavailability of Hydroxycinnamic Acids from Crepidiastrum denticulatum Using Simulated Digestion and Caco-2 Intestinal Cells. J. Agric. Food Chem. 2014, 62, 5290–5295. [Google Scholar] [CrossRef]
- Kern, S.M.; Bennett, R.N.; Needs, P.W.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.-T. Characterization of Metabolites of Hydroxycinnamates in the in Vitro Model of Human Small Intestinal Epithelium Caco-2 Cells. J. Agric. Food Chem. 2003, 51, 7884–7891. [Google Scholar] [CrossRef]
- Di Pede, G.; Mena, P.; Bresciani, L.; Achour, M.; Lamuela-Raventós, R.M.; Estruch, R.; Landberg, R.; Kulling, S.E.; Wishart, D.; Rodriguez-Mateos, A.; et al. A Systematic Review and Comprehensive Evaluation of Human Intervention Studies to Unravel the Bioavailability of Hydroxycinnamic Acids. Antioxid. Redox Signal. 2023, 40, 510–541. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Gu, T.; Yan, Y.; Guo, J.; Zhang, X.; Pang, H.; Chen, J. The Metabolic Characteristics and Bioavailability of Resveratrol Based on Metabolic Enzymes. Nutr. Rev. 2025, 83, 749–770. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Wolfe, B.; Jha, P.; Heubi, J.E. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014, 5, 491–501. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef] [PubMed]
- Lineberger, C.G.; Bowers, L.W.; Ford, N.A.; Rossi, E.L.; Kimler, B.K.; Fabian, C.J.; Hursting, S.D. Abstract 231: The polyphenolic plant lignan secoisolariciresinol diglycoside reduces mammary tumor growth, possibly via inhibition of local inflammatory signaling. Cancer Res. 2017, 77, 231. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br. J. Nutr. 2008, 99, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Milder, I.E.J.; Feskens, E.J.M.; Arts, I.C.W.; de Mesquita, H.B.B.; Hollman, P.C.H.; Kromhout, D. Intake of the Plant Lignans Secoisolariciresinol, Matairesinol, Lariciresinol, and Pinoresinol in Dutch Men and Women1. J. Nutr. 2005, 135, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Yoder, S.C.; Lancaster, S.M.; Hullar, M.A.J.; Lampe, J.W. Chapter 7-Gut Microbial Metabolism of Plant Lignans: Influence on Human Health. In Diet-Microbe Interactions in the Gut; Tuohy, K., Del Rio, D., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 103–117. [Google Scholar]
- Kattah, F.M.; Figueiredo, N.; Bezerra, K.K.; Oliveira, E.S.; Melo, C.C.; Lima, G.B.; Cavalcante, J.P.R.; Benetti, B.; Lima, G.C.; Mota, J.F.; et al. Curcumin Supplementation Improves Gastrointestinal Symptoms in Women with Severe Obesity: A Double-Blind, Randomized, Placebo-Controlled Trial-A Pilot Study. Nutrients 2025, 17, 2064. [Google Scholar] [CrossRef]
- Sudeep, V.H.; Gouthamchandra, K.; Chandrappa, S.; Naveen, P.; Reethi, B.; Venkatakrishna, K.; Shyamprasad, K. In vitro gastrointestinal digestion of a bisdemethoxycurcumin-rich Curcuma longa extract and its oral bioavailability in rats. Bull. Natl. Res. Cent. 2021, 45, 84. [Google Scholar] [CrossRef]
- Hashemi, N.; Krøyer Rasmussen, M.; Tsochatzis, E.D.; Corredig, M. In vitro digestion and intestinal absorption of curcumin-loaded zein nanoparticles. J. Funct. Foods 2023, 110, 105818. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, F. Curcuminoid metabolism and its contribution to the pharmacological effects. Curr. Drug Metab. 2013, 14, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.; Malhotra Mukhtyar, R.; Theofanous, D.; Pepper, C.; Thomas, A.; Brown, K.; Khan, S. A Systematic Review Assessing Clinical Utility of Curcumin with a Focus on Cancer Prevention. Mol. Nutr. Food Res. 2021, 65, 2000977. [Google Scholar] [CrossRef]
