Hair Growth and Health Promoting Effects of Standardized Ageratum conyzoides Extract in Human Follicle Dermal Papilla Cells and in C57BL/6 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Standardized Ageratum conyzoides Extract and Phytochemical Analysis
2.2. Cell Culture
2.3. Cell Viability Assays
2.4. Quantitative Real-Time PCR (qPCR)
2.5. Immunoblotting
2.6. ALDH2 Activity Assay
2.7. Animal Experiments
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
3.1. Cell Viability of ACE in Human Follicle Dermal Papilla Cells
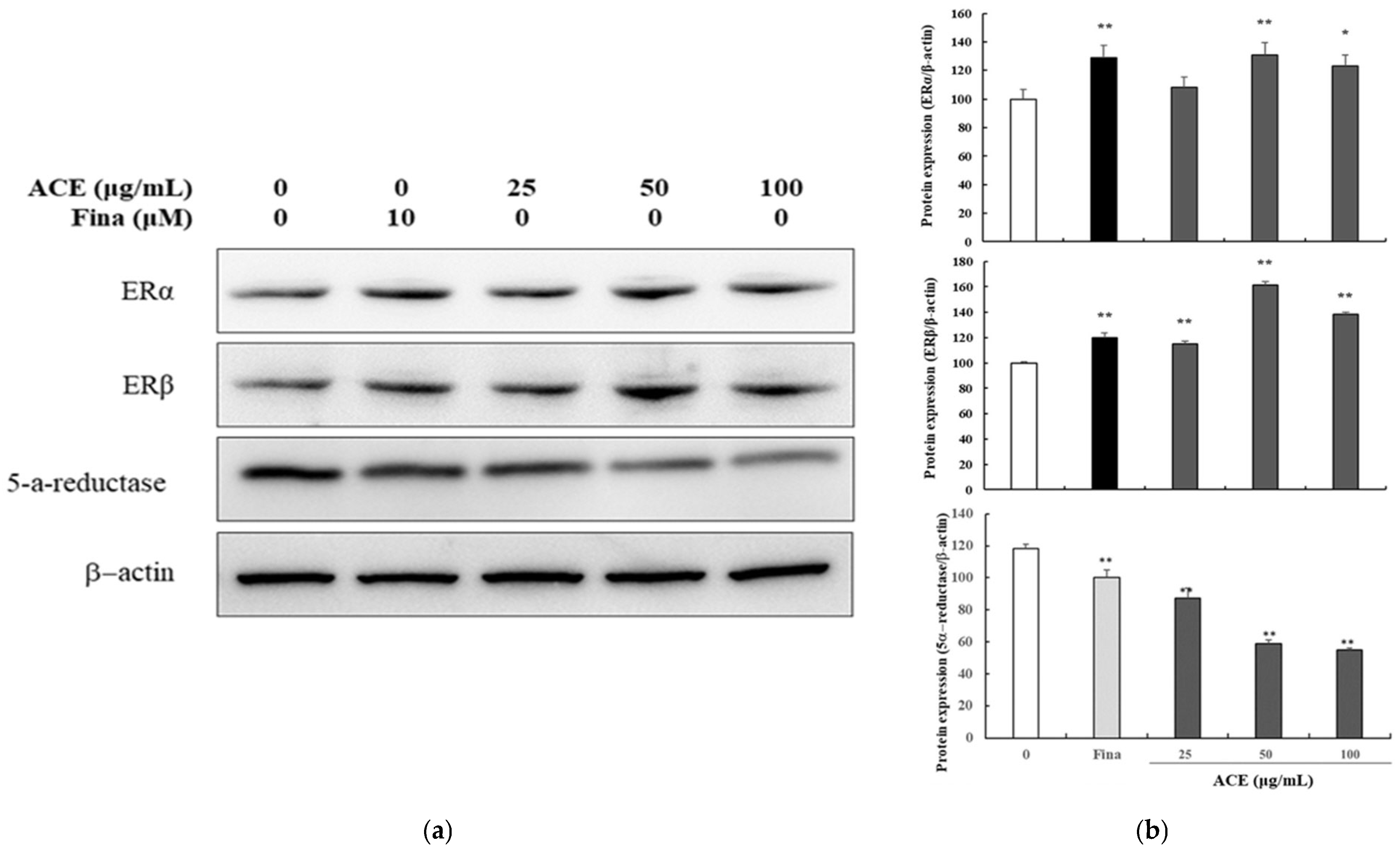
3.2. Inhibition of 5α-Reductase 2 and Modulation of Estrogen Receptors by ACE in Human Follicle Dermal Papilla Cells
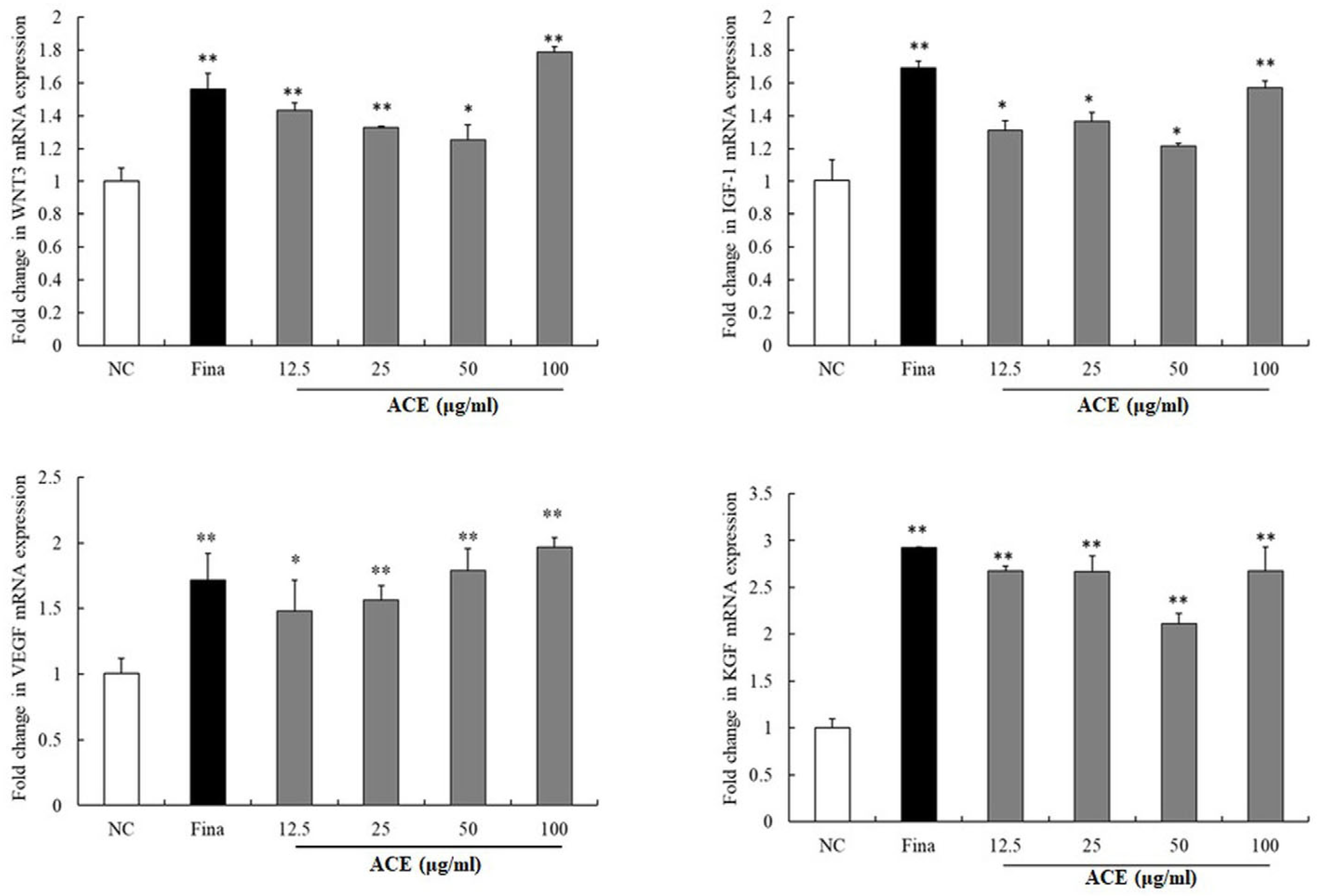
3.3. Modulation of Hair Growth Pathways by ACE in HFDPCs
3.4. Enhancement of Antioxidant Enzyme Expression by ACEs
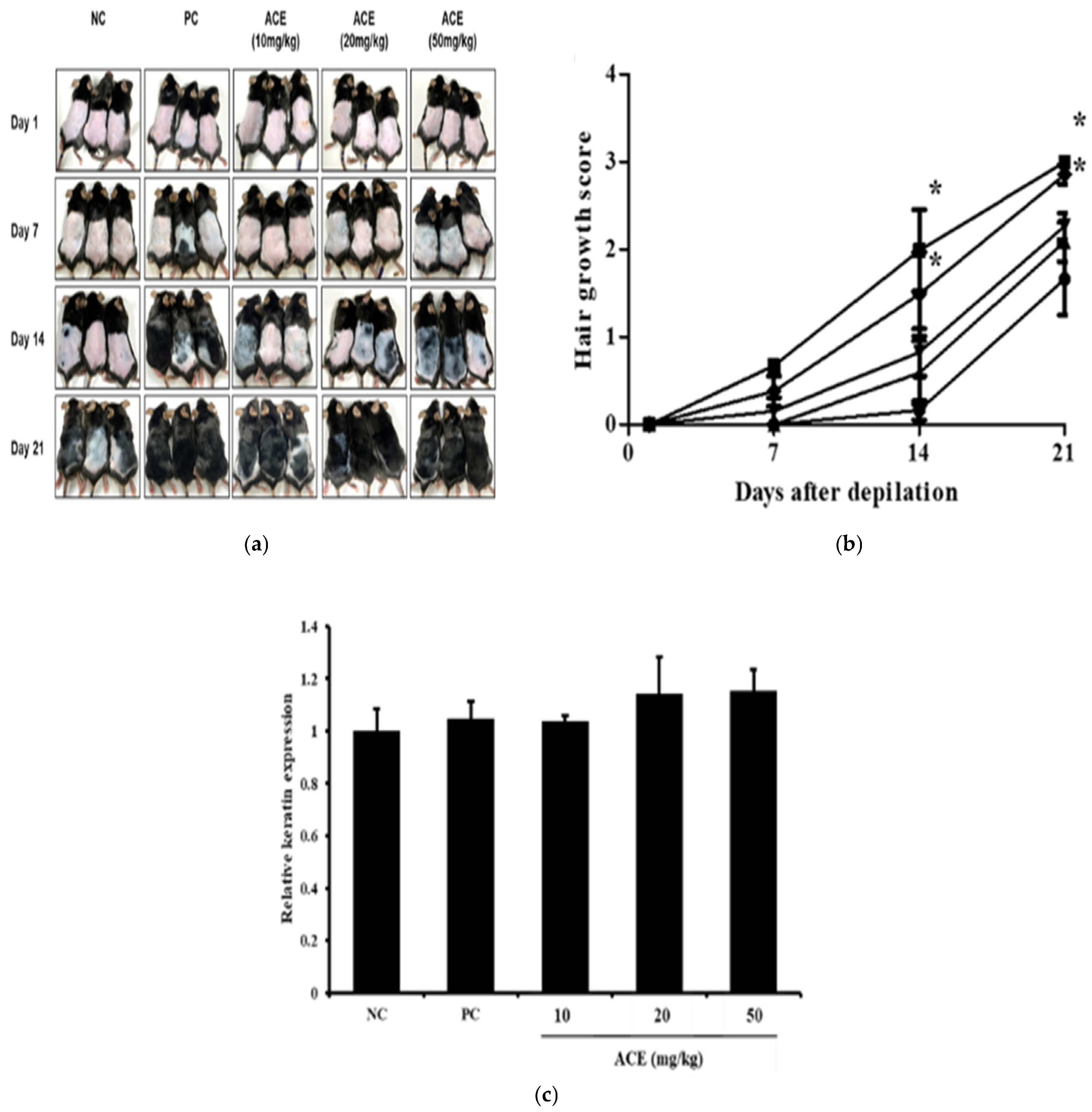
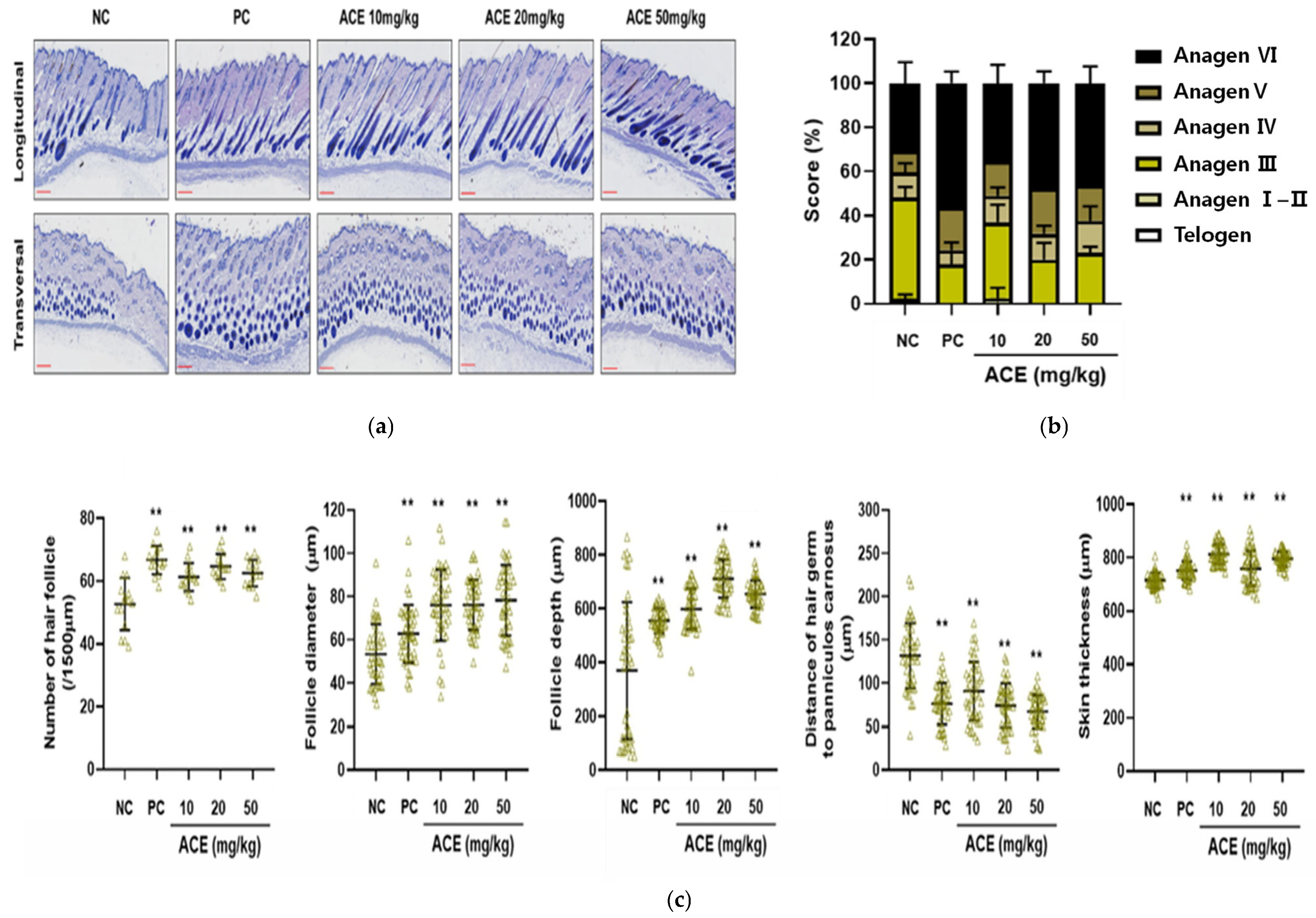
3.5. Hair Growth Promotion by ACE in C57BL/6 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Ageratum conyzoides extract |
| HFDPCs | Human follicle dermal papilla cells |
| ER | Estrogen receptor |
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factor |
| DKK-1 | Dickkopf-related protein-1 |
