Association of a Serum Uric Acid-Related Dietary Pattern with Metabolic Syndrome Among Guangzhou Children Aged 9–17 Years: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Definition of MetS
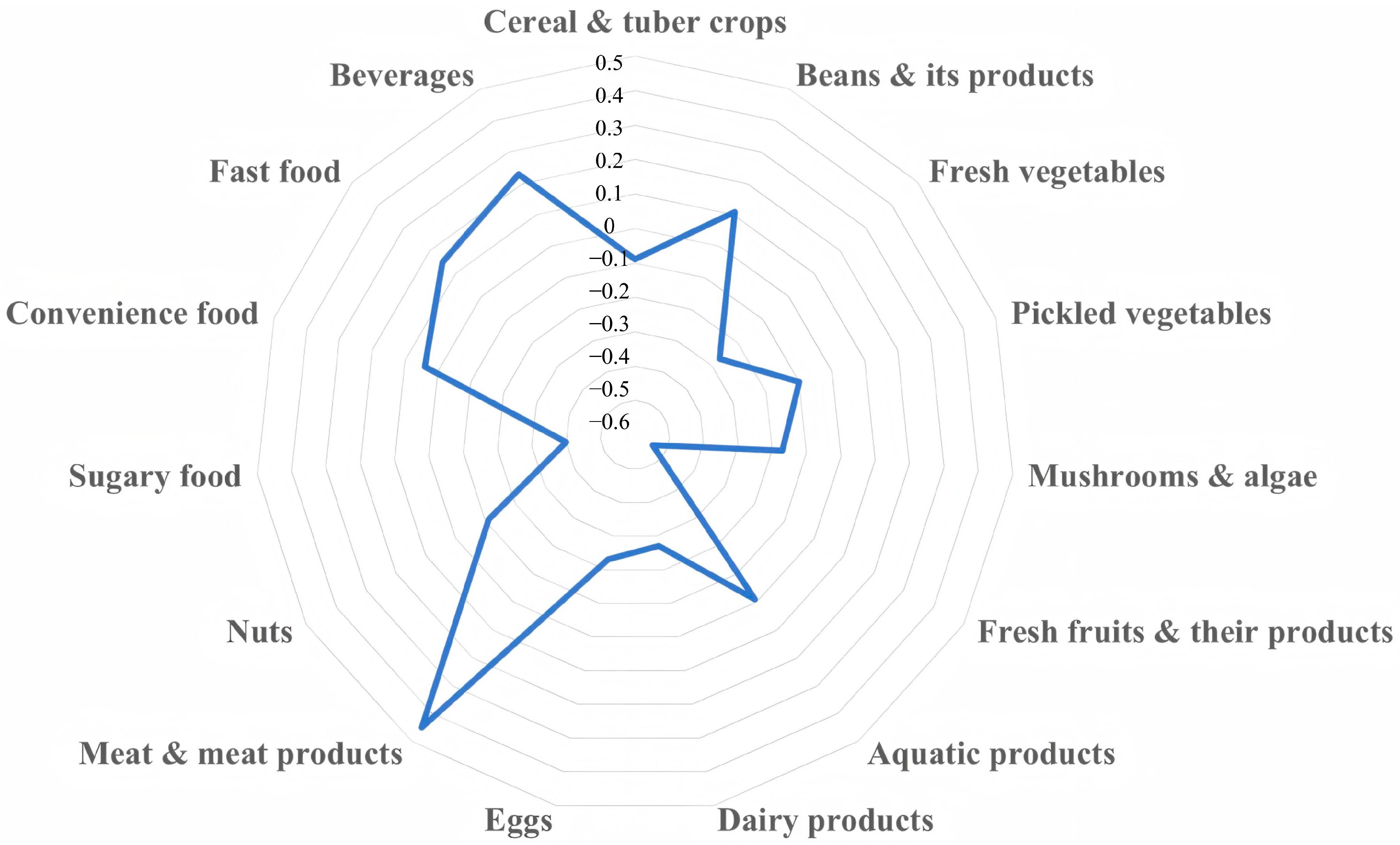
2.4. SUA-Related Dietary Pattern
2.5. Statistical Analyses
3. Results
3.1. Demographic and Basic Characteristics of the Study Population
3.2. SUA-Related Dietary Pattern and Its Characteristics
3.3. Relationship Between the SUA-Related Dietary Pattern Scores and MetS
3.3.1. Analysis of the SUA-Related Dietary Pattern Scores and MetS
3.3.2. Analysis of the SUA-Related Dietary Pattern Scores and Childhood MetS in Children of Different Physical Activity Sufficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MetS | Metabolic syndrome |
| SUA | Serum uric acid |
| RRR | Reduced-rank regression |
| DASH | Dietary Approaches to Stop Hypertension |
| FFQ | Food frequency questionnaire |
| FBG | Blood glucose |
| TG | Triglycerides |
| TC | Total cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| non-HDL-C | Non-high-density lipoprotein cholesterol |
| P90 | The 90th percentile |
| P95 | The 95th percentile |
| OR | Odds ratio |
| CI | Confidence interval |
| ChREBP | Carbohydrate-responsive element-binding protein |
| FAS | Fatty acid synthase |
| RAAS | Renin–angiotensin–aldosterone system |
References
- Christian Flemming, G.M.; Bussler, S.; Körner, A.; Kiess, W. Definition and Early Diagnosis of Metabolic Syndrome in Children. J. Pediatr. Endocrinol. Metab. JPEM 2020, 33, 821–833. [Google Scholar] [CrossRef] [PubMed]
- The Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases, The Society of Pediatrics, Chinese Medical Association; National Clinical Research Center for Child Health; China Clinical Practice Guideline Alliance. Clinical Practice Guideline for the Screening and Treatment of Pediatric Metabolic Syndrome (2025). Chin. J. Pediatr. 2025, 63, 6–14. [Google Scholar] [CrossRef]
- Al-Hamad, D.; Raman, V. Metabolic Syndrome in Children and Adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, Regional, and Country Estimates of Metabolic Syndrome Burden in Children and Adolescents in 2020: A Systematic Review and Modelling Analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhai, F.; Kok, F.J.; Zhao, W.; Piao, J.; Zhang, J.; Cui, Z.; Ma, G. Prevalence of the Metabolic Syndrome in Chinese Adolescents. Br. J. Nutr. 2008, 99, 565–570. [Google Scholar] [CrossRef]
- Shi, J.; He, L.; Yu, D.; Ju, L.; Guo, Q.; Piao, W.; Xu, X.; Zhao, L.; Yuan, X.; Cao, Q.; et al. Prevalence and Correlates of Metabolic Syndrome and Its Components in Chinese Children and Adolescents Aged 7–17: The China National Nutrition and Health Survey of Children and Lactating Mothers from 2016–2017. Nutrients 2022, 14, 3348. [Google Scholar] [CrossRef]
- Tragomalou, A.; Paltoglou, G.; Manou, M.; Kostopoulos, I.V.; Loukopoulou, S.; Binou, M.; Tsitsilonis, O.E.; Bacopoulou, F.; Kassari, P.; Papadopoulou, M.; et al. Non-Traditional Cardiovascular Risk Factors in Adolescents with Obesity and Metabolic Syndrome May Predict Future Cardiovascular Disease. Nutrients 2023, 15, 4342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Caserta, C.A.; Medeiros, C.C.M.; López-Bermejo, A.; Kollias, A.; Zhang, Q.; Pacifico, L.; Reinehr, T.; Litwin, M.; Bassols, J.; et al. Metabolic Syndrome, Clustering of Cardiovascular Risk Factors and High Carotid Intima-Media Thickness in Children and Adolescents. J. Hypertens. 2020, 38, 618–624. [Google Scholar] [CrossRef]
