Mismatch Between Perceived and Actual Dietary Nutrition in Hospitalized Cardiovascular Patients and Clinicians: A Cross-Sectional Assessment and Recommendations for Improvement
Highlights
- A mismatch was identified between clinicians’/patients’ perceived nutritional intake and the objectively assessed dietary intake, necessitating enhanced clinical nutrition monitoring.
- Both clinicians and patients demonstrated inadequate nutritional knowledge, highlighting the need for improved nutrition education, hospital dietary environment optimization, and dietary literacy enhancement.
- The study emphasized the critical need for strengthened dietitian–clinician collaboration to enable the scientific assessment of patients' nutritional status.
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Group
2.2. Survey Design
2.3. Nutritional Risk Screening
2.4. Global Leadership Initiative on Malnutrition Criteria
2.5. 24 h Dietary Recall
2.6. Blood Parameters Collection
2.7. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Nutritional Risk and Malnutrition Prevalence
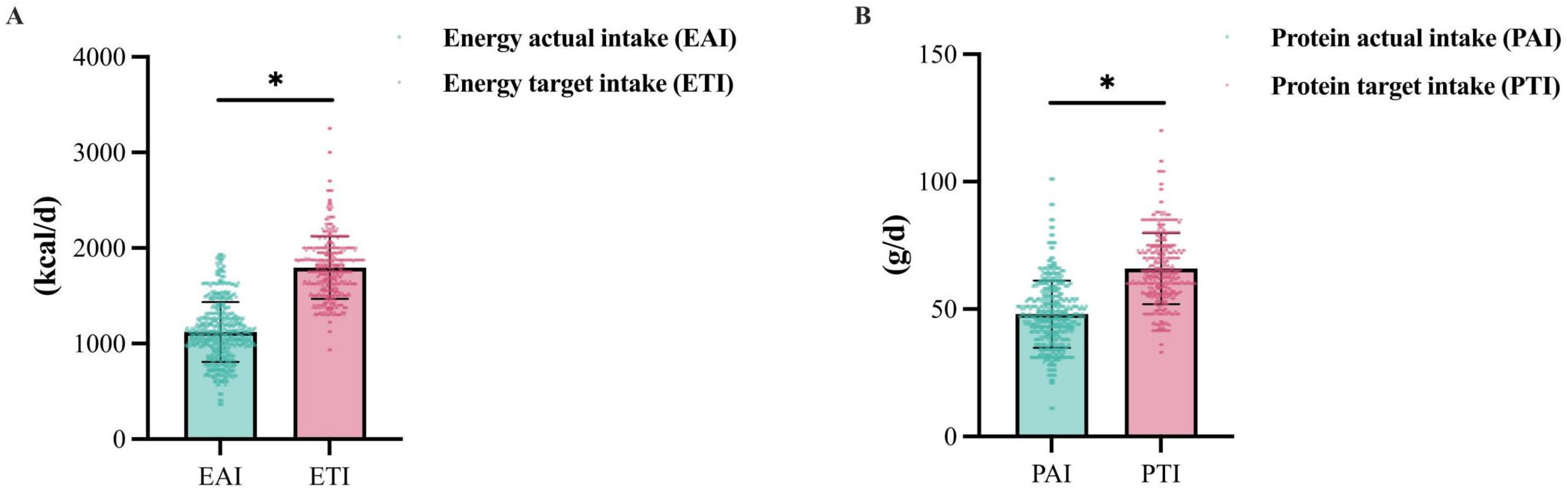
3.3. Dietary Survey
3.4. Dietary Awareness Among Cardiovascular Inpatients
3.5. Subjective Evaluation and Objective Situation
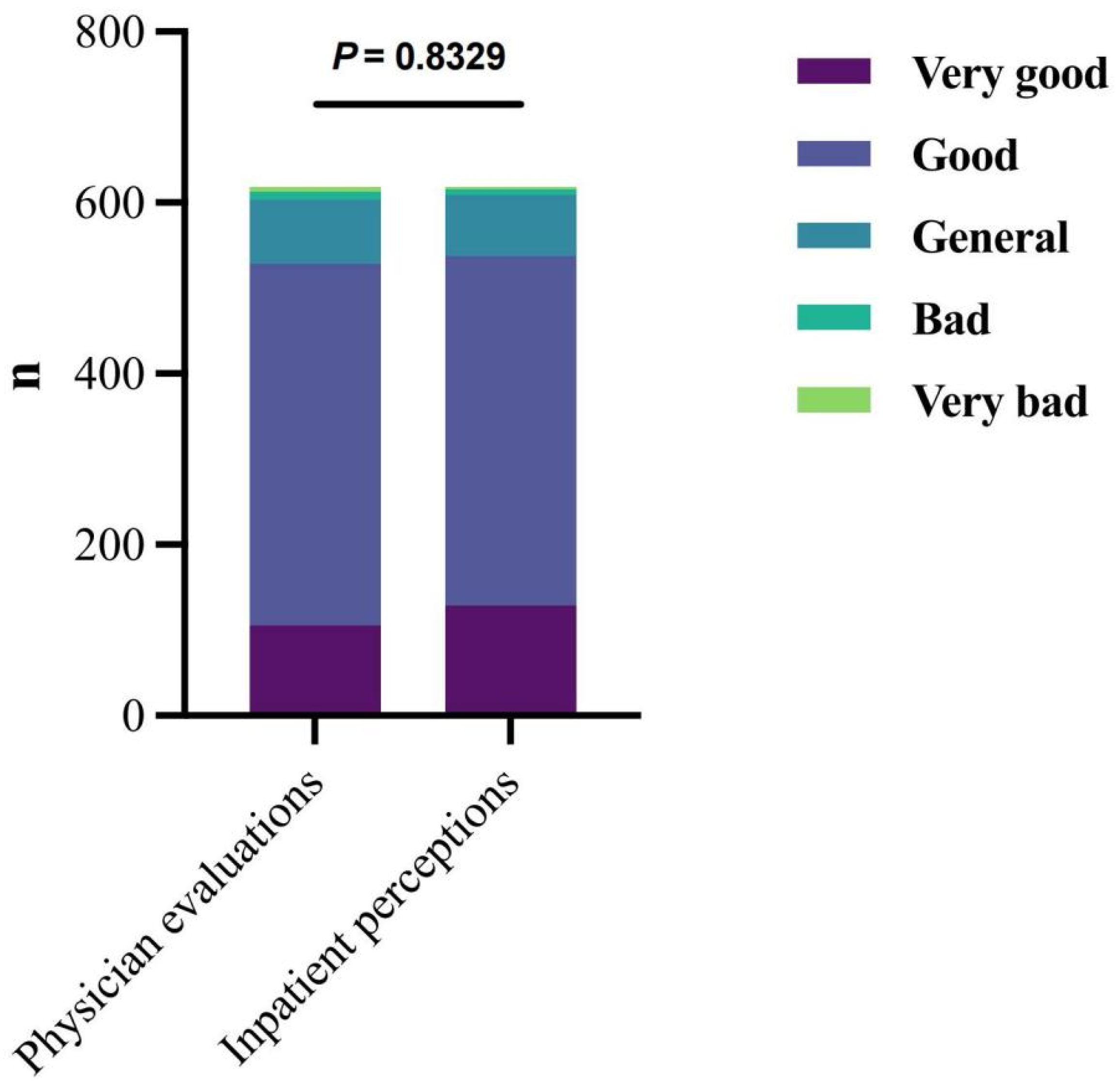
3.5.1. Physician Evaluations and Inpatient Perceptions
3.5.2. Dietary Intake and Inpatient Perceptions
3.5.3. Dietary Intake and Physician Evaluations
3.6. Blood Parameters
3.6.1. Blood Parameters and NRS 2002 Scores
3.6.2. Blood Parameters and GLIM Diagnosis
4. Discussion
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef]
- Gomez-Delgado, F.; Katsiki, N.; Lopez-Miranda, J.; Perez-Martinez, P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit. Rev. Food Sci. Nutr. 2021, 61, 1651–1669. [Google Scholar] [CrossRef]
- Zhao, D. Epidemiological Features of Cardiovascular Disease in Asia. JACC Asia 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Toth, P.P. Cardiovascular Disease Epidemiology and Risk Factors: General Concepts. In Nutraceuticals and Cardiovascular Disease: An Evidence-Based Approach for Clinical Practice; Cicero, A.F.G., Rizzo, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–22. [Google Scholar]
- Friars, D.; Walsh, O.; McNicholas, F. Assessment and management of cardiovascular complications in eating disorders. J. Eat. Disord. 2023, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.A.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560–2579. [Google Scholar] [CrossRef] [PubMed]
- Mutagwanya, R.; Nyago, C.M.; Nakwagala, F.N. Effect of diabetes nutrition education on the dietary feeding practices and lifestyle of type 2 diabetic patients. Eur. J. Clin. Nutr. 2022, 76, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shuremu, M.; Abate, K.H.; Belachew, T. Effect of nutrition education intervention to improve dietary diversity practice and nutritional status of the older people: A cluster randomized controlled trial. Food Sci. Nutr. 2023, 11, 7383–7395. [Google Scholar] [CrossRef]
- Jørgensen, T.; Jacobsen, R.K.; Toft, U.; Aadahl, M.; Glümer, C.; Pisinger, C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ Br. Med. J. 2014, 348, g3617. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aspry, K.E.; Garfield, K.; Kris-Etherton, P.; Seligman, H.; Velarde, G.P.; Williams, K.; Yang, E. “Food Is Medicine” Strategies for Nutrition Security and Cardiometabolic Health Equity. J. Am. Coll. Cardiol. 2024, 83, 843–864. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Dos Santos, K.; Moreira, T.M.; Belfort, G.P.; Da Silva, C.F.d.M.; Padilha, P.d.C.; De Barros, D.C.; Saunders, C. Adaptação da dieta DASH (Dietary Approaches to Stop Hypertension) para cuidado nutricional no período pós-parto, no âmbito da Atenção Básica. Rev. Bras. Epidemiol. 2019, 22, e190035. [Google Scholar] [CrossRef]
- Rychter, A.M.; Ratajczak, A.E.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Non-Systematic Review of Diet and Nutritional Risk Factors of Cardiovascular Disease in Obesity. Nutrients 2020, 12, 814. [Google Scholar] [CrossRef]