- De Jesus, N.Z.T.; Falcão, H.d.S.; Gomes, I.F.; Leite, T.J.d.A.; Lima, G.R.d.M.; Barbosa-Filho, J.M.; Tavares, J.F.; Silva, M.S.d.; Athayde-Filho, P.F.d.; Batista, L.M. Tannins, Peptic Ulcers and Related Mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. [Google Scholar] [CrossRef]
- Abraham, K.; Pfister, M.; Wöhrlin, F.; Lampen, A. Relative bioavailability of coumarin from cinnamon and cinnamon-containing foods compared to isolated coumarin: A four-way crossover study in human volunteers. Mol. Nutr. Food Res. 2011, 55, 644–653. [Google Scholar] [CrossRef]
- Ritschel, W.A.; Brady, M.E.; Tan, H.S. First-pass effect of coumarin in man. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 99–103. [Google Scholar]
- Lake, B.G.; Gray, T.J.B.; Evans, J.G.; Lewis, D.F.V.; Beamand, J.A.; Hue, K.L. Studies on the mechanism of coumarin-induced toxicity in rat hepatocytes: Comparison with dihydrocoumarin and other coumarin metabolites. Toxicol. Appl. Pharmacol. 1989, 97, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; López-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Goñi, I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006, 94, 442–447. [Google Scholar] [CrossRef]
- Godos, J.; Marventano, S.; Mistretta, A.; Galvano, F.; Grosso, G. Dietary sources of polyphenols in the Mediterranean healthy Eating, Aging and Lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017, 68, 750–756. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Silberberg, M.; Morand, C.; Thierry, M.; Besson, C.; Manach, C.; Scalbert, A.; Rémésy, C. The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur. J. Nutr. 2006, 45, 88–96. [Google Scholar] [CrossRef]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, L.; Huang, B.; Lu, Q.; Liu, R. 3,4-Dihydroxyphenylacetic acid ameliorates gut barrier dysfunction via regulation of MAPK-MLCK pathway in type 2 diabetes mice. Life Sci. 2022, 305, 120742. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid. Based Complement. Alternat Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Braune, A.; Gütschow, M.; Engst, W.; Blaut, M. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef] [PubMed]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic Degradation of Flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, X.L.; Li, T.Y.; Li, M.Y.; Huang, R.M.; Li, W.; Yang, R.L. 3-(4-Hydroxyphenyl)propionic acid, a major microbial metabolite of procyanidin A2, shows similar suppression of macrophage foam cell formation as its parent molecule. RSC Adv. 2018, 8, 6242–6250. [Google Scholar] [CrossRef]
- Braune, A.; Gütschow, M.; Blaut, M. An NADH-Dependent Reductase from Eubacterium ramulus Catalyzes the Stereospecific Heteroring Cleavage of Flavanones and Flavanonols. Appl. Environ. Microbiol. 2019, 85, e01233-19. [Google Scholar] [CrossRef]
- Lee, C.C.; Kim, J.H.; Kim, J.S.; Oh, Y.S.; Han, S.M.; Park, J.H.Y.; Lee, K.W.; Lee, C.Y. 5-(3′,4′-Dihydroxyphenyl-γ-valerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion. Int. J. Mol. Sci. 2017, 18, 1363. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, P.; Cui, Y.; Hu, X.; Chen, F.; Ma, C. Protective Effects of Hydroxyphenyl Propionic Acids on Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 1043. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kim, M.-Y.; Cho, J.Y. Immunopharmacological Activities of Luteolin in Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 2136. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Zhang, L.; Arango-Argoty, G.; Tomasula, P.; Liu, L.; Xiao, W.; Yam, K. Apigenin Impacts the Growth of the Gut Microbiota and Alters the Gene Expression of Enterococcus. Molecules 2017, 22, 1292. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Kulozik, U.; Schwarz, K.; Richling, E. Encapsulation of anthocyanins from bilberries—Effects on bioavailability and intestinal accessibility in humans. Food Chem. 2018, 248, 217–224. [Google Scholar] [CrossRef]