- Valizadeh, M.; Tasdighi, E.; Barzin, M.; Hariri, R.; Mahdavi, M.; Dehghan, P.; Momeni Moghaddam, A.; Azizi, F.; Hosseinpanah, F. Association of Childhood Metabolic Syndrome and Metabolic Phenotypes with the Carotid Intima-Media Thickness (CIMT) in Early Adulthood: Tehran Lipid and Glucose Study. Int. J. Cardiol. 2022, 348, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic Syndrome and Cardiovascular Diseases: Going beyond Traditional Risk Factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle Recommendations for the Prevention and Management of Metabolic Syndrome: An International Panel Recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef]
- Teixeira, B.; Afonso, C.; Rodrigues, S.; Oliveira, A. Healthy and Sustainable Dietary Patterns in Children and Adolescents: A Systematic Review. Adv. Nutr. 2022, 13, 1144–1185. [Google Scholar] [CrossRef]
- Ducharme-Smith, K.; Caulfield, L.E.; Brady, T.M.; Rosenstock, S.; Mueller, N.T.; Garcia-Larsen, V. Higher Diet Quality in African-American Adolescents Is Associated with Lower Odds of Metabolic Syndrome: Evidence from the NHANES. J. Nutr. 2021, 151, 1609–1617. [Google Scholar] [CrossRef]
- Behrooz, M.; Ostadrahimi, A.; Hajjarzadeh, S.; Mousavi, M.; Behbahani, A.G.; Shiva, S. The Association of Dietary Approaches to Stop Hypertension Measured by the Food Frequency Questionnaire with Metabolic Syndrome and Some Inflammatory Biomarkers in Adolescents with Obesity: A Case-Control Study. J. Health Popul. Nutr. 2025, 44, 12. [Google Scholar] [CrossRef]
- Ocké, M.C. Evaluation of Methodologies for Assessing the Overall Diet: Dietary Quality Scores and Dietary Pattern Analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef]
- Newby, P.K.; Tucker, K.L. Empirically Derived Eating Patterns Using Factor or Cluster Analysis: A Review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A Review of Statistical Methods for Dietary Pattern Analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wen, Q.; Lv, J.; Sun, D.; Ma, Y.; Man, S.; Yin, J.; Tong, M.; Wang, B.; Yu, C.; et al. Association between Dietary Patterns Reflecting C-Reactive Protein and Metabolic Syndrome in the Chinese Population. Nutrients 2022, 14, 2566. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Noh, H.; Lee, S. Association of a Dietary Pattern Related to Serum Vitamin D Levels with Metabolic Syndrome Risk among Korean Adults: Based on the Korean National Health and Nutrition Examination Survey. Eur. J. Nutr. 2024, 63, 2767–2778. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Zhao, J.; Liang, J.; Gao, X.; Gao, Q.; He, S.; Wang, T. Association between Nutrient Patterns and Hyperuricemia: Mediation Analysis Involving Obesity Indicators in the NHANES. BMC Public Health 2022, 22, 1981. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a New Statistical Method to Derive Dietary Patterns in Nutritional Epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Oda, E. Serum Uric Acid Is an Independent Predictor of Metabolic Syndrome in a Japanese Health Screening Population. Heart Vessel. 2014, 29, 496–503. [Google Scholar] [CrossRef]
- Goli, P.; Riahi, R.; Daniali, S.S.; Pourmirzaei, M.; Kelishadi, R. Association of Serum Uric Acid Concentration with Components of Pediatric Metabolic Syndrome: A Systematic Review and Meta-Analysis. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2020, 25, 43. [Google Scholar] [CrossRef]
