A Systematic Review and Meta-Analysis of the Effects of Vitamin D on Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Analysis
3. Results
3.1. Selection of Studies and Characteristics of Studies Included
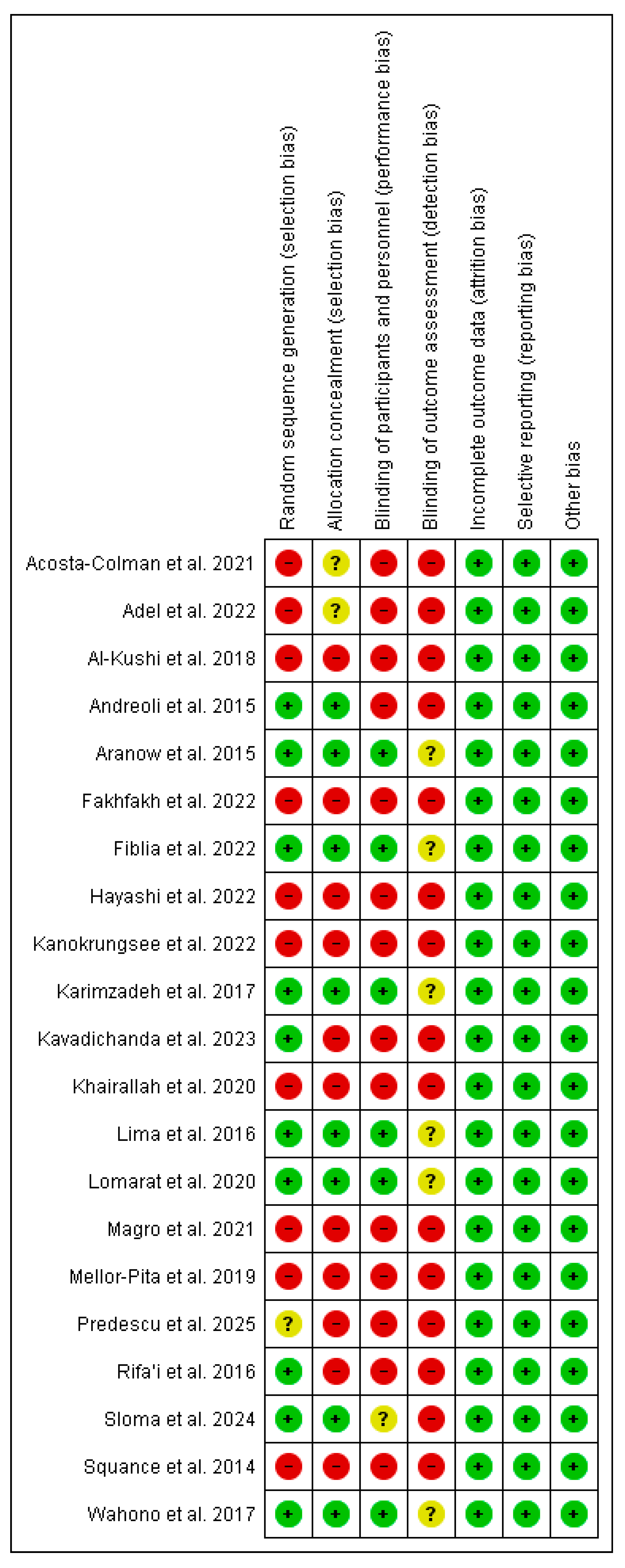
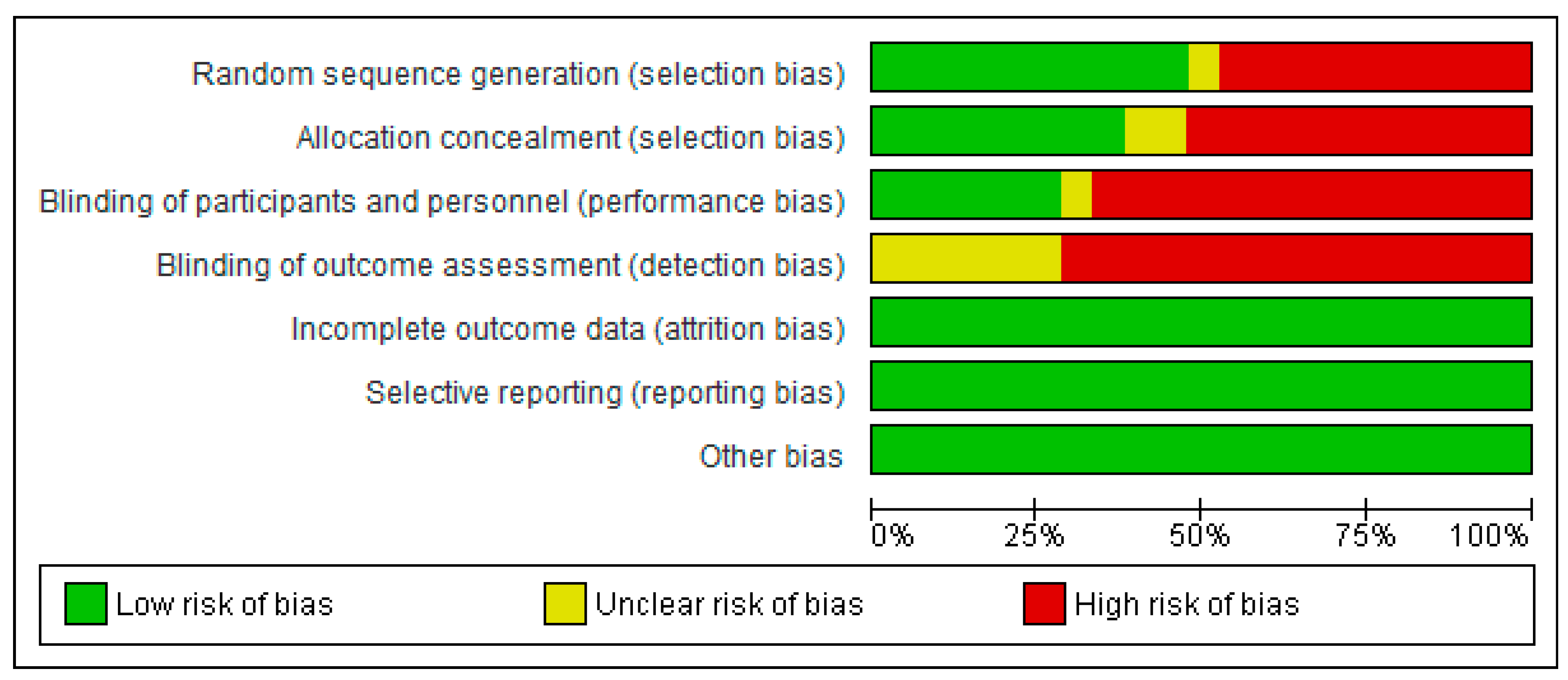
3.2. Assessment of Study Quality
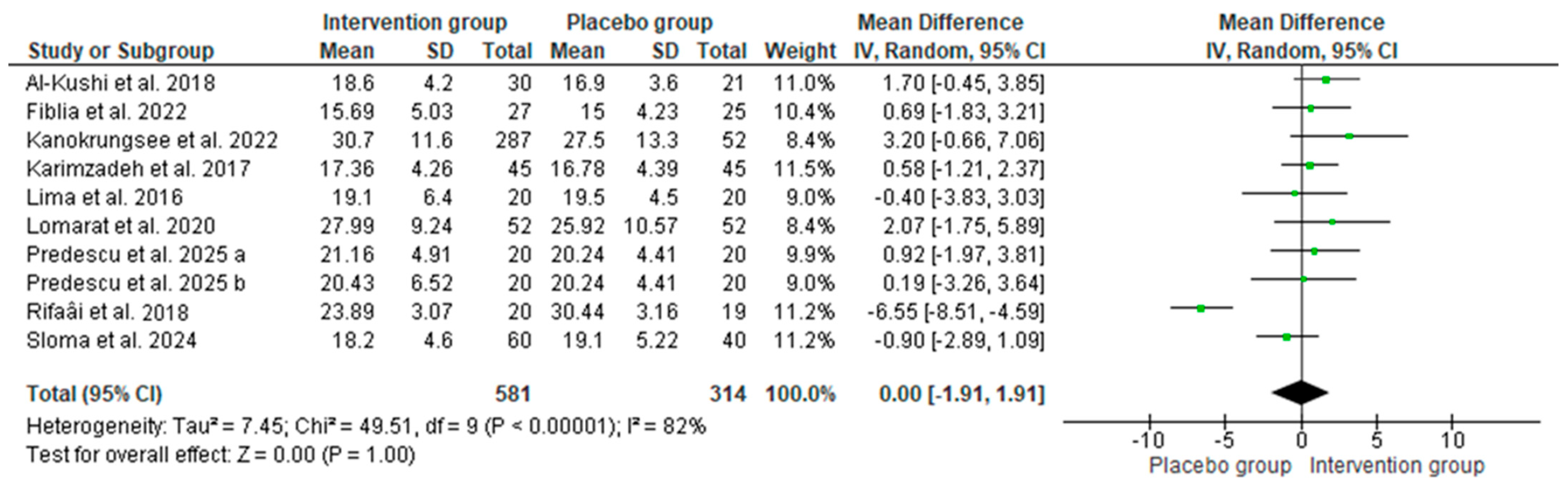
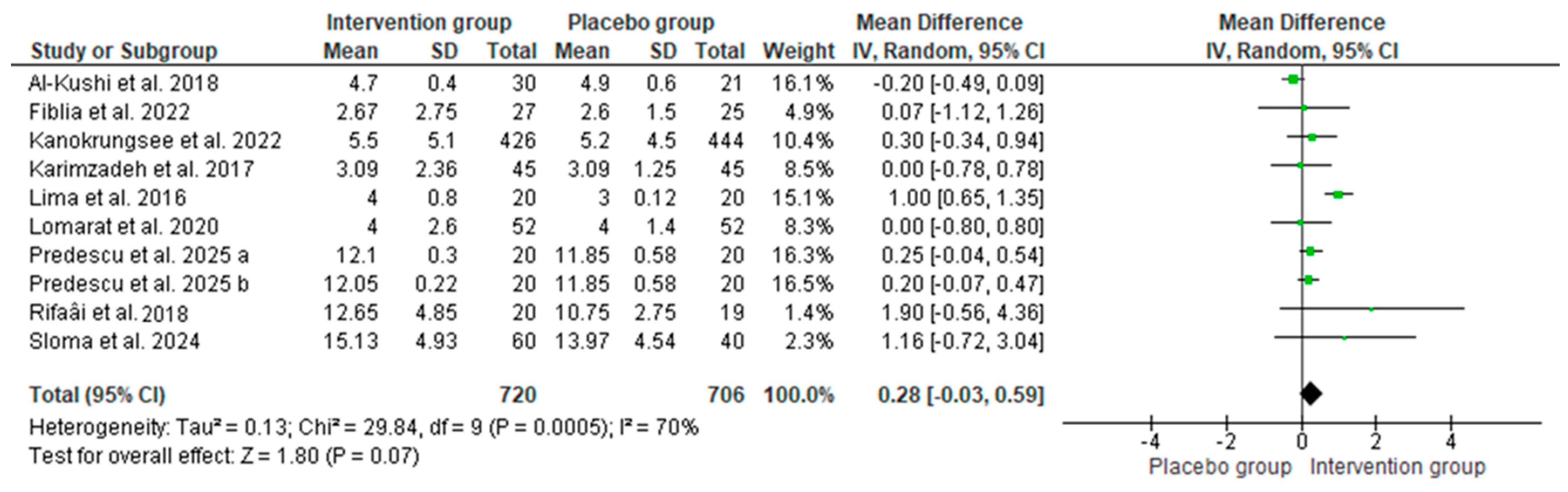
3.3. Effect of Vitamin D Supplementation on Serum 25-OH Vitamin D
3.3.1. The Results of the Systematic Review
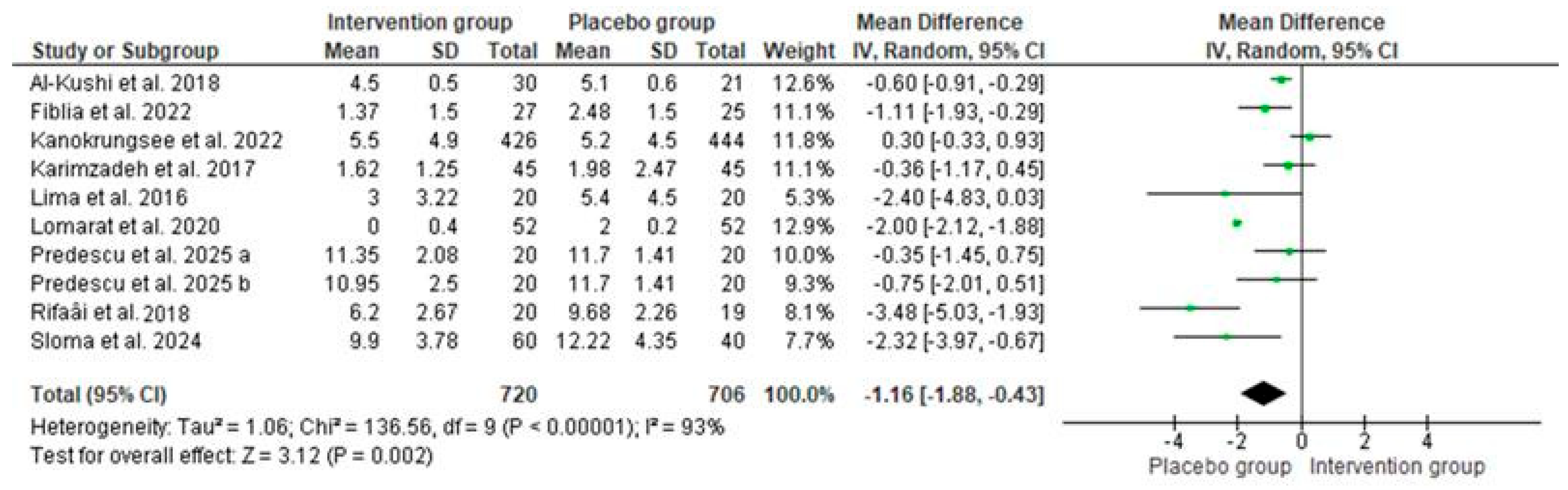
3.3.2. The Results of the Meta-Analyses
3.4. Sensitivity Analyses
3.5. Publication Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, X.; Yu, H.; Lei, Q.; Chen, X.; Tong, Y.; Zhang, Z.; Yang, W.; Guo, Y.; Lin, L. Systemic lupus erythematosus: Pathogenesis and targeted therapy. Mol. Biomed. 2024, 5, 54. [Google Scholar] [CrossRef]
- Albrecht, K.; Troll, W.; Callhoff, J.; Strangfeld, A.; Ohrndorf, S.; Mucke, J. Sex- and gender-related differences in systemic lupus erythematosus: A scoping review. Rheumatol. Int. 2025, 45, 160. [Google Scholar] [CrossRef]
- Gómez-Bañuelos, E.; Fava, A.; Andrade, F. An update on autoantibodies in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2023, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Accapezzato, D.; Caccavale, R.; Paroli, M.P.; Gioia, C.; Nguyen, B.L.; Spadea, L.; Paroli, M. Advances in the Pathogenesis and Treatment of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 6578. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Yuliasih, Y. Disentangling the Pathogenesis of Systemic Lupus Erythematosus: Close Ties between Immunological, Genetic and Environmental Factors. Medicina 2023, 59, 1033. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Reihl, A.M.; Luo, X.M. Breakdown of Immune Tolerance in Systemic Lupus Erythematosus by Dendritic Cells. J. Immunol. Res. 2016, 2016, 6269157. [Google Scholar] [CrossRef]
- Jung, J.; Suh, C. Incomplete clearance of apoptotic cells in systemic lupus erythematosus: Pathogenic role and potential biomarker. Int. J. Rheum. Dis. 2015, 18, 294–303. [Google Scholar] [CrossRef]
- Wu, H.; Fu, S.; Zhao, M.; Lu, L.; Lu, Q. Dysregulation of Cell Death and Its Epigenetic Mechanisms in Systemic Lupus Erythematosus. Molecules 2016, 22, 30. [Google Scholar] [CrossRef]
- Ma, K.; Du, W.; Wang, X.; Yuan, S.; Cai, X.; Liu, D.; Li, J.; Lu, L. Multiple Functions of B Cells in the Pathogenesis of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 6021. [Google Scholar] [CrossRef]
- Schilirò, D.; Silvagni, E.; Ciribè, B.; Fattorini, F.; Maccarrone, V.; Elefante, E.; Signorini, V.; Zucchi, D.; Cardelli, C.; Bortoluzzi, A.; et al. One year in review Systemic lupus erythematosus: One year in review 2024. Clin. Exp. Rheumatol. 2024, 42, 583–592. [Google Scholar]
- Lazar, S.; Kahlenberg, J.M. Systemic Lupus Erythematosus: New Diagnostic and Therapeutic Approaches. Annu. Rev. Med. 2023, 74, 339–352. [Google Scholar] [CrossRef]
