Ultra-Processed Food and Frailty: Evidence from a Prospective Cohort Study and Implications for Future Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diet Assessment
2.3. Frailty Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Frailty Analysis
3.2. Frality Components
4. Discussion
4.1. Our Findings in Context
4.2. Strengths and Limitations
4.3. Future Directions
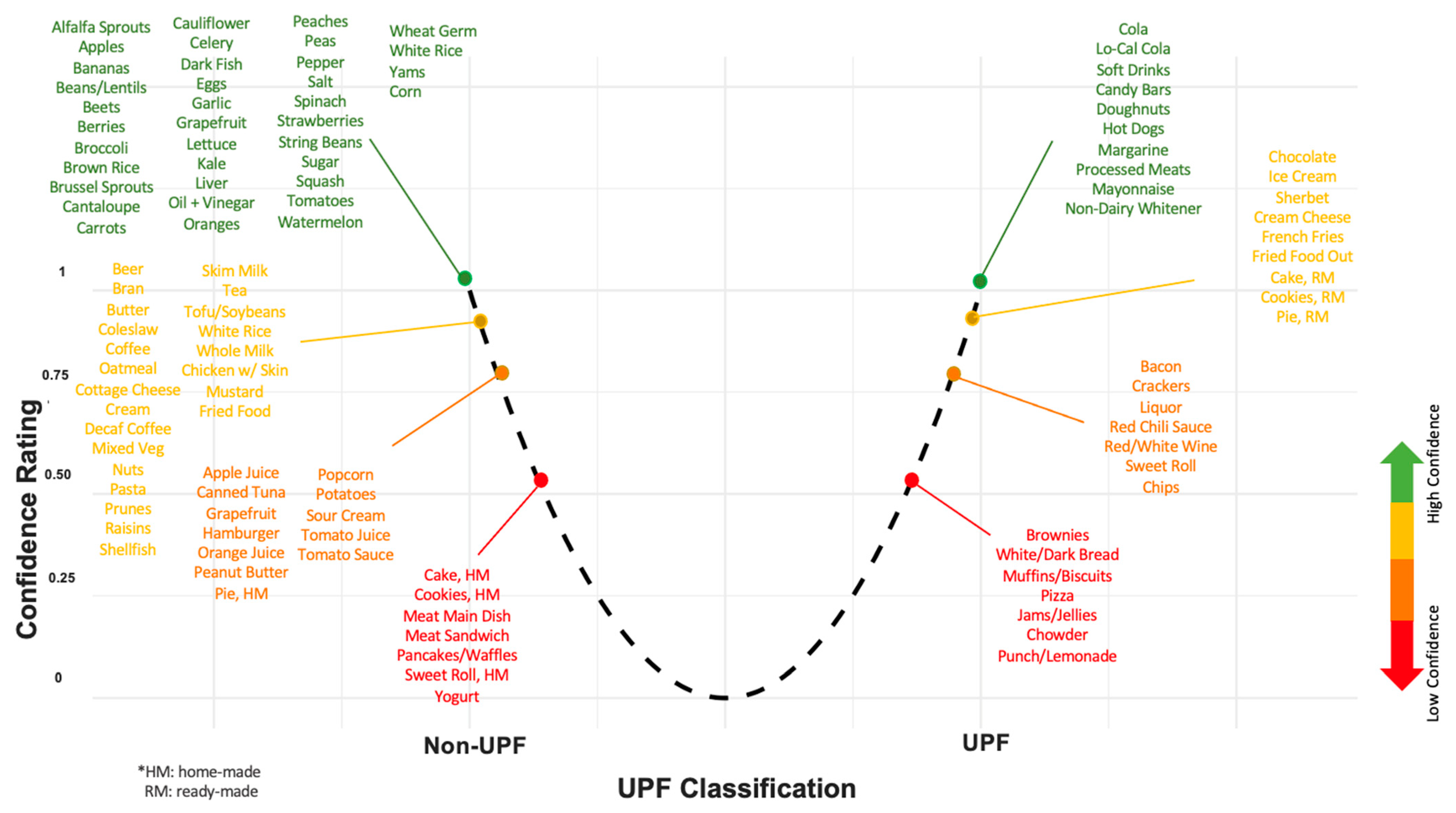
4.4. AI, UPF Research, and UPF Policy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017—Highlights; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Chen, X.; Mao, G. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. J. Am. Geriatr. Soc. 2010, 58, 681–687. [Google Scholar] [CrossRef]
- Klein, B.E.K.; Klein, R.; Knudtson, M.D.; Lee, K.E. Frailty, morbidity and survival. Arch. Gerontol. Geriatr. 2005, 41, 141–149. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of disabilities among community-dwelling older people: A systematic review and meta-analysis. Disabil. Rehabil. 2017, 39, 1897–1908. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.-L.; Walston, J.D.; Kasper, J.D. Frailty in Older Adults: A Nationally Representative Profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef]
- Young, A.C.M.; Glaser, K.; Spector, T.D.; Steves, C.J. The Identification of Hereditary and Environmental Determinants of Frailty in a Cohort of UK Twins. Twin Res. Hum. Genet. 2016, 19, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Murabito, J.M.; Yuan, R.; Lunetta, K.L. The Search for Longevity and Healthy Aging Genes: Insights From Epidemiological Studies and Samples of Long-Lived Individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67A, 470–479. [Google Scholar] [CrossRef]
- Livshits, G.; Ni Lochlainn, M.; Malkin, I.; Bowyer, R.; Verdi, S.; Steves, C.J.; Williams, F.M.K. Shared genetic influence on frailty and chronic widespread pain: A study from TwinsUK. Age Ageing 2018, 47, 119–125. [Google Scholar] [CrossRef]
- Dato, S.; Montesanto, A.; Lagani, V.; Jeune, B.; Christensen, K.; Passarino, G. Frailty phenotypes in the elderly based on cluster analysis: A longitudinal study of two Danish cohorts. Evidence for a genetic influence on frailty. AGE 2012, 34, 571–582. [Google Scholar] [CrossRef]
- Zoico, E.; Roubenoff, R. The Role of Cytokines in Regulating Protein Metabolism and Muscle Function. Nutr. Rev. 2002, 60, 39–51. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: Overlap of clinical features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; O’Neill, E.F.; Ryan, N.D.; Clements, K.M.; Solares, G.R.; Nelson, M.E.; Roberts, S.B.; Kehayias, J.J.; Lipsitz, L.A.; Evans, W.J. Exercise Training and Nutritional Supplementation for Physical Frailty in Very Elderly People. N. Engl. J. Med. 1994, 330, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Bartali, B.; Zhou, J.; Blaum, C.; Ko, C.-W.; Fried, L.P. Low Serum Micronutrient Concentrations Predict Frailty Among Older Women Living in the Community. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Rashidi Pour Fard, N.; Amirabdollahian, F.; Haghighatdoost, F. Dietary patterns and frailty: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 498–513. [Google Scholar] [CrossRef]
- Das, A.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Ribeiro, R.V.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Simpson, S.J.; Hirani, V. Prospective Associations Between Dietary Antioxidant Intake and Frailty in Older Australian Men: The Concord Health and Ageing in Men Project. J. Gerontol. Ser. A 2019, 75, 348–356. [Google Scholar] [CrossRef]