- Cantonese Dietary Pattern Expert Group; Guangdong Nutrition Society. An Introduction to the Cantonese Dietary Pattern (2023). Acta Nutr. Sin. 2023, 45, 417–421. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Zhu, B.; Zhang, H.; Zhang, X.; Ding, X. Demographic, Regional and Temporal Trends of Hyperuricemia Epidemics in Mainland China from 2000 to 2019: A Systematic Review and Meta-Analysis. Glob. Health Action 2021, 14, 1874652. [Google Scholar] [CrossRef]
- Tao, G.; Zeng, C.; Wan, J.; Zhong, W.; Su, Z.; Luo, S.; Huang, J.; Zhang, W.; Yuan, J.; Zhang, J.; et al. Meat-Carbohydrate Dietary Pattern and Elevated Serum Uric Acid in Children and Adolescents: Mediating Role of Obesity in a Cross-Sectional Study. Nutrients 2025, 17, 2090. [Google Scholar] [CrossRef]
- Zhan, S.; Ye, D.; Tan, H. Epidemiology; People’s Health Publishing House: Beijing, China, 2017; ISBN 978-7-117 24557-9. [Google Scholar]
- Gao, Y.; Zhang, L.; Zhu, W.; Wu, N.; Li, G.; Guo, S.; Song, H. Prevalence of metabolic syndrome in Chinese children and adolescents: A Meta-analysis. J. Math. Phys. Med. 2024, 37, 922–934. [Google Scholar] [CrossRef]
- Su, Z.; Zeng, C.; Huang, J.; Luo, S.; Guo, J.; Fu, J.; Zhang, W.; Zhang, Z.; Zhang, B.; Li, Y. Association of Dietary Patterns, C-Reactive Protein, and Risk of Obesity Among Children Aged 9–17 Years in Guangzhou, China: A Cross-Sectional Mediation Study. Nutrients 2024, 16, 3835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, K.; Wang, Y.; Wang, J.; Liu, A.; Chen, X.; Xu, J.; Yang, P.; Ding, C.; Wang, M.; et al. Physical Activity Guidelines for Chinese (2021). Chin. J. Public Health 2022, 38, 129–130. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Chen, M.; Ma, T.; Ma, Q.; Liu, J.; Dong, Y.; Song, Y.; Ma, J. Prevalence of Unhealthy Lifestyle in Chinese Han Children and Adolescents. Chin. J. Cardiovasc. Dis. 2022, 50, 1177–1185. [Google Scholar] [CrossRef]
- National Heath and Family Planning Commission of the People’s Republic of China. Chronic Disease and Nutrition Monitoring Work Plan for Chinese Residents (Trial). Available online: https://www.chinanutri.cn/tzgg_6537/tzgg_102/201412/t20141231_108847.html (accessed on 18 February 2025).
- Lv, R.; Huang, L.; He, L.; Zhang, H.; Yang, Z.; Lai, J.; Yuan, Z. A Systematic Review of the Reproducibility and Validity of Food Frequency Questionnaires among Chinese Pregnant Women, Children and Adolescents. Mod. Prev. Med. 2022, 49, 2335–2340+2359. [Google Scholar] [CrossRef]
- Huang, L.; Luo, X.; Tan, Y.; Su, Y. Study on Reproducibility and Validity of Food Frequency Questionnaire in Guangzhou Area. Chin. J. Dis. Control Prev. 2013, 17, 711–714. [Google Scholar]
- Yang, Y.X. China Food Composition Tables Standard, 6th ed.; Peking University Medical Press: Beijing, China, 2022. [Google Scholar]
- WST424-2013; Anthropometric measurements method in health surveillance. Ministry of Health of the People’s Republic of China: Beijing, China, 2013.
- The Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases, The Society of Pediatrics, Chinese Medical Association; The Subspecialty Group of Cardiology, The Society of Pediatrics, Chinese Medical Association; The Subspecialty Groups of Child Health Care, The Society of Pediatrics, Chinese Medical Association. The Definition of Metabolic Syndrome and Prophylaxis and Treatment Proposal in Chinese Children and Adolescents. Chin. J. Pediatr. 2012, 50, 420–422. [Google Scholar] [CrossRef]
- WS/T 611-2018; High Waist Circumference Screening Threshold Among Children and Adolescents Aged 7–18 Years. National Health Commission of the People’s Republic of China: Beijing, China, 2018.
