Nutritional Status Assessment Tools in Cardiovascular Patients
Abstract
1. Introduction
2. Methods
3. The Definition of Malnutrition
4. Anthropometric Measures
5. Biochemical and Laboratory Markers and Inflammation Indicators
6. Body Composition Methods as Clinical Support for Malnutrition Diagnosis
7. Dietary Assessment
8. Malnutrition Screening Scores
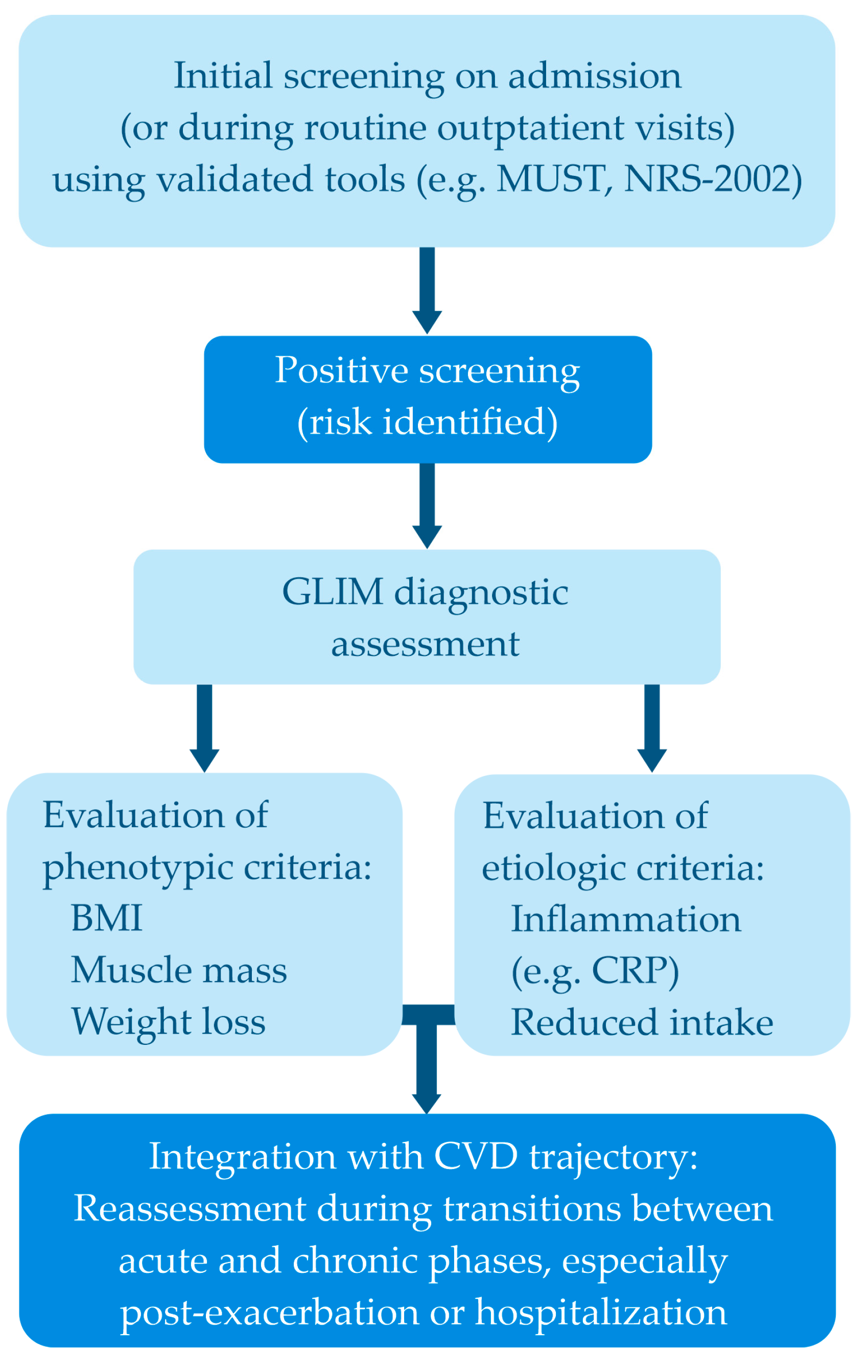
9. Suggested Integrated Clinical Assessment Strategy
10. Limitations and Research Gaps
11. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular diseases |
| HF | Heart Failure |
| BMI | Body Mass Index |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| WHO | World Health Organization |
| HFrEF | Heart failure and reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| NRS-2002 | Nutritional Risk Screening 2002 |
| MUST | The Malnutrition Universal Screening Tool |
| MNA | The Mini Nutritional Assessment |
| MNA-SF | The Mini Nutritional Assessment-Short Form |
| SGA | Subjective Global Assessment |
| CONUT | CONtrolling NUTritional status |
| WHTR | waist-to-height ratio |
| MUAC | mid-upper arm circumference |
| CC | Calf Circumference |
| SFT | Directory of open access journals |
| BIA | bioelectrical impedance analysis |
| MR | Magnetic Resonanse Imaging |
| CT | Computer Tomography Imaging |
| FFM | Fat-free mass |
| FM | Fat mass |
| TBW | Total body water |
| PA | Phase angel |
| XC | reactance |
| DXA | Dual-energy X-ray absorptiometry |
| LBM | Lean Body Mass |
| BMC | bone mineral content |
| ASPEN | the American Society of Parenteral and Enteral Nutrition |
| CRP | C-reactive protein |
| HPA | hypothalamic-pituitary-adrena |
| GLIM | Global Leadership Initiative on Malnutrition |
References
- Malone, A.; Mogensen, K.M. Key approaches to diagnosing malnutrition in adults. Nutr. Clin. Prac. 2022, 37, 23–34. [Google Scholar] [CrossRef]