- Aringer, M.; Toro-Domínguez, D.; Alarcón-Riquelme, M.E. Classification of systemic lupus erythematosus: From the development of classification criteria to a new taxonomy? Best Pract. Res. Clin. Rheumatol. 2023, 37, 101949. [Google Scholar] [CrossRef]
- Saegusa, K.; Tsuchida, Y.; Komai, T.; Tsuchiya, H.; Fujio, K. Advances in Targeted Therapy for Systemic Lupus Erythematosus: Current Treatments and Novel Approaches. Int. J. Mol. Sci. 2025, 26, 929. [Google Scholar] [CrossRef]
- Jiang, L.; Zhi, S.; Wei, C.; Rong, Z.; Zhang, H. Serum 25(OH)D levels are associated with disease activity and renal involvement in initial-onset childhood systemic lupus erythematosus. Front. Pediatr. 2023, 11, 1252594. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Eloi, M.; Horvath, D.V.; Ortega, J.C.; Prado, M.S.; Andrade, L.E.C.; Szejnfeld, V.L.; De Moura Castro, C.H. 25-Hydroxivitamin D Serum Concentration, Not Free and Bioavailable Vitamin D, Is Associated with Disease Activity in Systemic Lupus Erythematosus Patients. PLoS ONE 2017, 12, e0170323. [Google Scholar] [CrossRef]
- Nardin, M.; Verdoia, M.; Nardin, S.; Cao, D.; Chiarito, M.; Kedhi, E.; Galasso, G.; Condorelli, G.; De Luca, G. Vitamin D and Cardiovascular Diseases: From Physiology to Pathophysiology and Outcomes. Biomedicines 2024, 12, 768. [Google Scholar] [CrossRef]
- Hassanalilou, T.; Khalili, L.; Ghavamzadeh, S.; Shokri, A.; Payahoo, L.; Bishak, Y.K. Role of vitamin D deficiency in systemic lupus erythematosus incidence and aggravation. Autoimmun. Highlights 2018, 9, 1. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Gordo, S.; Olivares, N.; Egurbide, M.-V.; Aguirre, C. Changes in vitamin D levels in patients with systemic lupus erythematosus: Effects on fatigue, disease activity, and damage. Arthritis Care Res. 2010, 62, 1160–1165. [Google Scholar] [CrossRef]
- Attar, S.M.; Siddiqui, A.M. Vitamin d deficiency in patients with systemic lupus erythematosus. Oman Med. J. 2013, 28, 42–47. [Google Scholar] [CrossRef]
- Birmingham, D.J.; Hebert, L.A.; Song, H.; Noonan, W.T.; Rovin, B.H.; Nagaraja, H.N.; Yu, C.Y. Evidence that abnormally large seasonal declines in vitamin D status may trigger SLE flare in non-African Americans. Lupus 2012, 21, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Iruretagoyena, M.; Hirigoyen, D.; Naves, R.; Burgos, P.I. Immune Response Modulation by Vitamin D: Role in Systemic Lupus Erythematosus. Front. Immunol. 2015, 6, 513. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Ben-Zvi, I.; Aranow, C.; Mackay, M.; Stanevsky, A.; Kamen, D.L.; Marinescu, L.M.; Collins, C.E.; Gilkeson, G.S.; Diamond, B.; Hardin, J.A. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS ONE 2010, 5, e9193. [Google Scholar] [CrossRef]
- Ritterhouse, L.L.; Crowe, S.R.; Niewold, T.B.; Kamen, D.L.; Macwana, S.R.; Roberts, V.C.; Dedeke, A.B.; Harley, J.B.; Scofield, R.H.; Guthridge, J.M.; et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2011, 70, 1569–1574. [Google Scholar] [CrossRef]
