Associations of the MIND Diet with Human Health Outcomes: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Information Sources
2.3. Selection of Sources of Evidence
2.4. Data Charting Process
2.5. Synthesis of Results
3. Results
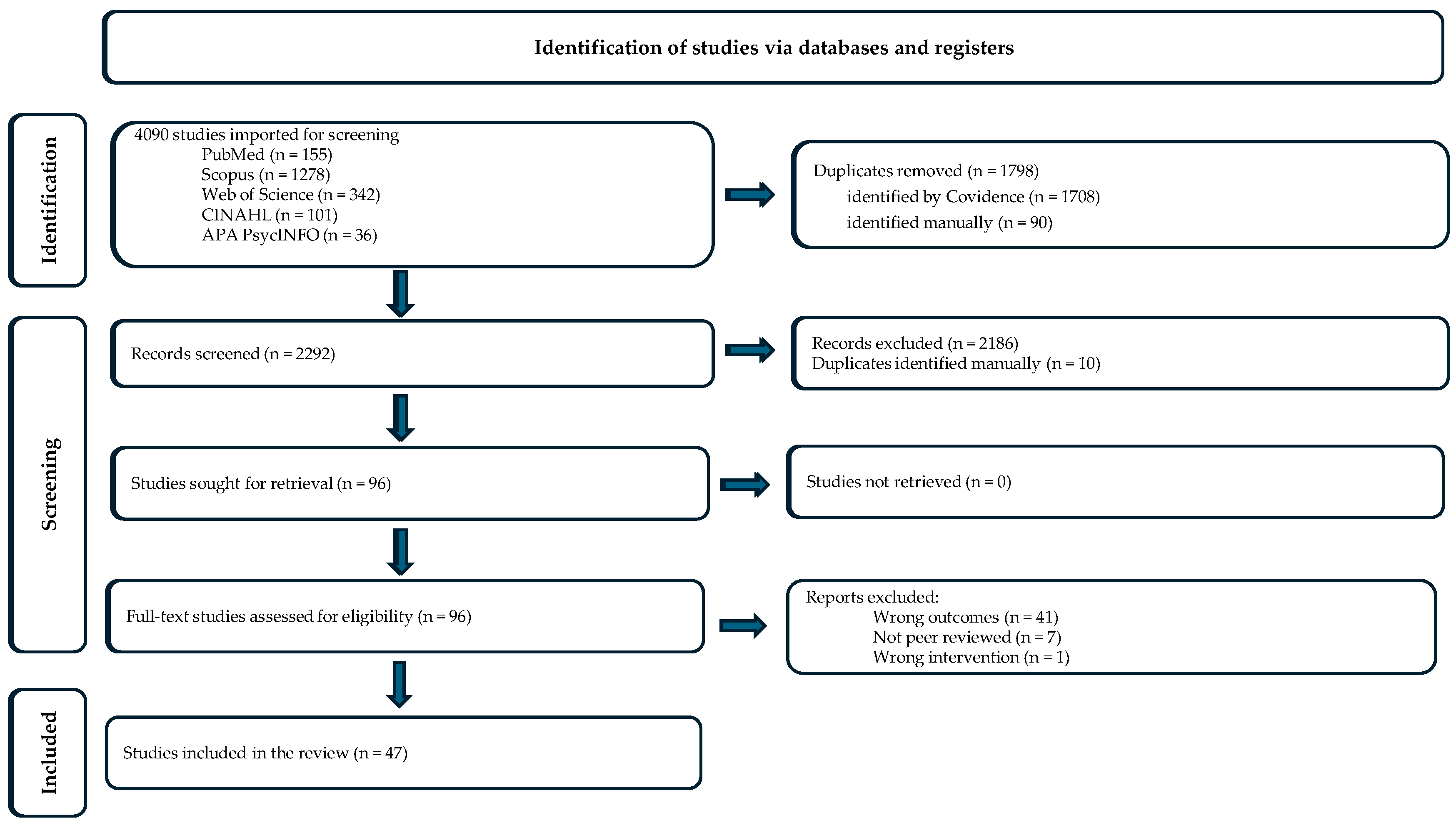
3.1. Identification and Selection
3.2. Study Characteristics
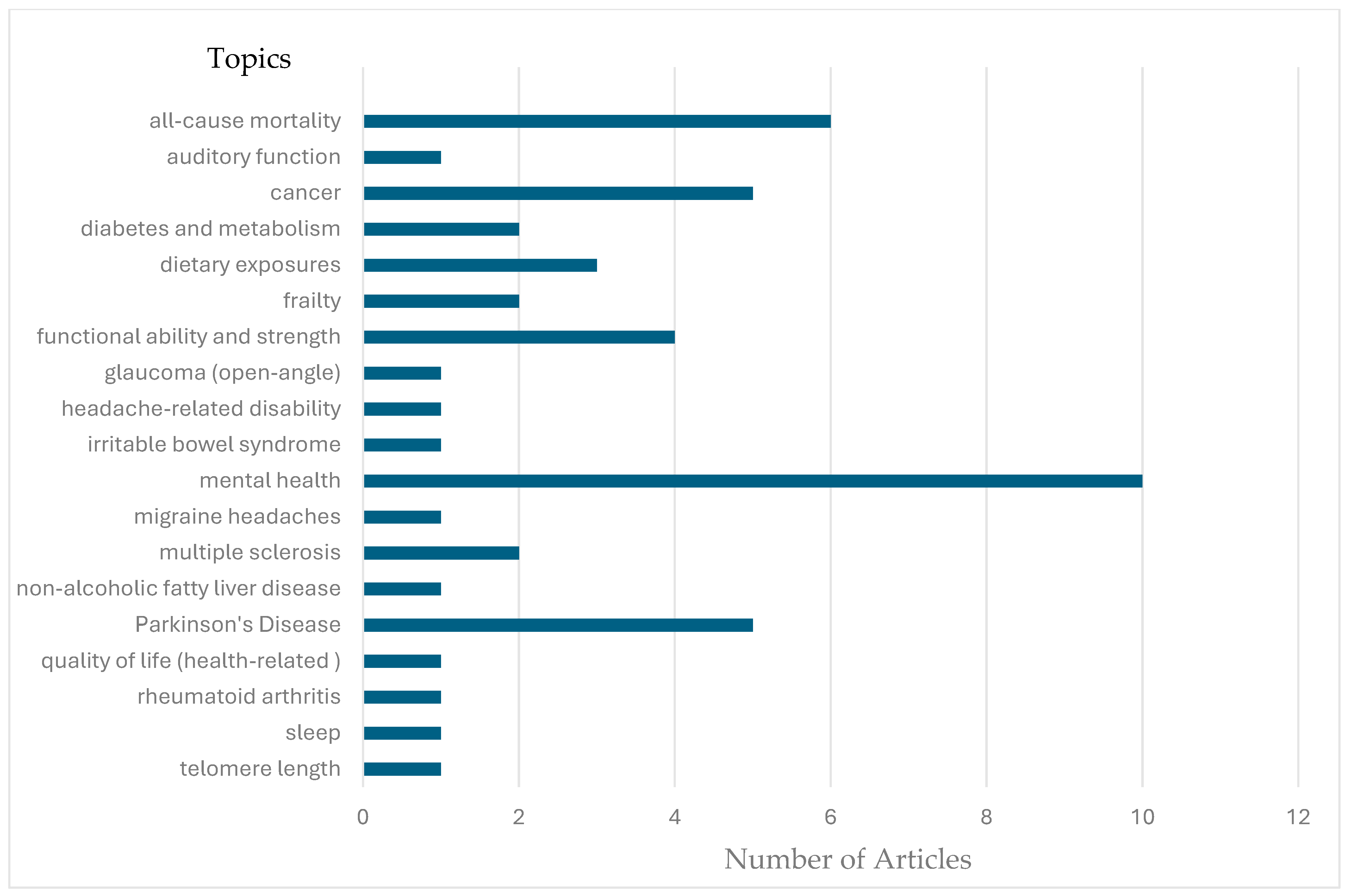
3.3. Evidence Synthesis of Similar Studies
3.3.1. All-Cause Mortality
3.3.2. Cancer/Oncology
Breast Cancer
Gliomas
3.3.3. Diabetes and Metabolism
3.3.4. Dietary Exposures
Sulfur
Selenium
Cadmium
3.3.5. Frailty
3.3.6. Functional Ability
3.3.7. Mental Health
Anxiety
Depression
Stress
3.3.8. Multiple Sclerosis
3.3.9. Parkinson’s Disease
3.4. Health Outcomes from Single Studies
3.4.1. Auditory Function
3.4.2. Glaucoma (Open-Angle)
3.4.3. Irritable Bowel Syndrome
3.4.4. Migraine Headaches
3.4.5. Non-Alcoholic Fatty Liver Disease
3.4.6. Quality of Life (Health-Related)
3.4.7. Rheumatoid Arthritis
3.4.8. Sleep
3.4.9. Telomere Length
4. Discussion
4.1. Associations with the MIND Dietary Pattern
4.2. What Is a High MDS?
4.3. Environmental Exposure
4.4. MIND Dietary Interventions
4.5. Suggestions for Future Research
4.6. Limitations of the Review
4.7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Maguire, B.; Brodaty, H.; O’Leary, F. Dietary Patterns and Cognitive Health in Older Adults: A Systematic Review. J. Alzheimers Dis. 2019, 67, 583–619. [Google Scholar] [CrossRef] [PubMed]
- Devranis, P.; Vassilopoulou, E.; Tsironis, V.; Sotiriadis, P.M.; Chourdakis, M.; Aivaliotis, M.; Tsolaki, M. Mediterranean Diet, Ketogenic Diet or MIND Diet for Aging Populations with Cognitive Decline: A Systematic Review. Life 2023, 13, 173. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. MIND diet and cognitive performance in older adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8059–8077. [Google Scholar] [CrossRef]
- van Soest, A.P.; Beers, S.; van de Rest, O.; de Groot, L.C. The Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet for the Aging Brain: A Systematic Review. Adv. Nutr. 2024, 15, 100184. [Google Scholar] [CrossRef]
- Akbar, Z.; Fituri, S.; Ouagueni, A.; Alalwani, J.; Sukik, A.; Al-Jayyousi, G.F.; Bassil, M.; Tayyem, R. Associations of the MIND Diet with Cardiometabolic Diseases and Their Risk Factors: A Systematic Review. Diabetes Metab. Syndr. Obes. 2023, 16, 3353–3371. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, H.; Gu, Y.; Shen, J.; Shen, T.; Ding, Y.; Lu, M.; Huang, L.; Yan, M.; Song, P.; et al. Healthy dietary patterns in relation to cognitive performance and Alzheimer’s disease mortality. J. Prev. Alzheimers Dis. 2025, 12, 100100. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C. Diet for the MIND: The Latest Science on What to Eat to Prevent Alzheimer’s and Cognitive Decline—From the Creator of the MIND Diet; Little, Brown Spark: New York, NY, USA, 2017. [Google Scholar]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Pallavi, S.; Bhushan, J.A.; Shanker, D.R.; Mohammad, P. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Robles-Vera, I.; Mañanes, D.; Galán, M.; Femenía-Muiña, M.; Redondo-Urzainqui, A.; Barrero-Rodríguez, R.; Papaioannou, E.; Amores-Iniesta, J.; Devesa, A.; et al. Imidazole propionate is a driver and therapeutic target in atherosclerosis. Nature 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Hu, F. Diet strategies for promoting healthy aging and longevity: An epidemiological perspective. J. Intern. Med. 2024, 295, 508–531. [Google Scholar] [CrossRef]
