The Role of Nutrition and Physical Activity in Modulating Disease Progression and Quality of Life in Multiple Sclerosis
Abstract
1. Introduction
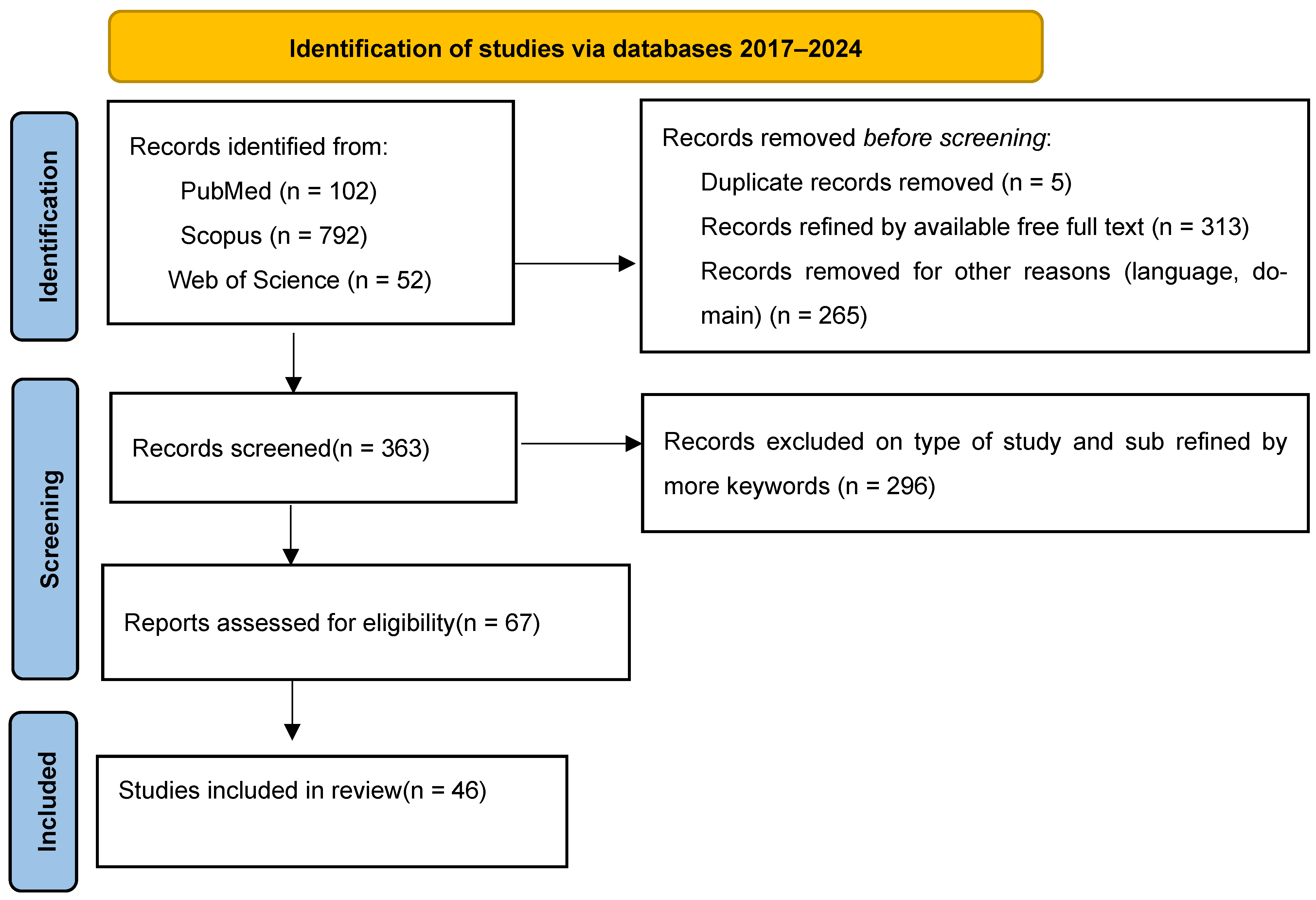
2. Materials and Methods
3. Benefits of the Mediterranean Diet in MS Patients
3.1. Reduction in MS Risk, Disease Progression, and Disability
3.2. Neuroprotection, Inflammation, and Gut Microbiota Modulation
3.3. Quality of Life, Mental and Cardiometabolic Health Outcome
4. Effects of the Ketogenic Diet in MS
4.1. Reduction in Inflammation and Neuroprotection
4.2. Improvements in Disability and Disease Progression
4.3. Impact on Fatigue and Quality of Life
4.4. Modulation of the Gut Microbiota and Metabolic Effects
4.5. Safety and Adherence Considerations
5. Effects of the Swank and Wahls Diets in MS
6. Effects of a Gluten-Free Diet in MS
7. Effects of Fasting in MS
7.1. Reduction in Inflammation and Autoimmunity
7.2. Promotion of Neuroprotection and Remyelination
7.3. Improvement in Symptoms and Quality of Life
7.4. Modulation of Metabolism and Gut Microbiota
7.5. Safety, Adherence, and Feasibility
8. Effects of Different Types of Exercises in MS
8.1. Aerobic Exercise
8.2. Resistance Training
8.3. Sensorimotor Training
8.4. Mind–Body Exercises: Yoga, Pilates, and Tai Chi
8.5. Combined Aerobic and Resistance Training
8.6. General Recommendations and Safety Considerations
9. Key Contraindications and Cautions
9.1. Ketogenic Diet (KD)
9.2. Fasting Protocols
9.3. Very Low-Fat Diets (e.g., Swank)
9.4. Wahls/Paleolithic Diet
9.5. Gluten-Free Diet (GFD)
9.6. Intensive Exercise Regimens
10. Discussions
11. Limitations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef]
- Sandroff, B.M.; Jones, C.D.; Baird, J.F.; Motl, R.W. Systematic Review on Exercise Training as a Neuroplasticity-Inducing Behavior in Multiple Sclerosis. Neurorehabil. Neural Repair 2020, 34, 575–588. [Google Scholar] [CrossRef]
- Abbasi, H.; Shakouri, F.; Mosaddeghi-Heris, R.; Gholipour-Khalili, E.; Jahanshahlou, F.; Sanaie, S.; Naseri, A.; Talebi, M. Mediterranean-like diets in multiple sclerosis: A systematic review. Rev. Neurol. 2024, 180, 1021–1030. [Google Scholar] [CrossRef]
- Cavalla, P.; Vercellino, M. May Mediterranean diet contribute to reduce risk of multiple sclerosis? Mult. Scler. 2023, 29, 045–1046. [Google Scholar] [CrossRef]
- Felicetti, F.; Tommasin, S.; Petracca, M.; De Giglio, L.; Gurreri, F.; Ianniello, A.; Nistri, R.; Pozzilli, C.; Ruggieri, S. Eating Hubs in Multiple Sclerosis: Exploring the Relationship Between Mediterranean Diet and Disability Status in Italy. Front. Nutr. 2022, 16, 882426. [Google Scholar] [CrossRef]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and Modified Mediterranean Diet as a Tool to Counteract Neuroinflammation in Multiple Sclerosis: Nutritional Suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef] [PubMed]