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I. Malnutrition in Adults. N. Engl. J. Med. 2024, 391, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Tevik, K.; Thürmer, H.; Husby, M.I.; De Soysa, A.K.; Helvik, A.-S. Nutritional risk screening in hospitalized patients with heart failure. Clin. Nutr. 2015, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Çalapkorur, S.; Bakır, B. Determination of the malnutrition risk in overweight and obese patients with cardiovascular disease. Clin. Sci. Nutr. 2021, 2, 74–80. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Huang, H.; He, Y.; Yu, Y.; Chen, G.; Liu, L.; Wang, B.; Li, Q.; Lai, W.; et al. Malnutrition in patients with coronary artery disease: Prevalence and mortality in a 46,485 Chinese cohort study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1186–1194. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Chrominski, T.; Szczasny, M.; Blaszczak, P. Nutritional Risk Screening 2002 score is a predictor of prolonged hospitalizations after cardiac ablation procedures. Eur. J. Cardiovasc. Nurs. 2024, 23, zvae098.114. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Yu, J.; Jia, Y.; Jiang, Y.; Chen, X.; Gao, Y.; Ye, L.; Wan, Z.; Cao, Y.; et al. Prognostic Value of the Nutritional Risk Screening 2002 Scale in Patients With Acute Myocardial Infarction: Insights From the Retrospective Multicenter Study for Early Evaluation of Acute Chest Pain. J. Cardiovasc. Nurs. 2021, 36, 546–555. [Google Scholar] [CrossRef]
- Raslan, M.; Gonzalez, M.C.; Torrinhas, R.S.M.M.; Ravacci, G.R.; Pereira, J.C.R.; Waitzberg, D.L. Complementarity of Subjective Global Assessment (SGA) and Nutritional Risk Screening 2002 (NRS 2002) for predicting poor clinical outcomes in hospitalized patients. Clin. Nutr. 2011, 30, 49–53. [Google Scholar] [CrossRef]
- Kootaka, Y.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Nakamura, T.; Yamashita, M.; Maekawa, E.; Reed, J.L.; Yamaoka-Tojo, M.; et al. The GLIM criteria for defining malnutrition can predict physical function and prognosis in patients with cardiovascular disease. Clin. Nutr. 2021, 40, 146–152. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Blaszczak, P. Nutritional Status of Coronary Artery Disease Patients—Preliminary Results. Int. J. Environ. Res. Public Health 2023, 20, 3464. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ito, M.; Sugiyama, H.; Iwagaitsu, S.; Nobata, H.; Kinashi, H.; Katsuno, T.; Banno, S.; Ito, Y.; Ando, M.; et al. Malnutrition according to the GLIM criteria with kidney dysfunction is associated with increased mortality in hospitalized patients with cardiovascular disease: A retrospective cohort study. Clin. Nutr. ESPEN 2023, 55, 167–173. [Google Scholar] [CrossRef]
- Ayob, R.; Vally, M.; Khan, R.; Orchard, A. Disparities in patients’ understanding of cardiovascular disease management. Cardiovasc. J. Afr. 2024, 35, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Sapała, A.-M.; Staśkiewicz-Bartecka, W.; Kiciak, A.; Kardas, M. Assessment of Nutritional Knowledge, Dietary Habits and Nutritional Status of Cardiology Patients, Considering Differences Between Individuals with Hypertension and Atherosclerosis and Those Without These Conditions. Nutrients 2025, 17, 754. [Google Scholar] [CrossRef] [PubMed]
- Harkin, N.; Johnston, E.; Mathews, T.; Guo, Y.; Schwartzbard, A.; Berger, J.; Gianos, E. Physicians’ Dietary Knowledge, Attitudes, and Counseling Practices: The Experience of a Single Health Care Center at Changing the Landscape for Dietary Education. Am. J. Lifestyle Med. 2019, 13, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kahan, S.; Manson, J.E. Nutrition Counseling in Clinical Practice: How Clinicians Can Do Better. JAMA 2017, 318, 1101–1102. [Google Scholar] [CrossRef]
- Johnston, E.; Mathews, T.; Aspry, K.; Aggarwal, M.; Gianos, E. Strategies to Fill the Gaps in Nutrition Education for Health Professionals through Continuing Medical Education. Curr. Atheroscler. Rep. 2019, 21, 13. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.L.E.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; De Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Institute of Nutrition and Food Safety, China CDC. China Food Composition Tables, 6th ed.; Peking University Medical Press: Beijing, China, 2019. [Google Scholar]