- Song, P.; Li, X.; Gasevic, D.; Flores, A.B.; Yu, Z. BMI, Waist Circumference Reference Values for Chinese School-Aged Children and Adolescents. Int. J. Environ. Res. Public. Health 2016, 13, 589. [Google Scholar] [CrossRef]
- WS/T 610-2018; Reference of Screening for Elevated Blood Pressure Among Children and Adolescents Aged 7~18 Years. National Health Commission of the People’s Republic of China: Beijing, China, 2018.
- Dong, X.C.; Zhang, M.J.; Guo, D.D.; Peng, S.S.; Yu, X.H.; Li, H.; Fang, A.P.; Zhao, Y.; Yu, Y.J. Association between Dietary Inflammatory Index and Metabolic Syndrome with Its Components among Children Aged 6–14 Years in Beijing City. Chin. J. Sch. Health 2023, 44, 1568–1573. [Google Scholar] [CrossRef]
- Wang, M.M.; Lou, X.H.; Xi, B. Comparison of the Prevalence of Metabolic Syndrome according to Three Definitions among Children and Adolescents aged 7–17 years in Urban Area of Jinan. Chin. J. Sch. Health 2017, 38, 1609–1613. [Google Scholar] [CrossRef]
- Yang, Y.; Piao, W.; Cai, S.; Huang, K.; Yuan, C.; Cheng, X.; Zhang, L.; Li, Y.; Zhao, L.; Yu, D. Comparison of Data-Driven Identified Hypertension-Protective Dietary Patterns among Chinese Adults: Based on a Nationwide Study. Eur. J. Nutr. 2023, 62, 2805–2825. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, X.; Li, R.; Zhang, Z.; Zhang, L.; Gao, X.; Yang, H.; Ma, Y. Association of Dietary Patterns with Blood Uric Acid Concentration and Hyperuricemia in Northern Chinese Adults. Nutr. J. 2022, 21, 42. [Google Scholar] [CrossRef]
- Firth, D. Bias Reduction of Maximum Likelihood Estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Alhajri, A.S.; Alanzi, T.; Alzahrani, W.; Alshehab, H.A.; Alanazi, E.; Alhajri, E.; Aljamaan, N.; Aldandan, F.A.A.; Almumttin, Z.; Alnwaisser, M.; et al. The Relationship between Physical Activity Level, Dietary Patterns, and Metabolic Syndrome: An Empirical Study in Saudi Arabia. Nutr. Health 2025, 2601060241305179. [CrossRef] [PubMed]
- Xiao, Y.; Xu, S.H.; Huang, H.Y.; Guo, L.; Lv, W.J.; Feng, N.X.; Wang, Q.F.; Mao, X.D.; Chen, G.F.; Liu, C. A Comparative Study of Different Diagnostic Criteria for Metabolic Syndrome in Adolescents in Changzhou Area. Int. J. Endocrinol. Metab. 2020, 40, 1–4. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, T.W.; Yu, M.H.; Kang, D.R.; Choi, S. Gender and Age Differences in the Prevalence and Associated Factors of Metabolic Syndrome among Children and Adolescents in South Korea. Child Health Nurs. Res. 2021, 27, 160–170. [Google Scholar] [CrossRef]
- Yasin, S.; Hasnain, M.; Khan, F.R.; Aslam, K.; Farooq, N.; Ihsan, A. Association between Obesity, Digital Screen Time, and Early-Onset Hypertension in Adolescents: A Prospective Cohort Study. Cureus 2025, 17, e79975. [Google Scholar] [CrossRef]
- Cui, H.; Xu, R.; Wan, Y.; Ling, Y.; Jiang, Y.; Wu, Y.; Guan, Y.; Zhao, Q.; Zhao, G.; Zaid, M. Relationship of Sleep Duration with Incident Cardiovascular Outcomes: A Prospective Study of 33,883 Adults in a General Population. BMC Public Health 2023, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Liu, F.; Zhang, S.; Wu, Y.; Li, Y.; Xiong, J.; Tang, Y.; Li, Y.; Yao, P. Associations between Dietary Patterns and Serum Uric Acid Concentrations in Children and Adolescents: A Cross-Sectional Study. Food Funct. 2023, 14, 9803–9814. [Google Scholar] [CrossRef] [PubMed]