- Pakchotanon, R.; Lomarat, W.; Narongroeknawin, P.; Chaiamnuay, S.; Asavatanabodee, P. Randomized double-blind controlled trial to evaluate efficacy of vitamin d supplementation among patients with systemic lupus erythematosus. J. Southeast Asian Med. Res. 2020, 4, 24–32. [Google Scholar] [CrossRef]
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef]
- Zheng, R.; Gonzalez, A.; Yue, J.; Wu, X.; Qiu, M.; Gui, L.; Zhu, S.; Huang, L. Efficacy and Safety of Vitamin D Supplementation in Patients with Systemic Lupus Erythematosus: A Meta-analysis of Randomized Controlled Trials. Am. J. Med. Sci. 2019, 358, 104–114. [Google Scholar] [CrossRef]
- Singgih Wahono, C.; Diah Setyorini, C.; Kalim, H.; Nurdiana, N.; Handono, K. Effect of Curcuma xanthorrhiza Supplementation on Systemic Lupus Erythematosus Patients with Hypovitamin D Which Were Given Vitamin D3 towards Disease Activity (SLEDAI), IL-6, and TGF-β1 Serum. Int. J. Rheumatol. 2017, 2017, 7687053. [Google Scholar] [CrossRef]
- Terrier, B.; Derian, N.; Schoindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. Ther. 2012, 14, R221. [Google Scholar] [CrossRef]
- Adel, Y.; Elgamal, M.; Adel Abdelsalam, S. Impact of vitamin D level and supplementation on systemic lupus erythematosus patients during COVID-19 pandemic. Arch. Rheumatol. 2022, 37, 288–299. [Google Scholar] [CrossRef]
- Al-Kushi, A.G.; Azzeh, F.S.; Header, E.A.; ElSawy, N.A.; Hijazi, H.H.; Jazar, A.S.; Ghaith, M.M.; Alarjah, M.A. Effect of Vitamin D and Calcium Supplementation in Patients with Systemic Lupus Erythematosus. Saudi J. Med. Med. Sci. 2018, 6, 137–142. [Google Scholar] [CrossRef]
- Furlan, A.D.; Pennick, V.; Bombardier, C.; van Tulder, M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009, 34, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Colman, I.; Morel, Z.; Paats, A.; Ortíz, N.; Román, L.; Vázquez, M.; Duarte, M.; Ávila-Pedretti, G.; Acosta, M.E.; Martínez De Filártiga, M.T. Association between vitamin D deficiency and disease activity in Paraguayan patients with systemic lupus erythematosus. Rev. Colomb. Reumatol. 2022, 29, 19–25. [Google Scholar] [CrossRef]
- Andreoli, L.; Dall’Ara, F.; Piantoni, S.; Zanola, A.; Piva, N.; Cutolo, M.; Tincani, A. A 24-month prospective study on the efficacy and safety of two different monthly regimens of vitamin D supplementation in pre-menopausal women with systemic lupus erythematosus. Lupus 2015, 24, 499–506. [Google Scholar] [CrossRef]
- Aranow, C.; Kamen, D.L.; Dall’Era, M.; Massarotti, E.M.; Mackay, M.C.; Koumpouras, F.; Coca, A.; Chatham, W.W.; Clowse, M.E.B.; Criscione-Schreiber, L.G.; et al. Randomized, Double-Blind, Placebo-Controlled Trial of the Effect of Vitamin D3 on the Interferon Signature in Patients with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 1848–1857. [Google Scholar] [CrossRef]
- Fakhfakh, R.; Feki, S.; Elleuch, A.; Neifar, M.; Marzouk, S.; Elloumi, N.; Hachicha, H.; Abida, O.; Bahloul, Z.; Ayadi, F.; et al. Vitamin D status and CYP27B1-1260 promoter polymorphism in Tunisian patients with systemic lupus erythematosus. Mol. Genet. Genom. Med. 2021, 9, e1618. [Google Scholar] [CrossRef]