- Expert Group of Chinese Society of Nephrology. Chinese guidelines for diagnosis and treatment of diabetic kidney disease. Chin. J. Nephrol. 2021, 37, 255–304. [Google Scholar]
- Li, L.; Junlong, Z.; Chaoting, W.; Youdong, L.; Jixian, Z.; Xin, X.; Jiaoyan, R. Comparison of Chinese and Foreign Guidelines and Expert Consensus on Nutrient Recommendation in Patients with Nephropathy. Mod. Food Sci. Amp Technol. 2023, 39, 386–400. [Google Scholar] [CrossRef]
- Wunderle, C.; Gomes, F.; Schuetz, P.; Stumpf, F.; Austin, P.; Ballesteros-Pomar, M.D.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; et al. ESPEN practical guideline: Nutritional support for polymorbid medical inpatients. Clin. Nutr. 2024, 43, 674–691. [Google Scholar] [CrossRef]
- Beavan, S.; Baker, R.; Sadler, H.; Collinson, A. Improving the nutritional intake of hospital patients: How far have we come? A re-audit. J. Hum. Nutr. Diet. 2019, 32, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.J.; Valaitis, R.; Laur, C.; McNicholl, T.; Nasser, R.; Keller, H. Low food intake in hospital: Patient, institutional, and clinical factors. Appl. Physiol. Nutr. Metab. 2018, 43, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Banks, M.D.; Mudge, A.M. Improving nutrition care and intake for older hospital patients through system-level dietary and mealtime interventions. Clin. Nutr. ESPEN 2018, 24, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, L.; Li, G.; Huang, Z.; Liu, J.; Wu, Z.; Wu, Y.; Lin, J.; Zhang, Y.; Yu, Y.; et al. Prevalence and prognostic significance of malnutrition in diabetic patients with coronary artery disease: A cohort study. Nutr. Metab. 2021, 18, 102. [Google Scholar] [CrossRef]
- Arya, R.; Antonisamy, B.; Kumar, S. Sample Size Estimation in Prevalence Studies. Indian J. Pediatr. 2012, 79, 1482–1488. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Zhang, X.N.; Jiang, Z.M.; Wu, H.S.; Lu, Q.; Yang, J.; Yu, K.; Li, Z. NRS 2002 Nutritional Risk Screening and GLIM Step 2 for diagnosis of malnutrition (without FFMI currently). Chin. J. Clin. Nutr. 2020, 28, 1–6. [Google Scholar]
- Bilancio, G.; Cavallo, P.; Ciacci, C.; Cirillo, M. Dietary Protein, Kidney Function and Mortality: Review of the Evidence from Epidemiological Studies. Nutrients 2019, 11, 196. [Google Scholar] [CrossRef]
- Tong, L. Interpretation of the Dietary Guidelines for Adults with Chronic Kidney Disease (2024 edition). Chin. J. Rural Med. Pharm. 2024, 31, 1–3. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Dietary Guidelines for Adults with Chronic Kidney Disease. Clin. Educ. Gen. Pract. 2024, 22, 196–199+207. [Google Scholar] [CrossRef]
- Chinese Society of Endocrinology. Expert Consensus on Medical Nutrition Therapy for Adult Diabetic Kidney Diseases in China. Chin. J. Endocrinol. Metab. 2022, 38, 927–936.39. [Google Scholar]
- Tang, W.; Sun, Y.; Wang, P.; Ou, J.; Yin, Y.; Tong, J.; Wang, L. Application of NRS-2002 in Nutritional Risk Screening of Hospitalized Patients with Cardiovascular Diseases and Analysis of Related Factors. Mod. Med. J. 2023, 51, 758–764. [Google Scholar]
- Wan, J.; Li, Q.; Yang, J.; Yuan, R.; Wang, C.; Chen, J.; Li, X. Relationship between Nutritional Risk and Depression in Hospitalized Patients with Cardiovascular Diseases. Mol. Cardiol. China 2021, 21, 4296–4299. [Google Scholar] [CrossRef]
- Hersberger, L.; Dietz, A.; Bürgler, H.; Bargetzi, A.; Bargetzi, L.; Kägi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, F.; Zhou, H.; Shu, L.; Wang, R.; Zhao, C. Clinical application of NRS-2002 in nutritional risk screening of tuberculosis inpatients. Ann. Palliat. Med. 2021, 10, 5322–5328. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Q.; Liu, H.; Liu, M.; Tang, C.; Wu, J.; Feng, G.; Wu, W. GLIM criteria using NRS-2002 and MUST as the first step adequately diagnose the malnutrition in Crohn’s disease inpatients: A retrospective study. Front. Nutr. 2023, 9, 1059191. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, S.; Ren, L.; Zhang, L.; Xu, Y.; Yuan, L.; Liu, C.; Zhao, B.; Xue, J. Prevalence and Diagnosis of Malnutrition in Patients with Chronic Kidney Disease: Evaluating the Value of NRS2002 and SGA Scores. Kidney Blood Press. Res. 2025, 50, 513–522. [Google Scholar] [CrossRef]