| SOD | Superoxide dismutase |
| HFs | Hair follicles |
| HHORSCs | Human hair outer root sheath cells |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| H&E | Hematoxylin and eosin |
| AGA | Androgenetic alopecia |
| FPHL | Female pattern hair loss |
| DHT | Dihydrotestosterone |
References
- Hunt, N.; McHale, S. The psychological impact of alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichology 2009, 1, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Tamashunas, N.L.; Bergfeld, W.F. Male and female pattern hair loss: Treatable and worth treating. Cleve. Clin. J. Med. 2021, 88, 173–182. [Google Scholar] [CrossRef]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Talukder, M.; Bamimore, M.A. Relative Efficacy of Minoxidil and the 5-α Reductase Inhibitors in Androgenetic Alopecia Treatment of Male Patients: A Network Meta-analysis. JAMA Dermatol. 2022, 158, 266–274. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.B.; Choe, S.J.; Lee, W.S. Adverse Sexual Effects of Treatment with Finasteride or Dutasteride for Male Androgenetic Alopecia: A Systematic Review and Meta-analysis. Acta. Derm. Venereol. 2019, 99, 12–17. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.; Reyes-Hadsall, S.; Martinez, J.; Heinrich, C.; Huang, K.; Mostaghimi, A. Evaluation of the Safety and Effectiveness of Nutritional Supplements for Treating Hair Loss: A Systematic Review. JAMA Dermatol. 2023, 159, 79–86. [Google Scholar] [CrossRef]
- Okunade, A.L. Ageratum conyzoides L. (Asteraceae). Fitoterapia 2002, 73, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Ganie, S.A.; Singh, B.; Chhillar, A.K.; Yadav, S.S. Phytochemical constituents and ethnopharmacological properties of Ageratum conyzoides L. Phytother. Res. 2019, 33, 2163–2178. [Google Scholar] [CrossRef]
- Detering, M.; Steels, E.; Koyyalamudi, S.R.; Allifranchini, E.; Bocchietto, E.; Vitetta, L. Ageratum conyzoides L. inhibits 5-alpha-reductase gene expression in human prostate cells and reduces symptoms of benign prostatic hypertrophy in otherwise healthy men in a double-blind randomized placebo controlled clinical study. Biofactors 2017, 43, 789–800. [Google Scholar] [CrossRef]
- Chung, E.H.; Kim, J.W.; Kim, J.H.; Jeong, J.S.; Lim, J.H.; Boo, S.Y.; Ko, J.W.; Kim, T.W. Ageratum conyzoides Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia via Inhibiting Proliferation, Inflammation of Prostates, and Induction of Apoptosis in Rats. Nutrients 2024, 16, 2267. [Google Scholar] [CrossRef]