- The Three-Generation Study of Women on Diets and Health Study Groups; Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S. Inverse association between dietary habits with high total antioxidant capacity and prevalence of frailty among elderly Japanese women: A multicenter cross-sectional study. J. Nutr. Health Aging 2014, 18, 827–836. [Google Scholar] [CrossRef]
- Millar, C.L.; Costa, E.; Jacques, P.F.; Dufour, A.B.; Kiel, D.P.; Hannan, M.T.; Sahni, S. Adherence to the Mediterranean-style diet and high intake of total carotenoids reduces the odds of frailty over 11 years in older adults: Results from the Framingham Offspring Study. Am. J. Clin. Nutr. 2022, 116, 630–639. [Google Scholar] [CrossRef]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2018, 66, 783–788. [Google Scholar] [CrossRef]
- Ward, R.E.; Orkaby, A.R.; Chen, J.; Hshieh, T.T.; Driver, J.A.; Gaziano, J.M.; Djousse, L. Association between Diet Quality and Frailty Prevalence in the Physicians’ Health Study. J. Am. Geriatr. Soc. 2020, 68, 770–776. [Google Scholar] [CrossRef]
- Shivappa, N.; Stubbs, B.; Hébert, J.R.; Cesari, M.; Schofield, P.; Soysal, P.; Maggi, S.; Veronese, N. The Relationship Between the Dietary Inflammatory Index and Incident Frailty: A Longitudinal Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 77–82. [Google Scholar] [CrossRef]
- Millar, C.L.; Dufour, A.B.; Shivappa, N.; Habtemariam, D.; Murabito, J.M.; Benjamin, E.J.; Hebert, J.R.; Kiel, D.P.; Hannan, M.T.; Sahni, S. A proinflammatory diet is associated with increased odds of frailty after 12-year follow-up in a cohort of adults. Am. J. Clin. Nutr. 2022, 115, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211. [Google Scholar] [CrossRef] [PubMed]
- Mariath, A.B.; Machado, A.D.; Ferreira, L.D.N.M.; Ribeiro, S.M.L. The possible role of increased consumption of ultra-processed food products in the development of frailty: A threat for healthy ageing? Br. J. Nutr. 2022, 128, 461–466. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; De Castro, I.R.R.; Cannon, G. Increasing consumption of ultra-processed foods and likely impact on human health: Evidence from Brazil. Public Health Nutr. 2010, 14, 5–13. [Google Scholar] [CrossRef]
- Louzada, M.L.D.C.; Martins, A.P.B.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.-C.; Cannon, G.; Monteiro, C.A. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev. Saúde Pública 2015, 49, 38. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; da Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019; Volume 48. [Google Scholar]
- Hao, J.; Zhou, P.; Qiu, H. Association between Ultra-Processed Food Consumption and Frailty in American Elder People: Evidence from a Cross-Sectional Study. J. Nutr. Health Aging 2022, 26, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Donghia, R.; Castellana, F.; Bortone, I.; De Nucci, S.; Sila, A.; Tatoli, R.; Lampignano, L.; Sborgia, G.; Panza, F.; et al. Ultra-processed food consumption and nutritional frailty in older age. GeroScience 2023, 45, 2229–2243. [Google Scholar] [CrossRef]