- Ran, Q.; Zhao, X.; Xu, W.; Liu, L.; Sun, H.; Luo, Y. Consistency of Mini-Nutritional Assessment short form and Nutritional Risk Screening 2002 in nutritional evaluation of diabetic foot patients. Front. Nutr. 2025, 12, 1596193. [Google Scholar] [CrossRef]
- Małgorzewicz, S.; Sliwińska, A. Nutritional status and muscle function in hospitalized diabetic patients. Clin. Nutr. ESPEN 2024, 63, 1090. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Wen-Ming, H.; Sheng, J.; Yan-Qing, X.; Si, C.; Li, J.-N. Comparing GLIM and SGA Nutritional Criteria for Malnutrition Assessment and Prognosis in Chronic Heart Failure Patients. Int. J. Gen. Med. 2025, 18, 1669–1679. [Google Scholar] [CrossRef]
- Agarwal, E.; Ferguson, M.; Banks, M.; Batterham, M.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: Results from the Nutrition Care Day Survey 2010. Clin. Nutr. 2013, 32, 737–745. [Google Scholar] [CrossRef]
- Agarwal, E.; Ferguson, M.; Banks, M.; Vivanti, A.; Batterham, M.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition, poor food intake, and adverse healthcare outcomes in non-critically ill obese acute care hospital patients. Clin. Nutr. 2019, 38, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.C.; Leong, L.P.; Lim, S.L. Nutrition intervention approaches to reduce malnutrition in oncology patients: A systematic review. Support. Care Cancer 2016, 24, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-H.; Lin, M.-C.; Liu, Y.-Y.; Lee, C.-L.; Chang, N.-J. Effect of Nutritional Intervention Programs on Nutritional Status and Readmission Rate in Malnourished Older Adults with Pneumonia: A Randomized Control Trial. Int. J. Environ. Res. Public Health 2019, 16, 4758. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Papathanail, I.; Brühlmann, J.; Vasiloglou, M.F.; Stathopoulou, T.; Exadaktylos, A.K.; Stanga, Z.; Münzer, T.; Mougiakakou, S. Evaluation of a Novel Artificial Intelligence System to Monitor and Assess Energy and Macronutrient Intake in Hospitalised Older Patients. Nutrients 2021, 13, 4539. [Google Scholar] [CrossRef]
- Albaladejo, L.; Giai, J.; Deronne, C.; Baude, R.; Bosson, J.-L.; Bétry, C. Assessing real-life food consumption in hospital with an automatic image recognition device: A pilot study. Clin. Nutr. ESPEN 2025, 68, 319–325. [Google Scholar] [CrossRef]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary supplement use among adults: United States, 2017–2018. NCHS Data Brief. 2021, 399, 1–8. [Google Scholar]
- Gong, W.; Liu, A.; Yao, Y.; Ma, Y.; Ding, C.; Song, C.; Yuan, F.; Zhang, Y.; Feng, G.; Chen, Z.; et al. Nutrient Supplement Use among the Chinese Population: A Cross-Sectional Study of the 2010–2012 China Nutrition and Health Surveillance. Nutrients 2018, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Wang, J.; Fang, Y.; Liu, Y.; Sun, M.; Wu, Q.; Wang, K.; Huang, Y.; Ling, Y.; Liu, Y.; et al. A multi-center survey on dietary knowledge and behavior among inpatients in oncology department. Support. Care Cancer 2018, 26, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.O.; Han, S.R.; Choi, S.I.; Lee, J.J.; Kim, S.H.; Ahn, H.S.; Lim, H. Effects of intensive nutrition education on nutritional status and quality of life among postgastrectomy patients. Ann. Surg. Treat. Res. 2015, 90, 79–88. [Google Scholar] [CrossRef]
- Paes-Barreto, J.G.; Barreto Silva, M.I.; Qureshi, A.R.; Bregman, R.; Cervante, V.F.; Carrero, J.J.; Avesani, C.M. Can Renal Nutrition Education Improve Adherence to a Low-Protein Diet in Patients With Stages 3 to 5 Chronic Kidney Disease? J. Ren. Nutr. 2013, 23, 164–171. [Google Scholar] [CrossRef]
- Hernández Morante, J.J.; Sánchez-Villazala, A.; Cutillas, R.C.; Fuentes, M.C.C. Effectiveness of a Nutrition Education Program for the Prevention and Treatment of Malnutrition in End-Stage Renal Disease. J. Ren. Nutr. 2014, 24, 42–49. [Google Scholar] [CrossRef]