- Liu, B.; Wu, X.; Wang, Y.; Hu, X. Association between cardiac cachexia and adverse outcomes in patients with heart failure: A meta-analysis of cohort studies. Heart 2025. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Singh, P.K.; Roy, P.; Kakar, S.S. Cardiac Cachexia: Unaddressed Aspect in Cancer Patients. Cells 2022, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Emami, A.; Saitoh, M.; Valentova, M.; Sandek, A.; Evertz, R.; Ebner, N.; Loncar, G.; Springer, J.; Doehner, W.; Lainscak, M.; et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: Results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur. J. Heart Fail. 2018, 20, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.; Kamel, A.; Ahmed, M.M.; Kramer, J. Pharmacological management of cardiac cachexia: A review of potential therapy options. Heart Fail. Rev. 2019, 24, 617–623. [Google Scholar] [CrossRef]
- Prokopidis, K.; Isanejad, M.; Akpan, A.; Stefil, M.; Tajik, B.; Giannos, P.; Venturelli, M.; Sankaranarayanan, R. Exercise and nutritional interventions on sarcopenia and frailty in heart failure: A narrative review of systematic reviews and meta-analyses. ESC Heart Fail. 2022, 9, 2787–2799. [Google Scholar] [CrossRef]
- Kida, K.; Miyajima, I.; Suzuki, N.; Greenberg, B.H.; Akashi, Y.J. Nutritional management of heart failure. J. Cardiol. 2023, 81, 283–291. [Google Scholar] [CrossRef]
- Rashid, M.; Kwok, C.S.; Gale, C.P.; Doherty, P.; Olier, I.; Sperrin, M.; Kontopantelis, E.; Peat, G.; Mamas, M.A. Impact of co-morbid burden on mortality in patients with coronary heart disease, heart failure, and cerebrovascular accident: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2017, 3, 20–36. [Google Scholar] [CrossRef]
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. Biomed. Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Magnesium for the prevention and treatment of cardiovascular disease. Open Heart 2018, 5, e000775. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Mollace, R.; Ritorto, G.; Ussia, S.; Altomare, C.; Tavernese, A.; Preianò, M.; Palma, F.; Muscoli, C.; Mollace, V.; et al. A Systematic Review of Thiamine Supplementation in Improving Diabetes and Its Related Cardiovascular Dysfunction. Int. J. Mol. Sci. 2025, 26, 3932. Available online: https://www.mdpi.com/1422-0067/26/9/3932 (accessed on 7 August 2025).
