Healthy Dietary Patterns and Risk of Sarcopenia in Adults Aged > 50 Years: A Systematic Review and Meta-Analysis Considering EWGSOP1 and EWGSOP2 Criteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection and Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis
3. Results
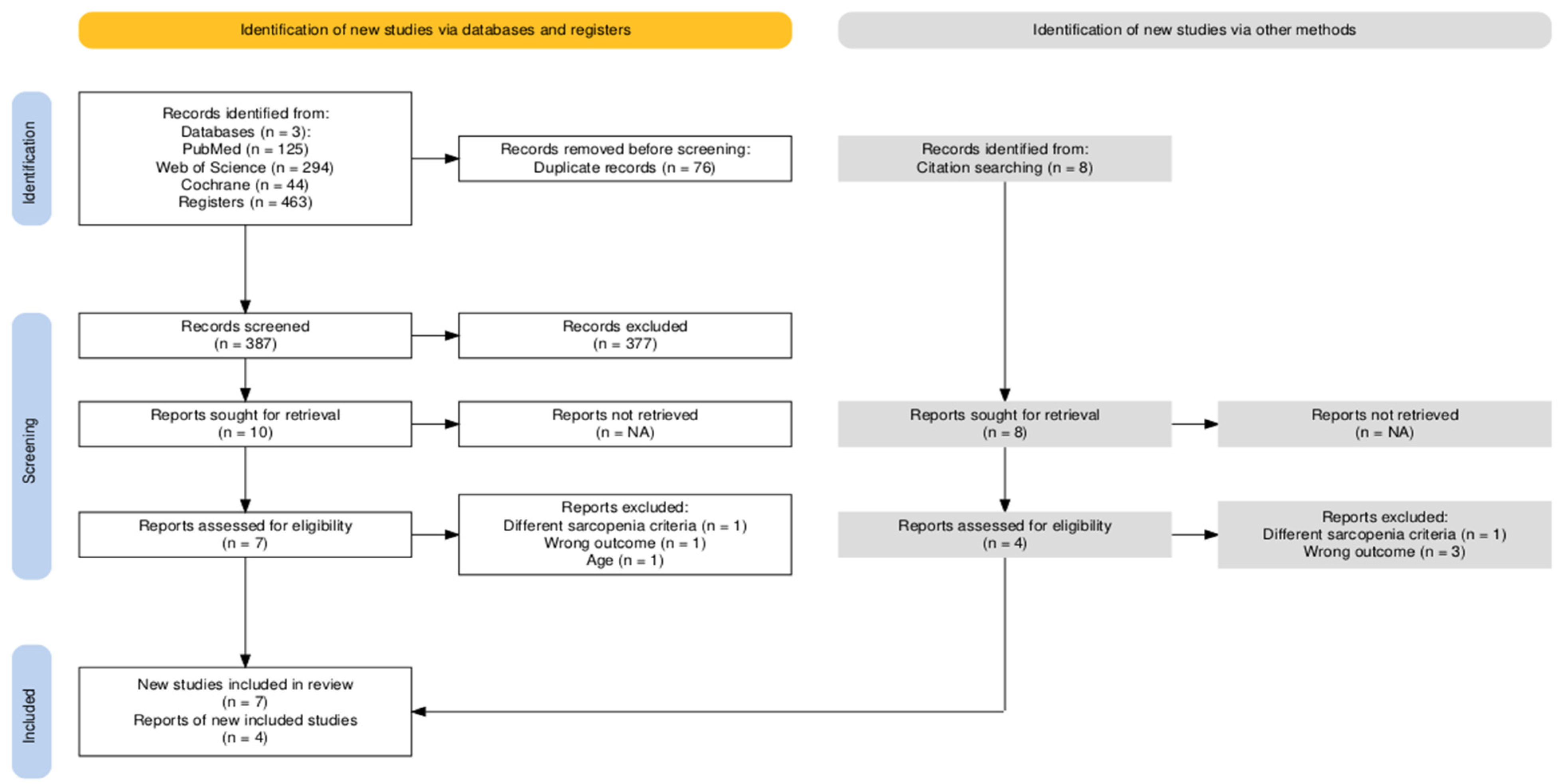
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Dietary Patterns
3.2.2. Sarcopenia Diagnosis
3.2.3. Methodological Quality of Included Studies
3.3. Meta-Analysis
3.3.1. A Priori and a Posteriori Healthy and Unhealthy Dietary Patterns and Sarcopenia Risk
3.3.2. A Priori Healthy Dietary Patterns and Sarcopenia
3.3.3. A Priori and a Posteriori Mediterranean Diet and Risk of Sarcopenia
4. Publication Bias
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHEI-2010 | Alternative Healthy Eating Index 2010 |
| aDGI | Australian Dietary Guideline Index |
| Anti | Anti-inflammatory dietary pattern |
| ASM | Appendicular Skeletal Muscle Mass |
| BSD | Baltic Sea Diet |
| Bread & Cheese | Bread & Cheese dietary pattern |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| CHAMP | Concord Health and Ageing in Men Project |
| Carbo-vit | Carbohydrate–Vitamin dietary pattern |
| DASH | Dietary Approaches to Stop Hypertension |
| DII | Dietary Inflammatory Index |
| DXA | Dual-energy X-ray Absorptiometry |
| EWGSOP1 | European Working Group on Sarcopenia in Older People 1 |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 |
| FFQ | Food Frequency Questionnaire |
| GS | Gait Speed |
| HGS | Handgrip Strength |
| JBI | The Joanna Briggs Institute checklist |
| LRM | Low Red Meat |
| LB | Low Butter |
| MD | Mediterranean diet |
| MDS | Mediterranean Diet Score |
| MED | Mediterranean Diet Score (Nordic-food-adapted version) |
| MEDAS | Mediterranean Diet Adherence Screener |
| mHDI | Modified Healthy Diet Index |
| Meat & Egg | Meat & Egg dietary pattern |
| Milk & Cereal | Milk & Cereal dietary pattern |
| mMDS | Modified Mediterranean Diet Score |
| MPR | Multiple Pass Recall |
| NRV | Nutrient Risk Variable |
| OSPTRE-FPS | Osteoporosis Risk Factor and Prevention-Fracture Prevention Study |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Pro-vit | Protein Vitamin pattern |
| PROSPERO | Prospective Register of Systematic Reviews |
| RSMI | Relative Skeletal Muscle Index |
| SARIR | Sarcopenia and its determinants among Iranian elderly |
| SMI | Skeletal Muscle Index |
| TUG | Timed Up and Go test |
| Traditional British | Traditional British dietary pattern |
| UPPSALA | Uppsala Longitudinal Study of Adult Men |
| Vegetable & Fruit | Vegetable & Fruit dietary pattern |
| Western | Western dietary pattern |
References
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Arnal-Gómez, A.; Cebrià IIranzo, M.A.; Tomas, J.M.; Tortosa-Chuliá, M.A.; Balasch-Bernat, M.; Sentandreu-Mañó, T.; Forcano, S.; Cezón-Serrano, N. Using the updated EWGSOP2 definition in diagnosing sarcopenia in Spanish older adults: Clinical approach. J. Clin. Med. 2021, 10, 1018. [Google Scholar] [CrossRef]
- Van Ancum, J.M.; Alcazar, J.; Meskers, C.G.M.; Nielsen, B.R.; Suetta, C.; Maier, A.B. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Arch. Gerontol. Geriatr. 2020, 90, 104125. [Google Scholar] [CrossRef]