- Sandoval-Insausti, H.; Blanco-Rojo, R.; Graciani, A.; López-García, E.; Moreno-Franco, B.; Laclaustra, M.; Donat-Vargas, C.; Ordovás, J.M.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Ultra-processed Food Consumption and Incident Frailty: A Prospective Cohort Study of Older Adults. J. Gerontol. Ser. A 2020, 75, 1126–1133. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rossato, S.L.; Chen, Z.; Khandpur, N.; Rodriguez-Artalejo, F.; Willett, W.C.; Struijk, E.A.; Lopez-Garcia, E. Ultraprocessed foods, unprocessed or minimally processed foods, and risk of frailty in a cohort of United States females. Am. J. Clin. Nutr. 2024, 120, 232–239. [Google Scholar] [CrossRef]
- Cordova, R.; Kliemann, N.; Huybrechts, I.; Rauber, F.; Vamos, E.P.; Levy, R.B.; Wagner, K.-H.; Viallon, V.; Casagrande, C.; Nicolas, G.; et al. Consumption of ultra-processed foods associated with weight gain and obesity in adults: A multi-national cohort study. Clin. Nutr. 2021, 40, 5079–5088. [Google Scholar] [CrossRef] [PubMed]
- Beslay, M.; Srour, B.; Méjean, C.; Allès, B.; Fiolet, T.; Debras, C.; Chazelas, E.; Deschasaux, M.; Wendeu-Foyet, M.G.; Hercberg, S.; et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: A prospective analysis of the French NutriNet-Santé cohort. PLoS Med. 2020, 17, e1003256. [Google Scholar] [CrossRef]
- Mendonça, R.D.D.; Pimenta, A.M.; Gea, A.; De La Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Lopes, A.C.S.; Bes-Rastrollo, M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016, 104, 1433–1440. [Google Scholar] [CrossRef]
- Canhada, S.L.; Luft, V.C.; Giatti, L.; Duncan, B.B.; Chor, D.; Fonseca, M.D.J.M.D.; Matos, S.M.A.; Molina, M.D.C.B.; Barreto, S.M.; Levy, R.B.; et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2020, 23, 1076–1086. [Google Scholar] [CrossRef]
- Willett, W.C.; Reynolds, R.D.; Cottrell-Hoehner, S.; Sampson, L.; Browne, M.L. Validation of a semi-quantitative food frequency questionnaire: Comparison with a 1-year diet record. J. Am. Diet. Assoc. 1987, 87, 43–47. [Google Scholar] [CrossRef]
- Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J.; Colditz, G.A.; Litin, L.B.; Willett, W.C. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. Am. J. Epidemiol. 1992, 135, 1114–1126. [Google Scholar] [CrossRef]
- Khandpur, N.; Rossato, S.; Drouin-Chartier, J.-P.; Du, M.; Steele, E.M.; Sampson, L.; Monteiro, C.; Zhang, F.F.; Willett, W.; Fung, T.T.; et al. Categorising ultra-processed foods in large-scale cohort studies: Evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J. Nutr. Sci. 2021, 10, e77. [Google Scholar] [CrossRef]
- Willett, W.; Howe, G.; Kushi, L. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Siefkas, A.C.; Millar, C.L.; Dufour, A.B.; Kiel, D.P.; Jacques, P.F.; Hannan, M.T.; Sahni, S. Dairy Food Intake Is Not Associated With Frailty in Adults From the Framingham Heart Study. J. Acad. Nutr. Diet. 2023, 123, 729–739.e1. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.; Millar, C.L.; Nguyen Lily, T.N.; Mukamal, K.J.; Kiel, D.P.; Lipsitz, L.A.; Hannan, M.T.; Sahni, S. Higher intake of dietary flavonols, specifically dietary quercetin, is associated with lower odds of frailty onset over 12 years of follow-up among adults in the Framingham Heart Study. Am. J. Clin. Nutr. 2023, 118, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Jacques, P.; Dufour, A.; Millar, C.; Kiel, D.; Hannan, M. Total Carotenoid Intake Reduces the Odds of Frailty over 9 Years in Older Adults: Results from the Framingham Offspring Study. Curr. Dev. Nutr. 2020, 4, nzaa040_072. [Google Scholar] [CrossRef]
- Millar, C.L.; Dufour, A.B.; Hebert, J.R.; Shivappa, N.; Okereke, O.I.; Kiel, D.P.; Hannan, M.T.; Sahni, S. Association of Proinflammatory Diet With Frailty Onset Among Adults With and Without Depressive Symptoms: Results From the Framingham Offspring Study. J. Gerontol. Ser. A 2023, 78, 250–257. [Google Scholar] [CrossRef]