- Santos, L. The impact of nutrition and lifestyle modification on health. Eur. J. Intern. Med. 2022, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lingerfelt, C.; Lee, C.; Lee, J.; Raynor, H.; Anderson, J. Association of adherence to high-intensity physical activity and the Mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay diet with cognition: A cross-sectional study. Int. J. Nurs. Stud. 2022, 131, 104243. [Google Scholar] [CrossRef]
- Salehin, S.; Rasmussen, P.; Mai, S.; Mushtaq, M.; Agarwal, M.; Hasan, S.M.; Salehin, S.; Raja, M.; Gilani, S.; Khalife, W.I. Plant based diet and its effect on cardiovascular disease. Int. J. Environ. Res. Public Health 2023, 20, 3337. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Gómez-Martínez, C.; Babio, N.; Júlvez, J.; Nishi, S.; Fernández-Aranda, F.; Martínez-González, M.; Cuenca-Royo, A.; Fernández, R.; Jiménez-Murcia, S.; Torre, R.d.l.; et al. Impulsivity is longitudinally associated with healthy and unhealthy dietary patterns in individuals with overweight or obesity and metabolic syndrome within the framework of the PREDIMED-Plus trial. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 101. [Google Scholar] [CrossRef]
- Ng, L.H.; Hart, M.; Dingle, S.E.; Milte, C.M.; Livingstone, K.M.; Shaw, J.E.; Magliano, D.J.; McNaughton, S.A.; Torres, S.J. Prospective associations between diet quality and health-related quality of life in the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Br. J. Nutr. 2023, 130, 83–92. [Google Scholar] [CrossRef]
- Tanaka, T.; Talegawkar, S.A.; Jin, Y.; Candia, J.; Tian, Q.; Moaddel, R.; Simonsick, E.M.; Ferrucci, L. Metabolomic profile of different dietary patterns and their association with frailty index in community-dwelling older men and women. Nutrients 2022, 14, 2237. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Ryan, C.P.; Caspi, A.; Liu, Z.; Moffitt, T.E.; Sugden, K.; Zhou, J.; Belsky, D.W.; Gu, Y. Diet, pace of biological aging, and risk of dementia in the Framingham Heart Study. Ann. Neurol. 2023, 95, 1069–1079. [Google Scholar] [CrossRef]
- Song, Y.; Chang, Z.g.; Song, C.; Cui, K.; Shi, B.; Zhang, R.; Dong, Q.; Dou, K. Association between MIND diet adherence and mortality: Insights from diabetic and non-diabetic cohorts. Nutr. Diabetes 2023, 13, 18. [Google Scholar] [CrossRef]
- Sand, I.K.; Fitzgerald, K.C.; Gu, Y.; Brandstadter, R.; Riley, C.S.; Buyukturkoglu, K.; Leavitt, V.M.; Krieger, S.; Miller, A.; Lublin, F.; et al. Dietary factors and MRI metrics in early multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 53, 103031. [Google Scholar] [CrossRef]
- Vergroesen, J.E.; de Crom, T.O.E.; van Duijn, C.M.; Voortman, T.; Klaver, C.C.W.; Ramdas, W.D. MIND diet lowers risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Nutr. 2023, 62, 477–487. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Holland, T.; Agarwal, P.; Aggarwal, N.; Morris, M.C. DASH and Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diets are associated with fewer depressive symptoms over time. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Corley, J. Adherence to the MIND diet is associated with 12-year all-cause mortality in older adults. Public Health Nutr. 2022, 25, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Fresán, U.; Bes-Rastrollo, M.; Segovia-Siapco, G.; Sanchez-Villegas, A.; Lahortiga, F.; de la Rosa, P.A.; Martínez-Gonzalez, M.A. Does the MIND diet decrease depression risk? A comparison with Mediterranean diet in the SUN cohort. Eur. J. Nutr. 2019, 58, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Jia, X.; Chen, Z.; Zhang, T.; Li, X.; Zhang, L.; Chen, F.; Zhang, J.; Zhang, Z.; Liu, Z.; et al. Dietary pattern, metabolomics and frailty in a large cohort of 120,000 participants. Food Funct. 2024, 15, 3174–3185. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Wang, C.; Chen, F.; Jiang, B.; Li, W. Adherence to healthy dietary patterns and glioma: A matched case-control study. Nutrients 2023, 15, 4886. [Google Scholar] [CrossRef]