- Chiu, W.C.; Kao, T.W.; Peng, T.C. Prevalence of sarcopenia in Asian older adults: A comparison of nine diagnostic criteria across different regions. Exp. Gerontol. 2025, 202, 112721. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Alcazar, J.; Aprahamian, I.; Batsis, J.A.; Yamada, Y.; Prado, C.M.; Reginster, J.Y.; Sanchez-Rodriguez, D.; Lim, W.S.; Sim, M.; et al. Health outcomes of sarcopenia: A consensus report by the outcome working group of the Global Leadership Initiative in Sarcopenia (GLIS). Aging Clin. Exp. Res. 2025, 37, 100. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.K.; et al. The conceptual definition of sarcopenia: Delphi consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Meng, S.; He, X.; Fu, X.; Zhang, X.; Tong, M.; Li, W.; Zhang, W.; Shi, X.; Liu, K. The prevalence of sarcopenia and risk factors in older adults in China: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1415398. [Google Scholar] [CrossRef]
- Reiss, J.; Iglseder, B.; Alzner, R.; Mayr-Pirker, B.; Pirich, C.; Kässmann, H.; Kreutzer, M.; Dovjak, P.; Reiter, R. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing 2019, 48, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Stuck, A.K.; Tsai, L.T.; Freystaetter, G.; Vellas, B.; Kanis, J.A.; Rizzoli, R.; Kressig, K.S.; Armbrecht, G.; Da Silva, J.A.P.; Dawson-Hughes, B. Comparing prevalence of sarcopenia using twelve sarcopenia definitions in a large multinational European population of community-dwelling older adults. J. Nutr. Health Aging 2023, 27, 205–212. [Google Scholar] [CrossRef]
- Miao, Y.; Xie, L.; Song, J.; Cai, X.; Yang, J.; Ma, X.; Chen, S.; Xie, P. Unraveling the causes of sarcopenia: Roles of neuromuscular junction impairment and mitochondrial dysfunction. Physiol. Rep. 2024, 12, e15917. [Google Scholar] [CrossRef]
- Kim, M.J.; Sinam, I.S.; Siddique, Z.; Jeon, J.H.; Lee, I.K. The link between mitochondrial dysfunction and sarcopenia: An update focusing on the role of pyruvate dehydrogenase kinase 4. Diabetes Metab. J. 2023, 47, 153–163. [Google Scholar] [CrossRef]
- Priego, T.; Martín, A.I.; González-Hedström, D.; Granado, M.; López-Calderón, A.; Cardalini, D. Role of hormones in sarcopenia. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2021; Volume 115, pp. 535–570. [Google Scholar] [CrossRef]
- Sung, J.Y.; Lee, M.J.; Kim, J. Relationship between lifestyle and physical fitness among older women with sarcopenia. Int. J. Mol. Sci. 2025, 26, 2205. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Coelho-Júnior, H.J.; Tosato, M.; Marzetti, E.; Landi, F. Diet for the prevention and management of sarcopenia. Metabolism 2023, 146, 155637. [Google Scholar] [CrossRef]
- Ely, I.A.; Phillips, B.E.; Smith, K.; Wilkinson, D.J.; Piasecki, M.; Breen, L.; Larsen, M.S.; Atherton, P.J. A focus on leucine in the nutritional regulation of human skeletal muscle metabolism in ageing, exercise and unloading states. Clin. Nutr. 2023, 42, 1849–1865. [Google Scholar] [CrossRef]
- Cailleaux, P.E.; Déchelotte, P.; Coëffier, M. Novel dietary strategies to manage sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 234–243. [Google Scholar] [CrossRef]