- Zhang, X.; Veliky, C.V.; Birru, R.L.; Barinas-Mitchell, E.; Magnani, J.W.; Sekikawa, A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases-From Molecular Mechanisms to Studies in Humans. Nutrients 2021, 13, 3739. [Google Scholar] [CrossRef]
- Rafii, F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERα transcriptional activation in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 110, 176–185. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Santamaría, L.; Cabrera-Rubio, R.; Plaza-Vinuesa, L.; Crispie, F.; de las Rivas, B.; Cotter, P.; Muñoz, R. A Diverse Range of Human Gut Bacteria Have the Potential To Metabolize the Dietary Component Gallic Acid. Appl. Environ. Microbiol. 2018, 84, e01558-18. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Brockmueller, A.; Ruiz de Porras, V.; Shakibaei, M. Microbiota and Resveratrol: How Are They Linked to Osteoporosis? Cells 2024, 13, 1145. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef]
- Cladis, D.P.; Simpson, A.M.R.; Cooper, K.J.; Nakatsu, C.H.; Ferruzzi, M.G.; Weaver, C.M. Blueberry polyphenols alter gut microbiota & phenolic metabolism in rats. Food Funct. 2021, 12, 2442–2456. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study123. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Molan, A.-L.; Liu, Z.; Plimmer, G. Evaluation of the Effect of Blackcurrant Products on Gut Microbiota and on Markers of Risk for Colon Cancer in Humans. Phytother. Res. 2014, 28, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; de Vos, W.M. Polyphenols as Drivers of a Homeostatic Gut Microecology and Immuno-Metabolic Traits of Akkermansia muciniphila: From Mouse to Man. Int. J. Mol. Sci. 2023, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Cait, A.; Li, Y.; Abolins-Thompson, H.; Gell, K.; Herst, P.M.; O’Sullivan, D.; Gasser, O. Practical Approach To Explore the Effects of Polyphenols on Aryl Hydrocarbon Receptor Regulated Immune Function. J. Agric. Food Chem. 2021, 69, 8625–8633. [Google Scholar] [CrossRef]
- Xue, Z.; Li, D.; Yu, W.; Zhang, Q.; Hou, X.; He, Y.; Kou, X. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct. 2017, 8, 1414–1437. [Google Scholar] [CrossRef]
- Cushing, K.; Alvarado, D.M.; Ciorba, M.A. Butyrate and Mucosal Inflammation: New Scientific Evidence Supports Clinical Observation. Clin. Transl. Gastroenterol. 2015, 6, e108. [Google Scholar] [CrossRef]
- Aghara, H.; Patel, M.; Chadha, P.; Parwani, K.; Chaturvedi, R.; Mandal, P. Unraveling the Gut–Liver–Brain Axis: Microbiome, Inflammation, and Emerging Therapeutic Approaches. Mediat. Inflamm. 2025, 2025, 6733477. [Google Scholar] [CrossRef] [PubMed]
- Talcott, S.; Talcott, S.; Sirven, M. Moderate to Severe Ulcerative Colitis Results in Differential Metabolism of Cranberry Polyphenols by the Colon Microbiome Ex Vivo. Curr. Dev. Nutr. 2020, 4, nzaa045_112. [Google Scholar] [CrossRef]
- Ashwin, K.; Pattanaik, A.K.; Howarth, G.S. Polyphenolic bioactives as an emerging group of nutraceuticals for promotion of gut health: A review. Food Biosci. 2021, 44, 101376. [Google Scholar] [CrossRef]
- Havlik, J.; Edwards, C.A. Non-extractable Polyphenols and the Gut Microbiome. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, J., Eds.; The Royal Society of Chemistry: London, UK, 2018; pp. 241–262. [Google Scholar]
- Petersen, K.; Mansell, T.J. Unveiling the prebiotic potential of polyphenols in gut health and metabolism. Curr. Opin. Biotechnol. 2025, 95, 103338. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Pan, Y.; Qin, R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 2022, 300, 121831. [Google Scholar] [CrossRef]