- Kannel, W.B. Some Health Benefits of Physical Activity: The Framingham Study. Arch. Intern. Med. 1979, 139, 857. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Zhang, Y.; Cheng, H.; Gao, W.; Lai, C.-Q.; Gabriel, S.; Gupta, N.; Vasan, R.S.; Levy, D.; et al. Diet Quality Scores Are Positively Associated with Whole Blood–Derived Mitochondrial DNA Copy Number in the Framingham Heart Study. J. Nutr. 2022, 152, 690–697. [Google Scholar] [CrossRef]
- Fung, T.T. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch. Intern. Med. 2008, 168, 713. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Relative validity and reproducibility of a diet history questionnaire in Spain. I. Foods. EPIC Group of Spain. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26, 91S–99S. [CrossRef] [PubMed]
- Braesco, V.; Souchon, I.; Sauvant, P.; Haurogné, T.; Maillot, M.; Féart, C.; Darmon, N. Ultra-processed foods: How functional is the NOVA system? Eur. J. Clin. Nutr. 2022, 76, 1245–1253. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Schols, J.M.G.A.; Meijers, J.M.M.; Tan, F.E.S.; Verlaan, S.; Luiking, Y.C.; Morley, J.E.; Halfens, R.J.G. Instruments to Assess Sarcopenia and Physical Frailty in Older People Living in a Community (Care) Setting: Similarities and Discrepancies. J. Am. Med. Dir. Assoc. 2015, 16, 301–308. [Google Scholar] [CrossRef]
- Watanabe, D.; Kurotani, K.; Yoshida, T.; Nanri, H.; Watanabe, Y.; Date, H.; Itoi, A.; Goto, C.; Ishikawa-Takata, K.; Kimura, M.; et al. Diet quality and physical or comprehensive frailty among older adults. Eur. J. Nutr. 2022, 61, 2451–2462. [Google Scholar] [CrossRef]
- Struijk, E.A.; Hagan, K.A.; Fung, T.T.; Hu, F.B.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Diet quality and risk of frailty among older women in the Nurses’ Health Study. Am. J. Clin. Nutr. 2020, 111, 877–883. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Dietary Quality Is Related to Frailty in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 483–489. [Google Scholar] [CrossRef]
- Shikany, J.M.; Barrett-Connor, E.; Ensrud, K.E.; Cawthon, P.M.; Lewis, C.E.; Dam, T.-T.L.; Shannon, J.; Redden, D.T.; for the Osteoporotic Fractures in Men (MrOS) Research Group. Macronutrients, Diet Quality, and Frailty in Older Men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 695–701. [Google Scholar] [CrossRef]
- Parsons, T.J.; Papachristou, E.; Atkins, J.L.; Papacosta, O.; Ash, S.; Lennon, L.T.; Whincup, P.H.; Ramsay, S.E.; Wannamethee, S.G. Physical frailty in older men: Prospective associations with diet quality and patterns. Age Ageing 2019, 48, 355–360. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Godin, J.; Cahill, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Park, Y.-M.; Fung, T.T.; Rockwood, K. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. 2021, 19, 64. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Shim, J.-S.; Shim, S.Y.; Cha, H.-J.; Kim, J.; Kim, H.C. Association between Ultra-processed Food Consumption and Dietary Intake and Diet Quality in Korean Adults. J. Acad. Nutr. Diet. 2022, 122, 583–594. [Google Scholar] [CrossRef]
- Calixto Andrade, G.; Julia, C.; Deschamps, V.; Srour, B.; Hercberg, S.; Kesse-Guyot, E.; Allès, B.; Chazelas, E.; Deschasaux, M.; Touvier, M.; et al. Consumption of Ultra-Processed Food and Its Association with Sociodemographic Characteristics and Diet Quality in a Representative Sample of French Adults. Nutrients 2021, 13, 682. [Google Scholar] [CrossRef]
