The Characterization and Identification of Cyperus Protein: An In Vitro Study on Its Antioxidant and Anti-Inflammatory Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Experiment
2.1.1. Cell Culture
2.1.2. Cell Cytotoxicity Assay
2.1.3. Determination of Neutral Red Phagocytosis
2.1.4. Determination of Intracellular SOD and CAT Activities
2.1.5. Measurement of IL-6 and TNF-α Gene Expression
2.1.6. IL-6 and TNFα Quantification
2.2. Non-Targeted Metabolomics
2.2.1. Sample Preparation
2.2.2. LC–MS/MS Analysis
2.3. Isolation, Purification and Structure Identification of CAOP
2.3.1. Sephadex G-25 Medium
2.3.2. UPLC–MS/MS-Based Peptide Identification
2.4. CAOP Component Screening and Structure–Activity Relationships
2.4.1. Structure Activity Relationship Analysis of Peptides
2.4.2. Molecular Docking
2.5. Metabolomics and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
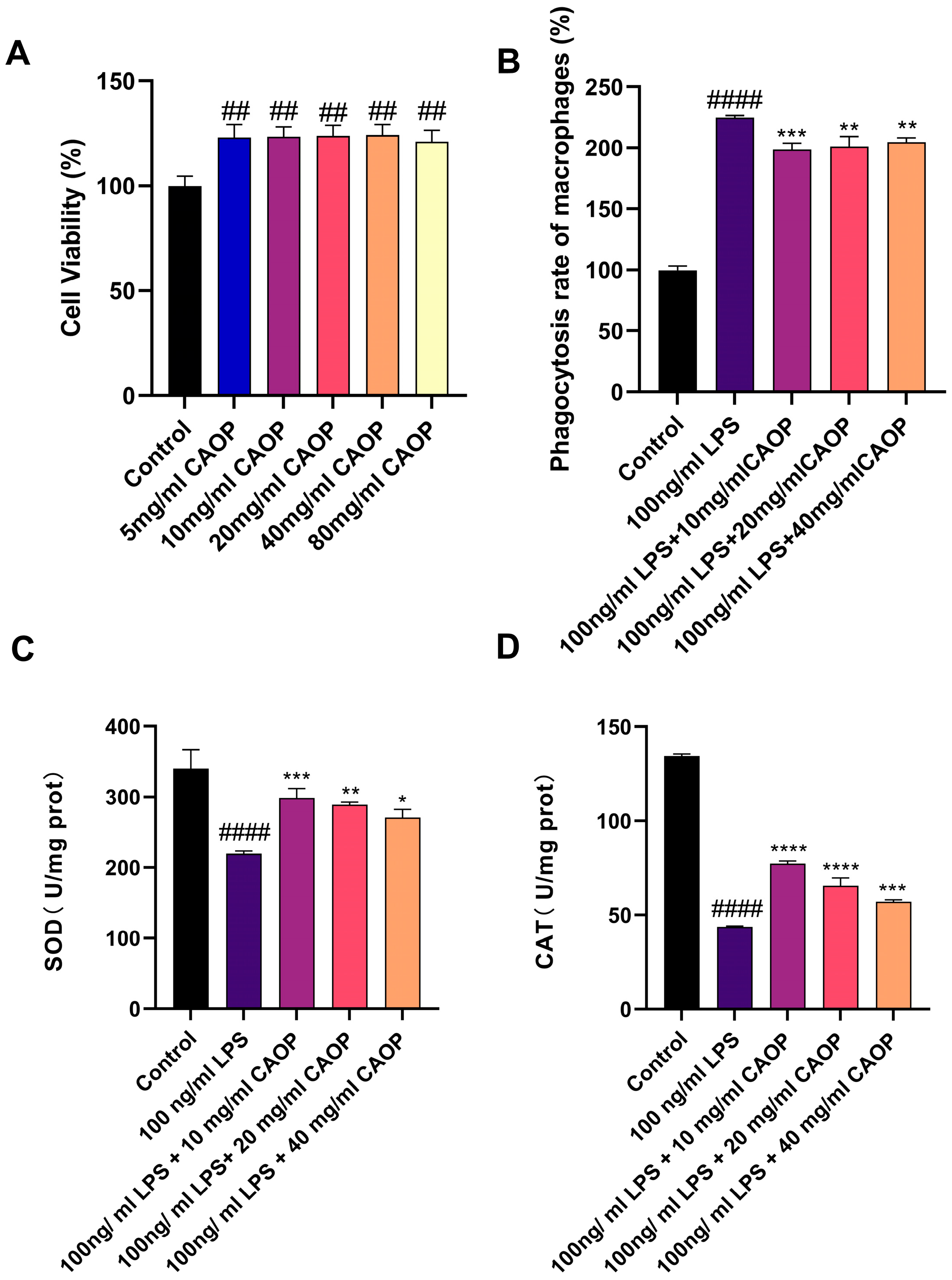
3.1. Cell Viability
3.2. Neutral Red Phagocytosis Assay
3.3. The Effect of CAOP on Cell SOD and MDA Content
3.4. Expression of Inflammatory Factors in RAW264.7 Cells
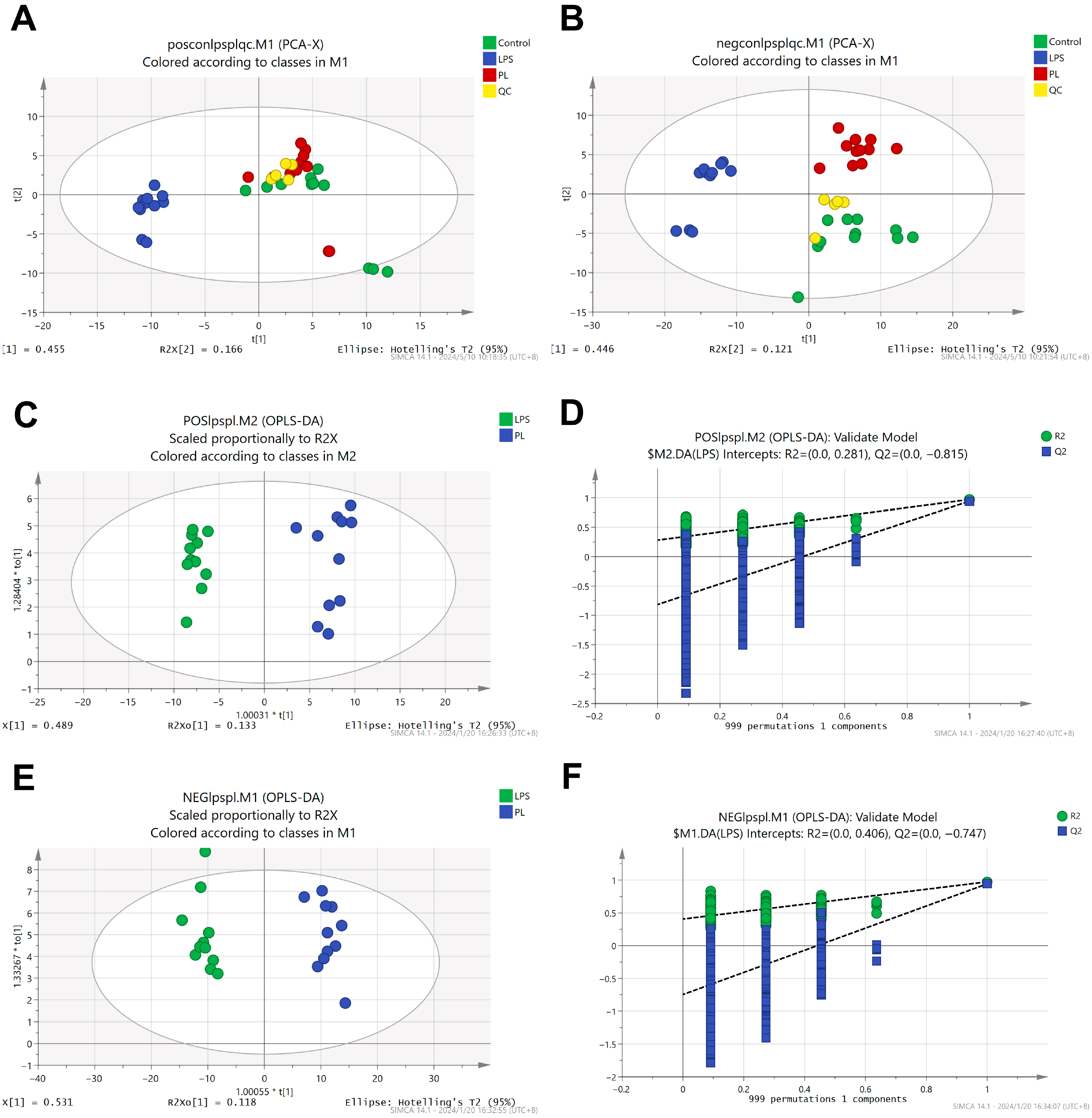
3.5. Metabolomics Analysis
3.5.1. Differentially Abundant Metabolite Analysis
3.5.2. Pathways Related to the Differentially Abundant Metabolites
3.6. Results of Sephadex G-25 Chromatography
3.7. Virtual Screening
3.8. Electronic Properties and Keap1 Binding Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| CAOP | Cyperus peptide |
| CAT | Catalase |
| β-actin | Beta-actin |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| Elisa | Enzyme-linked immunosorbent assay |
References
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Qu, P.; Cheng, Z.; Long, C.; Su, M.; Yang, D. Comprehensive development and utilization of oilseed resources. China Oil Fat. 2007, 9, 61–63. [Google Scholar]
- Shi, H.; Wang, L.; Wang, L.; Lai, C. Study on the nutritional value of oilseed meal and its effect on the feeding of growing pigs when replacing part of corn. Chin. J. Anim. Husb. 2022, 58, 178–182. [Google Scholar] [CrossRef]
- Hu, G. Synthesis of Anthraquinone Compounds and Study of Their Cytotoxic Activity Against Tumor Cells. Master’s Thesis, Lanzhou University, Lanzhou, China, 2014. [Google Scholar]