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Thiamine and Cardiovascular Disease: A Literature Review. Prog. Cardiovasc. Dis. 2018, 61, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.; Tangpricha, V. Vitamin D Deficiency and Risk for Cardiovascular Disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Pál, É.; Ungvári, Z.; Benyó, Z.; Várbíró, S. Role of Vitamin D Deficiency in the Pathogenesis of Cardiovascular and Cerebrovascular Diseases. Nutrients 2023, 15, 334. [Google Scholar] [CrossRef]
- Li, M. The role of vitamin D in chronic obstructive pulmonary disease with pulmonary hypertension. Pulm. Circ. 2023, 13, e12294. [Google Scholar] [CrossRef]
- Wleklik, M.; Uchmanowicz, I.; Jankowska-Polaå„Ska, B.; Andreae, C.; Regulska-Ilow, B. The Role of Nutritional Status in Elderly Patients with Heart Failure. J. Nutr. Health Aging 2018, 22, 581–588. [Google Scholar] [CrossRef]
- Szymański, F.M.; Bomba-Opoń, D.A.; Łęgosz, P.; Głogowska-Szeląg, J.; Baran, W.; Szepietowski, J.C.; Kos-Kudła, B.; Filipiak, K.J.; Kozłowska-Wojciechowska, M. Miejsce witaminy D w codziennej praktyce klinicznej—Interdyscyplinarne stanowisko ekspertów. Forum Med. Rodz. 2015, 9, 423–434. [Google Scholar]
- Eshak, E.S.; Arafa, A.E. Thiamine deficiency and cardiovascular disorders. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 965–972. [Google Scholar] [CrossRef]
- Voultsos, P.M.M.; Bazmpani, M.-A.M.M.; Papanastasiou, C.A.M.M.; Papadopoulos, C.E.; Efthimiadis, G.; Karvounis, H.; Kalogeropoulos, A.P.; Karamitsos, T.D. Magnesium Disorders and Prognosis in Heart Failure: A Systematic Review. Cardiol. Rev. 2022, 30, 281–285. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Undas, A.; Perła-Kaján, J.; Głowacki, R. Homocysteine in adult patients with cardiovascular disease: Is it clinically relevant in 2025? A tribute to Hieronim Jakubowski (1946–2025). Pol. Arch. Intern. Med. 2025, 135, 17012. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.R. Mechanisms of homocysteine-induced atherothrombosis. J. Thromb. Haemost. 2005, 3, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, R.; Wang, K.; Wang, S.; Chow, A.; Yang, A.K.; Lu, Y.; Leo, C. Effects of B Vitamins on Homocysteine Lowering and Thrombotic Risk Reduction-A Review of Randomized Controlled Trials Published Since January 1996. Nutrients 2025, 17, 1122. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Demir, M.C.; Kucukdemirci, O. Investigation of Vitamin B12 Deficiency in Patients with Acute Coronary Syndrome and its Relationship with Gensini Score. Clin. Lab. 2022, 68, 307–313. [Google Scholar] [CrossRef]
- Zhou, F.; He, Y.; Xie, X.; Guo, N.; Chen, W.; Zhao, Y. Homocysteine and Multiple Health Outcomes: An Outcome-Wide Umbrella Review of Meta-analyses and Mendelian Randomization Studies. Adv. Nutr. 2025, 16, 100434. [Google Scholar] [CrossRef]
- Habaybeh, D.; De Moraes, M.B.; Slee, A.; Avgerinou, C. Nutritional interventions for heart failure patients who are malnourished or at risk of malnutrition or cachexia: A systematic review and meta-analysis. Heart Fail. Rev. 2021, 26, 1103–1118. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Kalisz, G.; Zembala, M. The Application of Bioelectrical Impedance Analysis Phase Angle in Cardiac Surgery. Nutrients 2025, 17, 1914. [Google Scholar] [CrossRef]
- Martínez-Ortega, A.J.; Piñar-Gutiérrez, A.; Serrano-Aguayo, P.; González-Navarro, I.; Remón-Ruíz, P.J.; Pereira-Cunill, J.L.; García-Luna, P.P. Perioperative Nutritional Support: A Review of Current Literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef]
- Bae, M.I.; Shim, J.-K.; Lee, H.S.; Jeon, S.; Kwak, Y.-L. Predictive value of postoperative prognostic nutritional index trajectory for mortality outcomes after off-pump coronary artery bypass surgery: A retrospective cohort study. Front. Nutr. 2025, 12, 1530651. [Google Scholar] [CrossRef]
- Abe, T.; Inao, T.; Shingu, Y.; Yamada, A.; Takada, S.; Fukushima, A.; Oyama-Manabe, N.; Yokota, I.; Wakasa, S.; Kinugawa, S.; et al. Associations of sarcopenia and malnutrition with 30-day in-hospital morbidity and mortality after cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2024, 67, ezae456. [Google Scholar] [CrossRef]
- Pavone, N.; Cammertoni, F.; Bruno, P.; Cutrone, G.; Chiariello, G.; Calabrese, M.; Grandinetti, M.; Nesta, M.; Marzetti, E.; Calvani, R.; et al. Does a Poor Preoperative Nutritional Status Impact outcomes of Heart Valve Surgery? J. Frailty Aging 2024, 13, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Tonet, E.; Campana, R.; Caglioni, S.; Gibiino, F.; Fiorio, A.; Chiaranda, G.; Zagnoni, S.; Casella, G.; Campo, G. Tools for the Assessment of the Malnutrition Status and Possible Interventions in Elderly with Cardiovascular Diseases. J. Clin. Med. 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Mirzai, S.; Carbone, S.; Batsis, J.A.; Kritchevsky, S.B.; Kitzman, D.W.; Shapiro, M.D. Sarcopenic Obesity and Cardiovascular Disease: An Overlooked but High-Risk Syndrome. Curr. Obes. Rep. 2024, 13, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Zaghloul, A.; Halawani, S.H.; Fatani, B.A.; Alshareef, B.; Almalki, A.; Alsharif, E.; Alhadhrami, S.; Elmoneim, H.M.A.; ALhothaly, Q.A. Prevalence of Overweight/Obesity Associated with Anemia Among Female Medical Students at Umm Al-Qura University in Makkah, Saudi Arabia: A Cross-Sectional Study. Cureus 2024, 16. Available online: https://pubmed.ncbi.nlm.nih.gov/38681278/ (accessed on 7 August 2025).