- Kim, E.Y.; Ham, S.K.; Shigenaga, M.K.; Han, O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J. Nutr. 2008, 138, 1647–1651. [Google Scholar] [CrossRef]
- Ma, Q.; Kim, E.Y.; Han, O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J. Nutr. 2010, 140, 1117–1121. [Google Scholar] [CrossRef]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Bhamre Vaibhav, G.; Deore Pranjal, D.; Amrutkar Rakesh, D.; Patil Vinod, R. Polyphenols: The interactions with CYP 450 isoenzymes and effect on pharmacokinetics of drugs. Curr. Trends Pharm. Pharm. Chem. 2022, 4, 13–23. [Google Scholar] [CrossRef]
- Korobkova, E.A. Effect of Natural Polyphenols on CYP Metabolism: Implications for Diseases. Chem. Res. Toxicol. 2015, 28, 1359–1390. [Google Scholar] [CrossRef]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef]
- Taylor, J.R.; Wilt, V.M. Probable antagonism of warfarin by green tea. Ann. Pharmacother. 1999, 33, 426–428. [Google Scholar] [CrossRef]
- Knop, J.; Misaka, S.; Singer, K.; Hoier, E.; Müller, F.; Glaeser, H.; König, J.; Fromm, M.F. Inhibitory Effects of Green Tea and (–)-Epigallocatechin Gallate on Transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS ONE 2015, 10, e0139370. [Google Scholar] [CrossRef]
- Hegazy, S.K. The Effect of Green Tea on Sildenafil Pharmacokinetics in Egyptian Healthy Volunteers. J. Pharm. Res. Int. 2013, 4, 289–300. [Google Scholar] [CrossRef]
- Abdelkawy, K.S.; Abdelaziz, R.M.; Abdelmageed, A.M.; Donia, A.M.; El-Khodary, N.M. Effects of Green Tea Extract on Atorvastatin Pharmacokinetics in Healthy Volunteers. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Shin, K.H.; Park, J.E.; Kim, M.G.; Yun, Y.M.; Choi, D.H.; Kwon, K.J.; Lee, J. Effect of green tea catechins on the pharmacokinetics of digoxin in humans. Drug Des. Devel. Ther. 2018, 12, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Chandrareddy, A.; Muneyyirci-Delale, O.; McFarlane, S.I.; Murad, O.M. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complement. Ther. Clin. Pract. 2008, 14, 132–135. [Google Scholar] [CrossRef]
- Hutchins, A.M.; McIver, I.E.; Johnston, C.S. Hypertensive crisis associated with high dose soy isoflavone supplementation in a post-menopausal woman: A case report [ISRCTN98074661]. BMC Women’s Health 2005, 5, 9. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.-L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: Mechanism and structure–activity relationship. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Diab, F.; Khalil, M.; Lupidi, G.; Zbeeb, H.; Salis, A.; Damonte, G.; Bramucci, M.; Portincasa, P.; Vergani, L. Influence of Simulated In Vitro Gastrointestinal Digestion on the Phenolic Profile, Antioxidant, and Biological Activity of Thymbra spicata L. Extracts. Antioxidants 2022, 11, 1778. [Google Scholar] [CrossRef]
- Bandele, O.J.; Osheroff, N. Bioflavonoids as Poisons of Human Topoisomerase IIα and IIβ. Biochemistry 2007, 46, 6097–6108. [Google Scholar] [CrossRef] [PubMed]
- Bandele, O.J.; Clawson, S.J.; Osheroff, N. Dietary Polyphenols as Topoisomerase II Poisons: B Ring and C Ring Substituents Determine the Mechanism of Enzyme-Mediated DNA Cleavage Enhancement. Chem. Res. Toxicol. 2008, 21, 1253–1260. [Google Scholar] [CrossRef]
- Vanhees, K.; de Bock, L.; Godschalk, R.W.L.; van Schooten, F.J.; van Waalwijk van Doorn-Khosrovani, S.B. Prenatal Exposure to Flavonoids: Implication for Cancer Risk. Toxicol. Sci. 2011, 120, 59–67. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption2. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef]
| Year | First Author | Main Topic | Details | Comparison with the Present Review |
|---|---|---|---|---|