- Yuguang, L.; Chang, Y.; Li, H.; Li, F.; Zou, Q.; Liu, X.; Chen, X.; Cui, J. Inflammation Mediates the Relationship between Diet Quality Assessed by Healthy Eating Index-2015 and Metabolic Syndrome. Front. Endocrinol. 2024, 15, 1293850. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-W.; Chin, Y.-T.; Lin, W.-T.; Tsai, S.; Lee, C.-Y.; Tsai, W.-C.; Seal, D.W.; Lee, C.-H. Fructose Intake, Endogenous Biomarkers and Latent Metabolic Construct in Adolescents: Exploring Path Associations and Mediating Effects. Pediatr. Obes. 2024, 20, e13176. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Chang, H.Y.; Wu, H.C.; Stanaway, F.F.; Pan, W.H. High Sugar-Sweetened Beverage Intake Frequency Is Associated with Smoking, Irregular Meal Intake and Higher Serum Uric Acid in Taiwanese Adolescents. J. Nutr. Sci. 2020, 9, e7. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Zhang, P.; Zhong, F.; Cai, J.; Ma, A. Association of Dietary Fiber Intake with Hyperuricemia in U.S. Adults. Food Funct. 2019, 10, 4932–4940. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Lubawy, M.; Formanowicz, D. High-Fructose Diet-Induced Hyperuricemia Accompanying Metabolic Syndrome-Mechanisms and Dietary Therapy Proposals. Int. J. Environ. Res. Public. Health 2023, 20, 3596. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Lanaspa, M.A.; Sanchez-Lozada, L.G.; Tolan, D.; Nakagawa, T.; Ishimoto, T.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Stenvinkel, P. The Fructose Survival Hypothesis for Obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ye, F.; Li, X.C. Research Progress on the Interaction Between Hyperuricemia and Lipid Accumulation. Chin. J. Clin. Nutr. 2024, 32, 378–384. [Google Scholar] [CrossRef]
- Tian, L.; Xue, H. Research Progress on the Mechanism of Obesity-Induced Cardiovascular Disease. Chin. J. Gen. Pract. 2024, 23, 305–308. [Google Scholar] [CrossRef]
- Gallardo-Alfaro, L.; Bibiloni, M.D.M.; Mascaró, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality Are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients 2020, 12, 1013. [Google Scholar] [CrossRef]
- Ceraudo, F.; Caparello, G.; Galluccio, A.; Avolio, E.; Augimeri, G.; De Rose, D.; Vivacqua, A.; Morelli, C.; Barone, I.; Catalano, S.; et al. Impact of Mediterranean Diet Food Choices and Physical Activity on Serum Metabolic Profile in Healthy Adolescents: Findings from the DIMENU Project. Nutrients 2022, 14, 881. [Google Scholar] [CrossRef]
- Winpenny, E.M.; van Sluijs, E.M.F.; Forouhi, N.G. How Do Short-Term Associations Between Diet Quality and Metabolic Risk Vary with Age? Eur. J. Nutr. 2021, 60, 517–527. [Google Scholar] [CrossRef]
- Gardner, A.W.; Parker, D.E. Association between Arterial Compliance and Age in Participants 9 to 77 Years Old. Angiology 2010, 61, 37–41. [Google Scholar] [CrossRef] [PubMed]
| Metabolic Syndrome | Z/χ2 | p-Value | ||
|---|---|---|---|---|
| NO (n = 3837) | YES (n = 86) | |||
| Age in years, M (P25, P75) | 13.89 (11.73, 15.96) | 13.83 (11.56, 15.88) | 0.05 | 0.821 |
| Sex, n (%) | 11.65 | <0.001 | ||
| Male | 2031 (97.04) | 62 (2.96) | ||
| Female | 1806 (98.69) | 24 (1.31) | ||
| The education of father, n (%) | 0.25 | 0.881 | ||
| Junior high school and below | 1138 (97.93) | 24 (2.07) | ||
| Senior high school | 1201 (97.64) | 29 (2.36) | ||
| College degree or above | 1498 (97.84) | 33 (2.16) | ||
| The education of mother, n (%) | 2.47 | 0.291 | ||
| Junior high school and below | 1244 (98.34) | 21 (1.66) | ||
| Senior high school | 1129 (97.58) | 28 (2.42) | ||
| College degree or above | 1464 (97.53) | 37 (2.47) | ||
| Boarding, n (%) | 0.79 | 0.374 | ||
| Yes | 2258 (98.00) | 46 (2.00) | ||
| No | 1579 (97.53) | 40 (2.47) | ||
| Insufficient physical activity, n (%) | 1.95 | 0.163 | ||
| Yes | 2186 (98.11) | 42 (1.89) | ||
| No | 1651 (97.40) | 44 (2.60) | ||
| Screen time (min/d), M (P25, P75) | 60.00 (34.29, 102.86) | 68.57 (34.29, 102.86) | 0.35 | 0.552 |