- Bowen, M.E.; Cavanaugh, K.L.; Wolff, K.; Davis, D.; Gregory, R.P.; Shintani, A.; Eden, S.; Wallston, K.; Elasy, T.; Rothman, R.L. The diabetes nutrition education study randomized controlled trial: A comparative effectiveness study of approaches to nutrition in diabetes self-management education. Patient Educ. Couns. 2016, 99, 1368–1376. [Google Scholar] [CrossRef]
- Li, Z.; Jin, H.; Chen, W.; Sun, Z.; Jing, L.; Zhao, X.; Zhu, S.; Guo, X.; Study Group, C.N. Influencing Factors of Knowledge, Attitude, and Practice regarding Medical Nutrition Therapy in Patients with Diabetes: A National Cross-Sectional Study in Urban China. J. Diabetes Res. 2017, 2017, 8948452. [Google Scholar] [CrossRef]
- Brunner, S.; Mayer, H.; Blum, K.; Breidert, M.; Dietrich, M.; Dahl, E.; Müller, M. Nutrition-related care needs of older patients in hospital: A qualitative multimethod study. Int. J. Nurs. Knowl. 2023, 34, 148–160. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Hu, R.; Long, H.; Wang, N.; Wang, Q.; Mao, Z. Trends and Associated Factors of Dietary Knowledge among Chinese Older Residents: Results from the China Health and Nutrition Survey 2004–2015. Int. J. Environ. Res. Public Health 2020, 17, 8029. [Google Scholar] [CrossRef]
- Vrkatić, A.; Grujičić, M.; Jovičić-Bata, J.; Novaković, B. Nutritional Knowledge, Confidence, Attitudes towards Nutritional Care and Nutrition Counselling Practice among General Practitioners. Healthcare 2022, 10, 2222. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Katsouda, A.; Lekka, K.; Tsantekidis, K.; Bouras, E.; Kasapidou, E.; Poulia, K.-A.; Chourdakis, M. Is continuing medical education sufficient? Assessing the clinical nutrition knowledge of medical doctors. Nutrition 2019, 57, 69–73. [Google Scholar] [CrossRef] [PubMed]
- DiMaria-Ghalili, R.A.; Mirtallo, J.M.; Tobin, B.W.; Hark, L.; Van Horn, L.; Palmer, C.A. Challenges and opportunities for nutrition education and training in the health care professions: Intraprofessional and interprofessional call to action1234. Am. J. Clin. Nutr. 2014, 99, 1184S–1193S. [Google Scholar] [CrossRef] [PubMed]
- Gyeongsil, L.; Seung-Won, O. Guidelines for nutrition counseling in primary healthcare clinics. J. Korean Med. Assoc./Taehan Uisa Hyophoe Chi 2024, 67, 278–284. [Google Scholar] [CrossRef]
- Crowley, J.; Ball, L.; Hiddink, G.J. Nutrition in medical education: A systematic review. Lancet Planet. Health 2019, 3, e379–e389. [Google Scholar] [CrossRef]
- Marshall, A.P.; Takefala, T.; Williams, L.T.; Spencer, A.; Grealish, L.; Roberts, S. Health practitioner practices and their influence on nutritional intake of hospitalised patients. Int. J. Nurs. Sci. 2019, 6, 162–168. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, W.; Gao, C.; Zhang, C.; Yan, L.; Wu, Y.; XIE, Y. Optimization and application of multidisciplinary information consultation platform for outpatient service led by nurses. Chin. Nurs. Res. 2025, 39, 135–140. [Google Scholar] [CrossRef]
- Xie, J.; Jin, Y.; Shen, M.; Chen, L.; Zhang, S. A Patient-Centric, Coordinated Care Model for Rare Diseases: The Multidisciplinary Consultation Program at Peking Union Medical College Hospital. NEJM Catalyst 2023, 4, 477–487. [Google Scholar] [CrossRef]
- Jia, J.; Sun, T.; Tang, J.; Sun, K.; Meng, Z.; Zhu, H.; Huang, X. Participation in Multidisciplinary Teams Among Healthcare Professionals: A Discrete Choice Experiment in Tertiary Public Hospitals in China. J. Multidiscip. Healthc. 2024, 17, 4397–4409. [Google Scholar] [CrossRef]
- Yang, M.; Yan, Y.; Xu, Z.; Liu, H.; Ran, J.; Zheng, Y.; Cai, Z.; Liu, Z.; Gong, K. The status and challenges of online consultation service in internet hospitals operated by physical hospitals in China: A large-scale pooled analysis of multicenter data. BMC Health Serv. Res. 2025, 25, 611. [Google Scholar] [CrossRef]
- Wang, H. Clinical analysis of blood biochemical indexes in sub-health population. Chin. Community Dr. 2021, 37, 128–129. [Google Scholar] [CrossRef]
- Bodor, G.S. Biochemical Markers of Myocardial Damage. Ejifcc 2016, 27, 95–111. [Google Scholar] [PubMed]
- Chien, S.-C.; Chandramouli, C.; Lo, C.-I.; Lin, C.-F.; Sung, K.-T.; Huang, W.-H.; Lai, Y.-H.; Yun, C.-H.; Su, C.-H.; Yeh, H.-I.; et al. Associations of obesity and malnutrition with cardiac remodeling and cardiovascular outcomes in Asian adults: A cohort study. PLoS Med. 2021, 18, e1003661. [Google Scholar] [CrossRef]