- Kazemi, A.; Speakman, J.R.; Soltani, S.; Djafarian, K. Effect of calorie restriction or protein intake on circulating levels of insulin-like growth factor I in humans: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 1705–1716. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Dawson-Hughes, B.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: A narrative review. Maturitas 2020, 132, 57–64. [Google Scholar] [CrossRef]
- Phillips, N.; Gray, S.R.; Combet, E.; Witard, O.C. Long-chain n-3 polyunsaturated fatty acids for the management of age- and disease-related declines in skeletal muscle mass, strength and physical function. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef]
- Robinson, S.; Granic, A.; Cruz-Jentoft, A.J.; Sayer, A.A. The role of nutrition in the prevention of sarcopenia. Am. J. Clin. Nutr. 2023, 118, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Sabir, Z.; Dierkes, J.; Hjartåker, A.; Rosendahl-Riise, H. The association of dietary patterns with muscle mass and strength in old age: The Hordaland Health Study. Eur. J. Nutr. 2023, 62, 2739–2750. [Google Scholar] [CrossRef]
- Wingrove, K.; Lawrence, M.A.; McNaughton, S.A. A systematic review of the methods used to assess and report dietary patterns. Front. Nutr. 2022, 9, 892351. [Google Scholar] [CrossRef]
- Davis, J.A.; Mohebbi, M.; Collier, F.; Loughman, A.; Staudacher, H.; Shivappa, N.; Hébert, J.R.; Pasco, J.A.; Jacka, F.N. The role of diet quality and dietary patterns in predicting muscle mass and function in men over a 15-year period. Osteoporos. Int. 2021, 32, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Pizato, N.; da Mata, F.; Figueiredo, A.; Ito, M.; Pereira, M.G. Mediterranean diet and musculoskeletal-functional outcomes in community-dwelling older people: A systematic review and meta-analysis. J. Nutr. Health Aging 2018, 22, 655–663. [Google Scholar] [CrossRef]
- Álvarez-Bustos, A.; Coelho-Junior, H.J.; Carnicero, J.A.; García-García, F.J.; Marzetti, E.; Rodriguez-Mañas, L. Adherence to the Mediterranean diet and physical activity in relation to sarcopenia: A cross-sectional epidemiological cohort study. Aging Clin. Exp. Res. 2025, 37, 164. [Google Scholar] [CrossRef]
- Boushey, C.; Ard, J.; Bazzano, L.; Heymsfield, S.; Mayer-Davis, E.; Sabaté, J.; Snetselaar, L.; Van Horn, L.; Schneeman, B.; English, L.K.; et al. Dietary Patterns and Sarcopenia: A Systematic Review; USDA: Alexandria, VA, USA, 2020. [Google Scholar] [CrossRef]
- Van Elswyk, M.E.; Teo, L.; Lau, C.S.; Shanahan, C.J. Dietary patterns and the risk of sarcopenia: A systematic review and meta-analysis. Curr. Dev. Nutr. 2022, 6, nzac001. [Google Scholar] [CrossRef]
- Peña-Ordóñez, G.G.; Bustamante-Montes, L.P.; Ramírez-Duran, N.; Sánchez-Castellano, C.; Cruz-Jentoft, A.J. Populations and outcome measures used in ongoing research in sarcopenia. Aging Clin. Exp. Res. 2017, 29, 695–700. [Google Scholar] [CrossRef]
- Ramadas, A.; Law, H.H.; Krishnamoorthy, R.; Ku, J.W.S.; Mohanty, P.; Lim, M.Z.C.; Shyam, S. Diet quality and measures of sarcopenia in developing economies: A systematic review. Nutrients 2022, 14, 868. [Google Scholar] [CrossRef]
- Carvalho do Nascimento, P.R.; Bilodeau, M.; Poitras, S. How do we define and measure sarcopenia? A meta-analysis of observational studies. Age Ageing 2021, 50, 1906–1913. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Healthy Diet; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 17 February 2025).