- Askarpour, M.; Yarizadeh, H.; Sheikhi, A.; Khorsha, F.; Mirzaei, K. Associations between adherence to MIND diet and severity, duration and frequency of migraine headaches among migraine patients. BMC Res. Notes 2020, 13, 341. [Google Scholar] [CrossRef]
- Noormohammadi, M.; Ghorbani, Z.; Moghadasi, A.N.; Saeedirad, Z.; Shahemi, S.; Ghanaatgar, M.; Rezaeimanesh, N.; Hekmatdoost, A.; Ghaemi, A.; Jahromi, S.R. MIND diet adherence might be associated with a reduced odds of multiple sclerosis: Results from a case-control study. Neurol. Ther. 2022, 11, 397–412. [Google Scholar] [CrossRef]
- Mokhtari, E.; Jamshidi, S.; Farhadnejad, H.; Teymoori, F.; Rashidkhani, B.; Mirmiran, P.; Tehrani, F.R.; Heidari, Z. The relationship between Mediterranean-DASH diet intervention for the neurodegenerative delay (MIND) Diet and risk of breast Cancer: A case-control study among iranian adult women. BMC Nutr. 2022, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Urbano, T.; Verzelloni, P.; Malavolti, M.; Sucato, S.; Polledri, E.; Agnoli, C.; Sieri, S.; Natalini, N.; Marchesi, C.; Fustinoni, S.; et al. Influence of dietary patterns on urinary excretion of cadmium in an Italian population: A cross-sectional study. J. Trace Elem. Med. Biol. 2023, 80, 127298. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chang, Z.g.; Cui, K.; Song, C.; Cai, Z.; Shi, B.; Dong, Q.; Dou, K. The value of the MIND diet in the primary and secondary prevention of hypertension: A cross-sectional and longitudinal cohort study from NHANES analysis. Front. Nutr. 2023, 10, 1129667. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Sardone, R.; Donghia, R.; Castellana, F.; Lampignano, L.; Bortone, I.; Misciagna, G.; De Pergola, G.; Panza, F.; Lozupone, M.; et al. Traditional dietary patterns and risk of mortality in a longitudinal cohort of the Salus in Apulia study. Nutrients 2020, 12, 1070. [Google Scholar] [CrossRef]
- Chan, R.S.M.; Yu, B.W.M.; Leung, J.; Lee, J.S.W.; Auyeung, T.W.; Kwok, T.; Woo, J. How dietary patterns are related to inflammaging and mortality in community-dwelling older Chinese adults in Hong Kong—A prospective analysis. J. Nutr. Health Aging 2019, 23, 181–194. [Google Scholar] [CrossRef]
- Jin, Y.; Tanaka, T.; Reed, N.S.; Tucker, K.L.; Ferrucci, L.; Talegawkar, S.A. Associations between dietary indices and hearing status among middle-older aged adults—Results from the Baltimore Longitudinal Study of Aging. Am. J. Clin. Nutr. 2024, 119, 1338–1345. [Google Scholar] [CrossRef]
- Aghamohammadi, V.; Salari-Moghaddam, A.; Benisi-Kohansal, S.; Taghavi, M.; Azadbakht, L.; Esmaillzadeh, A. Adherence to the MIND diet and risk of breast cancer: A case-control study. Clin. Breast Cancer 2021, 21, E158–E164. [Google Scholar] [CrossRef]
- Sheikhhossein, F.; Imani, H.; Amini, M.R.; Hosseini, F.; Shab-Bidar, S. The association between adherence to MIND diet and risk of breast cancer: A case-control study. Int. J. Clin. Pract. 2021, 75, e14780. [Google Scholar] [CrossRef]
- Soltani, S.; Shayanfar, M.; Benisi-Kohansal, S.; Mohammad-Shirazi, M.; Sharifi, G.; Djazayeri, A.; Esmaillzadeh, A. Adherence to the MIND diet in relation to glioma: A case-control study. Nutr. Neurosci. 2022, 25, 771–778. [Google Scholar] [CrossRef]