- Jing, S.Q.; Wang, S.S.; Zhong, R.M.; Zhang, J.Y.; Wu, J.Z.; Tu, Y.X.; Pu, Y.; Yan, L.J. Neuroprotection of Cyperus esculentus L. orientin against cerebral ischemia/reperfusion induced brain injury. Neural Regen. Res. 2020, 15, 548–556. [Google Scholar] [CrossRef]
- Seo, E.J.; Lee, D.U.; Kwak, J.H.; Lee, S.M.; Kim, Y.S.; Jung, Y.S. Antiplatelet effects of Cyperus rotundus and its component (+)-nootkatone. J. Ethnopharmacol. 2011, 135, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Raut, N.A.; Gaikwad, N.J. Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 2006, 77, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Meng, X.; Wang, S.; Bao, Y.; Cui, Y. The biological activity of Cyperus rotundus essential oil and its GC-MS analysis. Chin. J. Exp. Formul. 2015, 21, 32–35. [Google Scholar] [CrossRef]
- Jebasingh, D.; Jackson, D.; Venkataraman, S.; Emerald, B. Physiochemical Toxicol. Stud. Med. Plant Cyperus rotundus L (Cyperaceae). Int. J. Appl. Res. Nat. Prod. 2012, 5, 1–8. [Google Scholar]
- Kamala, A.; Middha, S.K.; Karigar, C.S. Plants in traditional medicine with special reference to Cyperus rotundus L.: A review. 3 Biotech 2018, 8, 309. [Google Scholar] [CrossRef]
- Fang, F.; Jing, S.; Ma, Z.; Wang, Y.; Chen, Z. Nutritional evaluation of oilseed protein. Food Technol. 2013, 38, 69–73. [Google Scholar] [CrossRef]
- Yin, H.; Liu, Z.; Zhang, S.; An, Q. Response surface optimization of ultrasonic-assisted enzymatic extraction of ACE inhibitory peptides from oilseed rape. Food Ind. Technol. 2021, 42, 182–187. [Google Scholar] [CrossRef]
- Yang, Z. Structural Characterization and Mechanism Study of ACE Inhibitory Peptides Modified by Plastein Reaction from Oilseed Rape. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2022. [Google Scholar]
- Ma, C.; Wu, X. Cyperus peptide SFRWQ inhibits oxidation and inflammation in RAW264.7 cell model. Int. J. Biol. Macromol. 2024, 267, 131272. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wu, X. Preparation and stability study of antioxidant peptides from oilseed meal. J. Chin. Grain Oil 2023, 38, 80–89. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Xiao, G.; Vargas-De-La-Cruz, C.; Simal-Gandara, J.; Xiao, J.; Wang, Q. Active sites of peptides Asp-Asp-Asp-Tyr and Asp-Tyr-Asp-Asp protect against cellular oxidative stress. Food Chem. 2022, 366, 130626. [Google Scholar] [CrossRef]