- Fiblia, F.; Rengganis, I.; Purnamasari, D.; Widhani, A.; Karjadi, T.H.; Shatri, H.; Putranto, R. Effect of Cholecalciferol Supplementation on Disease Activity and Quality of Life of Systemic Lupus Erythematosus Patients: A Randomized Clinical Trial Study. Acta Med. Indones. 2022, 54, 406–413. [Google Scholar]
- Hayashi, K.; Sada, K.-E.; Asano, Y.; Katayama, Y.; Ohashi, K.; Morishita, M.; Miyawaki, Y.; Watanabe, H.; Katsuyama, T.; Narazaki, M.; et al. Real-world data on vitamin D supplementation and its impacts in systemic lupus erythematosus: Cross-sectional analysis of a lupus registry of nationwide institutions (LUNA). PLoS ONE 2022, 17, e0270569. [Google Scholar] [CrossRef]
- Kanokrungsee, S.; Patcharapojanart, C.; Suchonwanit, P.; Chanprapaph, K. High Prevalence of Hypovitaminosis D in Cutaneous and Systemic Lupus Erythematosus Patients and Its Associated Factors: A Cross-Sectional Study in Thailand. CCID 2022, 15, 1663–1671. [Google Scholar] [CrossRef]
- Karimzadeh, H.; Shirzadi, M.; Karimifar, M. The effect of Vitamin D supplementation in disease activity of systemic lupus erythematosus patients with Vitamin D deficiency: A randomized clinical trial. J. Res. Med. Sci. 2017, 22, 4. [Google Scholar] [CrossRef]
- Kavadichanda, C.; Singh, P.; Maurya, S.; Tota, S.; Kiroubagarin, A.; Kounassegarane, D.; Anand, S.; Negi, V.S.; Aggarwal, A. Clinical and serological association of plasma 25-hydroxyvitamin D (25(OH)D) levels in lupus and the short-term effects of oral vitamin D supplementation. Arthritis Res. Ther. 2023, 25, 2. [Google Scholar] [CrossRef]
- Khairallah, M.K.; Makarem, Y.S.; Dahpy, M.A. Vitamin D in active systemic lupus erythematosus and lupus nephritis: A forgotten player. Egypt. J. Intern. Med. 2020, 32, 16. [Google Scholar] [CrossRef]
- Lima, G.L.; Paupitz, J.; Aikawa, N.E.; Takayama, L.; Bonfa, E.; Pereira, R.M.R. Vitamin D Supplementation in Adolescents and Young Adults with Juvenile Systemic Lupus Erythematosus for Improvement in Disease Activity and Fatigue Scores: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care Res. 2016, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Magro, R.; Saliba, C.; Camilleri, L.; Scerri, C.; Borg, A.A. Vitamin D supplementation in systemic lupus erythematosus: Relationship to disease activity, fatigue and the interferon signature gene expression. BMC Rheumatol. 2021, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Mellor-Pita, S.; Tutor-Ureta, P.; Rosado, S.; Alkadi, K.; Granado, F.; Jimenez-Ortiz, C.; Castejon, R. Calcium and vitamin D supplement intake may increase arterial stiffness in systemic lupus erythematosus patients. Clin. Rheumatol. 2019, 38, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Predescu, O.R.; Vreju, F.A.; Musetescu, A.E.; Florescu, A.; Dinescu, S.C.; Bita, C.E.; Barbulescu, A.L.; Ciurea, P.L. Effects of Vitamin D Supplementation on Fatigue and Disease Activity in Systemic Lupus Erythematosus. Cureus 2025, 17, e78830. [Google Scholar] [CrossRef]