- Dakanalis, A.; Tryfonos, C.; Pavlidou, E.; Vadikolias, K.; Papadopoulou, S.K.; Alexatou, O.; Vorvolakos, T.; Chrysafi, M.; Fotiou, D.; Mentzelou, M.; et al. Associations between Mediterranean Diet Adherence, Quality of Life, and Mental Health in Patients with Multiple Sclerosis: A Cross-Sectional Study. J. Pers. Med. 2024, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I.; Levy, S.; Fitzgerald, K.; Sorets, T.; Sumowski, J.F. Mediterranean diet is linked to less objective disability in multiple sclerosis. Mult. Scler. 2023, 29, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Elbahrawi, R.; Abdukadir, A.; Rabeh, N.; Aljoudi, S.; Dimassi, Z.; Hamdan, H. Ketogenic Diet: Implications on Multiple Sclerosis. In Exploring the Effects of Diet on the Development and Prognosis of Multiple Sclerosis (MS), 1st ed.; Hamdan, H., Ed.; Springer: Singapore, 2024; pp. 195–205. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Wetmore, E.; Lehner-Gulotta, D.; Florenzo, B.; Banwell, B.; Bergqvist, A.G.C.; Coleman, R.; Conaway, M.; Goldman, M.D.; Brenton, J.N. Ketogenic diet in relapsing multiple sclerosis: Patient perceptions, post-trial diet adherence & outcomes. Clin. Nutr. 2023, 42, 1427–1435. [Google Scholar] [CrossRef]
- Brenton, J.N.; Lehner-Gulotta, D.; Woolbright, E.; Banwell, B.; Bergqvist, A.G.C.; Chen, S.; Coleman, R.; Conaway, M.; Goldman, M.D. Phase II study of ketogenic diets in relapsing multiple sclerosis: Safety, tolerability and potential clinical benefits. J. Neurol. Neurosurg. Psychiatry 2022, 93, 637–644. [Google Scholar] [CrossRef]
- Lin, W.S.; Lin, S.J.; Liao, P.Y.; Suresh, D.; Hsu, T.R.; Wang, P.Y. Role of Ketogenic Diets in Multiple Sclerosis and Related Animal Models: An Updated Review. Adv. Nutr. 2022, 13, 2002–2014. [Google Scholar] [CrossRef]
- Oh, U.; Woolbright, E.; Lehner-Gulotta, D.; Coleman, R.; Conaway, M.; Goldman, M.D.; Brenton, J.N. Serum neurofilament light chain in relapsing multiple sclerosis patients on a ketogenic diet. Mult. Scler. Relat. Disord. 2023, 73, 104670. [Google Scholar] [CrossRef]
- Brockhoff, J.D.; Bereswill, S.; Heimesaat, M.M. The impact of ketogenic diet on the onset and progression of multiple sclerosis. Eur. J. Microbiol. Immunol. 2023, 13, 29–36. [Google Scholar] [CrossRef]
- Ortí, J.E.R.; Cuerda-Ballester, M.; Sanchis-Sanchis, C.E.; Lajara Romance, J.M.; Navarro-Illana, E.; García Pardo, M.P. Exploring the impact of ketogenic diet on multiple sclerosis: Obesity, anxiety, depression, and the glutamate system. Front. Nutr. 2023, 10, 1227431. [Google Scholar] [CrossRef]
- Wahls, T.L.; Titcomb, T.J.; Bisht, B.; Eyck, P.T.; Rubenstein, L.M.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; Kamholz, J.; Snetselaar, L.G. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 20552173211035399. [Google Scholar] [CrossRef] [PubMed]
- Chenard, C.A.; Rubenstein, L.M.; Snetselaar, L.G.; Wahls, T.L. Nutrient Composition Comparison between a Modified Paleolithic Diet for Multiple Sclerosis and the Recommended Healthy U.S.-Style Eating Pattern. Nutrients 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Titcomb, T.J.; Brooks, L.; Smith, K.L.; Ten Eyck, P.; Rubenstein, L.M.; Wahls, T.L.; Snetselaar, L.G. Change in Micronutrient Intake among People with Relapsing-Remitting Multiple Sclerosis Adapting the Swank and Wahls Diets: An Analysis of Weighed Food Records. Nutrients 2021, 13, 3507. [Google Scholar] [CrossRef] [PubMed]