- de Fátima Garcia, B.; de Barros, M.; de Souza Rocha, T. Bioactive peptides from beans with the potential to decrease the risk of developing noncommunicable chronic diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 2003–2021. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef]
- Zhang, D.D.; Lo, S.C.; Sun, Z.; Habib, G.M.; Lieberman, M.W.; Hannink, M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J. Biol. Chem. 2005, 280, 30091–30099. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, D.S.; Kim, T.H.; Kang, T.C. Glutathione Regulates GPx1 Expression during CA1 Neuronal Death and Clasmatodendrosis in the Rat Hippocampus following Status Epilepticus. Antioxidants 2022, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L. The effects of resveratrol powder on blood glucose levels and superoxide dismutase, catalase, glutathione peroxidase, and malondialdehyde in patients with type 2 diabetes. Hebei Tradit. Chin. Med. 2017, 39, 19–22+75. [Google Scholar]
- Stanhope, S.C.; Brandwine-Shemmer, T.; Blum, H.R.; Doud, E.H.; Jannasch, A.; Mosley, A.L.; Minke, B.; Weake, V.M. Proteome-wide quantitative analysis of redox cysteine availability in the Drosophila melanogaster eye reveals oxidation of phototransduction machinery during blue light exposure and age. Redox Biol. 2023, 63, 102723. [Google Scholar] [CrossRef]
- Banerjee, P.; Gaddam, N.; Chandler, V.; Chakraborty, S. Oxidative Stress-Induced Liver Damage and Remodeling of the Liver Vasculature. Am. J. Pathol. 2023, 193, 1400–1414. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Yeo, J.H.; Park, S.; Bae, Y.J.; Kwon, I.J.; Seong, S.H.; Lee, J.; Oh, S.H. ISG15-USP18 Dysregulation by Oxidative Stress Promotes IFN-γ Secretion from CD8+ T Cells in Vitiligo. J. Investig. Dermatol. 2024, 144, 273–283.e211. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.; Li, Y.; Duan, Y.; Lu, Y.; Sun, J. Antioxidant activity and stability of lotus seed shell polyphenols. Chin. J. Food Sci. 2019, 19, 89–95. [Google Scholar] [CrossRef]