- Rifa’i, A.; Kalim, H.; Kusworini, K.; Wahono, C.S. Effect of vitamin D supplementation on disease activity (SLEDAI) and fatigue in Systemic Lupus Erythematosus patients with hipovitamin D: An Open Clinical Trial. Indones. J. Rheum. 2018, 8, 231053. [Google Scholar] [CrossRef]
- Sloma, A.R.G.; Abdelkareem, M.I.M.; Hashim, A.M.; Azeem, H.A. Effect of Vitamin D Supplementation on Disease Activity and Fatigue In Systemic Lupus Erythematosis Patients with Hypovitaminosis D: A Randomized Controlled Trial. Al-Azhar Int. Med. J. 2024, 5, 46. [Google Scholar] [CrossRef]
- Squance, M.L.; Reeves, G.E.M.; Tran, H.A. Vitamin D Levels Are Associated with Expression of SLE, but Not Flare Frequency. Int. J. Rheumatol. 2014, 2014, 362834. [Google Scholar] [CrossRef][Green Version]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Gu, Y.; Cai, Z.; Wei, D.; Feng, X.; Yang, C. Analysis of the molecular mechanism of inosine monophosphate deposition in Jingyuan chicken muscles using a proteomic approach. Poult. Sci. 2022, 101, 101741. [Google Scholar] [CrossRef]
- De Medeiros, M.C.S.; Medeiros, J.C.A.; De Medeiros, H.J.; Leitão, J.C.G.D.C.; Knackfuss, M.I. Dietary intervention and health in patients with systemic lupus erythematosus: A systematic review of the evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 2666–2673. [Google Scholar] [CrossRef]
- Guan, S.-Y.; Cai, H.Y.; Wang, P.; Lv, T.T.; Liu, L.N.; Mao, Y.M.; Zhao, C.N.; Wu, Q.; Dan, Y.L.; Sam, N.B.; et al. Association between circulating 25-hydroxyvitamin D and systemic lupus erythematosus: A systematic review and meta-analysis. Rev. Int. Des Mal. Rhum. 2019, 22, 1803–1813. [Google Scholar] [CrossRef]
- Jiao, H.; Acar, G.; Robinson, G.A.; Ciurtin, C.; Jury, E.C.; Kalea, A.Z. Diet and Systemic Lupus Erythematosus (SLE): From Supplementation to Intervention. IJERPH 2022, 19, 11895. [Google Scholar] [CrossRef]
- Md Yusof, M.Y.; Vital, E.M. Early intervention in systemic lupus erythematosus: Time for action to improve outcomes and health-care utilization. Rheumatol. Adv. Pract. 2022, 6, rkab106. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E.; Young, K.A.; Kamen, D.L.; Guthridge, J.M.; Niewold, T.B.; Costenbader, K.H.; Weisman, M.H.; Ishimori, M.L.; Wallace, D.J.; Gilkeson, G.S.; et al. Discerning Risk of Disease Transition in Relatives of Systemic Lupus Erythematosus Patients Utilizing Soluble Mediators and Clinical Features. Arthritis Rheumatol. 2017, 69, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.; Saccon, F.; Zen, M.; Iaccarino, L.; Doria, A. Preclinical and early systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101422. [Google Scholar] [CrossRef] [PubMed]
- Irfan, S.A.; Ali, A.A.; Shabbir, N.; Altaf, H.; Ahmed, A.; Thamara Kunnath, J.; Divya Boorle, N.V.L.; Miguel, A.K.; Loh, C.C.; Gandrakota, N.; et al. Effects of Vitamin D on Systemic Lupus Erythematosus Disease Activity and Autoimmunity: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e25896. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Mahmood, S.B.Z.; Ayaz, A.; Al Karim Manji, A.; Ahuja, A.K. Association of vitamin D deficiency and disease activity in systemic lupus erythematosus patients: Two-year follow-up study. Arch. Rheumatol. 2021, 36, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Gallagher, J.C.; Suiter, C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: A randomized clinical trial. J. Steroid Biochem. Mol. Biol. 2017, 173, 317–322. [Google Scholar] [CrossRef]