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- World Health Organization. Malnutrition—Questions and Answers. Available online: https://www.who.int/news-room/questions-and-answers/item/malnutrition (accessed on 1 August 2025).
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J. Parenter. Enter. Nutr. 2010, 34, 156–159. [Google Scholar] [CrossRef]
- Madini, N.; Vincenti, A.; Beretta, A.; Santero, S.; Viroli, G.; Cena, H. Addressing Inflammaging and Disease-Related Malnutrition: Adequacy of Oral Nutritional Supplements in Clinical Care. Nutrients 2024, 16, 4141. [Google Scholar] [CrossRef]
- Stumpf, F.; Keller, B.; Gressies, C.; Schuetz, P. Inflammation and Nutrition: Friend or Foe? Nutrients 2023, 15, 1159. [Google Scholar] [CrossRef]
- Hegazi, R.; Miller, A.; Sauer, A. Evolution of the diagnosis of malnutrition in adults: A primer for clinicians. Front. Nutr. 2024, 11, 1169538. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Kwak, M.J.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lin, S.; Cao, Z.; Yu, R.; Fan, Y.; Chen, J. The role of chronic low-grade inflammation in the development of sarcopenia: Advances in molecular mechanisms. Int. Immunopharmacol. 2025, 147, 114056. [Google Scholar] [CrossRef]
- Wojzischke, J.; Van Wijngaarden, J.; van den Berg, C.; Yavuz, A.C.; Diekmann, R.; Luiking, Y.; Bauer, J. Nutritional status and functionality in geriatric rehabilitation patients: A systematic review and meta-analysis. Eur. Geriatr. Med. 2020, 11, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández, A.; Villar-Taibo, R.; Alejo, M.; Arroyo, D.; Palomas, J.L.B.; Cachero, M.; Joaquin, C.; Bailón, M.M.; Pérez-Rivera, J.Á.; Romero-Vigara, J.C.; et al. Diagnosis and Management of Malnutrition in Patients with Heart Failure. J. Clin. Med. 2023, 12, 3320. [Google Scholar] [CrossRef]
- Cereda, E.; Pedrolli, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Turri, A.; Rondanelli, M.; Caccialanza, R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA ®. Clin. Nutr. 2016, 35, 1282–1290. [Google Scholar] [CrossRef]
- Locks, L.M.; Parekh, A.; Newell, K.; Dauphinais, M.R.; Cintron, C.; Maloomian, K.; A Yu, E.; Finkelstein, J.L.; Mehta, S.; Sinha, P. The ABCDs of Nutritional Assessment in Infectious Diseases Research. J. Infect. Dis. 2025, 231, 562–572. [Google Scholar] [CrossRef]
- Gołacki, J.; Witczak, K.; Górecki, K.; Szafraniec-Porada, A.; Wronecki, J.; Porada, D.; Matyjaszek-Matuszek, B. Bioimpedance Body Composition Analysis in Estimating Insulin Resistance in Women with Overweight and Obesity (LUCAS 1.1): A Retrospective Analysis. Clin. Diabetol. 2025, 14, 5–11. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Khanna, D.; Peltzer, C.; Kahar, P.; Parmar, M.S. Body Mass Index (BMI): A Screening Tool Analysis. Cureus 2022, 14, e22119. [Google Scholar] [CrossRef]
- Busetto, L.; Dicker, D.; Frühbeck, G.; Halford, J.C.G.; Sbraccia, P.; Yumuk, V.; Goossens, G.H. A new framework for the diagnosis, staging and management of obesity in adults. Nat. Med. 2024, 30, 2395–2399. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Chrominski, T.; Szczasny, M.; Blaszczak, P. Nutritional Status Predicts the Length of Stay and Mortality in Patients Undergoing Electrotherapy Procedures. Nutrients 2024, 16, 843. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Blaszczak, P. Nutritional Status of Coronary Artery Disease Patients-Preliminary Results. Int. J. Environ. Res. Public. Health 2023, 20, 3464. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Rovere, L.D.; Fernández-Jiménez, R.; Hardy-Añón, C.; Herola-Cobos, C.; Garcia-Olivares, M.; Fernández, J.A.; Sánchez, F.H.; Jiménez, V.M.; Aguilar, I.V.; et al. The usefulness of the updated bioelectrical impedance vector analysis references for assessing malnutrition, sarcopenia and predicting mortality in hospitalized patients. Clin. Nutr. 2025, 47, 187–195. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.G.-V.; Weerink, L.B.; Milovanovic, M.; Haveman, J.-W.; Hemmer, P.H.; Dijkstra, G.; Lindeboom, R.; Campmans-Kuijpers, M.J. Bioelectrical Impedance Analysis and Mid-Upper Arm Muscle Circumference Can Be Used to Detect Low Muscle Mass in Clinical Practice. Nutrients 2021, 13, 2350. [Google Scholar] [CrossRef]