- Moubarac, J.-C.; Batal, M.; Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017, 108, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Steele, E.M.; Li, Y.; Karageorgou, D.; Micha, R.; Monteiro, C.A.; Mozaffarian, D. Consumption of Ultraprocessed Foods and Diet Quality Among U.S. Children and Adults. Am. J. Prev. Med. 2022, 62, 252–264. [Google Scholar] [CrossRef]
- Lauria, F.; Dello Russo, M.; Formisano, A.; De Henauw, S.; Hebestreit, A.; Hunsberger, M.; Krogh, V.; Intemann, T.; Lissner, L.; Molnar, D.; et al. Ultra-processed foods consumption and diet quality of European children, adolescents and adults: Results from the I.Family study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3031–3043. [Google Scholar] [CrossRef]
- Buchner, D.M.; Cress, M.E.; Esselman, P.C.; Margherita, A.J.; De Lateur, B.J.; Campbell, A.J.; Wagner, E.H. Factors Associated With Changes in Gait Speed in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51A, M297–M302. [Google Scholar] [CrossRef]
- Perera, S.; Patel, K.V.; Rosano, C.; Rubin, S.M.; Satterfield, S.; Harris, T.; Ensrud, K.; Orwoll, E.; Lee, C.G.; Chandler, J.M.; et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 63–71. [Google Scholar] [CrossRef]
- Shinkai, S. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing 2000, 29, 441–446. [Google Scholar] [CrossRef]
- Studenski, S. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Yousef, N.S.; Dilian, O.; Iktilat, K.; Agmon, M. CRP, but not fibrinogen, is associated with gait speed as early as middle age, in females but not males. Sci. Rep. 2023, 13, 15571. [Google Scholar] [CrossRef]
- Baptista, G.; Dupuy, A.-M.; Jaussent, A.; Durant, R.; Ventura, E.; Sauguet, P.; Picot, M.-C.; Jeandel, C.; Cristol, J.P. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Radic. Res. 2012, 46, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Oh-Park, M.; Derby, C.A.; Lipton, R.B.; Wang, C. Inflammatory Markers and Gait Speed Decline in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66A, 1083–1089. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, Y.; Rayamajhi, S.; Thapa, A.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Zhang, T.; Wang, X.; et al. Ultra-processed food intake is associated with grip strength decline in middle-aged and older adults: A prospective analysis of the TCLSIH study. Eur. J. Nutr. 2022, 61, 1331–1341. [Google Scholar] [CrossRef]
- Payette, H.; Hanusaik, N.; Boutier, V.; Morais, J.; Gray-Donald, K. Muscle strength and functional mobility in relation to lean body mass in free-living frail elderly women. Eur. J. Clin. Nutr. 1998, 52, 45–53. [Google Scholar] [CrossRef][Green Version]
- Schaap, L.A.; Van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. Ser. A 2018, 73, 1199–1204. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kong, W.; Xie, Y.; Hu, J.; Ding, W.; Cao, C. Higher ultra processed foods intake is associated with low muscle mass in young to middle-aged adults: A cross-sectional NHANES study. Front. Nutr. 2024, 11, 1280665. [Google Scholar] [CrossRef]
- Jung, S.; Seo, J.; Kim, J.Y.; Park, S. Associations of Ultra-Processed Food Intake with Body Fat and Skeletal Muscle Mass by Sociodemographic Factors. Diabetes Metab. J. 2024, 48, 780–789. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Steele, E.M.; Yang, X.; Gao, R.; Wang, C.; Liu, J. Association of ultra-processed food consumption with muscle mass among young and middle-aged US adults. Eur. J. Nutr. 2024, 63, 2621–2629. [Google Scholar] [CrossRef]