- Food and Agriculture Organization of the United Nations; World Health Organization. Sustainable Healthy Diets: Guiding Principles; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2019. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public. Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Ghoreishy, S.M.; Koujan, S.E.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Relationship between healthy eating index and sarcopenia in elderly people. BMC Geriatr. 2023, 23, 3734. [Google Scholar] [CrossRef]
- Das, A.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Ribeiro, R.V.; Simpson, S.J.; Hirani, V. Associations between nutrient intakes and dietary patterns with different sarcopenia definitions in older Australian men: The Concord Health and Ageing in Men Project. Public. Health Nutr. 2021, 24, 4490–4505. [Google Scholar] [CrossRef]
- Isanejad, M.; Sirola, J.; Mursu, J.; Rikkonen, T.; Kröger, H.; Tuppurainen, M.; Erkkilä, A.T. Association of the Baltic Sea and Mediterranean diets with indices of sarcopenia in elderly women: The OSTPRE-FPS study. Eur. J. Nutr. 2018, 57, 1435–1448. [Google Scholar] [CrossRef]
- Soltani, S.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Association of dietary approaches to stop hypertension eating style and risk of sarcopenia. Sci. Rep. 2020, 10, 19375. [Google Scholar] [CrossRef]
- Bagheri, A.; Soltani, S.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr. J. 2020, 19, 133. [Google Scholar] [CrossRef]
- Karlsson, M.; Becker, W.; Michaëlsson, K.; Cederholm, T.; Sjögren, P. Associations between dietary patterns at age 71 and the prevalence of sarcopenia 16 years later. Clin. Nutr. 2020, 39, 1077–1084. [Google Scholar] [CrossRef]
- Bagheri, A.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Patterns of nutrient intake in relation to sarcopenia and its components. Front. Nutr. 2021, 8, 645072. [Google Scholar] [CrossRef]
- Granic, A.; Mendonça, N.; Sayer, A.A.; Hill, T.R.; Davies, K.; Siervo, M.; Mathers, J.C.; Jagger, C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: The Newcastle 85+ study. Clin. Nutr. 2020, 39, 166–173. [Google Scholar] [CrossRef]
- Karlsson, M.; Becker, W.; Cederholm, T.E.; Byberg, L. A posteriori dietary patterns in 71-year-old Swedish men and the prevalence of sarcopenia 16 years later. Br. J. Nutr. 2022, 128, 909–920. [Google Scholar] [CrossRef]
- Mazza, E.; Ferro, Y.; Maurotti, S.; Micale, F.; Boragina, G.; Russo, R.; Lascala, L.; Sciacqua, A.; Gazzaruso, C.; Montalcini, T.; et al. Association of dietary patterns with sarcopenia in adults aged 50 years and older. Eur. J. Nutr. 2024, 63, 1651–1662. [Google Scholar] [CrossRef]
- Hashemi, R.; Motlagh, A.D.; Heshmat, R.; Esmaillzadeh, A.; Payab, M.; Yousefinia, M.; Siassi, F.; Pasalar, P.; Baygi, F. Diet and its relationship to sarcopenia in community-dwelling Iranian elderly: A cross-sectional study. Nutrition 2015, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Merkies, I.S.; Schmitz, P.I.; Samijn, J.P.; Meché, F.G.; Toyka, K.V.; van Doorn, P.A. Assessing grip strength in healthy individuals and patients with immune-mediated polyneuropathies. Muscle Nerve 2000, 23, 1393–1401. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Detopoulou, P.; Voulgaridou, G.; Tsoumana, D.; Spanoudaki, M.; Sadikou, F.; Papadopoulou, V.G.; Zidrou, C.; Chatziprodromidou, I.P.; Giaginis, C.; et al. Mediterranean diet and sarcopenia features in apparently healthy adults over 65 years: A systematic review. Nutrients 2023, 15, 1104. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary patterns, skeletal muscle health, and sarcopenia in older adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D.; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE). Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- Sharma, B.; Dabur, R. Role of pro-inflammatory cytokines in regulation of skeletal muscle metabolism: A systematic review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, G.E.; Martínez-Gómez, L.E.; Martínez-Armenta, C.; Pineda, C.; Martínez-Nava, G.A.; Lopez-Reyes, A. Molecular mechanisms of inflammation in sarcopenia: Diagnosis and therapeutic update. Cells 2022, 11, 2359. [Google Scholar] [CrossRef]
- Tessier, A.J.; Wang, F.; Korat, A.A.; Eliassen, A.H.; Chavarro, J.; Grodstein, F.; Li, J.; Liang, L.; Willett, W.C.; Sun, Q.; et al. Optimal dietary patterns for healthy aging. Nat. Med. 2025, 31, 1644–1652. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Cooper, R.; Robinson, S.M. Nutrition in the prevention and treatment of skeletal muscle ageing and sarcopenia: A single nutrient, a whole food, and a whole diet approach. Proc. Nutr. Soc. 2024, 83, E54. [Google Scholar] [CrossRef]
- Bai, A.; Xu, W.; Liang, Y.; Jiang, Y.; Lin, Z. Dietary patterns from mid-through later-life in relation to sarcopenia risk over 20 years among Chinese community-dwelling oldest old individuals. Clin. Nutr. 2023, 42, 2569–2577. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Ognenoska, V.; Trajkovska-Broach, A. Mediterranean diet: From ancient traditions to modern science—A sustainable way towards better health, wellness, longevity, and personalized nutrition. Sustainability 2025, 17, 4187. [Google Scholar] [CrossRef]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Foods of the Mediterranean diet: Tomato, olives, chili pepper, wheat flour and wheat germ. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E4–E11. [Google Scholar] [CrossRef]
- Yu, X.; Pu, H.; Voss, M. Overview of anti-inflammatory diets and their promising effects on non-communicable diseases. Br. J. Nutr. 2024, 132, 898–918. [Google Scholar] [CrossRef]
- Moresi, V.; Renzini, A.; Cavioli, G.; Seelaender, M.; Coletti, D.; Gigli, G.; Cedola, A. Functional nutrients to ameliorate neurogenic muscle atrophy. Metabolites 2022, 12, 1149. [Google Scholar] [CrossRef]
- Prokopidis, K.; Chambers, E.; Ni Lochlainn, M.; Witard, O.C. Mechanisms linking the gut-muscle axis with muscle protein metabolism and anabolic resistance: Implications for older adults at risk of sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef]
- McColl, T.J.; Clarke, D.C. Kinetic modeling of leucine-mediated signaling and protein metabolism in human skeletal muscle. iScience 2023, 27, 108634. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M. The role of dietary plant and animal protein intakes on mitigating sarcopenia risk. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 425–429. [Google Scholar] [CrossRef]
- Qi, P.; Fu, X.; Zhao, D.; Li, C.; Lu, Y.; Li, N. Effects of vitamin D supplementation on muscle strength in middle-aged and elderly individuals: A retrospective, propensity score-matched study. Front. Nutr. 2024, 11, 1450265. [Google Scholar] [CrossRef]
- Bird, J.K.; Troesch, B.; Warnke, I.; Calder, P.C. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: A scoping systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 46, 73–86. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef]
- Centonze, M.; Caruso, E.A.; De Nunzio, V.; Cofano, M.; Saponara, I.; Pinto, G.; Notarnicola, M. The antiaging potential of dietary plant-based polyphenols: A review on their role in cellular senescence modulation. Nutrients 2025, 17, 1716. [Google Scholar] [CrossRef]