- Rezmuves, M.G.; Nastasa, E.; Demian, S. Vitamin D Toxicity Due to Self-Medication During the COVID-19 Pandemic—A Case Report. J. Interdiscip. Med. 2023, 8, 11–15. [Google Scholar] [CrossRef]
- Alkundi, A.; Momoh, R.; Musa, A.; Nwafor, N. Vitamin D intoxication and severe hypercalcaemia complicating nutritional supplements misuse. BMJ Case Rep. 2022, 15, e250553. [Google Scholar] [CrossRef]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef]
- Jiang, L.; Rong, Z.; Zhang, H. The changes of Treg and Th17 cells relate to serum 25(OH)D in patients with initial-onset childhood systemic lupus erythematosus. Front. Pediatr. 2023, 11, 1228112. [Google Scholar] [CrossRef]
- Parks, C.G.; De Souza Espindola Santos, A.; Barbhaiya, M.; Costenbader, K.H. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2017, 31, 306–320. [Google Scholar] [CrossRef]
- Mok, C.C. Vitamin D and systemic lupus erythematosus: An update. Expert. Rev. Clin. Immunol. 2013, 9, 453–463. [Google Scholar] [CrossRef]
- Touma, Z.; Kayaniyil, S.; Parackal, A.; Bonilla, D.; Su, J.; Johnston, A.; Gahn, J.; Hille, E.D.; Ohsfeldt, R.; Chandran, S. Modelling long-term outcomes for patients with systemic lupus erythematosus. Semin. Arthritis Rheum. 2024, 68, 152507. [Google Scholar] [CrossRef]


| PICOS Elements | Details |
|---|---|
| Participants | Patients diagnosed with SLE |
| Interventions | Monitoring vitamin D supplement dose in patients with SLE |
| Comparisons | A patient with vitamin D supplement compared to a patient without a vitamin D supplement (placebo) |
| Outcomes | Pre–post intervention changes in vitamin D levels and disease activity |
| Study designs | Case controls, cohorts, cross-sections, nRCTs, nRnCTs, RCTs |
| Study | Country | Sample Size | Sex | Age (Means ± SD) or Range | Disease Duration (Years) | Supplement Duration | Vit D Supplement Doses | According to Diagnostic Criteria | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Acosta-Colman et al. (2021) [37] | Paraguay | 100 | Men and women | 27.5 ± 9.8 | 1.6 | 24 weeks | 2000 IU/day | SLEDAI | Increasing levels of serum VD with supplementation (p = 0.0224), with a statistically significant association with disease activity |
| Adel et al. (2022) [32] | Egypt | 38 | Men and women | 49.2 ± 8.1 | 6.2 ± 3.7 | 8 weeks | 800 IU/day | SLEDAI | No significant differences in baseline vitamin D levels between the patients who were adherent or non-adherent to vitamin D supplementation (p = 0.1) |
| Al-Kushi et al. (2018) [33] | Saudi Arabia | 81 | Men and women | Gr 1: 36.4 ± 7.6 Gr 2: 35.2 ± 8.7 Gr 3: 37.7 ± 8.9 | 12.4 ± 3.4 13.9 ± 4.9 13.4 ± 2.9 | 6 months | 1400 IU/day | SLEDAI | Vitamin D and calcium supplementation did not attenuate immune markers or disease activity but improved the bone mineral density in vitamin D-deficient SLE patients |