| 2016 | Tomás-Barberán et al. [14] | Interactions of gut microbiota with dietary polyphenols | Focus on interindividual variability, metabotypes, and implications for clinical trials. | We covered variability, but it adds dose dependence, broader dietary sources, and adverse effects. |
| 2016 | Ozdal et al. [15] | Reciprocal interactions between polyphenols and gut microbiota | Two-way microbiota–polyphenol relationship; effects on bioavailability and health. | We covered bidirectional interaction, but with more detail on classes, GI metabolism, and safety. |
| 2020 | Luca et al. [16] | Bioactivity of dietary polyphenols: role of metabolites | Low bioavailability/high bioactivity paradox; focuses on specific compounds’ metabolites. | We covered broader compounds, including dose, adverse effects, and microbiota modulation. |
| 2020 | Scazzocchio et al. [17] | Curcumin–gut microbiota interaction | Curcumin-specific review; bidirectional effects explain the paradox of low bioavailability. | We reported polyphenols; curcumin is just one example. |
| 2021 | Wan et al. [18] | Dietary polyphenol impact on gut health and microbiota | Structural diversity, gut accumulation, immune modulation, and barrier function. | We included these mechanisms, plus dose-related aspects and a full classification of polyphenols. |
| 2023 | Vivarelli et al. [19] | From bioavailability to bioactivity in chronic diseases | Polyphenol metabolism, microbiota interaction, delivery systems, and clinical trials. | We included mechanistic and GI-focused aspects rather than a clinical focus. |
| 2023 | Yang et al. [20] | cyanidin-3-O-glucoside: biotransformation, bioactivity, nano-encapsulation | Anthocyanin-specific nano-delivery strategies to enhance bioavailability. | We covered broader compounds, classes, and health targets. |
| 2023 | Wang et al. [21] | Flavonols: biotransformation and microbiota-mediated bioactivity | Flavonol-specific; low bioavailability paradox and metabolites’ bioactivity. | We covered broader compounds, covering all polyphenol classes. |
| 2023 | Cuciniello et al. [22] | Antioxidant effect of bioactives via gut microbiota | Includes carbohydrates, polyphenols, and PUFAs; Nrf2 pathway focus. | We included polyphenol-specific compounds with broader GI/dose coverage. |
| 2023 | Gade and Kumar [23] | Gut microbial metabolites of dietary polyphenols | Classification, metabolism, and pharmacology: highlights marine polyphenols. | We add dose, adverse effects, and full polyphenol classification. |
| 2024 | Oumeddour et al. [24] | Cyanidin-3-O-glucoside in obesity-related metabolic disorders | Anthocyanin-specific; detailed mechanisms in metabolism and inflammation. | We included general polyphenolic compounds; cyanidin-3-O-glucoside is one compound among many. |
| 2024 | Láng et al. [25] | Polyphenols and the gut–brain axis in aging | Aging-specific modulation of gut–brain axis, and neural health. | We covered the gut–liver–brain axis partially, not aging-specific. |
| 2025 | Gao et al. [26] | Anthocyanins: bioavailability, gut/system health, industry | Anthocyanin-focused; translational and industrial applications. | We covered general aspects rather than industrial and personalized nutrition aspects. |
| 2025 | Tang [27] | Cyanidin-3-O-glucoside in atherosclerosis via gut microbiota | Anthocyanin-specific; endothelial and lipid effects, microbiota metabolites. | We included more polyphenol classes and a GI tract focus. |
| Major Area | Minor Area | Chemical/Biological Features | Example/Mechanisms | |
|---|---|---|---|---|
| Dietary polyphenols | Structure | Flavonoids | Flavonols: quercetin Flavanones: naringenin Flavanols: epicatechin Flavones: apigenin Anthocyanins: cyanidin Isoflavones: daidzein | [28] |