| Sleep duration (h/d), M (P25, P75) | 8.95 (8.17, 9.83) | 8.90 (8.18, 10.03) | 0.79 | 0.374 |
| MetS components, M (P25, P75) | ||||
| Waist circumference (cm) | 64.00 (59.20, 69.20) | 85.90 (82.40, 90.68) | 214.09 | <0.001 |
| Systolic blood pressure (mmHg) | 110.50 (102.00, 118.00) | 122.50 (114.00, 131.75) | 81.31 | <0.001 |
| Diastolic blood pressure (mmHg) | 67.00 (62.50, 72.00) | 72.00 (68.00, 79.38) | 44.49 | <0.001 |
| FBG (mmol/L) | 4.91 (4.65, 5.16) | 5.15 (4.93, 5.61) | 40.55 | <0.001 |
| TG (mmol/L) | 0.82 (0.65, 1.08) | 1.68 (1.47, 2.11) | 155.10 | <0.001 |
| HDL-C (mmol/L) | 1.35 (1.18, 1.53) | 1.13 (1.00, 1.29) | 59.72 | <0.001 |
| non-HDL-C (mmol/L) | 2.57 (2.17, 2.99) | 3.43 (2.75, 3.96) | 77.77 | <0.001 |
| SUA (mg/dL), M (P25, P75) | 6.25 (5.34, 7.29) | 8.09 (6.85, 9.02) | 76.90 | <0.001 |
| Central Obesity | p-Value | Elevated Blood Pressure | p-Value | Hyperglycemia | p-Value | Hypertriglyceridemia | p-Value | Dyslipidemia | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO (n = 3466) | YES (n = 457) | NO (n = 3481) | YES (n = 442) | NO (n = 3755) | YES (n = 168) | NO (n = 3551) | YES (n = 372) | NO (n = 3360) | YES (n = 603) | ||||||
| Age in years, M (P25, P75) | 13.87 (11.75, 15.94) | 14.02 (11.60, 16.01) | 0.589 | 13.81 (11.65, 15.92) | 14.39 (12.77, 16.12) | <0.001 | 13.96 (11.79, 15.97) | 12.76 (11.29, 14.13) | <0.001 | 13.90 (11.74, 15.95) | 13.74 (11.61, 15.96) | 0.738 | 13.94 (11.74, 15.96) | 13.66 (11.69, 15.91) | 0.172 |
| Sex, n (%) | <0.001 | <0.001 | 0.214 | 0.741 | <0.001 | ||||||||||
| Male | 1803 (86.14) | 290 (13.86) | 1816 (86.77) | 277 (13.23) | 1995 (95.32) | 98 (4.68) | 1891 (90.35) | 202 (9.65) | 1752 (83.71) | 341 (16.29) | |||||
| Female | 1663 (90.87) | 167 (9.13) | 1665 (90.98) | 165 (9.02) | 1760 (96.17) | 70 (3.83) | 1660 (90.71) | 170 (9.29) | 1608 (87.87) | 222 (12.13) | |||||
| The education of father, n (%) | 0.164 | 0.939 | 0.181 | 0.034 | 0.189 | ||||||||||
| Junior high school and below | 1028 (88.47) | 134 (11.53) | 1028 (88.47) | 134 (11.53) | 1118 (96.21) | 44 (3.79) | 1039 (89.41) | 123 (10.59) | 977 (84.08) | 185 (15.92) | |||||
| Senior high school | 1102 (89.59) | 128 (10.41) | 1092 (88.78) | 138 (11.22) | 1183 (96.18) | 47 (3.82) | 1103 (89.67) | 127 (10.33) | 1063 (86.42) | 167 (13.58) | |||||
| College degree or above | 1336 (87.26) | 195 (12.74) | 1361 (88.90) | 170 (11.10) | 1454 (94.97) | 77 (5.03) | 1409 (92.03) | 122 (7.97) | 1320 (86.22) | 211 (13.78) | |||||
| The education of mother, n (%) | 0.059 | 0.373 | 0.282 | 0.057 | 0.944 | ||||||||||
| Junior high school and below | 1140 (90.12) | 125 (9.88) | 1121 (88.62) | 144 (11.38) | 1219 (96.36) | 46 (3.64) | 1134 (89.64) | 131 (10.36) | 1085 (85.77) | 180 (14.23) | |||||
| Senior high school | 1013 (87.55) | 144 (12.45) | 1016 (87.81) | 141 (12.19) | 1108 (95.76) | 49 (4.24) | 1037 (89.63) | 120 (10.37) | 993 (85.83) | 164 (14.17) | |||||
| College degree or above | 1313 (87.48) | 188 (12.52) | 1344 (89.54) | 157 (10.46) | 1428 (95.14) | 73 (4.86) | 1380 (91.94) | 121 (8.06) | 1282 (85.41) | 219 (14.59) | |||||
| Boarding, n (%) | 0.848 | 0.407 | <0.001 | 0.660 | 0.010 | ||||||||||
| Yes | 2038 (88.45) | 266 (11.55) | 2053 (89.11) | 251 (10.89) | 2232 (96.88) | 72 (3.12) | 2090 (90.71) | 214 (9.29) | 1945 (84.42) | 359 (15.58) | |||||
| No | 1428 (88.20) | 191 (11.80) | 1428 (88.20) | 191 (11.80) | 1523 (94.07) | 96 (5.93) | 1461 (90.24) | 158 (9.76) | 1415 (87.40) | 204 (12.60) | |||||
| Insufficient physical activity, n (%) | 0.267 | 0.385 | 0.156 | 0.282 | 0.505 | ||||||||||