- Tirani, S.; Poursalehi, D.; Lotfi, K.; Shahdadian, F.; Hajhashemy, Z.; Rouhani, P.; Saneei, P. Adherence to Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet in relation to serum brain-derived neurotrophic factor concentrations and metabolic health status in adults. Curr. Dev. Nutr. 2024, 8, 102082. [Google Scholar] [CrossRef]
- Tison, S.E.; Shikany, J.M.; Long, D.L.; Carson, A.P.; Cofield, S.S.; Pearson, K.E.; Howard, G.; Judd, S.E. Differences in the association of select dietary measures with risk of incident type 2 diabetes. Diabetes Care 2022, 45, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Passafiume, A.; Rossetti, A.; Vescovi, L.; Malavolti, M.; Baraldi, C.; Rovesti, S.; Vinceti, M.; Filippini, T. Sulfur content in foods consumed in an Italian population and impact of diet quality on sulfur intake. J. Food Compost. Anal. 2023, 123, 105543. [Google Scholar] [CrossRef]
- Urbano, T.; Filippini, T.; Malavolti, M.; Fustinoni, S.; Michalke, B.; Wise, L.A.; Vinceti, M. Adherence to the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet and exposure to selenium species: A cross-sectional study. Nutr. Res. 2024, 122, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Bennett, D.A.; Morris, M.C. Dietary patterns and self-reported incident disability in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2019, 74, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Pasdar, Y.; Moradi, S.; Saedi, S.; Moradinazar, M.; Rahmani, N.; Hamzeh, B.; Najafi, F. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet in relation to age-associated poor muscle strength; a cross-sectional study from the Kurdish cohort study. Sci. Rep. 2022, 12, 11866. [Google Scholar] [CrossRef]
- Talegawkar, S.; Jin, Y.; Simonsick, E.; Tucker, K.; Ferrucci, L.; Tanaka, T. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet is associated with physical function and grip strength in older men and women. Am. J. Clin. Nutr. 2022, 115, 625–632. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Sin, D.; Yu, R.; Leung, J.; Woo, J. Dietary patterns and intrinsic capacity in community-dwelling older adults: A cross-sectional study. J. Nutr. Health Aging 2022, 26, 174–182. [Google Scholar] [CrossRef]
- Nouri-Majd, S.; Salari-Moghaddam, A.; Keshteli, A.H.; Esmaillzadeh, A.; Adibi, P. The association between adherence to the MIND diet and irritable bowel syndrome. Dig. Dis. 2022, 40, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, R.; Namayandeh, M.; Mirzaei, M.; Sohouli, M.H.; Hosseinzadeh, M. The relation between MIND diet with psychological disorders and psychological stress among Iranian adults. BMC Psychiatry 2022, 22, 496. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Parastouei, K.; Samadi, M.; Taghdir, M.; Eskandari, E. Adherence to the MIND dietary pattern and sleep quality, sleep related outcomes and mental health in male adults: A cross-sectional study. BMC Psychiatry 2022, 22, 167. [Google Scholar] [CrossRef]
- Seifollahi, A.; Sardari, L.; Yarizadeh, H.; Mirzababaei, A.; Shiraseb, F.; Clark, C.; Mirzaei, K. Associations between adherence to the MIND diet and prevalence of psychological disorders, and sleep disorders severity among obese and overweight women: A cross-sectional study. Nutr. Health 2022, 30, 513–519. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Keshteli, A.H.; Mousavi, S.M.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to the MIND diet and prevalence of psychological disorders in adults. J. Affect. Disord. 2019, 256, 96–102. [Google Scholar] [CrossRef]