| Andreoli et al. (2015) [38] | Italy | 34 | Women | 32.5 (19–44) | 7 ± 2.3 | 24 months | 25,000 IU/months | SLEDAI | Neither regimen of vitamin D supplementation affected SLE serology |
| Aranow et al. (2015) [39] | USA | 54 | Women | G1: 38.7 ± 12.27 G2: 36.5 ± 10.90 G3: 38.3 ± 12.88 | 10.1 ± 7.2 | 12 weeks | 2000 IU/day Or 400 IU/day | ACR SLEDAI | No significant difference between patients receiving a vitamin D3 supplement and those receiving a placebo |
| Fakhfakh et al. (2021) [40] | Tunisia | 106 | Men and women | 37.8 (21–63) | 24 months | 2000 IU/day | ACR SLEDAI | A significant difference in the mean levels of 25[OH]D between vitamin D-supplemented SLE patients and controls was observed but was not associated with changes in SLEDAI | |
| Fiblia et al. (2022) [41] | Indonesia | 60 | Women | 18–60 years | 1–5 years | 12 weeks | 5000 IU/day | MEX- SLEDAI | Supplementation with cholecalciferol increased vitamin D levels and improved disease activity |
| Hayashi et al. (2022) [42] | Japan | 870 | Men and women | 45 ± 14 | 12.75 ± 10.08 | No data | ACR SLEDAI | Vitamin D supplementation did not change disease activity | |
| Kanokrungsee et al. (2022) [43] | Thailand | 414 | Men | than 18 years | 7 ± 3.4 | 3 months | 10,000 IU of vitamin D2/week | SLEDAI-2K | Vitamin D replacement therapy increased serum vitamin D levels in 45% of patients |
| Karimzadeh et al. (2017) [44] | Iran | 90 | Men and women | IV: 33.78 ± 6.2 PB: 35.69 ± 6.8 | IV: 9.53 ± 3.8 PB: 10.98 ± 3.5 | 12 weeks | 50,000 IU/month | SLEDAI | The mean values of SLEDAI were not different before and after vitamin D supplementation in intervention and placebo groups |
| Kavadichand a et al. (2023) [45] | India | 702 | Men and women | 29.44 ± 10.7 | G 1: 1.6 G 2: 1.3 | 6 months | 30,000 IU/Day | ACR SLEDAI | High-dose oral vitamin D supplementation may be safe and effective in improving vitamin D levels in SLE but had a weak correlation with disease activity |
| Khairallah et al. (2020) [46] | Egypt | 100 | Men and women | IV: 28.30 ± 8.9 PB: 25.32 ± 6.98 | 10 ± 3.2 years | SLEDAI | Vitamin D supplements do not appear to significantly decrease the positivity of anti-dsDNA and SLE activity | ||
| Lima et al. (2016) [47] | Brazil | 40 | Men and women | IV: 18.5 6 3.5 PB: 19.3 6 3.3 | 2.5 ± 1.5 | 24 weeks | 50,000 IU/week | SLEDAI | Cholecalciferol supplementation decreased disease activity and improved fatigue in juvenile-onset SLE patients |
| Pakchotanon et al. (2020) [27] | Bangkok Thailand | 91 | Men and women | 42.41 ± 13.25 | 6 ± 1.5 | 6 months | D2: 100,000 IU/ week/ 4 weeks 40,000 IU/Week For 20 weeks D3: 800 IU/day/24 week | ACR SLEDAI | Study was inconclusive in demonstrating the efficacy of high-dose ergocalciferol in controlling SLE disease activity |
| Magro et al. (2021) [48] | Malta | 31 | Women | 47.9 ± 13.7 | 14.1 ± 8 | 12 months | vitamin D insufficiency: 8000 IU/day for 4 weeks/ followed by 2000 IU/day vitamin D deficiency: 8000 IU/day/8 weeks followed by 2000 IU daily | ACR SLIC SLEDAI | Improved disease activity and fatigue were noted |