- Prado, C.M.; Landi, F.; Chew, S.T.H.; Atherton, P.J.; Molinger, J.; Ruck, T.; Gonzalez, M.C. Advances in muscle health and nutrition: A toolkit for healthcare professionals. Clin. Nutr. 2022, 41, 2244–2263. [Google Scholar] [CrossRef]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet quality and sarcopenia in older adults: A systematic review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef]
- Diao, H.; Yan, F.; He, Q.; Li, M.; Zheng, Q.; Zhu, Q.; Fang, F.; Cui, W. Association between dietary inflammatory index and sarcopenia: A meta-analysis. Nutrients 2023, 15, 219. [Google Scholar] [CrossRef]
- Pu, R.; Man, Q.; Song, S.; Jia, S.; Liu, Z.; Zhang, X.; Zhang, J.; Song, P. The dietary inflammatory index and sarcopenia in older adults in four Chinese provinces: A cross-sectional study. Nutrients 2025, 17, 478. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. The influence of the Mediterranean dietary pattern on osteoporosis and sarcopenia. Nutrients 2023, 15, 3224. [Google Scholar] [CrossRef]
- Itsiopoulos, C.; Mayr, H.L.; Thomas, C.J. The anti-inflammatory effects of a Mediterranean diet: A review. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Smith, L.; Ragusa, F.S.; Schirò, P.; Di Bella, G.; Barbagallo, M. Associations between adherence to the Mediterranean diet and incident sarcopenia in prospective cohort studies. Nutrients 2025, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, A.; García-Villanova, B.; Guerra-Hernández, E.J.; Amiano, P.; Azpiri, M.; Molina-Montes, E. Description of indexes based on the adherence to the Mediterranean dietary pattern: A review. Nutr. Hosp. 2015, 32, 1872–1884. [Google Scholar] [CrossRef]
- Cacciatore, S.; Calvani, R.; Marzetti, E.; Picca, A.; Coelho-Júnior, H.J.; Martone, A.M.; Massaro, C.; Tosato, M.; Landi, F. Low adherence to Mediterranean diet is associated with probable sarcopenia in community-dwelling older adults: Results from the Longevity Check-Up (Lookup) 7+ project. Nutrients 2023, 15, 1026. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Picca, A.; Cacciatore, S.; Tosato, M.; Landi, F.; Marzetti, E. Combined aerobic training and Mediterranean diet is not associated with a lower prevalence of sarcopenia in Italian older adults. Nutrients 2023, 15, 2963. [Google Scholar] [CrossRef]
- Hong, S.H.; Bae, Y.J. Association of dietary vegetable and fruit consumption with sarcopenia: A systematic review and meta-analysis. Nutrients 2024, 16, 1707. [Google Scholar] [CrossRef]
- Pilleron, S.; Pérès, K.; Jutand, M.A.; Helmer, C.; Dartigues, J.F.; Samieri, C.; Féart, C. Dietary patterns and risk of self-reported activity limitation in older adults from the Three-City Bordeaux Study. Br. J. Nutr. 2018, 120, 549–556. [Google Scholar] [CrossRef] [PubMed]



| Author (Ref) | Country | Study Name | n, % Sex, Age in Years (mean) | Dietary Intake Assessment Method | Dietary Pattern Type | Dietary Pattern Name |
|---|---|---|---|---|---|---|
| Ghoreishy et al. [43] | Iran | SARIR Protocol | 300, 50% women, ≥55 (66.8) | 117-item FFQ | A priori | AHEI-2010 |
| Das et al. [44] | Australia | CHAMP | 1705, 100% men, ≥70 (81) | Diet history questionnaire | A priori | Australian DGI, MDS, NRV |
| Isanejad et al. [45] | Finland | OSPTRE-EPS | 3432, 100% women, ≥65 (67.8) | 3-day food record | A priori | BSD, MED Score |
| Soltani et al. [46] | Iran | SARIR Protocol | 300, 50% women, ≥55 (66.8) | 117-item FFQ | A priori | DASH diet score |
| Bagueri et al. [47] | Iran | SARIR Protocol | 300, 50% women, ≥55 (66.8) | 117-item FFQ | A priori | DII |