- Rudakoff, L.C.S.; Magalhães, E.I.D.S.; Viola, P.C.D.A.F.; De Oliveira, B.R.; Da Silva Coelho, C.C.N.; Bragança, M.L.B.M.; Arruda, S.P.M.; Cardoso, V.C.; Bettiol, H.; Barbieri, M.A.; et al. Ultra-processed food consumption is associated with increase in fat mass and decrease in lean mass in Brazilian women: A cohort study. Front. Nutr. 2022, 9, 1006018. [Google Scholar] [CrossRef]
- Wallace, J.I.; Schwartz, R.S. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int. J. Cardiol. 2002, 85, 15–21. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Garry, P.J.; Hunt, W.C.; Rhyne, R.L. Distributions of serial changes in stature and weight in a healthy elderly population. Hum. Biol. 1988, 60, 917–925. [Google Scholar] [PubMed]
- DiMilia, P.R.; Mittman, A.C.; Batsis, J.A. Benefit-to-Risk Balance of Weight Loss Interventions in Older Adults with Obesity. Curr. Diab. Rep. 2019, 19, 114. [Google Scholar] [CrossRef]
- McMinn, J.; Steel, C.; Bowman, A. Investigation and management of unintentional weight loss in older adults. BMJ 2011, 342, d1732. [Google Scholar] [CrossRef]
- Rothman, M.D.; Leo-Summers, L.; Gill, T.M. Prognostic Significance of Potential Frailty Criteria. J. Am. Geriatr. Soc. 2008, 56, 2211–2216. [Google Scholar] [CrossRef]
- Han, T.S.; Tajar, A.; Lean, M.E.J. Obesity and weight management in the elderly. Br. Med. Bull. 2011, 97, 169–196. [Google Scholar] [CrossRef]
- Leonberg, K.E.; Maski, M.R.; Scott, T.M.; Naumova, E.N. Ultra-Processed Food and Chronic Kidney Disease Risk: A Systematic Review, Meta-Analysis, and Recommendations. Nutrients 2025, 17, 1560. [Google Scholar] [CrossRef]
- Leonberg, K.E.; Maski, M.R.; Scott, T.M.; Chen, Y.; Zhou, B.; Naumova, E.N. Trends in chronic kidney disease and calories from ultra-processed foods: NHANES at the highly granular level. Discov. Public Health 2025, 22, 169. [Google Scholar] [CrossRef]
- Huffman, G.B. Evaluating and treating unintentional weight loss in the elderly. Am. Fam. Physician 2002, 65, 640–650. [Google Scholar]
- Sahyoun, N.R.; Jacques, P.F.; Zhang, X.L.; Juan, W.; McKeown, N.M. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am. J. Clin. Nutr. 2006, 83, 124–131. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Yoshida, M.; Shea, M.K.; Jacques, P.F.; Lichtenstein, A.H.; Rogers, G.; Booth, S.L.; Saltzman, E. Whole-Grain Intake and Cereal Fiber Are Associated with Lower Abdominal Adiposity in Older Adults. J. Nutr. 2009, 139, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Maras, J.E.; Newby, P.K.; Bakun, P.J.; Ferrucci, L.; Tucker, K.L. Whole grain intake: The Baltimore Longitudinal Study of Aging. J. Food Compos. Anal. 2009, 22, 53–58. [Google Scholar] [CrossRef]
- Monge, A.; Silva Canella, D.; López-Olmedo, N.; Lajous, M.; Cortés-Valencia, A.; Stern, D. Ultraprocessed beverages and processed meats increase the incidence of hypertension in Mexican women. Br. J. Nutr. 2021, 126, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Khandpur, N.; Katsikari, A.; Biessy, C.; Torres-Mejía, G.; Ángeles-Llerenas, A.; Alvarado-Cabrero, I.; Sánchez, G.I.; Maldonado, M.E.; Porras, C.; et al. Consumption of industrial processed foods and risk of premenopausal breast cancer among Latin American women: The PRECAMA study. BMJ Nutr. Prev. Health 2022, 5, 1–9. [Google Scholar] [CrossRef]