- Zhang, S.; Chou, S.; Cui, H.; Wang, Z.; Liu, X.; Li, B. The effect of root bark glycoside on the rheological properties, antioxidant activity, and microstructure of low-ester pectin under acidic conditions. Food Sci. 2020, 41, 43–49. [Google Scholar]
- Zhu, K.; Zhu, H.; He, S.; Zhang, Y.; Tan, L.; Ma, S. Separation and characterization of crude polysaccharides from wintergreen tea and study of their antioxidant activity. J. Trop. Crop Sci. 2016, 37, 2014–2019. [Google Scholar]
- Zhang, W.; Zhang, J.; Zhou, H.; Zhao, D.; Li, H.; Liu, X.; Xiao, L. Preparation of small molecule antioxidant active peptides from flaxseed meal. Food Sci. 2020, 41, 36–44. [Google Scholar]
- Basilicata, M.G.; Pepe, G.; Sommella, E.; Ostacolo, C.; Manfra, M.; Sosto, G.; Pagano, G.; Novellino, E.; Campiglia, P. Peptidome profiles and bioactivity elucidation of buffalo-milk dairy products after gastrointestinal digestion. Food Res. Int. 2018, 105, 1003–1010. [Google Scholar] [CrossRef]
- Cao, X.; Lyu, Y.; Ning, J.; Tang, X.; Shen, X. Synthetic peptide, Ala-Arg-Glu-Gly-Glu-Met, abolishes pro-proliferative and anti-apoptotic effects of high glucose in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2017, 485, 215–220. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Ren, X.J.; Deng, S.G.; Wu, C.W. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem. 2015, 168, 662–667. [Google Scholar] [CrossRef]
- Luo, X.; Fei, Y.; Xu, Q.; Lei, T.; Mo, X.; Wang, Z.; Zhang, L.; Mou, X.; Li, H. Isolation and identification of antioxidant peptides from tartary buckwheat albumin (Fagopyrum tataricum Gaertn.) and their antioxidant activities. J. Food Sci. 2020, 85, 611–617. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, N.; Yang, J.; Kuang, W.; Xia, X.; Chen, X.; Liu, Z.; Huang, R. Puerarin Induces Molecular Details of Ferroptosis-Associated Anti-Inflammatory on RAW264.7 Macrophages. Metabolites 2022, 12, 653. [Google Scholar] [CrossRef]