- Clayton, P.; Bogoda, N.; Rao, A. Efficacy of an Oral Ageratum Conyzoides Formulation on Increasing Hair Growth and Decreasing Hair Loss in Males and Females: A Randomised Double-Blind Placebo-Controlled Study. Trichology Cosmetol. Open J. 2023, 6, 1–6. [Google Scholar] [CrossRef]
- Hwang, S.B.; Park, H.J.; Lee, B.H. Hair-Growth-Promoting Effects of the Fish Collagen Peptide in Human Dermal Papilla Cells and C57BL/6 Mice Modulating Wnt/β-Catenin and BMP Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 11904. [Google Scholar] [CrossRef]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef]
- Kesika, P.; Sivamaruthi, B.S.; Thangaleela, S.; Bharathi, M.; Chaiyasut, C. Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals 2023, 16, 206. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W. The Molecular Mechanism of Natural Products Activating Wnt/β-Catenin Signaling Pathway for Improving Hair Loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.M.; D’Amore, P.A. Wnt signaling in the vasculature. Angiogenesis 2002, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Cadoret, A.; Blivet-Van Eggelpoël, M.J.; Bertrand, F.; Cherqui, G.; Perret, C.; Capeau, J. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 2001, 20, 252–259. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, W.; Wang, S.; Zhang, T.; Si, H.; Yang, F.; Li, G. Epidermal Growth Factor Promotes Proliferation and Migration of Follicular Outer Root Sheath Cells via Wnt/β-Catenin Signaling. Cell. Physiol. Biochem. 2016, 39, 360–370. [Google Scholar] [CrossRef]
- Sohn, K.M.; Jeong, K.H.; Kim, J.E.; Park, Y.M.; Kang, H. Hair growth-promotion effects of different alternating current parameter settings are mediated by the activation of Wnt/β-catenin and MAPK pathway. Exp. Dermatol. 2015, 24, 958–963. [Google Scholar] [CrossRef]
- Lee, Y.R.; Bae, S.; Kim, J.Y.; Lee, J.; Cho, D.H.; Kim, H.S.; An, I.S.; An, S. Monoterpenoid Loliolide Regulates Hair Follicle Inductivity of Human Dermal Papilla Cells by Activating the Akt/β-Catenin Signaling Pathway. J. Microbiol. Biotechnol. 2019, 29, 1830–1840. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef]
- Porter, R.M. Mouse models for human hair loss disorders. J. Anat. 2003, 202, 125–131. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-H.; Yi, C.; Chung, E.-H.; Jeong, J.-S.; Kim, J.-H.; Boo, S.-Y.; Lee, S.-H.; Ko, J.-W.; Kim, T.-W.; Kim, Y.