- Dinu, M.; Bonaccio, M.; Martini, D.; Madarena, M.P.; Vitale, M.; Pagliai, G.; Esposito, S.; Ferraris, C.; Guglielmetti, M.; Rosi, A.; et al. Reproducibility and validity of a food-frequency questionnaire (NFFQ) to assess food consumption based on the NOVA classification in adults. Int. J. Food Sci. Nutr. 2021, 72, 861–869. [Google Scholar] [CrossRef]
- Ravandi, B.; Ispirova, G.; Sebek, M.; Mehler, P.; Barabási, A.-L.; Menichetti, G. Prevalence of processed foods in major US grocery stores. Nat. Food 2025, 6, 296–308. [Google Scholar] [CrossRef]
- Côté, M.; Lamarche, B. Artificial intelligence in nutrition research: Perspectives on current and future applications. Appl. Physiol. Nutr. Metab. 2022, 47, 1–8. [Google Scholar] [CrossRef]
- Menichetti, G.; Ravandi, B.; Mozaffarian, D.; Barabási, A.-L. Machine learning prediction of the degree of food processing. Nat. Commun. 2023, 14, 2312. [Google Scholar] [CrossRef]
- Hu, G.; Flexner, N.; Tiscornia, M.V.; L’Abbé, M.R. Accelerating the Classification of NOVA Food Processing Levels Using a Fine-Tuned Language Model: A Multi-Country Study. Nutrients 2023, 15, 4167. [Google Scholar] [CrossRef]
- Elbassuoni, S.; Ghattas, H.; Ati, J.E.; Shmayssani, Z.; Katerji, S.; Zoughbi, Y.; Semaan, A.; Akl, C.; Gharbia, H.B.; Sassi, S. DeepNOVA: A Deep Learning NOVA Classifier for Food Images. IEEE Access 2022, 10, 128523–128535. [Google Scholar] [CrossRef]
- Kumu. Relationship Mapping Software; Kumu: Mandaluyong City, Philippines, 2025. [Google Scholar]
- Naumova, E.N. Causal AI for public health research and policy: A journey back to the future. J. Public Health Policy 2024, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sgaier, S.K.; Huang, V.; Charles, G. The Case for Causal AI. Stanf. Soc. Innov. Rev. 2020, 18, 5055. [Google Scholar] [CrossRef]


| Characteristics | Included Participants | Participants Excluded Due to Lack of Frailty Follow-Up Assessment | ||
|---|---|---|---|---|
| Mean (SD) or n (%) | n | Mean (SD) or n (%) | n | |
| Follow-Up Time (years) | 10.8 (2.7) | 2547 | - | - |
| Age (years) | 60.3 (8.9) | 2547 | 66.7 (9.6) | 431 |
| Female, n (%) | 1402 (55.1) | 2547 | 215 (49.9) | 431 |
| Education, n (%) | 2547 | 431 | ||
| Less than High School | 87 (3.4) | 18 (4.2) | ||
| High School Graduate | 708 (27.8) | 144 (33.4) | ||
| Some College | 723 (28.4) | 131 (30.4) | ||
| College Graduate | 990 (38.9) | 113 (26.2) | ||
| Missing | 39 (1.5) | 25 (5.8) | ||
| Current Smoking, n (%) | 283 (11.1) | 2547 | 68 (15.8) | 430 |
| Health Status, n (%) | 2547 | 430 | ||
| Excellent | 1231 (48.5) | 127 (39.5) | ||
| Good/Very Good | 1187 (46.7) | 260 (60.5) | ||
| Fair/Poor | 123 (4.8) | 43 (10.0) | ||
| UPF Intake (servings/day) | 7.2 (2.9) | 2547 | 7.3 (2.7) | 431 |
| Energy Intake (kcal/day) | 1840.4 (594.3) | 2547 | 1788.4 (587.8) | 431 |
| DASH Score | 24.2 (5.2) | 2547 | 23.7 (5.2) | 431 |
| Multivitamin Use, n (%) | 1331 (52.3) | 2545 | 220 (51.2) | 430 |
| BMI (kg/m2) | 28.1 (5.3) | 2547 | 28.5 (5.1) | 431 |
| Physical Activity Index | 37.9 (6.2) | 2510 | 37.8 (6.6) | 418 |
| Grip Strength (kg) | 33.6 (12.9) | 1905 | 30.4 (11.9) | 290 |
| Gait Speed (m/s) | 1.3 (0.3) | 2084 | 1.1 (0.3) | 304 |
| Exhaustion, n (%) | 130 (5.1) | 2533 | 28 (6.6) | 427 |
| Weight Loss, n (%) | 47 (1.9) | 2547 | 8 (1.9) | 431 |
| History of CVD, n (%) | 257 (10.1) | 2547 | 102 (23.7) | 431 |
| History of Cancer, n (%) | 212 (8.3) | 2547 | 69 (16.0) | 431 |
| History of Diabetes, n (%) | 261 (10.3) | 2547 | 89 (20.7) | 431 |