| Non-flavonoids | Lignans: secoisolariciresinol Phenolic acids: hydroxycinnamic (caffeic acid) hydroxybenzoic acids (gallic acid) Stilbenes: resveratrol | |||
| Other Polyphenols | Curcuminoids, tannins, and coumarins | |||
| Structure-activity | Antioxidants | Radical scavenging Enhance intrinsic antioxidant defence | [29] | |
| Covalent and non-covalent binding with food matrix | Binding with macronutrients | Fibers Protein | [30] | |
| Polyphenols- digestion | Intake | Polyphenol amount determines the biological activity | High in plant-based diets | [46] |
| Stability | Cooking type determines the stability | High in fresh fruits and vegetables | [47] | |
| Oral digestion | Limited digestion; bound to macronutrients | Polyphenols interact with salivary proteins | [48] | |
| Gastric digestion | An acidic environment begins to release some compounds | Polyphenols are released from the food matrix and hydrolysed by the acidic pH environment | [49] | |
| Intestinal digestion | Digestion of intact or partially digested polyphenols | Brush-border LPH or cytosolic glucosidases hydrolyze flavonoid glycosides, breaking down polyphenol conjugates into aglycones (non-bound forms) | [31] | |
| Bioavailability | Transport via the luminal intestine | Absorbed polyphenols/metabolites | Aglycones can cross the intestinal epithelium by:
| [46] |
| Absorption into circulation | Across the intestinal barriers to the liver via the portal vein | 5–10% of total polyphenolic compounds may be absorbed in the small intestine Absorbed polyphenols undergo phase II metabolism (methylation, glucuronidation, sulfation) in enterocytes/liver Many conjugated metabolites circulate, bound to albumin, with slow clearance | [32] | |
| Local beneficial effects of polyphenol intake | High local concentrations in the gut modulate gut microbiota, epithelial barrier, and immune cells before systemic distribution | Prebiotic effects Suppression of pathogenic bacteria Reduction of endotoxins | [33] | |
| Gut-polyphenol interaction | Polyphenols-microbiota | Non-absorbed polyphenols reach the colon | Change in microbial composition Change in metabolites (SCFAs) Mucus production | [33,34,50] |
| Microbiota-polyphenols | Metabolism and biotransformation | Microbial enzymes de-glycosylate and degrade polyphenols into bioactive metabolites (SCFAs, phenolic acids, equol) | [34] | |
| Intestinal immune system and inflammation | Role of polyphenols in the adaptive immune system/microbiota Role of polyphenols in the innate immune system/microbiota | Inhibit NF-κB signaling (e.g., via TLR4/MyD88) → reduce cytokines/inflammation Influence both innate (macrophages, dendritic cells) and adaptive responses (T/B-cell regulation) | [35] | |
| Health-polyphenols | Targeting gut microbiota for host health by dietary polyphenols | Diabetes | Improve insulin sensitivity via SCFAs Reduce inflammation | [36] |
| Cancer | Gut metabolites modulate proliferation/apoptosis, reduce inflammation | [37,38] | ||
| Obesity | Appetite regulation via microbial signals, improved fat metabolism | [39,40,41] | ||
| Cardiovascular diseases | Better lipid profile, endothelial function, and reduced oxidative stress | [40,42] | ||
| Aging | Antioxidant/anti-inflammatory systemic effects | [43] | ||
| MASLD | Enhanced hepatic lipid metabolism, reduced liver inflammation | [44,45] | ||
| Dyslipidemia | Lower LDL-C, increased HDL-C, and improved SCFA production | [51] | ||
| Gastrointestinal diseases | Improve gut microbiota, intestinal inflammation and permeability | [51,52] |
| Phenolic Class | Main Subtypes | Chemical Structure | Dietary Source | Daily Dosage Intake | Beneficial Effects | References |
|---|---|---|---|---|---|---|