- Torabynasab, K.; Shahinfar, H.; Jazayeri, S.; Effatpanah, M.; Azadbakht, L.; Abolghasemi, J. Adherence to the MIND diet is inversely associated with odds and severity of anxiety disorders: A case-control study. BMC Psychiatry 2023, 23, 330. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Xu, Y.; Gibson, R.; Williams, C.; Lawrence, A.J.; Nosarti, C.; Dazzan, P.; Rodriguez-Mateos, A. Plant-based dietary patterns and their association with mood in healthy individuals. Food Funct. 2022, 14, 2326–2337. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Feizi, A.; Esmaillzadehc, A.; Keshtelid, A.; Roohafzaf, H.; Afshar, H.; Adibi, P. The MIND (Mediterranean-DASH Diet Intervention for Neurodegenerative Delay) and Mediterranean Diets are differently associated with psychosomatic complaints profile in adults: Results from SEPAHAN cross-sectional study. Med. J. Nutr. Metab. 2020, 13, 341–359. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Carrasco-Marin, F.; Boonpor, J.; Parra-Soto, S.; Shannon, O.; Malcomson, F.; Phillips, N.; Jain, M.; Deo, S.; Livingstone, K.M.; et al. Association of five diet scores with severe NAFLD incidence: A prospective study from UK Biobank. Diabetes Obes. Metab. 2024, 26, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. MIND diet associated with reduced Incidence and delayed progression of parkinsonism in old age. J. Nutr. Health Aging 2018, 22, 1211–1215. [Google Scholar] [CrossRef]
- Metcalfe-Roach, A.; Cirstea, M.; Yu, A.; Golz, E.; Sundvick, K.; Kliger, D.; Finlay, B.; Appel-Cresswell, S. Higher adherence to MIND diet associated with later onset of Parkinson’s disease. Mov. Disord. 2020, 35, S335–S336. [Google Scholar]
- Fox, D.J.; Park, S.J.; Mischley, L.K. Comparison of associations between MIND and Mediterranean diet scores with patient-reported outcomes in Parkinson’s disease. Nutrients 2022, 14, 5185. [Google Scholar] [CrossRef]
- Keramati, M.; Kheirouri, S.; Etemadifar, M. Dietary approach to stop hypertension (DASH), but not Mediterranean and MIND, dietary pattern protects against Parkinson’s disease. Food Sci. Nutr. 2024, 12, 943–951. [Google Scholar] [CrossRef]
- Lawrie, S.; Coe, S.; Mansoubi, M.; Welch, J.; Hu, M.T.; Dawes, H. Dietary patterns and nonmotor symptoms in Parkinson’s disease: A cross-sectional analysis. J. Am. Nutr. Assoc. 2023, 42, 393–402. [Google Scholar] [CrossRef]
- Safaei, M.; Kheirouri, S.; Alizadeh, M.; Pirovi, A. Association between Mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay diet and biomarkers of oxidative stress, metabolic factors, disease severity, and odds of disease in rheumatoid arthritis patients. Food Sci. Nutr. 2024, 12, 3973–3981. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Tang, N.; Woo, J. Dietary patterns and telomere length in community-dwelling Chinese older men and women: A cross-sectional analysis. Eur. J. Nutr. 2020, 59, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Vahdat, S.; Hojati, A.; Moradi, H.; Tousi, A.Z.; Ebrahimzadeh, F.; Farhangi, M.A. Evaluating the association between the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, mental health, and cardio-metabolic risk factors among individuals with obesity. BMC Endocr. Disord. 2023, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef]
- Rothman, S.M.; Griffioen, K.J.; Wan, R.; Mattson, M.P. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann. N. Y. Acad. Sci. 2012, 1264, 49–63. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Cadmium in Food and Foodwares. Available online: https://www.fda.gov/food/environmental-contaminants-food/cadmium-food-and-foodwares (accessed on 13 June 2025).