- Irish, A.K.; Erickson, C.M.; Wahls, T.L.; Snetselaar, L.G.; Darling, W.G. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: A pilot study. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Shemirani, F.; Titcomb, T.J.; Saxby, S.M.; Eyck, P.T.; Rubenstein, L.M.; Hoth, K.F.; Snetselaar, L.G.; Wahls, T.L. Association of serum homocysteine, folate, and vitamin B12 and mood following the Swank and Wahls elimination dietary interventions in relapsing-remitting multiple sclerosis: Secondary analysis of the WAVES trial. Mult. Scler. Relat. Disord. 2023, 75, 104743. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Yogev, N.; Hauptmann, J.; Nikolaev, A.; Pickert, G.; Heib, V.; Fittler, N.; Steven, S.; Luessi, F.; Neerukonda, M.; et al. Dietary wheat amylase trypsin inhibitors exacerbate CNS inflammation in experimental multiple sclerosis. Gut 2023, 73, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases. Nutrients 2020, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.L.; Jessen, E.B.; Passali, M.; Frederiksen, J.L. The role of gluten in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2019, 27, 156–163. [Google Scholar] [CrossRef]
- Morales-Suarez-Varela, M.; Collado Sánchez, E.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef]
- Bai, M.; Wang, Y.; Han, R.; Xu, L.; Huang, M.; Zhao, J.; Lin, Y.; Song, S.; Chen, Y. Intermittent caloric restriction with a modified fasting-mimicking diet ameliorates autoimmunity and promotes recovery in a mouse model of multiple sclerosis. J. Nutr. Biochem. 2021, 87, 108493. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Bhargava, P.; Smith, M.D.; Vizthum, D.; Henry-Barron, B.; Kornberg, M.D.; Cassard, S.D.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. EBioMedicine 2022, 82, 104124. [Google Scholar] [CrossRef] [PubMed]
- Lorefice, L.; Pitzalis, M.; Zoledziewska, M. Intermittent and periodic fasting—Evidence and perspectives in multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 88, 105744. [Google Scholar] [CrossRef]
- Hansen, B.; Roomp, K.; Ebid, H.; Schneider, J.G. Perspective: The Impact of Fasting and Caloric Restriction on Neurodegenerative Diseases in Humans. Adv. Nutr. 2024, 15, 100197. [Google Scholar] [CrossRef]
- Wingo, B.C.; Rinker, J.R., 2nd; Green, K.; Peterson, C.M. Feasibility and acceptability of time-restricted eating in a group of adults with multiple sclerosis. Front. Neurol. 2023, 13, 1087126. [Google Scholar] [CrossRef]