- Ceolin, C.; Acunto, V.; Simonato, C.; Cazzavillan, S.; Vergadoro, M.; Papa, M.V.; Trapella, G.S.; Sermasi, R.; Noale, M.; De Rui, M.; et al. New Perspectives in the Association between Anthropometry and Mortality: The Role of Calf Circumference. J. Frailty Aging 2024, 13, 108–115. [Google Scholar] [CrossRef]
- Ishida, Y.; Maeda, K.; Nonogaki, T.; Shimizu, A.; Yamanaka, Y.; Matsuyama, R.; Kato, R.; Mori, N. Impact of edema on length of calf circumference in older adults. Geriatr. Gerontol. Int. 2019, 19, 993–998. [Google Scholar] [CrossRef]
- Ashtekar, S.V.; Padhyegurjar, M.S.; Padhyegurjar, S.B.; Powar, J.D. Nutritional assessment with skinfold thickness and body- fat proportion in tribal and urban schoolchildren in Nashik district: A cross sectional study. J. Fam. Med. Prim. Care 2022, 11, 3148–3155. [Google Scholar] [CrossRef]
- Yin, L.; Fan, Y.; Lin, X.; Zhang, L.; Li, N.; Liu, J.; Guo, J.; Zhang, M.; He, X.; Liu, L.; et al. Fat mass assessment using the triceps skinfold thickness enhances the prognostic value of the Global Leadership Initiative on Malnutrition criteria in patients with lung cancer. Br. J. Nutr. 2022, 127, 1506–1516. [Google Scholar] [CrossRef]
- Ulijaszek, S.J.; Kerr, D.A. Anthropometric measurement error and the assessment of nutritional status. Br. J. Nutr. 1999, 82, 165–177. [Google Scholar] [CrossRef]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; the ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Chiba, T.; Yokota, J.; Takahashi, R.; Sasaki, K.; Suzuki, H. Prealbumin level is a predictor of activities of daily living at discharge in older patients with heart failure who became ADL-independent after hospitalization. Jpn. J. Compr. Rehabilitation Sci. 2023, 14, 69–77. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Dellière, S.; Cynober, L. Is transthyretin a good marker of nutritional status? Clin. Nutr. 2017, 36, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Cabré, M.; Ferreiro, C.; Arus, M.; Roca, M.; Palomera, E.; Serra-Prat, M. Evaluation of conut for clinical malnutrition detection and short-term prognostic assessment in hospitalized elderly people. J. Nutr. Health Aging 2015, 19, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, Y.; Sota, T.; Kamitani, H.; Nakayama, N.; Nakamura, K.; Hirai, M.; Yanagihara, K.; Kato, M.; Ono, T.; Takahashi, M.; et al. Diagnostic performance of nutritional indicators in patients with heart failure. ESC Heart Fail. 2022, 9, 2096–2106. [Google Scholar] [CrossRef]
- Raat, W.; Nees, L.; Vaes, B. Diagnostic accuracy of signs and symptoms in acute coronary syndrome and acute myocardial infarction: A diagnostic meta-analysis. Scand. J. Prim. Health Care 2025, 43, 111–119. [Google Scholar] [CrossRef]
- Lai, A.R.; Warrier, M.; Ng, E.Z.; Lin, C.; Chin, Y.H.; Kong, G.; Anand, V.V.; Lee, E.C.; Lai, H.; Ng, H.W.; et al. Cardiovascular Outcomes in Acute Coronary Syndrome and Malnutrition. JACC Adv. 2023, 2, 100635. [Google Scholar] [CrossRef]
- Salinas, G.L.A.; Cepas-Guillén, P.; León, A.M.; Jiménez-Méndez, C.; Lozano-Vicario, L.; Martínez-Avial, M.; Díez-Villanueva, P. The Impact of Geriatric Conditions in Elderly Patients with Coronary Heart Disease: A State-of-the-Art Review. J. Clin. Med. 2024, 13, 1891. [Google Scholar] [CrossRef]
- Hersberger, L.; Dietz, A.; Bürgler, H.; Bargetzi, A.; Bargetzi, L.; Kägi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2307–2319. [Google Scholar] [CrossRef]
- Charkiewicz, M.; Wojszel, Z.B.; Kasiukiewicz, A.; Magnuszewski, L.; Wojszel, A. Association of Chronic Heart Failure with Frailty, Malnutrition, and Sarcopenia Parameters in Older Patients—A Cross-Sectional Study in a Geriatric Ward. J. Clin. Med. 2023, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Ręka, G.; Olszewska, A.; Warchulińska, J.; Piecewicz-Szczęsna, H. Methods of assessing body composition and anthropometric measurements—A review of the literature. J. Educ. Health Sport. 2021, 11, 18–27. [Google Scholar] [CrossRef]