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Curhan, S.G.; Halpin, C.; Wang, M.; Eavey, R.D.; Curhan, G.C. Prospective study of dietary patterns and hearing threshold elevation. Am. J. Epidemiol. 2019, 189, 204–214. [Google Scholar] [CrossRef]
- The WHOQOL Group. The World Health Organization Quality of Life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Karam, G.; Agarwal, A.; Sadeghirad, B.; Jalink, M.; Hitchcock, C.L.; Ge, L.; Kiflen, R.; Ahmed, W.; Zea, A.M.; Milenkovic, J.; et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: Systematic review and network meta-analysis. BMJ 2023, 380, e072003. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Leary, S.; Niu, J.; Perry, R.; Papadaki, A. The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials. Nutrients 2023, 15, 2099. [Google Scholar] [CrossRef]
- González-Palacios Torres, C.; Barrios-Rodríguez, R.; Muñoz-Bravo, C.; Toledo, E.; Dierssen, T.; Jiménez-Moleón, J.J. Mediterranean diet and risk of breast cancer: An umbrella review. Clin. Nutr. 2023, 42, 600–608. [Google Scholar] [CrossRef]
- Milenkovic, T.; Bozhinovska, N.; Macut, D.; Bjekic-Macut, J.; Rahelic, D.; Velija Asimi, Z.; Burekovic, A. Mediterranean diet and type 2 diabetes mellitus: A perpetual inspiration for the scientific world. A review. Nutrients 2021, 13, 1307. [Google Scholar] [CrossRef]
- Poursalehi, D.; Lotfi, K.; Saneei, P. Adherence to the Mediterranean diet and risk of frailty and pre-frailty in elderly adults: A systematic review and dose-response meta-analysis with GRADE assessment. Ageing Res. Rev. 2023, 87, 101903. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Peng, Y.; Lin, Z.; Gong, Y. Association between Mediterranean diet adherence and Parkinson’s disease: A systematic review and meta-analysis. J. Nutr. Health Aging 2025, 29, 100451. [Google Scholar] [CrossRef] [PubMed]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Guglielmetti, M.; Ferraris, C.; Frias-Toral, E.; Domínguez Azpíroz, I.; Lipari, V.; Di Mauro, A.; Furnari, F.; Castellano, S.; Galvano, F.; et al. Mediterranean diet and quality of life in adults: A systematic review. Nutrients 2025, 17, 577. [Google Scholar] [CrossRef]
- López-Gil, J.F.; Victoria-Montesinos, D.; García-Hermoso, A.; López-Moreno, M.; Ezzatvar, Y.; Gutiérrez-Espinoza, H.; Quesada-Fernández, G.; Stubbs, B.; Smith, L.; Kales, S.N. Is greater adherence to the Mediterranean diet related to higher health-related quality of life among children and adolescents? A systematic review and meta-analysis. Eur. J. Pediatr. 2025, 184, 498. [Google Scholar] [CrossRef] [PubMed]
- Romero-Robles, M.A.; Ccami-Bernal, F.; Ortiz-Benique, Z.N.; Pinto-Ruiz, D.F.; Benites-Zapata, V.A.; Casas Patiño, D. Adherence to Mediterranean diet associated with health-related quality of life in children and adolescents: A systematic review. BMC Nutr. 2022, 8, 57. [Google Scholar] [CrossRef]
- Hu, P.; Lee, E.K.; Li, Q.; Tam, L.S.; Wong, S.Y.; Poon, P.K.; Yip, B.H. Mediterranean diet and rheumatoid arthritis: A nine-year cohort study and systematic review with meta-analysis. Eur. J. Clin. Nutr. 2025. [Google Scholar] [CrossRef]
- Arab, A.; Lempesis, I.G.; Garaulet, M.; Scheer, F. Sleep and the Mediterranean diet: A systematic review and meta-analysis. Sleep. Med. Rev. 2025, 80, 102071. [Google Scholar] [CrossRef]
- Roldán-Ruiz, A.; Bertotti, G.; López-Moreno, M. Effects of dietary interventions in patients with migraine: A systematic review. Nutr. Rev. 2025, 83, e1815–e1827. [Google Scholar] [CrossRef]
- Madani, S.; Ahmadi, A.; Shoaei-Jouneghani, F.; Moazen, M.; Sasani, N. The relationship between the Mediterranean diet and Axis I disorders: A systematic review of observational studies. Food Sci. Nutr. 2022, 10, 3241–3258. [Google Scholar] [CrossRef]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate wine consumption and health: A narrative review. Nutrients 2022, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. Guidance Cadmium: General Information. Available online: https://www.gov.uk/government/publications/cadmium-properties-incident-management-and-toxicology/cadmium-general-information (accessed on 26 June 2025).