- Homma, T.; Fujii, J. Application of Glutathione as Anti-Oxidative and Anti-Aging Drugs. Curr. Drug Metab. 2015, 16, 560–571. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Regulation of redox glutathione levels and gene transcription in lung inflammation: Therapeutic approaches. Free Radic. Biol. Med. 2000, 28, 1405–1420. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yao, W.; Li, J.; He, Q.; Shao, Y.; Huang, F. Acetyl-CoA from inflammation-induced fatty acids oxidation promotes hepatic malate-aspartate shuttle activity and glycolysis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E496–E510. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.B.; Mora, A.; Wang, T.J.; Santeford, A.; Usmani, D.; Ligon, M.M.; Mysorekar, I.U.; Apte, R.S. Loss of stearoyl-CoA desaturase 2 disrupts inflammatory response in macrophages. mBio 2023, 14, e0092523. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bai, J.; Du, Y.; Tan, P.; Zheng, T.; Chen, Y.; Cheng, Y.; Cai, T.; Huang, M.; Fu, W.; et al. Thiamine pretreatment improves endotoxemia-related liver injury and cholestatic complications by regulating galactose metabolism and inhibiting macrophage activation. Int. Immunopharmacol. 2022, 108, 108892. [Google Scholar] [CrossRef]
- Smith, T.J.; Johnson, C.R.; Koshy, R.; Hess, S.Y.; Qureshi, U.A.; Mynak, M.L.; Fischer, P.R. Thiamine deficiency disorders: A clinical perspective. Ann. N. Y. Acad. Sci. 2021, 1498, 9–28. [Google Scholar] [CrossRef]
- Yadav, U.C.; Kalariya, N.M.; Srivastava, S.K.; Ramana, K.V. Protective role of benfotiamine, a fat-soluble vitamin B1 analogue, in lipopolysaccharide-induced cytotoxic signals in murine macrophages. Free Radic. Biol. Med. 2010, 48, 1423–1434. [Google Scholar] [CrossRef]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Choi, Y.S.; Lee, J.K.; Lee, B.J.; Kim, W.K.; Kang, H. Anti-Inflammatory Activity of Citric Acid-Treated Wheat Germ Extract in Lipopolysaccharide-Stimulated Macrophages. Nutrients 2017, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, M.J.; McDonough, K.L.; Pituch, J.J.; Christopherson, P.L.; Cornell, T.T.; Selewski, D.T.; Shanley, T.P.; Blatt, N.B. Citrate modulates lipopolysaccharide-induced monocyte inflammatory responses. Clin. Exp. Immunol. 2015, 180, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.; Omara, E.A.; Sleem, A.A. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef]



 —van der Waals;
—van der Waals;  —conventional hydrogen bond;
—conventional hydrogen bond;  —carbon hydrogen bond;
—carbon hydrogen bond;  —pi–alkyl.