| Mellor-Pita et al. (2019) [49] | Spain | 47 | Women | 48.8 (21–65) | 10.85 ± 7.9 | 3 months | 400 to 800 IU/ day with 500 to 1000 mg of calcium | ACR SLEDAI | No significant association between 25(OH)D serum levels and cardiovascular risk factors and disease activity |
| Predescu et al. (2025) [50] | Romania | 60 | Men and women | 3.23 ± 12.65 | IV1: 9.3 ± 3.48 IV2: 9.83 ± 3.97 PB: 9.83 ± 3.97 | 6 months | IV1: 4000 IU/Day IV2: 8000 IU/Day | SELENA-SLEDAI | Significant increases in vitamin D levels and serum complement levels in the supplementation groups. Slight reduction in SELENA-SLEDAI scores in the treated groups |
| Rifa’i et al. (2018) [51] | Indonesia Malang | 39 | Women | IV: 28.25 ± 6.97 PB: 27.75 ± 6.86 | ˂1 years | 3 months | 1200 IU/day | ACR SLEDAI | Supplementation with vitamin D improved disease activity and degree of fatigue |
| Sloma et al. (2024) [52] | Romania | 100 | Women | IV: 26.80 ± 4.57 PB: 28.15 ± 5.99 | IV: 3.86 ±1.78 PB: 4.25 ± 1.93 | 3 months | 2000 IU/day | SLEDAI | The average SLEDAI score was reduced after three months of supplementation |
| Squance et al. (2014) [53] | Australia | 80 | Women | PB: 49.8 ± 12.4 IV: 47.7 ± 13.5 | 7.7 ± 6.2 years | 3 months | ACR | Vitamin D supplementation along with regular monitoring should be a consideration as part of individual patient health management plans | |
| Wahono et al. (2017) [30] | Indonesia | 40 | Women | IV: 29.1 ± 8.95 PB: 30.3 ± 10.0 | 2.12 ± 1.5 | 3 months | 400 IU/day | ACR SLIC SLEDA | No significant differences in SLEDAI reduction, decreased serum levels of IL-6, and increased levels of TGF-β1 serum among groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kababi, S.; El Ouali, E.M.; Kartibou, J.; Lamiri, A.; Deblij, S.; Supriya, R.; Saiedi, A.; Del Coso, J.; Laher, I.; Zouhal, H. A Systematic Review and Meta-Analysis of the Effects of Vitamin D on Systemic Lupus Erythematosus. Nutrients 2025, 17, 2794. https://doi.org/10.3390/nu17172794
El Kababi S, El Ouali EM, Kartibou J, Lamiri A, Deblij S, Supriya R, Saiedi A, Del Coso J, Laher I, Zouhal H. A Systematic Review and Meta-Analysis of the Effects of Vitamin D on Systemic Lupus Erythematosus. Nutrients. 2025; 17(17):2794. https://doi.org/10.3390/nu17172794
Chicago/Turabian StyleEl Kababi, Samira, El Mokhtar El Ouali, Jihan Kartibou, Abderrahman Lamiri, Sanae Deblij, Rashmi Supriya, Ayoub Saiedi, Juan Del Coso, Ismail Laher, and Hassane Zouhal. 2025. "A Systematic Review and Meta-Analysis of the Effects of Vitamin D on Systemic Lupus Erythematosus" Nutrients 17, no. 17: 2794. https://doi.org/10.3390/nu17172794
APA StyleEl Kababi, S., El Ouali, E. M., Kartibou, J., Lamiri, A., Deblij, S., Supriya, R., Saiedi, A., Del Coso, J., Laher, I., & Zouhal, H. (2025). A Systematic Review and Meta-Analysis of the Effects of Vitamin D on Systemic Lupus Erythematosus. Nutrients, 17(17), 2794. https://doi.org/10.3390/nu17172794