- EUR-Lex: Access to European Union law. Maxiumum Levels for Certain Contaminants in Food. Available online: https://eur-lex.europa.eu/EN/legal-content/summary/maximum-levels-for-certain-contaminants-in-food.html?fromSummary=30 (accessed on 26 June 2025).
- Liu, X.; Morris, M.; Dhana, K.; Ventrelle, J.; Johnson, K.; Bishop, L.; Hollings, C.; Boulin, A.; Laranjo, N.; Stubbs, B.; et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study: Rationale, design and baseline characteristics of a randomized control trial of the MIND diet. Contemp. Clin. Trials 2021, 102, 106270. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Stead, T.S.; Ganti, L. What’s the risk: Differentiating risk ratios, odds ratios, and hazard ratios? Cureus 2020, 12, e10047. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Marlow, M.L.; Lavie, C.J. Controversy and debate: Memory-based methods paper 1: The fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J. Clin. Epidemiol. 2018, 104, 113–124. [Google Scholar] [CrossRef]
| 10 Healthy Foods | Servings/Frequency | Examples |
| Leafy green vegetables | 6+/week | Kale, spinach, romaine lettuce |
| Other vegetables | 1+/day | Broccoli, green beans, squash |
| Whole grains | 3+/day | Oats, farro, wheat, brown rice |
| Berries | 2+/week | Blueberries, strawberries |
| Nuts | 5+/week | Walnuts, almonds, peanuts |
| Seafood | 1+/week | Fish, shrimp, scallops (fresh or saltwater fish) |
| Poultry | 2+/week | Chicken, turkey |
| Beans & legumes | 4+/week | Black beans, lentils |
| Olive oil as primary oil | ||
| Wine * | 1/day for women 1–2/day for men | 5 ounces of wine, 12 ounces of beer (5% alcohol), or 1.5 ounces of liquor |
| 5 Unhealthy Foods to Limit | Servings/Frequency | Examples |
| Sweets & pastries | <5/week | Cake, candy, ice cream, pie |
| Red meats & red-meat products | <4/week | Beef, pork, bacon, corn beef hash |
| Fried/fast foods | <1/week | Fries, burgers, chicken |
| Whole-fat cheese | <1/week (1 to 2 ounces per week) | Cheddar, Colby, Swiss, American |
| Butter or trans-fat margarine | <1 pat/day | Butter, margarine |
| “MIND diet” |
| “MIND dietary pattern” |
| “Mediterranean DASH Intervention for Neurodegenerative Delay” |
| “Mediterranean Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay” |
| Filters applied: Publication Date from 2015 to 2024 |
| Number of Studies | Health Outcome | Favorable n (%) | Unfavorable n (%) | No Association n (%) | Study Reference(s) |
|---|---|---|---|---|---|
| 6 | All-Cause mortality: | 4 (67%) | 0 | 2 (33%) * | Song [26]; Song [38]; Thomas [25]; Zupo [39]; Corley [30]; Chan [40] |
| All-cause mortality in a diabetic population | 1 | 0 | 0 | Song [26] | |
| All-cause mortality (non-diabetic population) | 1 | 0 | 0 | Song [26] | |
| All-cause mortality in a hypertensive population | 1 | 0 | 0 | Song [38] | |
| 1 | Auditory function | 0 | 0 | 1 (100%) * | Jin [41] |
| 5 | Cancer (Risk of): | ||||
| Breast cancer | 2 (67%) | 0 | 1 (33%) | Mokhtari [36]; Aghamohammadi [42]; Sheikhhossein [43] | |
| Glioma | 2 (100%) | 0 | 0 | Zhang [33]; Soltani [44] | |
| 2 | Diabetes and metabolism: | ||||
| Metabolic health status | 1 (100%) | 0 | 0 | Tirani [45] | |
| Hypertension | 1 (100%) | 0 | 0 | Tirani [45] | |
| Hypertriglyceridemia | 1 (100%) | 0 | 0 | Tirani [45] | |
| Serum brain-derived neurotrophic concentrations | 0 | 0 | 1 (100%) | Tirani [45] | |
| Incident diabetes | 1 (100%) | 0 | 0 | Tison [46] | |
| 3 | Dietary exposures: | ||||
| Sulfur content in food | 1 (100%) | 0 | 0 | Passafiume [47] | |
| Selenium exposure | 1 (100%) | 0 | 0 | Urbano [48] | |
| Cadmium exposure | 0 | 1 (100%) | 0 | Urbano [37] | |
| 2 | Frailty (risk of) | 2 (100%) | 0 | 0 | Yao [32]; Tanaka [24] |
| 6 | Functional ability: | ||||
| ADL disability | 1 (100%) | 0 | 0 | Agarwal [49] | |