| Flavonoids | Flavonols | 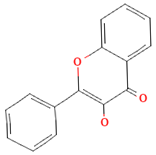 | Onions, kale, broccoli, apples, beans, berries, blackcurrants | 14.4 mg/day in the United States | ↓Glycemia ↓Obesity | [57,58,59,60] |
| Flavones | 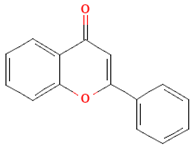 | Parsley and celery, onions, and tea | 0.5 to 4 mg/day in European adults | ↓Platelet action | [61,62,63,64,65] | |
| Flavanones | 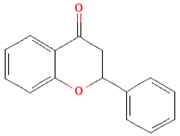 | Oranges, grapefruits, lemons, and their juices | 130.9 mg/day for men and 97 mg/day for women in the United Kingdom | ↑Intestinal integrity | [63,66,67,68] | |
| Flavanols | 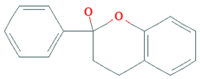 | Tea, apples, pears, and cocoa-based products | 241, 283, and 449 mg/day in Southern, Northern, and Central Europe, respectively | ↓Hypertension ↑Endothelial function | [69,70,71,72] | |
| Anthocyanins | 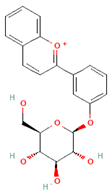 | Berries, red vegetables and fruits, and sumac | 19.8 to 64.9 mg/day in Europe | ↓Glycemia ↓ Lipid accumulation | [73,74,75] [76,77] | |
| Isoflavones | 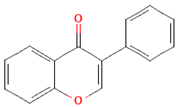 | Soybeans and soy-based products | 3.1 mg/day in the United States, 38.1 mg/day in Japan | ↑Radioprotection, ↑Phytoestrogenic activity | [78,79,80] | |
| Phenolic acids | Hydroxybenzoic acids | 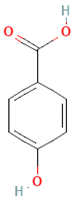 | Coffee | Comprises 55.3% to 80.7% of total phenolic acid intake in European populations | ↓Oxidative stress ↑Antimicrobial effect, ↑Endothelial protection | [81,82,83] |
| Hydroxycinnamic acids | 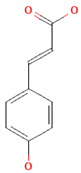 | Fruits, vegetables, cereals, coffee, tea, and wine | 123.2 mg/day in Greece to 1265.5 mg/day in Denmark | ↑Neuroprotection ↓Oxidative stress | [81,84,85] | |
| Stilbenes | Resveratrol | 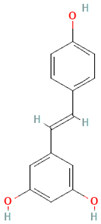 | Grapes, peanuts, strawberries, blueberries, pistachios, red mulberries, cranberries, and tomatoes | Between 30 mg and 150 mg/day | ↓Tumor ↓Glycemia ↓ Lipid accumulation | [86,87,88,89] |
| Lignans | Secoisolariciresinol diglucoside | 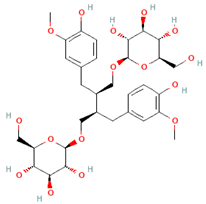 | Flaxseeds | At least 500 mg/day | ↓ Lipid accumulation | [90,91,92] |
| Matairesinol | 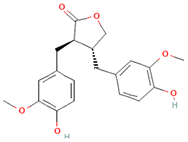 | Flaxseeds, sesame seeds, oats, rye, barley, berries, and broccoli | 25% of lignan intake | ↓Tumor | [93,94] | |
| Other polyphenols | Curcuminoids | 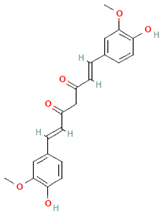 | Turmeric | 1500 mg/day | ↓Inflammation | [95,96] |
| Tannins | 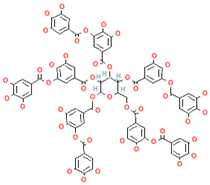 | Berries, nuts, seeds, tea, and wine | 0.1–0.5 g/day | ↑Chemosensitizer ↓Oxidative stress ↓Inflammation | [97,98,99] | |
| Coumarins | 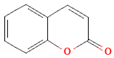 | Cassia cinnamon and Ceylon cinnamon | Limit of </= 0.1 mg/kg body weight | ↓Tumor ↓Inflammation | [100,101] |
| Polyphenols Subclasses | Stability | Bioaccessibility/Bioavailability | Absorption and Metabolism | References |
|---|---|---|---|---|
| Flavonols | Stable at acidic pH | Enhanced after gastric passage | Absorbed in the small intestine as glucosides; undergo microbial metabolism in the colon | [102,104,106] |
| Flavones | Degradable at high temperatures | Varies by glycoside form | Absorbed in the small intestine; conjugated (glucuronidation/sulfation); further metabolized by gut microbes | [106,108,110] |
| Flavanones | Stable in acidic pH | Increased with enzymatic hydrolysis | Absorbed in the small intestine; conjugated; further processed by gut microbes | [115,116,118] |
| Flavanols | Stable in acidic pH and low temperatures | Higher for monomers; reduced for oligomers due to protein interactions | Monomers absorbed in the small intestine; oligomers metabolized in the colon | [122,124,126] |
| Anthocyanins | Stable in acidic pH and low temperatures | Low | Absorbed in jejunum/ileum; undergo phase II conjugation | [127,128,130] |