| Yes | 1980 (88.87) | 248 (11.13) | 1986 (89.14) | 242 (10.86) | 2142 (96.14) | 86 (3.86) | 2027 (90.98) | 201 (9.02) | 1916 (86.00) | 312 (14.00) | |||||
| No | 1486 (87.67) | 209 (12.33) | 1495 (88.20) | 200 (11.80) | 1613 (95.16) | 82 (4.84) | 1524 (89.91) | 171 (10.09) | 1444 (85.19) | 251 (14.81) | |||||
| Screen time (min/d), M (P25, P75) | 60.00 (34.29, 102.86) | 68.57 (34.29, 107.14) | 0.009 | 60.00 (34.29, 102.86) | 68.57 (34.29, 111.43) | 0.032 | 60.00 (34.29, 102.86) | 51.43 (25.71, 102.86) | 0.087 | 60.00 (34.29, 102.86) | 56.43 (34.29, 101.79) | 0.327 | 60.00 (34.29, 102.86) | 60.00 (34.29, 102.86) | 0.803 |
| Sleep duration (h/d), M (P25, P75) | 8.96 (8.17, 9.83) | 8.93 (8.14, 9.75) | 0.440 | 9.00 (8.17, 9.85) | 8.76 (8.17, 9.62) | 0.011 | 8.93 (8.17, 9.79) | 9.49 (8.52, 10.33) | <0.001 | 8.93 (8.17, 9.79) | 9.08 (8.33, 10.00) | 0.009 | 8.93 (8.17, 9.80) | 9.07 (8.29, 9.95) | 0.006 |
| SUA (mg/dL), M (P25, P75) | 6.16 (5.29, 7.14) | 7.14 (6.20, 8.55) | <0.001 | 6.20 (5.31, 7.22) | 6.97 (5.98, 8.11) | <0.001 | 6.28 (5.36, 7.34) | 6.07 (5.30, 7.15) | 0.352 | 6.23 (5.33, 7.27) | 6.65 (5.59, 8.10) | <0.001 | 6.25 (5.32, 7.26) | 6.47 (5.52, 7.85) | <0.001 |
| SUA-Related Dietary Pattern Scores | H/χ2 | p-Value | |||
|---|---|---|---|---|---|
| T1 (n = 1308) | T2 (n = 1308) | T3 (n = 1307) | |||
| Age in years, M (P25, P75) | 13.16 (11.21, 14.57) | 13.96 (11.84, 15.96) | 14.90 (12.97, 16.18) | 224.33 | <0.001 |
| Sex, n (%) | 146.65 | <0.001 | |||
| Male | 539 (25.75) | 707 (33.78) | 847 (40.47) | ||
| Female | 769 (42.02) | 601 (32.84) | 460 (25.14) | ||
| The education of father, n (%) | 4.73 | 0.317 | |||
| Junior high school and below | 369 (31.76) | 383 (32.96) | 410 (35.28) | ||
| Senior high school | 433 (35.20) | 402 (32.68) | 395 (32.12) | ||
| College degree or above | 506 (33.05) | 523 (34.16) | 502 (32.79) | ||
| The education of mother, n (%) | 3.33 | 0.504 | |||
| Junior high school and below | 402 (31.78) | 426 (33.68) | 437 (34.54) | ||
| Senior high school | 386 (33.36) | 396 (34.23) | 375 (32.41) | ||
| College degree or above | 520 (34.64) | 486 (32.38) | 495 (32.98) | ||
| Boarding, n (%) | 106.12 | <0.001 | |||
| Yes | 629 (27.30) | 790 (34.29) | 885 (38.41) | ||
| No | 679 (41.94) | 518 (32.00) | 422 (26.06) | ||
| Insufficient physical activity, n (%) | 1.66 | 0.436 | |||
| Yes | 725 (32.54) | 757 (33.98) | 746 (33.48) | ||
| No | 583 (34.40) | 551 (32.51) | 561 (33.09) | ||
| Screen time (min/d), M (P25, P75) | 51.43 (25.71, 94.29) | 64.29 (34.29, 102.86) | 68.57 (38.57, 115.71) | 90.61 | <0.001 |
| Sleep duration (h/d), M (P25, P75) | 9.25 (8.43, 10.11) | 9.00 (8.25, 9.79) | 8.58 (8.00, 9.46) | 160.06 | <0.001 |
| SUA-Related Dietary Pattern Scores | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Metabolic Syndrome | ||||
| T1 | 1.00 | — | 1.00 | — |
| T2 | 1.36 (0.79–2.39) | 0.266 | 1.31 (0.75–2.31) | 0.344 |
| T3 | 1.55 (0.91–2.68) | 0.106 | 1.43 (0.82–2.55) | 0.211 |
| Linear for 1 unit | 1.30 (1.04–1.64) | 0.022 | 1.27 (1.00–1.62) | 0.049 |
| Central Obesity | ||||
| T1 | 1.00 | — | 1.00 | — |
| T2 | 1.56 (1.21–2.02) | <0.001 | 1.50 (1.16–1.96) | 0.002 |
| T3 | 1.87 (1.46–2.40) | <0.001 | 1.72 (1.33–2.25) | <0.001 |
| Linear for 1 unit | 1.29 (1.16–1.43) | <0.001 | 1.24 (1.11–1.38) | <0.001 |
| Elevated Blood Pressure | ||||
| T1 | 1.00 | — | 1.00 | — |
| T2 | 1.45 (1.13–1.87) | 0.003 | 1.31 (1.02–1.70) | 0.036 |
| T3 | 1.36 (1.06–1.76) | 0.016 | 1.12 (0.85–1.46) | 0.420 |
| Linear for 1 unit | 1.12 (1.01–1.24) | 0.027 | 1.03 (0.92–1.15) | 0.634 |
| Hyperglycemia | ||||
| T1 | 1.00 | — | 1.00 | — |
| T2 | 0.64 (0.44–0.93) | 0.021 | 0.71 (0.48–1.03) | 0.070 |