- Nienaber-Rousseau, C.; Swanepoel, B.; Dolman, R.C.; Pieters, M.; Conradie, K.R.; Towers, G.W. Interactions between C-Reactive Protein Genotypes with Markers of Nutritional Status in Relation to Inflammation. Nutrients 2014, 6, 5034–5050. [Google Scholar] [CrossRef]
- Mottalib, A.; Salsberg, V.; Mohd-Yusof, B.-N.; Mohamed, W.; Carolan, P.; Pober, D.M.; Mitri, J.; Hamdy, O. Effects of nutrition therapy on HbA1c and cardiovascular disease risk factors in overweight and obese patients with type 2 diabetes. Nutr. J. 2018, 17, 42. [Google Scholar] [CrossRef]
- Pourhassan, M.; Böttger, S.; Janssen, G.; Sieske, L.; Wirth, R. The Association of Inflammation with Food Intake in Older Hospitalized Patients. J. Nutr. Health Aging 2018, 22, 589–593. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Zeng, Y.; Dong, J.; Li, Y.; Zhang, P.; Wang, K. Correlation Between Nutritional Status and Serum C-reactive Protein in Patients with Cancer Pain. Cancer Res. Prev. Treat. 2020, 47, 372–375. [Google Scholar] [CrossRef]
- Liang, P.; Wu, J. Association between nutritional risk and CRP levels in patients with acute exacerbation of chronic obstructive pulmonary disease. Front. Med. 2025, 12, 1611981. [Google Scholar] [CrossRef]
- Bargetzi, A.; Emmenegger, N.; Wildisen, S.; Nickler, M.; Bargetzi, L.; Hersberger, L.; Segerer, S.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; et al. Admission kidney function is a strong predictor for the response to nutritional support in patients at nutritional risk. Clin. Nutr. 2021, 40, 2762–2771. [Google Scholar] [CrossRef]
- Miličević, T.; Kolčić, I.; Đogaš, T.; Živković, P.M.; Radman, M.; Radić, J. Nutritional Status and Indicators of 2-Year Mortality and Re-Hospitalizations: Experience from the Internal Clinic Departments in Tertiary Hospital in Croatia. Nutrients 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Zhao, B.; Gao, F.; Yuan, X.; Zhu, Y.; Liu, D. The relationship of nutritional risk and NRS2002 score with disease progression and prognosis in patients with COVID-19. Front. Nutr. 2023, 10, 1089972. [Google Scholar] [CrossRef] [PubMed]
- Guligowska, A.; Stephenson, S.S.; Cieślak-Skubel, A.; Kravchenko, G.; Korycka-Błoch, R.; Kostka, T.J.; Chrzastek, Z.; Sołtysik, B.K. Low total cholesterol levels are associated with a high risk of malnutrition in older adults. Clin. Nutr. ESPEN 2023, 58, 471. [Google Scholar] [CrossRef]
- Oh, S.E.; Park, J.S.; Jeung, H.C. Pre-treatment Nutritional Risk Assessment by NRS-2002 Predicts Prognosis in Patients With Advanced Biliary Tract Cancer: A Single Center Retrospective Study. Clin. Nutr. Res. 2022, 11, 183–193. [Google Scholar] [CrossRef]
- Hirose, S.; Matsue, Y.; Kamiya, K.; Kagiyama, N.; Hiki, M.; Dotare, T.; Sunayama, T.; Konishi, M.; Saito, H.; Saito, K.; et al. Prevalence and prognostic implications of malnutrition as defined by GLIM criteria in elderly patients with heart failure. Clin. Nutr. 2021, 40, 4334–4340. [Google Scholar] [CrossRef]
- Oguri, M.; Ishii, H.; Yasuda, K.; Sumi, T.; Takahashi, H.; Murohara, T. Combined prognostic value of malnutrition using GLIM criteria and renal insufficiency in elderly heart failure. ESC Heart Fail. 2022, 9, 704–711. [Google Scholar] [CrossRef]
- Pourhassan, M.; Cederholm, T.; Trampisch, U.; Volkert, D.; Wirth, R. Inflammation as a diagnostic criterion in the GLIM definition of malnutrition—What CRP-threshold relates to reduced food intake in older patients with acute disease? Eur. J. Clin. Nutr. 2022, 76, 397–400. [Google Scholar] [CrossRef]
- Xie, H.; Yuan, K.; Ruan, G.; Wei, L.; Zhang, H.; Ge, Y.; Lin, S.; Song, M.; Wang, Z.; Liu, C.; et al. Improving the assessment of malnutrition in cancer: Using systemic inflammation markers as a supplement to the inflammation items of the GLIM criteria. Clin. Nutr. 2023, 42, 2036–2044. [Google Scholar] [CrossRef]

| Classification | Patient Category | Recommendation | |
|---|---|---|---|
| Target energy intake | Bedridden patients | 22 kcal·kg−1·d−1 | |
| Ambulatory patients | 25 kcal·kg−1·d−1 | ||