| Cumulative Logistic Regression | Mixed Logistic Regression | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Model 1 a | 1.04 (0.98, 1.10) | 0.16 | 0.97 (0.90, 1.04) | 0.35 |
| Model 2 b | 1.01 (0.95, 1.07) | 0.81 | 0.98 (0.91, 1.10) | 0.94 |
| Model 3 c | 0.98 (0.92, 1.05) | 0.55 | 0.93 (0.84, 1.02) | 0.14 |
| Cumulative Logistic Regression | Mixed Logistic Regression | |||
|---|---|---|---|---|
| OR (95% CI) a | P (Interaction) b | OR (95% CI) a | P (Interaction) b | |
| Sex | ||||
| Men (n = 1145) | 0.95 (0.86, 1.06) | 0.39 | 0.99 (0.81, 1.21) | 0.70 |
| Women (n = 1402) | 1.07 (1.04, 1.09) | 0.94 (0.83, 1.05) | ||
| Baseline Age | ||||
| Baseline Age < 60 years (n = 1253) | 1.05 (0.93, 1.20) | 0.17 | 0.94 (0.78, 1.12) | 0.68 |
| Baseline Age ≥ 60 years (n = 1294) | 0.95 (0.88, 1.03) | 0.96 (0.85, 1.08) | ||
| β (95% CI) | p Value | |
|---|---|---|
| Annualized Change in Grip Strength, kg/year (n = 1872) | ||
| Model 1 a | −0.01 (−0.02, 0.00) | 0.14 |
| Model 2 b | −0.01 (−0.02, 0.01) | 0.23 |
| Model 3 c | −0.01 (−0.02, 0.01) | 0.30 |
| Annualized Change in Gait Speed, m/s/year (n = 2033) | ||
| Model 1 | −0.001 (−0.001, −0.002) | 0.01 * |
| Model 2 | −0.001 (−0.001, −0.0001) | 0.03 * |
| Model 3 | −0.001 (−0.001, −0.0001) | 0.03 * |
| Annualized Change in Weight, lb/year (n = 2545) | ||
| Model 1 | −0.02 (−0.05, 0.00) | 0.09 |
| Model 2 | −0.02 (−0.05, 0.01) | 0.17 |
| Model 3 | −0.02 (−0.05, 0.01) | 0.28 |
| β (95% CI) a | p (Interaction) b | |
|---|---|---|
| Sex | ||
| Annualized Change in Grip Strength, kg/year | ||
| Men (n = 848) | −0.02 (−0.05, −0.001) | 0.01 * |
| Women (n = 1024) | 0.01 (−0.01, 0.03) | |
| Annualized Change in Gait Speed, m/s/year | ||
| Men (n = 917) | −0.0004 (−0.001, 0.0004) | 0.35 |
| Women (n = 1116) | −0.001 (−0.002, −0.0003) | |
| Annualized Change in Weight, lb/year | ||
| Men (n = 1145) | −0.02 (−0.06, 0.02) | 0.98 |
| Women (n = 1400) | −0.01 (−0.06, 0.03) | |
| Baseline Age | ||
| Annualized Change in Grip Strength, kg/year | ||
| <60 years (n = 959) | −0.02 (−0.04, −0.001) | 0.14 |
| ≥60 years (n = 913) | 0.01 (−0.01, 0.03) | |
| Annualized Change in Gait Speed, m/s/year | ||
| <60 years (n = 1027) | −0.0004 (−0.001, 0.0002) | 0.64 |
| ≥60 years (n = 1006) | −0.0008 (−0.002, 0.000) | |
| Annualized Change in Weight, lb/year | ||
| <60 years (n = 1253) | −0.03 (−0.08, 0.01) | 0.71 |
| ≥60 years (n = 1292) | 0.001 (−0.04, 0.04) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczynski, E.M.; Sahni, S.; Jacques, P.F.; Naumova, E.N. Ultra-Processed Food and Frailty: Evidence from a Prospective Cohort Study and Implications for Future Research. Nutrients 2025, 17, 2631. https://doi.org/10.3390/nu17162631
Konieczynski EM, Sahni S, Jacques PF, Naumova EN. Ultra-Processed Food and Frailty: Evidence from a Prospective Cohort Study and Implications for Future Research. Nutrients. 2025; 17(16):2631. https://doi.org/10.3390/nu17162631
Chicago/Turabian StyleKonieczynski, Elsa M., Shivani Sahni, Paul F. Jacques, and Elena N. Naumova. 2025. "Ultra-Processed Food and Frailty: Evidence from a Prospective Cohort Study and Implications for Future Research" Nutrients 17, no. 16: 2631. https://doi.org/10.3390/nu17162631
APA StyleKonieczynski, E. M., Sahni, S., Jacques, P. F., & Naumova, E. N. (2025). Ultra-Processed Food and Frailty: Evidence from a Prospective Cohort Study and Implications for Future Research. Nutrients, 17(16), 2631. https://doi.org/10.3390/nu17162631