| T3 | 0.63 (0.43–0.91) | 0.016 | 0.76 (0.51–1.12) | 0.171 |
| Linear for 1 unit | 0.83 (0.72–0.97) | 0.014 | 0.90 (0.77–1.06) | 0.193 |
| Hypertriglyceridemia | ||||
| T1 | 1.00 | — | 1.00 | — |
| T2 | 1.19 (0.92–1.54) | 0.189 | 1.22 (0.93–1.59) | 0.146 |
| T3 | 0.98 (0.75–1.29) | 0.896 | 1.03 (0.77–1.37) | 0.845 |
| Linear for 1 unit | 1.02 (0.91–1.13) | 0.785 | 1.04 (0.92–1.17) | 0.541 |
| Dyslipidemia | ||||
| T1 | 1.00 | — | 1.00 | — |
| T2 | 1.12 (0.90–1.40) | 0.293 | 1.09 (0.88–1.37) | 0.432 |
| T3 | 1.01 (0.81–1.26) | 0.949 | 0.97 (0.76–1.22) | 0.779 |
| Linear for 1 unit | 1.00 (0.92–1.10) | 0.939 | 0.98 (0.89–1.09) | 0.758 |
| SUA-Related Dietary Pattern Scores | |||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | Linear for 1 unit | ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Insufficient physical activity group | — | — | — | — | |||
| Metabolic Syndrome | 1.00 | 3.07 (1.23–8.92) | 0.015 | 3.74 (1.49–10.89) | 0.004 | 1.69 (1.18–2.42) | 0.004 |
| Central Obesity | 1.00 | 2.28 (1.57–3.38) | <0.001 | 2.64 (1.80–3.92) | <0.001 | 1.39 (1.20–1.62) | <0.001 |
| Elevated Blood Pressure | 1.00 | 1.29 (0.91–1.84) | 0.155 | 1.23 (0.86–1.77) | 0.268 | 1.07 (0.92–1.24) | 0.396 |
| Hyperglycemia | 1.00 | 0.72 (0.43–1.20) | 0.213 | 0.61 (0.34–1.07) | 0.087 | 0.83 (0.68–1.03) | 0.089 |
| Hypertriglyceridemia | 1.00 | 1.44 (0.99–2.11) | 0.058 | 1.47 (0.99–2.18) | 0.058 | 1.17 (0.99–2.11) | 0.060 |
| Dyslipidemia | 1.00 | 0.88 (0.65–1.18) | 0.370 | 0.88 (0.65–1.21) | 0.442 | 1.00 (0.88–1.14) | 0.991 |
| Sufficient physical activity group | — | — | — | — | |||
| Metabolic Syndrome | 1.00 | 0.69 (0.32–1.48) | 0.325 | 0.69 (0.32–1.48) | 0.338 | 0.97 (0.70–1.36) | 0.870 |
| Central Obesity | 1.00 | 0.97 (0.67–1.42) | 0.894 | 1.13 (0.78–1.64) | 0.516 | 1.09 (0.93–1.28) | 0.315 |
| Elevated Blood Pressure | 1.00 | 1.32 (0.91–1.93) | 0.141 | 0.98 (0.66–1.46) | 0.914 | 0.97 (0.83–1.14) | 0.721 |
| Hyperglycemia | 1.00 | 0.67 (0.37–1.18) | 0.170 | 0.96 (0.55–1.67) | 0.899 | 0.99 (0.79–1.26) | 0.949 |
| Hypertriglyceridemia | 1.00 | 1.03 (0.71–1.50) | 0.877 | 0.67 (0.43–1.02) | 0.062 | 0.91 (0.77–1.07) | 0.246 |
| Dyslipidemia | 1.00 | 1.41 (1.01–1.97) | 0.047 | 1.05 (0.73–1.51) | 0.778 | 0.95 (0.82–1.10) | 0.499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Luo, S.; Tao, G.; Wan, J.; Fu, J.; Zeng, C.; Huang, J.; Chen, X.; Deng, N.; Zhang, W.; et al. Association of a Serum Uric Acid-Related Dietary Pattern with Metabolic Syndrome Among Guangzhou Children Aged 9–17 Years: A Cross-Sectional Study. Nutrients 2025, 17, 2618. https://doi.org/10.3390/nu17162618
Zhong W, Luo S, Tao G, Wan J, Fu J, Zeng C, Huang J, Chen X, Deng N, Zhang W, et al. Association of a Serum Uric Acid-Related Dietary Pattern with Metabolic Syndrome Among Guangzhou Children Aged 9–17 Years: A Cross-Sectional Study. Nutrients. 2025; 17(16):2618. https://doi.org/10.3390/nu17162618
Chicago/Turabian StyleZhong, Wanzhen, Shiyun Luo, Guixian Tao, Jiayi Wan, Jinhan Fu, Cunzi Zeng, Jie Huang, Xi Chen, Nali Deng, Weiwei Zhang, and et al. 2025. "Association of a Serum Uric Acid-Related Dietary Pattern with Metabolic Syndrome Among Guangzhou Children Aged 9–17 Years: A Cross-Sectional Study" Nutrients 17, no. 16: 2618. https://doi.org/10.3390/nu17162618
APA StyleZhong, W., Luo, S., Tao, G., Wan, J., Fu, J., Zeng, C., Huang, J., Chen, X., Deng, N., Zhang, W., Gu, J., & Li, Y. (2025). Association of a Serum Uric Acid-Related Dietary Pattern with Metabolic Syndrome Among Guangzhou Children Aged 9–17 Years: A Cross-Sectional Study. Nutrients, 17(16), 2618. https://doi.org/10.3390/nu17162618