| Isoflavones | Stable in acidic pH and low temperatures | Enhanced after β-glucosidase hydrolysis | Converted to aglycones; undergo phase II conjugation; participate in enterohepatic recirculation | [132,133,135] |
| Hydroxybenzoic Acids | Stable in gastric and duodenal pH | Increased after microbial hydrolysis | Absorbed in jejunum via MCT1; undergo phase II metabolism; further metabolized in the colon and liver | [138,139,140] |
| Hydroxycinnamic Acids | Stable in gastric and duodenal pH | Lower in esterified forms | Hydrolyzed in the intestine; absorbed; conjugated and excreted | [127,142,143] |
| Resveratrol | Stable in acidic pH and low temperatures | Low | Undergoes extensive phase II metabolism; transformed by gut microbes into dihydroresveratrol and lunularin | [86,146] |
| Secoisolariciresinol | Stability enhanced by protein binding | Enhanced by microbial metabolism to SECO | Hydrolyzed to SECO; metabolized by microbiota into bioactive lignans | [148,149] |
| Matairesinol | Stable at acidic pH | Moderate | Converted in the colon to enterolignans; absorbed systemically | [153,154,155] |
| Curcuminoids | Stable in dairy matrices (e.g., buttermilk and yogurt) | Low | Absorbed in the small intestine; undergo phase I and II metabolism | [95,158] |
| Tannins | Stable in acidic pH and low temperatures | Low if highly polymerized; enhanced after hydrolysis | Hydrolyzed in the intestine; metabolites absorbed systemically | [97,162] |
| Coumarins | Stable in acidic pH and low temperatures | Low | Absorbed in the small intestine; metabolized in the colon to bioavailable derivatives | [163,164] |
| Study/Cohort | Country/Region | Total Polyphenols (mg/day) | Main Findings | Reference |
|---|---|---|---|---|
| MEAL | Italy | ~664 mg/day (362.7 mg phenolic acids, 258.7 mg flavonoids) | Nuts (28%), tea, coffee, fruits, vegetables; olive oil/wine/chocolate contribute too. | [169] |
| EPIC | Mediterranean countries (Greece, Italy, Spain, and S. France) | 584–1786 mg/day | Indicates large regional variation across Europe. | [170] |
| PREDIMED | Spain | ~820 mg/day (443 mg flavonoids, 304 mg phenolic acids) | Fruits’ primary source; flavanols mainly from red wine/apples; olive oil and olives provide ~11%. | [167] |
| Spanish Mediterranean diet | Spain | 1171 mg/day | About 68% of the total dietary antioxidant capacity (TDAC) came from beverages, and 20% from fruits and vegetables. | [168] |
| SUN cohort | Spain | ~785 mg/day (436 mg flavonoids, 305 mg phenolic acids) | Key contributors: Chocolate, apples, pears, coffee, and olives. | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdi, L.; Graziani, A.; Baffy, G.; Mitten, E.K.; Portincasa, P.; Khalil, M. Unlocking Polyphenol Efficacy: The Role of Gut Microbiota in Modulating Bioavailability and Health Effects. Nutrients 2025, 17, 2793. https://doi.org/10.3390/nu17172793
Mahdi L, Graziani A, Baffy G, Mitten EK, Portincasa P, Khalil M. Unlocking Polyphenol Efficacy: The Role of Gut Microbiota in Modulating Bioavailability and Health Effects. Nutrients. 2025; 17(17):2793. https://doi.org/10.3390/nu17172793
Chicago/Turabian StyleMahdi, Laura, Annarita Graziani, Gyorgy Baffy, Emilie K. Mitten, Piero Portincasa, and Mohamad Khalil. 2025. "Unlocking Polyphenol Efficacy: The Role of Gut Microbiota in Modulating Bioavailability and Health Effects" Nutrients 17, no. 17: 2793. https://doi.org/10.3390/nu17172793
APA StyleMahdi, L., Graziani, A., Baffy, G., Mitten, E. K., Portincasa, P., & Khalil, M. (2025). Unlocking Polyphenol Efficacy: The Role of Gut Microbiota in Modulating Bioavailability and Health Effects. Nutrients, 17(17), 2793. https://doi.org/10.3390/nu17172793