| Instrumental ADL disability | 1 (100%) | 0 | 0 | Agarwal [49] | |
| Mobility disability | 1 (100%) | 0 | 0 | Agarwal [49] | |
| Grip strength | 2 (100%) | 0 | 0 | Pasdar [50]; Talegawkar [51] | |
| Physical function | 1 (100%) | 0 | 0 | Talegawkar [51] | |
| Psychological function | 1 (100%) | 0 | 0 | Yeung [52] | |
| 1 | Glaucoma (open-angle) | 1 (100%) | 0 | 0 | Vergroesen [28] |
| 1 | Irritable bowel syndrome | 0 | 0 | 1 (100%) | Nouri-Majd [53] |
| 10 | Mental health: | ||||
| Anxiety | 2 (40%) | 0 | 3 (60%) | Barkhordari [54]; Rostami [55]; Seifollahi [56]; Salari-Moghaddam [57]; Torabynasab [58] | |
| Depression | 3 (50%) | 0 | 3 (50%) * | Barkhordari [54]; Cherian [29]; Fresan [31]; Seifollahi [56] *; Salari-Moghaddam [57]; Rostami [55] | |
| Impulsivity | 0 | 0 | 1 (100%) | Gomez-Martinez [22] | |
| Mood | 0 | 0 | 1 (100%) | Ma [59] | |
| Somatization | 1 (100%) | 0 | 0 | Haghighatdoost [60] | |
| Stress | 1 (20%) | 0 | 4 (80%) | Barkhordari [54]; Rostami [55]; Salari-Moghaddam [57]; Seifollahi [56] | |
| 1 | Migraine headaches: | ||||
| Severity | 1 (100%) | 0 | 0 | Askarpour [34] | |
| Frequency | 1 (100%) | 0 | 0 | Askarpour [34] | |
| Duration | 1 (100%) | 0 | 0 | Askarpour [34] | |
| Disability | 0 | 0 | 1 (100%) | Askarpour [34] | |
| 2 | Multiple sclerosis: | ||||
| Reduced odds of MS | 1 (100%) | 0 | 0 | Noormohammadi [35] | |
| Higher thalamic volume | 1 (100%) | 0 | 0 | Noormohammadi [35] | |
| Lesion volume | 0 | 0 | 1 (100%) | Katz Sand [27] | |
| Gray matter volume | 0 | 0 | 1 (100%) | Katz Sand [27] | |
| Normal appearing white matter | 0 | 0 | 1 (100%) | Katz Sand [27] | |
| 1 | Non-alcoholic fatty liver disease | 1(100%) | 0 | 0 | Petermann-Rocha [61] |
| 5 | Parkinson’s disease: | ||||
| Incidence | 1 (100%) | 0 | 0 | Agarwal [62] | |
| Later onset | 1 (100%) | 0 | 0 | Metcalfe-Roach [63] | |
| Motor symptoms | 1 (100%) | 0 | 0 | Fox [64] | |
| Nonmotor symptoms | 1 (100%) | 0 | 0 | Fox [64] | |
| Progression | 1 (100%) | 0 | 0 | Agarwal [62] | |
| Risk | 0 | 0 | 1 (100%) | Keramati [65] | |
| Severity | 0 | 0 | 2 (100%) | Keramati [65]; Lawrie [66] | |
| Total symptoms | 1 (100%) | 0 | 0 | Fox [64] | |
| 1 | Quality of Life (health-related) | 1 (100%) | 0 | 0 | Ng [23] |
| 1 | Rheumatoid arthritis: | ||||
| Oxidative stress indicators | 0 | 0 | 1 (100%) | Safaei [67] | |
| Metabolic factors | 1 (100%) | 0 | 0 | Safaei [67] | |
| Disease activity | 1 (100%) | 0 | 0 | Safaei [67] | |
| Odds of disease | 1 (100%) | 0 | 0 | Safaei [67] | |
| 1 | Sleep: | ||||
| Sleep quality | 1 (100%) | 0 | 0 | Rostami [55] | |
| Insomnia | 1 (100%) | 0 | 0 | Rostami [55] | |
| Sleepiness (daytime) | 1 (100%) | 0 | 0 | Rostami [55] | |
| 1 | Telomere length | 0 | 0 | 1 (100%) | Chan [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, K.H.; Lee, M.L.; Barroso, C.S.; Anderson, J.G.; Lott, S.; Reth, D.; Horn, C.; Dixson, M. Associations of the MIND Diet with Human Health Outcomes: A Scoping Review. Nutrients 2025, 17, 2687. https://doi.org/10.3390/nu17162687
Morgan KH, Lee ML, Barroso CS, Anderson JG, Lott S, Reth D, Horn C, Dixson M. Associations of the MIND Diet with Human Health Outcomes: A Scoping Review. Nutrients. 2025; 17(16):2687. https://doi.org/10.3390/nu17162687
Chicago/Turabian StyleMorgan, Katherine Hope, Michelle Lanphere Lee, Cristina S. Barroso, Joel G. Anderson, Shelley Lott, Danielle Reth, Chelsea Horn, and Melanie Dixson. 2025. "Associations of the MIND Diet with Human Health Outcomes: A Scoping Review" Nutrients 17, no. 16: 2687. https://doi.org/10.3390/nu17162687
APA StyleMorgan, K. H., Lee, M. L., Barroso, C. S., Anderson, J. G., Lott, S., Reth, D., Horn, C., & Dixson, M. (2025). Associations of the MIND Diet with Human Health Outcomes: A Scoping Review. Nutrients, 17(16), 2687. https://doi.org/10.3390/nu17162687