- Gudden, J.; Arias Vasquez, A.; Bloemendaal, M. The Effects of Intermittent Fasting on Brain and Cognitive Function. Nutrients 2021, 13, 3166. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Gao, Y. The effects of intermittent fasting for patients with multiple sclerosis (MS): A systematic review. Front. Nutr. 2024, 10, 1328426. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Vizthum, D.; Henry-Barron, B.; Schweitzer, A.; Cassard, S.D.; Kossoff, E.; Hartman, A.L.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.N.; Fitzgerald, K.C.; Beier, M.; Mowry, E.M. Safety and feasibility of various fasting-mimicking diets among people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 42, 102149. [Google Scholar] [CrossRef]
- Bellisario, V.; Squillacioti, G.; Ghelli, F.; Monti, M.C.; Correale, L.; Montomoli, C.; Bono, R. Inflammation and physical activity in multiple sclerosis patients. A systematic review and meta-analysis. Complement. Ther. Med. 2024, 82, 103040. [Google Scholar] [CrossRef]
- Razazian, N.; Kazeminia, M.; Moayedi, H.; Daneshkhah, A.; Shohaimi, S.; Mohammadi, M.; Jalali, R.; Salari, N. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: A systematic review and meta-analysis. BMC Neurol. 2020, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Reina-Gutiérrez, S.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Núñez de Arenas-Arroyo, S.; López-Muñoz, P.; Álvarez-Bueno, C.; Guzmán-Pavón, M.J.; Torres-Costoso, A. The type of exercise most beneficial for quality of life in people with multiple sclerosis: A network meta-analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101578. [Google Scholar] [CrossRef]
- Proschinger, S.; Kuhwand, P.; Rademacher, A.; Walzik, D.; Warnke, C.; Zimmer, P.; Joisten, N. Fitness, physical activity, and exercise in multiple sclerosis: A systematic review on current evidence for interactions with disease activity and progression. J. Neurol. 2022, 269, 2922–2940. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult. Scler. J. 2020, 26, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Bahr, L.S.; Bock, M.; Liebscher, D.; Bellmann-Strobl, J.; Franz, L.; Prüß, A.; Schumann, D.; Piper, S.K.; Kessler, C.S.; Steckhan, N.; et al. Ketogenic diet and fasting diet as Nutritional Approaches in Multiple Sclerosis (NAMS): Protocol of a randomized controlled study. Trials 2020, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Temperley, I.A.; Seldon, A.N.; Reckord, M.A.; Yarad, C.A.; Islam, F.T.; Duncanson, K.; Lea, R.A.; Lechner-Scott, J.; Maltby, V.E. Dairy and gluten in disease activity in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2023, 9, 20552173231218107. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Z.; Wang, S.; Ma, D.; Zhu, M.; Feng, J. Role of Gut Microbiota in Multiple Sclerosis and Potential Therapeutic Implications. Curr. Neuropharmacol. 2022, 20, 1413–1426. [Google Scholar] [CrossRef]
- Lee, A.R.; Wolf, R.L.; Lebwohl, B.; Ciaccio, E.J.; Green, P.H.R. Persistent Economic Burden of the Gluten Free Diet. Nutrients 2019, 11, 399. [Google Scholar] [CrossRef]
- Edwards, T.; Pilutti, L.A. The effect of exercise training in adults with multiple sclerosis with severe mobility disability: A systematic review and future research directions. Mult. Scler. Relat. Disord. 2017, 16, 31–39. [Google Scholar] [CrossRef]
