Nitrogen Requirements in Healthy Adults: A Systematic Review and Meta-Analysis of Nitrogen Balance Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of the Literature
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Study Risk of Bias Assessment
2.5. Nitrogen Requirement
2.6. Statistical Analysis
3. Results
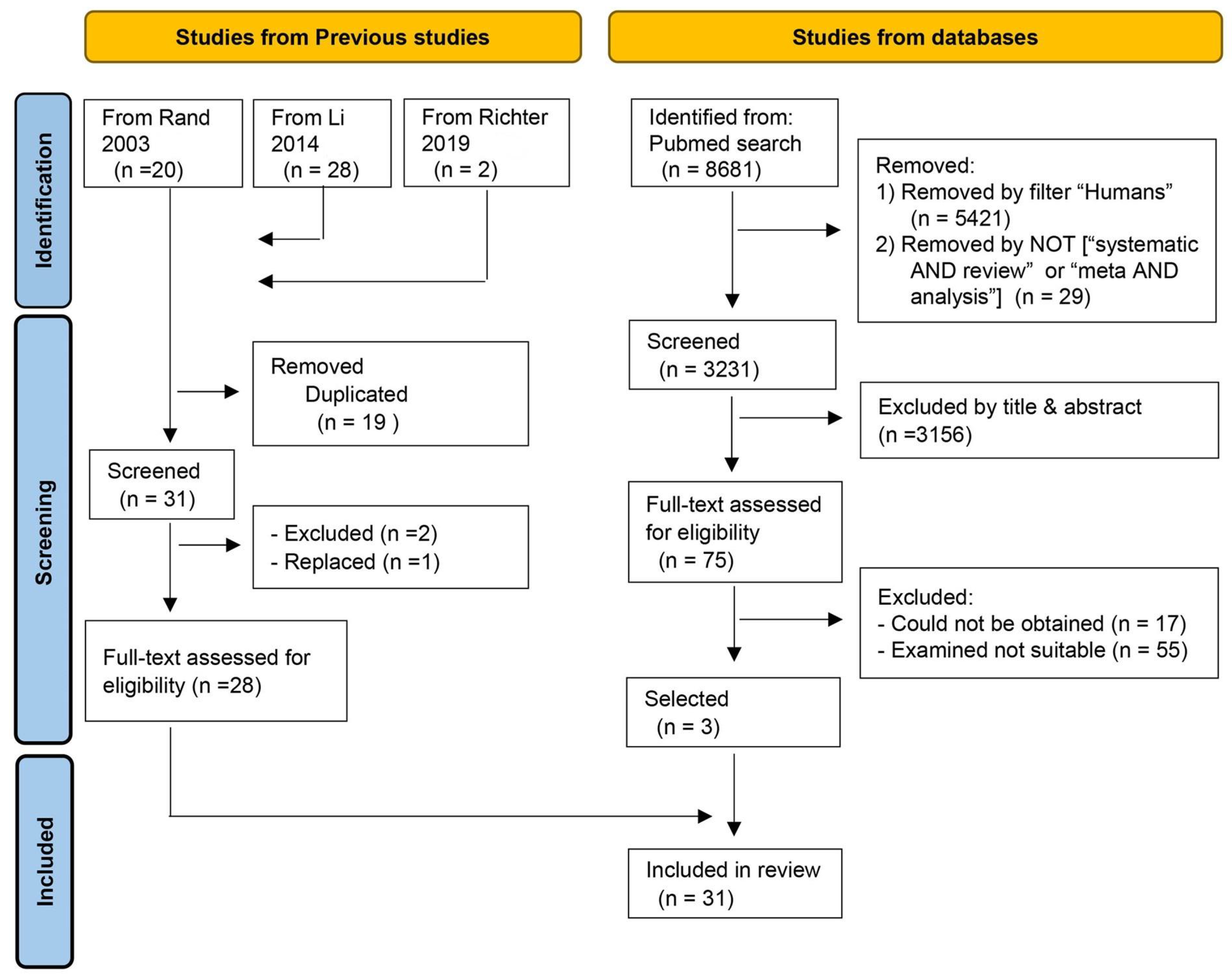
3.1. Literature Selection and Data Extraction
3.1.1. Literature Selection from Previous Studies
- One paper [15] from Li et al.’s review [14] reported individual protein requirements calculated using regression equations, with miscellaneous losses set at 8 mg/kg/day. However, nitrogen intake, excretion, and the regression equations themselves were not reported, making it impossible to adjust the requirement to 4.8 mg/kg/day based on the study site’s template climate.
- The remaining 28 studies had their metadata extracted, nitrogen requirements obtained, and were included in this review.
3.1.2. Literature Selection from the PubMed Search
3.2. Metadata
- One study [48] was classified as conducted in a “Tropical” climate based on the 2014 review [14], which referred to researchers from the University of São Paulo (Brazil). However, the original text states that the experiment was conducted during a period with average temperatures of 23.1–24.0 °C. Therefore, the climate classification was revised to “Temperate.”