- Ward, L.C.; Brantlov, S. Bioimpedance basics and phase angle fundamentals. Rev. Endocr. Metab. Disord. 2023, 24, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, F.; Petrella, L.; Cavaliere, G.; Ambrosio, K.; Trinchese, G.; Monda, V.; D’angelo, M.; Di Giacomo, C.; Sacconi, A.; Messina, G.; et al. A Bioelectrical Impedance Analysis in Adult Subjects: The Relationship between Phase Angle and Body Cell Mass. J. Funct. Morphol. Kinesiol. 2023, 8, 107. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Szczygiel, K. Bioelectrical Impedance Analysis and Body Composition in Cardiovascular Diseases. Curr. Probl. Cardiol. 2023, 48, 101911. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Kalisz, G. Malnutrition assessed with phase angle and mortality risk in heart failure—Meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2025. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Messina, C.; Albano, D.; Gitto, S.; Tofanelli, L.; Bazzocchi, A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quant. Imaging Med. Surg. 2020, 10, 1687–1698. [Google Scholar] [CrossRef]
- Ceniccola, G.D.; Castro, M.G.; Piovacari, S.M.F.; Horie, L.M.; Corrêa, F.G.; Barrere, A.P.N.; Toledo, D.O. Current technologies in body composition assessment: Advantages and disadvantages. Nutrition 2019, 62, 25–31. [Google Scholar] [CrossRef]
- Kuriyan, R. Body composition techniques. Indian. J. Med. Res. 2018, 148, 648–658. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Hoogendijk, E.O.; Visvanathan, R.; Wright, O.R.L. Malnutrition Screening and Assessment in Hospitalised Older People: A Review. J. Nutr. Health Aging 2019, 23, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Hollings, M.; Blaszczak, P. Nutritional risk score predicts the length of stay in patients undergoing coronary angiography. Nutr Diet 2025, 1–9. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Błaszczak, P. The impact of nutritional risk on the length of stay in patients undergoing percutaneous coronary interventions. Kardiol. Pol. 2025. [Google Scholar] [CrossRef]
- Murphy, J.; Mayor, A.; Forde, E. Identifying and treating older patients with malnutrition in primary care: The MUST screening tool. Br. J. Gen. Pract. 2018, 68, 344–345. [Google Scholar] [CrossRef]
- Brown, F.; Fry, G.; Cawood, A.; Stratton, R. Economic Impact of Implementing Malnutrition Screening and Nutritional Management in Older Adults in General Practice. J. Nutr. Health Aging 2020, 24, 305–311. [Google Scholar] [CrossRef]
- Oshima, T.; Tsutsumi, R. The Malnutrition Universal Screening Tool (MUST) Predicts Postoperative Declines in Activities of Daily Living (ADL) in Patients Undergoing Cardiovascular Open-Heart Surgery. Nutrients 2025, 17, 1120. [Google Scholar] [CrossRef]
- Schrader, E.; Grosch, E.; Bertsch, T.; Sieber, C.C.; Volkert, D. Nutritional and functional status in geriatric day hospital patients–MNA short form versus full MNA. J. Nutr. Health Aging 2016, 20, 918–926. [Google Scholar] [CrossRef]
- Guillén, R.L.; Pla, M.A.; García, A.M.; de Miguel, Á.D.; Santana, E.G.; Sanchis, S.M.; Torres, J.F.M. Nutritional Assessment in Outpatients with Heart Failure. Nutrients 2024, 16, 2853. [Google Scholar] [CrossRef]