-H. Hair Growth and Health Promoting Effects of Standardized Ageratum conyzoides Extract in Human Follicle Dermal Papilla Cells and in C57BL/6 Mice. Nutrients 2025, 17, 2617. https://doi.org/10.3390/nu17162617
Lim J-H, Yi C, Chung E-H, Jeong J-S, Kim J-H, Boo S-Y, Lee S-H, Ko J-W, Kim T-W, Kim Y-H. Hair Growth and Health Promoting Effects of Standardized Ageratum conyzoides Extract in Human Follicle Dermal Papilla Cells and in C57BL/6 Mice. Nutrients. 2025; 17(16):2617. https://doi.org/10.3390/nu17162617
Chicago/Turabian StyleLim, Jong-Hwan, Chunsik Yi, Eun-Hye Chung, Ji-Soo Jeong, Jin-Hwa Kim, So-Young Boo, Su-Ha Lee, Je-Won Ko, Tae-Won Kim, and Young-Hun Kim. 2025. "Hair Growth and Health Promoting Effects of Standardized Ageratum conyzoides Extract in Human Follicle Dermal Papilla Cells and in C57BL/6 Mice" Nutrients 17, no. 16: 2617. https://doi.org/10.3390/nu17162617
APA StyleLim, J.-H., Yi, C., Chung, E.-H., Jeong, J.-S., Kim, J.-H., Boo, S.-Y., Lee, S.-H., Ko, J.-W., Kim, T.-W., & Kim, Y.-H. (2025). Hair Growth and Health Promoting Effects of Standardized Ageratum conyzoides Extract in Human Follicle Dermal Papilla Cells and in C57BL/6 Mice. Nutrients, 17(16), 2617. https://doi.org/10.3390/nu17162617