| Target protein intake | General patients | 1.0 g·kg−1·d−1 | |
| CKD patients | eGFR < 15 mL·min−1·(1.73 m2)−1 (dialysis-dependent) | 1.0 g·kg−1·d−1 | |
| eGFR < 30 mL·min−1·(1.73 m2)−1 (non-dialysis) | 0.6 g·kg−1·d−1 | ||
| eGFR 30–89 mL·min−1·(1.73 m2)−1 | 0.8 g·kg−1·d−1 | ||
| Groups | β | SE | Wald χ2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Inpatient Perceptions | ||||||
| Very good a | - | - | - | - | - | - |
| Good | 2.650 | 0.575 | 21.234 | 0.000 | 14.157 | 4.586–43.706 |
| General | −0.738 | 0.542 | 1.852 | 0.174 | 0.478 | 0.165–1.384 |
| Bad | −2.850 | 0.709 | 16.160 | 0.000 | 0.058 | 0.014–0.232 |
| Very bad | −3.555 | 0.867 | 16.826 | 0.000 | 0.029 | 0.005–0.156 |
| Dietary Intake | ||||||
| Achievement rate of target energy intake | 0.041 | 0.012 | 12.307 | 0.000 | 1.042 | 1.018–1.066 |
| Achievement rate of target protein intake | −0.019 | 0.009 | 4.016 | 0.045 | 0.982 | 0.964–1.000 |
| Code | Variable | Assignment |
|---|---|---|
| Y1 | Inpatient perceptions | Very good = 5; Good = 4; General = 3; Bad = 2; Very bad = 1 |
| Y2 | Physician evaluations | Very good = 5; Good = 4; General = 3; Bad = 2; Very bad = 1 |
| X1 | Energy intake achievement levels | Fully achieved = 3; Partially achieved = 2; Not achieved = 1 |
| X2 | Protein intake achievement levels | Fully achieved = 3; Partially achieved = 2; Not achieved = 1 |
| Factor | β | SE | Wald χ2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Inpatient Perceptions | ||||||
| Very good a | - | - | - | - | - | - |
| Good | 0.084 | 0.572 | 0.022 | 0.883 | 1.088 | 0.355–3.337 |
| General | −3.356 | 0.616 | 29.668 | 0.000 | 0.035 | 0.010–0.117 |
| Bad | −5.482 | 0.772 | 50.470 | 0.000 | 0.004 | 0.001–0.019 |
| Very bad | −6.191 | 0.920 | 45.326 | 0.000 | 0.002 | 0.000–0.012 |
| Energy Intake Achievement Degree | ||||||
| Fully achieved a | - | - | - | - | - | - |
| Partially achieved | −2.627 | 0.739 | 12.633 | 0.000 | 0.072 | 0.017–0.308 |
| Not achieved | −2.571 | 0.772 | 11.079 | 0.001 | 0.076 | 0.017–0.348 |
| Protein Intake Achievement Degree | ||||||
| Fully achieved a | - | - | - | - | - | - |
| Partially achieved | 1.397 | 0.552 | 6.400 | 0.011 | 4.044 | 1.370–11.936 |
| Not achieved | 0.610 | 0.640 | 0.908 | 0.341 | 1.841 | 0.525–6.457 |
| Groups | β | SE | Wald χ2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Physician evaluations | ||||||
| Very good a | - | - | - | - | - | - |
| Good | 2.225 | 0.574 | 15.032 | 0.000 | 9.250 | 3.004–28.482 |
| General | −1.263 | 0.554 | 5.199 | 0.023 | 0.283 | 0.096–0.838 |
| Bad | −3.230 | 0.691 | 21.846 | 0.000 | 0.040 | 0.010–0.153 |
| Very bad | −4.162 | 0.881 | 22.335 | 0.000 | 0.016 | 0.003–0.088 |
| Dietary intake | ||||||
| Achievement rate of target energy intake | 0.034 | 0.012 | 8.887 | 0.003 | 1.035 | 1.012–1.059 |
| Achievement rate of target protein intake | −0.022 | 0.009 | 5.356 | 0.021 | 0.979 | 0.961–0.997 |
| Factor | β | SE | Wald χ2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Physician Evaluations | ||||||
| Very good a | - | - | - | - | - | - |
| Good | 1.087 | 0.567 | 3.669 | 0.055 | 2.965 | 0.975–9.016 |
| General | −2.391 | 0.592 | 16.297 | 0.000 | 0.092 | 0.029–0.292 |
| Bad | −4.368 | 0.718 | 37.062 | 0.000 | 0.013 | 0.003–0.052 |
| Very bad | −5.303 | 0.903 | 34.522 | 0.000 | 0.005 | 0.001–0.029 |
| Energy Intake Achievement Degree | ||||||
| Fully achieved a | - | - | - | - | - | - |
| Partially achieved | −1.584 | 0.660 | 5.759 | 0.016 | 0.205 | 0.056–0.748 |
| Not achieved | −1.956 | 0.706 | 7.680 | 0.006 | 0.141 | 0.035–0.564 |
| Protein intake Achievement Degree | ||||||
| Fully achieved a | - | - | - | - | - | - |
| Partially achieved | 1.178 | 0.521 | 5.112 | 0.024 | 3.247 | 1.170–9.013 |
| Not achieved | 1.337 | 0.637 | 4.404 | 0.036 | 3.809 | 1.092–13.283 |
| Classification | Parameters | Results | Normal Reference Range | r | p |
|---|---|---|---|---|---|
| Blood lipids | TC (mmol/L) | 4.08 ± 1.16 | 2.90–6.20 | −0.116 | 0.124 |
| TG (mmol/L) | 1.58 ± 1.35 | 0.45–1.70 | −0.085 | 0.241 | |
| HDL (mmol/L) | 1.07 ± 0.32 | 1.03–1.55 | −0.002 | 0.980 | |
| LDL (mmol/L) | 2.23 ± 0.86 | 1.90–4.10 | −0.110 | 0.130 | |