—pi–alkyl.
 —van der Waals;
—van der Waals;  —conventional hydrogen bond;
—conventional hydrogen bond;  —carbon hydrogen bond;
—carbon hydrogen bond;  —pi–alkyl.
—pi–alkyl.
| Time | Buffer A | Buffer B |
|---|---|---|
| 0 | 96% | 4% |
| 3 | 92% | 8% |
| 89 | 72% | 28% |
| 109 | 60% | 40% |
| 110 | 5% | 95% |
| 120 | 5% | 95% |
| CAOP | Fractions a | Fractions b | |
|---|---|---|---|
| DPPH clearance rate | 88.69% | 81.26% | 90.63% |
| Peptide Sequence | Theoretical Mass-to-Charge Ratio | PeptideRanker Scores | Rate of Repetition | Scavenger Score | |
|---|---|---|---|---|---|
| 1 | LWGR | 530.6243 | 0.912846 | 75.00% | 0.49713 |
| 2 | GHPWG | 552.599 | 0.945357 | 60.00% | 0.61598 |
| 3 | SFRWQ | 722.799 | 0.891748 | 40.00% | 0.43137 |
| 4 | TLGHPWG | 766.8586 | 0.608396 | 42.86% | 0.58287 |
| 5 | DLHMFVWS | 1034.1933 | 0.613035 | 37.50% | 0.41534 |
| 6 | FAY | 399.4395 | 0.871389 | 33.33% | 0.46884 |
| 7 | YLW | 480.5595 | 0.948405 | 33.33% | 0.55369 |
| 8 | WSY | 454.4795 | 0.82987 | 33.33% | 0.51467 |
| 9 | FHL | 415.4895 | 0.88902 | 33.33% | 0.47922 |
| 10 | IIWYTL | 807.9738 | 0.707751 | 33.33% | 0.50076 |
| 11 | WLH | 454.5295 | 0.904459 | 33.33% | 0.5521 |
| 12 | IWY | 480.5595 | 0.886712 | 33.33% | 0.54823 |
| 13 | VWF | 450.5395 | 0.975754 | 33.33% | 0.47109 |
| 14 | WGI | 374.4395 | 0.941714 | 33.33% | 0.48014 |
| 15 | QPRYLP | 772.8938 | 0.76001 | 33.33% | 0.50282 |
| 16 | IPW | 414.4995 | 0.959086 | 33.33% | 0.49862 |
| 17 | AYF | 399.4395 | 0.915352 | 33.33% | 0.48733 |
| 18 | LAW | 388.4595 | 0.879554 | 33.33% | 0.44419 |
| 19 | WGW | 447.4995 | 0.996392 | 33.33% | 0.57219 |
| 20 | FVW | 450.5395 | 0.974429 | 33.33% | 0.4388 |
| 21 | WIY | 480.5595 | 0.884482 | 33.33% | 0.52127 |
| 22 | MYL | 425.5395 | 0.816343 | 33.33% | 0.49952 |
| 23 | TWL | 418.4895 | 0.776305 | 33.33% | 0.45991 |
| 24 | IVW | 416.5195 | 0.603901 | 33.33% | 0.41524 |
| 25 | LGHPWGNAPG | 1005.1028 | 0.713675 | 30.00% | 0.51702 |
| Sequence of Peptide | HOMO (ev) | LUMO (ev) | Molecular Energy (a.u.) | -ICE | |
|---|---|---|---|---|---|
| 1 | DLHMFVWS | −5.4507 | −1.1325 | −3809.8757 | 62.8072 |
| 2 | LGHPWGNAPG | −5.5236 | −0.6152 | −3459.8023 | 57.4345 |
| 3 | SFRWQ | −6.9920 | −0.1101 | −2472.8600 | 49.3525 |