| Karlsson et al. [48] | Sweden | UPSALA | 254, 100% men ≥60, (70.9) | 7-day diet record | A priori | mHDI, mMDS |
| Bagueri et al. [49] | Iran | SARIR Protocol | 300, 50% women, ≥55 (66.8) | 117-item FFQ | A posteriori | Anti, Carbo-vit, Pro-vit |
| Granic et al. [50] | UK | Newcastle 85+ Study | 757, 61% women, ≥85 (85) | 24 h MPR | A posteriori | Low Red Meat, Traditional British, Low Butter |
| Karlsson et al. [51] | Sweden | UPSALA | 257, 100% men, ≥60 (70.9) | 7-day dietary record | A posteriori | Milk & Cereal, Vegetable & Fruit, Bread & Cheese, Meat & Egg |
| Mazza et al. [52] | Italy | NR | 568, 62% women, ≥50 (61) | FFQ, 24 h recall | A posteriori | Mediterranean |
| Hashemi et al. [53] | Iran | SARIR Protocol | 296, 50% women, ≥55 (66.8) | 117-item FFQ | A posteriori | Mediterranean, Western, Mixed |
| Sarcopenia Components and Cutoff Points | ||||||
|---|---|---|---|---|---|---|
| Author | Method (% Sarcopenia) | Muscle Mass | Muscle Strength | Physical Performance | Results | Adjusted Variables |
| Ghoreishy et al. [43] | EWGSOP1 (NR) | DXA ASM < 5.45 kg/m2 (W) ASM < 7.26 kg/m2 (M) | HGS [54] | GS (4 m) <0.8 m/s | AHEI-2010 OR T3 vs. T1: 0.55 (95% CI: 0.22–1.37) | Age, BMI, sex, energy intake, physical activity, smoking, alcohol, medications. |
| EWGSOP2 (10.3) | DXA ASM < 5.45 kg/m2 (W) ASM < 7.26 kg/m2 (M) | AHEI-2010 OR T3 vs. T1: 0.39 (95% CI: 0.10–1.51) | ||||
| Das et al. [44] | EWGSOP1 (12.9) | DXA ASM < 7.25 kg/m2 (M) | HGS <30 kg | GS (6 m) <0.8 m/s | aDGI OR 1.00 (95% CI: 0.97–1.03) MDS OR 0.55 (95% CI: 0.28–1.09) NRV OR 1.44 (95% CI: 0.72–2.87) | Age, BMI, energy intake, alcohol, physical activity, smoking, MMSE score, marital status, living arrangement, income, SRH, meal service, able to shop for groceries, meal preparation, co-morbidities. |
| EWGSOP2 (19.6) | DXA ASM < 7.0 kg/m2 (M) | HGS <27 kg | aDGI OR 1.01 (95% CI: 0.98–1.03) MDS OR 1.05 (95% CI: 0.90–1.22) NRV OR 0.97 (95% CI: 0.47–2.01) | |||
| Isanejad et al. [45] | EWGSOP1 (23.2) | DXA RSMI Lowest quartile (M) | HGS Lowest quartile | GS (10 m) Lowest quartile | BSD OR Q4 vs. Q1 1.05 (95% CI: 0.44–1.66) MED Score OR Q4 vs. Q1 0.93 (95% CI: 0.38–1.48) | Age, energy intake, physical activity, smoking, hormone therapy, osteoporosis, rheumatoid arthritis, coronary heart disease, fat mass percentage and income. |
| Soltani et al. [46] | EWGSOP1 (NR) | DXA ASM < 5.45 kg/m2 (W) ASM < 7.26 kg/m2 (M) | HGS <20 kg (W) <30 kg (M) | GS (4 m) <0.8 m/s | DASH OR T3 vs. T1 0.78 (95% CI: 0.36–1.67) | Age, sex, energy intake, physical activity, smoking, alcohol, medication. |
| EWGSOP2 (10.3) | ASM < 5.5 kg/m2 (W) ASM < 7.0 kg/m2 (M) | DASH OR T3 vs. T1 1.04 (95% CI: 0.39–2.75) | ||||
| Bagueri et al. [47] | EWGSOP1 (17.6) | DXA ASM < 5.45 kg/m2 (W) ASM < 7.26 kg/m2 (M) | HGS [54] | GS (4 m) <0.8 m/s | DII OR T3 vs. T1 2.18 (95% CI: 1.01–4.74) | Age, sex, energy intake, physical activity, smoking, alcohol, medications, disease history. |
| Karlsson et al. [48] | EWGSOP1 (21) | DXA ASM < 7.26 kg/m2 (M) | HGS <30 kg | GS (4–10 m) <0.8 m/s | mHDI OR T3 vs. T1 0.47 (95% CI: 0.17–1.28) mMDS OR T3 vs. T1 0.33 (95% CI: 0.09–1.23) | Age, BMI, protein intake, physical activity, smoking, inflammation, morbidity, hospital stay, education, living alone. |
| Bagueri et al. [49] | EWGSOP1 (20) | DXA ASM < 5.45 kg/m2 (W) ASM < 7.26 kg/m2 (M) | HGS <20 kg (W) <30 kg (M) | GS (4 m) <0.8 m/s | Anti OR T3 vs. T1 0.25 (95% CI: 0.10–0.63) Carbo-vit OR T3 vs. T1 0.59 (95% CI: 0.25–1.36) Pro-vit OR T3 vs. T1 0.74 (95% CI: 0.31–1.76) | Age, sex, energy intake, physical activity, smoking, alcohol, medications, disease history. |