| Liver function | ALT (U/L) | 22.74 ± 24.84 | Male: 9–50, female: 7–40 | 0.021 | 0.771 |
| AST (U/L) | 22.82 ± 15.72 | Male: 15–40, female: 13–35 | 0.073 | 0.300 | |
| Renal function | BUN (mmol/L) | 6.11 ± 2.61 | 2.8–7.2 | 0.150 | 0.031 * |
| Cr (µmol/L) | 77.86 ± 39.58 | Male: 59–104, female: 45–84 | 0.133 | 0.057 | |
| eGFR (mL·min−1·(1.73 m2)−1) | 88.43 ± 20.27 | - | −0.353 | <0.001 * | |
| Glucose metabolism | Glu (mmol/L) | 5.85 ± 2.86 | 3.3–6.1 | 0.091 | 0.192 |
| HbA1c (%) | 7.14 ± 4.41 | 4.0–6.0 | 0.357 | <0.001 * | |
| Inflammation | CRP (mg/L) | 8.98 ± 26.03 | 0–10 | 0.446 | <0.001 * |
| Nutrition | ALB (g/L) | 41.29 ± 4.31 | Male: 40–50, female: 40–55 | −0.211 | 0.002 * |
| Hb (g/L) | 133.78 ± 18.97 | Male: 130–175, female: 115–150 | −0.197 | 0.005 * | |
| Electrolyte | Na (mmol/L) | 140.72 ± 2.76 | 137–147 | −0.067 | 0.340 |
| K (mmol/L) | 3.99 ± 0.40 | 3.5–5.3 | 0.006 | 0.933 | |
| Ca (mmol/L) | 2.28 ± 0.13 | 2.2–2.65 | −0.150 | 0.031 * | |
| IP (mmol/L) | 1.21 ± 0.22 | 0.80–1.45 | −0.182 | 0.009 * | |
| Metabolism | UA (µmol/L) | 335.89 ± 98.26 | Male: 208–428, female: 155–357 | 0.085 | 0.227 |
| Classification | Parameters | β | SE | Wald χ2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| GLIM diagnosis | Severe malnutrition a | - | - | - | - | - | - |
| Moderate malnutrition | 113.438 | 49.407 | 5.272 | 0.022 | 1.84 × 1049 | 1.62 × 107–2.09 × 1091 | |
| Non-malnutrition | 124.042 | 51.484 | 5.805 | 0.016 | 7.43 × 1053 | 1.11 × 1010–4.95 × 1097 | |
| Blood lipids | TC | 1.043 | 4.080 | 0.065 | 0.798 | 2.837 | 0.001–8.43 × 103 |
| TG | 0.673 | 0.929 | 0.524 | 0.469 | 1.960 | 0.317–12.110 | |
| HDL | 5.844 | 4.284 | 1.861 | 0.173 | 3.45 × 1002 | 0.078–1.53 × 106 | |
| LDL | −2.312 | 4.888 | 0.224 | 0.636 | 0.099 | 6.84 × 10−6–1.43 × 103 | |
| Liver function | ALT | 0.033 | 0.047 | 0.500 | 0.479 | 1.0336 | 0.943–1.134 |
| AST | 0.036 | 0.025 | 1.979 | 0.159 | 1.0367 | 0.986–1.089 | |
| Renal function | BUN | 0.559 | 0.377 | 2.199 | 0.138 | 1.749 | 0.835–3.666 |
| Cr | −0.072 | 0.051 | 1.978 | 0.160 | 0.931 | 0.842−1.028 | |
| eGFR | 0.038 | 0.071 | 0.278 | 0.598 | 1.039 | 0.903–1.193 | |
| Glucose metabolism | Glu | −0.428 | 0.275 | 2.425 | 0.119 | 0.652 | 0.381–1.117 |
| HbA1c | 0.029 | 0.178 | 0.026 | 0.872 | 1.029 | 0.726–1.458 | |
| Inflammation | CRP | 0.055 | 0.028 | 3.918 | 0.048 | 1.056 | 1.001–1.116 |
| Nutrition | ALB | 0.077 | 0.163 | 0.220 | 0.639 | 1.080 | 0.784–1.487 |
| Hb | −0.022 | 0.038 | 0.320 | 0.571 | 0.978 | 0.908–1.054 | |
| Electrolyte | Na | 0.768 | 0.331 | 5.395 | 0.020 | 2.155 | 1.127–4.121 |
| K | 5.552 | 2.380 | 5.440 | 0.020 | 2.58 × 102 | 2.428–2.74 × 104 | |
| Ca | −8.665 | 7.168 | 1.461 | 0.227 | 0.000 | 1.37 × 10−10–2.18 × 102 | |
| IP | −1.981 | 2.659 | 0.555 | 0.456 | 0.138 | 0.001–25.305 | |
| Metabolism | UA | 0.007 | 0.007 | 0.927 | 0.336 | 1.007 | 0.993–1.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Han, J.; Peng, Y.; Yu, X.; Xiao, Y.; Song, J.; Liu, P. Mismatch Between Perceived and Actual Dietary Nutrition in Hospitalized Cardiovascular Patients and Clinicians: A Cross-Sectional Assessment and Recommendations for Improvement. Nutrients 2025, 17, 2624. https://doi.org/10.3390/nu17162624
Li D, Han J, Peng Y, Yu X, Xiao Y, Song J, Liu P. Mismatch Between Perceived and Actual Dietary Nutrition in Hospitalized Cardiovascular Patients and Clinicians: A Cross-Sectional Assessment and Recommendations for Improvement. Nutrients. 2025; 17(16):2624. https://doi.org/10.3390/nu17162624
Chicago/Turabian StyleLi, Di, Jiaheng Han, Ye Peng, Xi Yu, Ying Xiao, Junxian Song, and Peng Liu. 2025. "Mismatch Between Perceived and Actual Dietary Nutrition in Hospitalized Cardiovascular Patients and Clinicians: A Cross-Sectional Assessment and Recommendations for Improvement" Nutrients 17, no. 16: 2624. https://doi.org/10.3390/nu17162624
APA StyleLi, D., Han, J., Peng, Y., Yu, X., Xiao, Y., Song, J., & Liu, P. (2025). Mismatch Between Perceived and Actual Dietary Nutrition in Hospitalized Cardiovascular Patients and Clinicians: A Cross-Sectional Assessment and Recommendations for Improvement. Nutrients, 17(16), 2624. https://doi.org/10.3390/nu17162624