- Lisiak, M.; Jędrzejczyk, M.; Wleklik, M.; Lomper, K.; Czapla, M.; Uchmanowicz, I. Nutritional risk, frailty and functional status in elderly heart failure patients. ESC Heart Fail. 2025. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N.; Keller, H.; Gramlich, L.; Allard, J.P.; Laporte, M.; Duerksen, D.R.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Nutritional assessment: Comparison of clinical assessment and objective variables for the prediction of length of hospital stay and readmission. Am. J. Clin. Nutr. 2015, 101, 956–965. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Ruszkowski, J.; Dębska-Ślizień, A.; Małgorzewicz, S. Nutritional Status, Uremic Toxins, and Metabo-Inflammatory Biomarkers as Predictors of Two-Year Cardiovascular Mortality in Dialysis Patients: A Prospective Study. Nutrients 2025, 17, 1043. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Passos, L.B.; De-Souza, D.A. Some considerations about the GLIM criteria—A consensus report for the diagnosis of malnutrition. Clin. Nutr. 2019, 38, 1482. [Google Scholar] [CrossRef] [PubMed]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Lewandowicz, M.; Deskur-Śmielecka, E.; Stachnik, K.; Wieczorowska-Tobis, K. Diagnostic Performance and Accuracy of the MNA-SF against GLIM Criteria in Community-Dwelling Older Adults from Poland. Nutrients 2021, 13, 2183. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, K.T.; Arslan, S.; Aydın, A.; Durmaz, D. Which screening tool performs best in identifying malnutrition risk among hospitalized older adults with cardiovascular disease? A diagnostic accuracy study comparing six different screening tools with GLIM criteria. Eur. Geriatr. Med. 2025. [Google Scholar] [CrossRef]
- Thanapholsart, J.; Khan, E.; Janwanishstaporn, S.; Thongma, P.; Naowapanich, S.; Pramyothin, P.; Chirakarnjanakorn, S.; Sethalao, P.; Tankumpuan, T.; Waldréus, N.; et al. The assessment of reliability and validity of the Thai Versions of the Thirst Distress Scale for patients with Heart Failure and the Simplified Nutritional Appetite Questionnaire in heart failure patients. J. Res. Nurs. 2024, 29, 622–636. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef]
- Cass, A.R.; Charlton, K.E. Prevalence of hospital-acquired malnutrition and modifiable determinants of nutritional deterioration during inpatient admissions: A systematic review of the evidence. J. Human. Nutrition Diet. 2022, 35, 1043–1058. [Google Scholar] [CrossRef]
- Driggin, E.; Cohen, L.P.; Gallagher, D.; Karmally, W.; Maddox, T.; Hummel, S.L.; Carbone, S.; Maurer, M.S. Nutrition Assessment and Dietary Interventions in Heart Failure. J. Am. Coll. Cardiol. 2022, 79, 1623–1635. [Google Scholar] [CrossRef]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J.E. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: A systematic review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef]
| Type of Malnutrition | Description | Clinical Effects |
|---|---|---|
| Disease-related malnutrition with inflammation | Malnutrition is associated with active disease with marked inflammation (e.g., malignancies, sepsis, COVID-19). Characterized by increased catabolism and weight loss despite energy intake. |
|
| Disease-related malnutrition without inflammation | Malnutrition is due to a chronic disease that interferes with the intake, digestion, or metabolism of nutrients but does not induce a significant inflammatory response (e.g., stroke, benign tumor, neurological disability). |
|
| Starvation-related malnutrition | Malnutrition resulting from long-term energy and/or nutrient deficiencies. Typical in cases of starvation, anorexia, nutritional neglect. |
|
| Method | Description | Advantages | Limitations in Cardiac Patients | References |
|---|---|---|---|---|
| BMI | Ratio of weight (kg) to height2 (m2) | Simple, widely used; available in both specialist and outpatient settings; correlates with risk of metabolic diseases. | Cannot assess body composition, fat distribution, or edema; predictive ability for metabolic diseases limited; racial differences may affect interpretation; less accurate than waist circumference or WHtR for CVD risk prediction. | [51,52,53,54,55,56,57,58] |
| MUAC | Measured at midpoint between shoulder and elbow (non-dominant arm). | Valid tool for detecting low muscle mass compared to BIA and CT; easy and non-invasive. | Overestimation possible in presence of peripheral edema; does not assess muscle quality or cause of low lean mass. | [59,61] |