- Baird, J.F.; Motl, R.W. Response Heterogeneity With Exercise Training and Physical Activity Interventions Among Persons With Multiple Sclerosis. Neurorehabil. Neural Repair 2019, 33, 3–14. [Google Scholar] [CrossRef]
- Silarova, A.; Hvid, L.G.; Hradílek, P.; Dalgas, U. Exercise-induced heat sensitivity in patients with multiple sclerosis: Definition, prevalence, etiology, and management-A scoping review. Mult. Scler. Relat. Disord. 2024, 90, 105827. [Google Scholar] [CrossRef]
- Ispoglou, T.; Ferentinos, P.; Prokopidis, K.; Blake, C.; Aldrich, L.; Elia, A.; Lees, M.; Hind, K. Exploring the impact of exercise and essential amino acid plus cholecalciferol supplementation on physical fitness and body composition in multiple sclerosis: A case study. Clin. Case Rep. 2023, 11, e7548. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Michońska, I. Macronutrients, vitamins and minerals in the diet of multiple sclerosis patients. Postep. Psychiatr. Neurol. 2022, 31, 128–137. [Google Scholar] [CrossRef]
- Allogmanny, S.; Stefoska-Needham, A.; Probst, Y. Exploring the Perspectives of Healthcare Consumers Towards the Integration of Nutrition in Routine Multiple Sclerosis Care: A Qualitative Study. J. Hum. Nutr. Diet. 2025, 38, e70042. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; Cheek, J.J.; Fox, S.S.; Healy, H.S.; Schweizer, M.L.; Bao, W.; Kamholz, J.; Titcomb, T.J. Efficacy of Diet on Fatigue and Quality of Life in Multiple Sclerosis: A Systematic Review and Network Meta-analysis of Randomized Trials. Neurology 2023, 100, e357–e366. [Google Scholar] [CrossRef] [PubMed]
- Opsommer, E.; Ribeiro, C.; Carrard, S.; Hilfiker, R.; Mbarga, J. Exploring the integration and patient engagement of balance home exercises in the daily management of multiple sclerosis: A comprehensive qualitative analysis. Disabil. Rehabil. 2024, 47, 3938–3946. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Study Type | Duration | Fasting Regimen | MS Patients (n) | MS Type | Adherence |
|---|---|---|---|---|---|---|
| Morales-Suarez-Varela et al., 2021 [26] | Systematic Review of RCTs | Varied | Intermittent fasting | Not specified | Multiple (not detailed) | Varied; not uniformly reported |
| Fitzgerald et al., 2022 [28] | Randomized Controlled Trial (RCT) | 12 weeks | Intermittent Calorie Restriction | 36 | Relapsing-remitting MS (RRMS) | High (monitored and reported) |
| Wingo et al., 2023 [31] | Feasibility Study | 8 weeks | Time-Restricted Eating | 19 | Not specified | Good (based on reported compliance) |
| Lin et al., 2024 [33] | Systematic review | Varied | Intermittent Fasting | Included studies (n not stated) | Primarily RRMS | Generally high in included studies |
| Fitzgerald et al., 2018 [34] | Randomized Controlled Trial | 8 weeks | Intermittent vs. Daily Calorie Restriction | 36 | Relapsing-remitting MS (RRMS) | Good (monitored and analyzed) |
| Roman et al., 2020 [35] | Feasibility Study | Short-term (5–7 days cycles) | Fasting-Mimicking Diets | 16 | Relapsing-remitting MS (RRMS) | Acceptable; tolerability reported |