| Granic et al. [50] | EWGSOP1 (19.2) | BIA SMI < 8.87 kg/m2 (M) SMI < 6.67 kg/m2 (W) | HGS <16 kg (W) <26 kg (M) | TUG <0.8 m/s | Low Red Meat vs. Low Butter OR 1.00 (95% CI: 0.51–1.96) Traditional British vs. Low Butter OR 1.20 (95% CI: 0.59–2.42) Low Butter (reference) | Age, sex, energy intake, physical activity, smoking, alcohol, medications. |
| Karlsson et al. [51] | EWGSOP1 (21) | DXA ASM < 7.26 kg/m2 (M) | HGS <30 kg | GS (4–10 m) <0.8 m/s | Milk & Cereal: OR T3 vs. T1 0.60 (95% CI: 0.26–1.40) Vegetable & Fruit: OR T3 vs. T1 1.05 (95% CI: 0.45–2.43) Bread & Cheese: OR T3 vs. T1 0.44 (95% CI: 0.14–1.35) Meat & Egg: OR T3 vs. T1 1.72 (95% CI: 0.74–4.02) | Age, BMI, energy intake, physical activity, education, smoking, morbidity. |
| EWGSOP2 (19) | DXA ASM < 7.0 kg/m2 (M) | HGS <27 kg | GS or 5x chair stand (4–10 m) >15 s or GS < 0.8 m/s | Milk & Cereal: OR T3 vs. T1 1.13 (95% CI: 0.48–2.70 Vegetable & Fruit: OR T3 vs. T1 0.40 (95% CI: 0.17–0.94) Bread & Cheese: OR T3 vs. T1 0.53 (95% CI: 0.17–1.70) Meat & Egg: OR T3 vs. T1 1.61 (95% CI: 0.67–3.87) | ||
| Mazza et al. [52] | EWGSOP2 (9) | BIA ASM < 15 kg (W) ASM < 20 kg (M) | HGS <16 kg (W) <27 kg (M) | NR | Mediterranean OR T3 vs. T1: 0.10 (95% CI: 0.015–0.71) | NR |
| Hashemi et al. [53] | EWGSOP1 (18.1) | DXA ASM < 5.45 kg/m2 (W) ASM < 7.26 kg/m2 (M) | HGS [54] | GS (4 m) <0.8 m/s | Mediterranean OR T3 vs. T1 0.40 (95% CI: 0.17–0.97) Western OR T3 vs. T1 0.51 (95% CI: 0.21–1.24) Mixed OR T3 vs. T1 1.45 (95% CI: 0.66–3.19) | Age, sex, energy intake, physical activity, smoking, alcohol, medications, disease history. |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Total Score | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Ghoreishy et al. [43] |  |  |  |  |  |  |  |  | 8 | High |
| Das et al. [44] |  |  |  |  |  |  |  |  | 6 | High |
| Isanejad et al. [45] |  |  |  |  |  |  |  |  | 4 | Low |
| Soltani et al. [46] |  |  |  |  |  |  |  |  | 8 | High |
| Bagueri et al. [47] |  |  |  |  |  |  |  |  | 7 | High |
| Karlsson et al. [48] |  |  |  |  |  |  |  |  | 7 | High |
| Bagueri et al. [49] |  |  |  |  |  |  |  |  | 7 | High |
| Granic et al. [50] |  |  |  |  |  |  |  |  | 5 | Low |
| Karlsson et al. [51] |  |  |  |  |  |  |  |  | 7 | High |
| Mazza et al. [52] |  |  |  |  |  |  |  |  | 5 | Low |
| Hashemi et al. [53] |  |  |  |  |  |  |  |  | 8 | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Valenzuela, R.E.; Artacho, R.; Ruiz-López, M.D.; Molina-Montes, E. Healthy Dietary Patterns and Risk of Sarcopenia in Adults Aged > 50 Years: A Systematic Review and Meta-Analysis Considering EWGSOP1 and EWGSOP2 Criteria. Nutrients 2025, 17, 2764. https://doi.org/10.3390/nu17172764
Ruiz-Valenzuela RE, Artacho R, Ruiz-López MD, Molina-Montes E. Healthy Dietary Patterns and Risk of Sarcopenia in Adults Aged > 50 Years: A Systematic Review and Meta-Analysis Considering EWGSOP1 and EWGSOP2 Criteria. Nutrients. 2025; 17(17):2764. https://doi.org/10.3390/nu17172764
Chicago/Turabian StyleRuiz-Valenzuela, Roxana E., Reyes Artacho, María Dolores Ruiz-López, and Esther Molina-Montes. 2025. "Healthy Dietary Patterns and Risk of Sarcopenia in Adults Aged > 50 Years: A Systematic Review and Meta-Analysis Considering EWGSOP1 and EWGSOP2 Criteria" Nutrients 17, no. 17: 2764. https://doi.org/10.3390/nu17172764
APA StyleRuiz-Valenzuela, R. E., Artacho, R., Ruiz-López, M. D., & Molina-Montes, E. (2025). Healthy Dietary Patterns and Risk of Sarcopenia in Adults Aged > 50 Years: A Systematic Review and Meta-Analysis Considering EWGSOP1 and EWGSOP2 Criteria. Nutrients, 17(17), 2764. https://doi.org/10.3390/nu17172764