| CC | Reflects subcutaneous fat and bone mass; proxy for muscle mass due to large muscle volume in legs. | Useful surrogate for diagnosing sarcopenia; easy and low-cost. | Influenced by reduced mobility during illness; edema leads to overestimation of muscle mass; limited assessment of muscle quality. | [60,61] |
| SFT | Measures subcutaneous fat thickness; commonly TSFT (triceps). | Simple, cost-effective; reliable in resource-limited settings; correlates with undernutrition; adds prognostic value (e.g., lung cancer mortality risk). | Susceptible to high measurement error (imprecision and inaccuracy); observer technique and inter-observer variability limit reliability; may be less useful in fluid-retaining cardiac patients. | [62,63,64] |
| Tool | Number of Items | Main Criteria | Advantages | Limitations |
|---|---|---|---|---|
| NRS 2002 | 2 parts (4 screening questions + nutritional status and disease severity) | Weight loss, reduced food intake, disease severity; +1 point if ≥70 years old | Widely used and recommended in hospitalized patients; incorporates disease severity | Less suited for community use; BMI- and weight-based criteria can be confounded by fluid overload; requires reliable body mass changes history |
| MUST | 3 items (+ final risk classification) | BMI, weight loss, acute disease effect on intake | Simple and quick; used in hospitals and community; effective in primary care; reduces healthcare costs when integrated in care pathways | May underestimate malnutrition risk in fluid-overloaded or obese patients; relies on accurate weight history |
| MNA | 18 items (screening + assessment) | Anthropometrics, dietary intake, lifestyle, medications, mobility, subjective perception | Designed for elderly; comprehensive; used for frailty and functional outcomes | Time-consuming; may overestimate normal status in overweight/obese elderly |
| MNA-SF | 6 screening questions | Food intake, weight loss, mobility, stress/acute illness, neuropsychological problems, BMI | Quick and easy to use; recommended for geriatric screening; suitable for frailty assessments | Moderate agreement with full MNA; underestimates at-risk patients compared to MNA; limited in details |
| SGA | 3 domains (history, exam, subjective judgment) | Weight change, dietary intake, gastrointestinal symptoms, functional capacity, muscle/fat loss, edema | Comprehensive; includes clinical judgment | Subjective; interobserver variability; predictive validity in CVD risk limited |
| CONUT | Based on 3 laboratory values | Albumin, total cholesterol, lymphocyte count | Objective, rapid, reproducible; based on routine labs | Influenced by inflammation, pharmacotherapy, and hydration; not specific for malnutrition in CVD |
| GLIM Criteria | 2-step (screening + diagnosis) | Phenotypic: weight loss, low BMI, reduced muscle mass; Etiologic: reduced intake/absorption, disease burden/inflammation | Provides diagnostic framework; endorsed internationally; allows grading of severity | Criticized for limited sensitivity; BMI/weight loss may be confounded by hydration; overlaps with other tools; may require body composition methods |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, I.; Gorecki, K.; Kalisz, G.; Popiolek-Kalisz, J. Nutritional Status Assessment Tools in Cardiovascular Patients. Nutrients 2025, 17, 2703. https://doi.org/10.3390/nu17162703
Jarosz I, Gorecki K, Kalisz G, Popiolek-Kalisz J. Nutritional Status Assessment Tools in Cardiovascular Patients. Nutrients. 2025; 17(16):2703. https://doi.org/10.3390/nu17162703
Chicago/Turabian StyleJarosz, Izabela, Kamil Gorecki, Grzegorz Kalisz, and Joanna Popiolek-Kalisz. 2025. "Nutritional Status Assessment Tools in Cardiovascular Patients" Nutrients 17, no. 16: 2703. https://doi.org/10.3390/nu17162703
APA StyleJarosz, I., Gorecki, K., Kalisz, G., & Popiolek-Kalisz, J. (2025). Nutritional Status Assessment Tools in Cardiovascular Patients. Nutrients, 17(16), 2703. https://doi.org/10.3390/nu17162703