| Diet Type | Type of Studies | Types of MS Form | Key Benefits | Risks/Limitations | References |
|---|---|---|---|---|---|
| Mediterranean | Observational, cross-section, narrative reviews | RRMS SPMS | ↓ Inflammation, ↑ BDNF, ↓ Disability (EDSS), ↑ QoL | Limited RCTs; adherence varies | Di Majo et al., 2022 [6]; Felicetti et al., 2022 [5]; Dakanalis et al., 2024 [8] |
| Ketogenic | Phase II clinical trial, narrative review, survey | RRMS | ↓ Inflammation, ↑ Mitochondrial function, ↓ Fatigue, ↑ Neuroprotection | GI issues, hard adherence, potential nutrient deficits | Brenton et al., 2022 [13]; Di Majo et al., 2022 [6]; Wetmore et al., 2023 [12] |
| Wahls Diet | RCT, nutritional intake analysis | RRMS | ↓ Fatigue, ↑ Mental health, ↑ Nutrient density | Highly restrictive, risk of calcium/B12 deficiency | Wahls et al., 2021 [18]; Titcomb et al., 2021 [20] |
| Swank Diet | Descriptive diet comparison, RCT | RRMS | ↓ Fatigue, slower disability progression | Risk of EFA & vitamin D deficiency | Chenard et al., 2019 [19]; Wahls et al., 2021 [18] |
| Gluten-Free | Review | RRMS | Possible ↓ Inflammation; ATI removal may help | Low fiber, ↑ weight gain risk, no strong evidence in non-celiac MS | Thomsen et al., 2019 [25]; Passali et al., 2020 [24] |
| Fasting | RCT, feasibility study | RRMS | ↓ Inflammation (Th1, Th17), ↑ BDNF, ↓ Fatigue | Hard to sustain, not for Type 1 diabetes, eating disorders | Bai et al., 2021 [27]; Fitzgerald et al., 2022 [28]; Wingo et al., 2023 [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, C.; Ignat, E.B.; Alexa, D.; Ciubotaru, A.; Leon, M.M.; Maștaleru, A.; Popescu, G.; Cumpăt, C.M.; Cucu, L.-E.; Smihor, M.I.; et al. The Role of Nutrition and Physical Activity in Modulating Disease Progression and Quality of Life in Multiple Sclerosis. Nutrients 2025, 17, 2713. https://doi.org/10.3390/nu17162713
Grosu C, Ignat EB, Alexa D, Ciubotaru A, Leon MM, Maștaleru A, Popescu G, Cumpăt CM, Cucu L-E, Smihor MI, et al. The Role of Nutrition and Physical Activity in Modulating Disease Progression and Quality of Life in Multiple Sclerosis. Nutrients. 2025; 17(16):2713. https://doi.org/10.3390/nu17162713
Chicago/Turabian StyleGrosu, Cristina, Emilian Bogdan Ignat, Daniel Alexa, Alin Ciubotaru, Maria Magdalena Leon, Alexandra Maștaleru, Gabriela Popescu, Carmen Marinela Cumpăt, Laura-Elena Cucu, Mădălina Irina Smihor, and et al. 2025. "The Role of Nutrition and Physical Activity in Modulating Disease Progression and Quality of Life in Multiple Sclerosis" Nutrients 17, no. 16: 2713. https://doi.org/10.3390/nu17162713
APA StyleGrosu, C., Ignat, E. B., Alexa, D., Ciubotaru, A., Leon, M. M., Maștaleru, A., Popescu, G., Cumpăt, C. M., Cucu, L.-E., Smihor, M. I., & Trofin, D. (2025). The Role of Nutrition and Physical Activity in Modulating Disease Progression and Quality of Life in Multiple Sclerosis. Nutrients, 17(16), 2713. https://doi.org/10.3390/nu17162713