| 4 | LWGR | −6.9546 | 0.1952 | −1789.8200 | 47.4082 |
| 5 | IIWYTL | −5.8627 | −0.5208 | −2696.6552 | 43.0278 |
| 6 | QPRYLP | −5.7266 | −1.1627 | −2629.4169 | 40.4706 |
| 7 | TLGHPWG | −5.5236 | −0.6152 | −2625.8286 | 38.9967 |
| 8 | AYF | −6.0262 | −0.4726 | −1355.3300 | 33.4841 |
| 9 | FVW | −5.5519 | −0.4343 | −1490.2817 | 32.4883 |
| 10 | YLW | −5.6167 | −0.4707 | −1604.7700 | 32.4435 |
| 11 | WGW | −5.4719 | −0.4188 | −1503.8900 | 31.9292 |
| 12 | FAY | −6.1293 | −0.7725 | −1355.3300 | 29.1102 |
| 13 | WSY | −5.2632 | −0.7638 | −1562.0300 | 28.9494 |
| 14 | IVW | −0.7721 | 3.4173 | −1368.4686 | 28.8689 |
| 15 | VWF | −5.5876 | −0.4090 | −1490.2800 | 29.5592 |
| 16 | GHPWG | −6.9375 | 0.1404 | −1899.5600 | 27.8538 |
| 17 | LAW | −5.5522 | −0.4441 | −1298.5900 | 27.9754 |
| 18 | MYL | −5.5745 | −0.8579 | −1719.0041 | 27.2575 |
| 19 | TWL | −5.9503 | −0.7564 | −1413.0840 | 27.0853 |
| 20 | WGI | −5.4961 | −0.8079 | −1259.2700 | 26.2893 |
| 21 | IPW | −5.4243 | −0.5412 | −1375.9600 | 26.2193 |
| 22 | IWY | −5.6442 | −0.4528 | −1604.7700 | 26.1046 |
| 23 | WIY | −5.3859 | −0.8465 | 1604.7711 | 25.0357 |
| 24 | WLH | −5.2948 | −1.1453 | −1523.5100 | 23.5987 |
| 25 | FHL | −6.5590 | −0.7031 | −1392.0000 | 19.8777 |
| TX6 | 17.7358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Ma, C.; Wu, X.; Hao, H. The Characterization and Identification of Cyperus Protein: An In Vitro Study on Its Antioxidant and Anti-Inflammatory Potential. Nutrients 2025, 17, 2633. https://doi.org/10.3390/nu17162633
Zhang Q, Ma C, Wu X, Hao H. The Characterization and Identification of Cyperus Protein: An In Vitro Study on Its Antioxidant and Anti-Inflammatory Potential. Nutrients. 2025; 17(16):2633. https://doi.org/10.3390/nu17162633
Chicago/Turabian StyleZhang, Qian, Chaoyue Ma, Xiaotong Wu, and Huifang Hao. 2025. "The Characterization and Identification of Cyperus Protein: An In Vitro Study on Its Antioxidant and Anti-Inflammatory Potential" Nutrients 17, no. 16: 2633. https://doi.org/10.3390/nu17162633
APA StyleZhang, Q., Ma, C., Wu, X., & Hao, H. (2025). The Characterization and Identification of Cyperus Protein: An In Vitro Study on Its Antioxidant and Anti-Inflammatory Potential. Nutrients, 17(16), 2633. https://doi.org/10.3390/nu17162633