| Data Source | Climate | Protein | Sex | n | Age | Requirement 2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Study ID | Ex 1 | Source | (y, Median) | Mean | SD | Ref. | |||
| 01_Clark HE | A | Temperate | Mixed | F | 1 | 24 | 66.8 | [46] | |
| 01_Clark HE | B | Temperate | Mixed | M | 4 | 24 | 92.2 | 9.9 | [46] |
| 02_Alford BB | A | Temperate | Plant-based | F | 14 | 21 | 123.7 | 20.9 | [50] |
| 03_Cheng AH | A | Temperate | Mixed | M | 7 | 24 | 148.2 | 27.3 | [51] |
| 03_Cheng AH | B | Temperate | Mixed | M | 7 | 68 | 138.2 | 16.3 | [51] |
| 04_Uauy R | A | Temperate | Animal-based | F | 7 | 74 | 145.4 | 34.1 | [47] |
| 04_Uauy R | B | Temperate | Animal-based | M | 6 | 71 | 102.5 | 41.6 | [47] |
| 05_Thomas MR | A | Temperate | Plant-based | F | 7 | 20 | 95.4 | 25.3 | [52] |
| 06_Fajardo LF | A | Tropical | Animal-based | F | 2 | 22.5 | 111.6 | 2.4 | [44] |
| 06_Fajardo LF | B | Tropical | Animal-based | M | 4 | 22.5 | 133.1 | 15.2 | [44] |
| 06_Fajardo LF | C | Tropical | Plant-based | M | 7 | 23 | 134.4 | 16.1 | [44] |
| 07_Inoue G | A | Temperate | Animal-based | M | 7 | 21 | 93.4 | 9.1 | [53] |
| 07_Inoue G | B | Temperate | Plant-based | M | 5 | 22 | 119.8 | 15.9 | [53] |
| 07_Inoue G | C | Temperate | Mixed | M | 8 | 21 | 97.7 | 21.1 | [53] |
| 08_Tontisirin K | A | Tropical | Animal-based | M | 13 | 23 | 138.1 | 18.8 | [54] |
| 09_Bourges H | A | Temperate | Plant-based | M | 8 | 20.5 | 113.6 | 20.0 | [55] |
| 09_Bourges H | B | Temperate | Animal-based | M | 3 | 21 | 102.6 | 19.8 | [55] |
| 10_Calloway DH | A | Temperate | Animal-based | F | 4 | 25 | 85.7 | 25.3 | [56] |
| 11_Huang PC | A | Temperate | Mixed | M | 7 | 24.5 | 127.4 | 21.7 | [57] |
| 11_Huang PC | B | Temperate | Animal-based | M | 5 | 24.5 | 95.4 | 13.1 | [57] |
| 12_Yanez E | A | Temperate | Animal-based | M | 8 | 25.5 | 98.4 | 5.8 | [58] |
| 12_Yanez E | B | Temperate | Mixed | M | 7 | 25 | 127.0 | 17.8 | [58] |
| 13_Istfan N | A | Temperate | Plant-based | M | 8 | 19.5 | 94.2 | 19.2 | [59] |
| 14_Scrimshaw NS | A | Temperate | Plant-based | M | 13 | 20 | 117.7 | 31.3 | [60] |
| 14_Scrimshaw NS | B | Temperate | Animal-based | M | 5 | 19 | 103.1 | 9.7 | [60] |
| 15_Vannucchi H | A | Tropical | Plant-based | M | 8 | 23 | 108.9 | 14.8 | [48] |
| 16_Agarwal KN | A | Tropical | Plant-based | F | 5 | 32 | 104.8 | 6.9 | [61] |
| 16_Agarwal KN | B | Tropical | Plant-based | M | 6 | 32 | 90.4 | 10.3 | [61] |
| 17_Dutra de Oliveira | A | Tropical | Plant-based | M | 8 | 26.5 | 115.3 | 17.2 | [45] |
| 18_Hussein MA | A | Tropical | Mixed | F | 8 | 22.5 | 86.0 | 6.5 | [62] |
| 19_Ozalp I | A | Temperate | Mixed | M | 11 | 23 | 98.5 | 15.3 | [63] |
| 20-2_Chen XC | A | Temperate | Mixed | M | 10 | 42 | 147.7 | 17.9 | [21] |
| 21_Young VR | A | Temperate | Plant-based | M | 8 | 19.4 | 112.2 | 15.2 | [64] |
| 21_Young VR | B | Temperate | Animal-based | M | 7 | 21.1 | 90.9 | 45.8 | [64] |
| 22_Atinmo T | A | Tropical | Mixed | U 3 | 14 | 20 | 119.6 | 20.4 | [49] |
| 23_Kaneko K | A | Temperate | Mixed | F | 12 | 21 | 103.0 | 21.4 | [65] |
| 24_De Unamuno | A | Tropical | Plant-based | M | 7 | 71 | 113.1 | 25.8 | [66] |
| 25_Egana JI | A | Tropical | Plant-based | M | 7 | 26 | 102.8 | 13.1 | [67] |
| 25_Egana JI | B | Tropical | Animal-based | M | 5 | 26 | 80.1 | 11.2 | [67] |
| 26_Egun GN | A | Tropical | Mixed | F | 12 | 23.5 | 81.4 | 2.8 | [68] |
| 28_Campbell WW | A | Temperate | Animal-based | M | 11 | 33.5 | 93.0 | 32.6 | [16] |
| 28_Campbell WW | B | Temperate | Animal-based | F | 11 | 33.5 | 106.1 | 18.8 | [16] |
| 28_Campbell WW | C | Temperate | Animal-based | M | 8 | 72 | 104.1 | 14.7 | [16] |
| 28_Campbell WW | D | Temperate | Animal-based | F | 10 | 72 | 87.6 | 14.5 | [16] |
| 29_Atinmo T | A | Tropical | Mixed | M | 18 | 23 | 108.6 | 15.4 | [17] |
| R12_Vargas E | A | Temperate | Plant-based | M | 19 | 28 | 92.4 | 11.8 | [43] |
| R12_Vargas E | B | Temperate | Mixed | M | 20 | 26.5 | 80.2 | 10.0 | [43] |
| R45_Pasricha S | A | Tropical | Mixed | F | 3 | 26 | 107.5 | 6.0 | [41] |
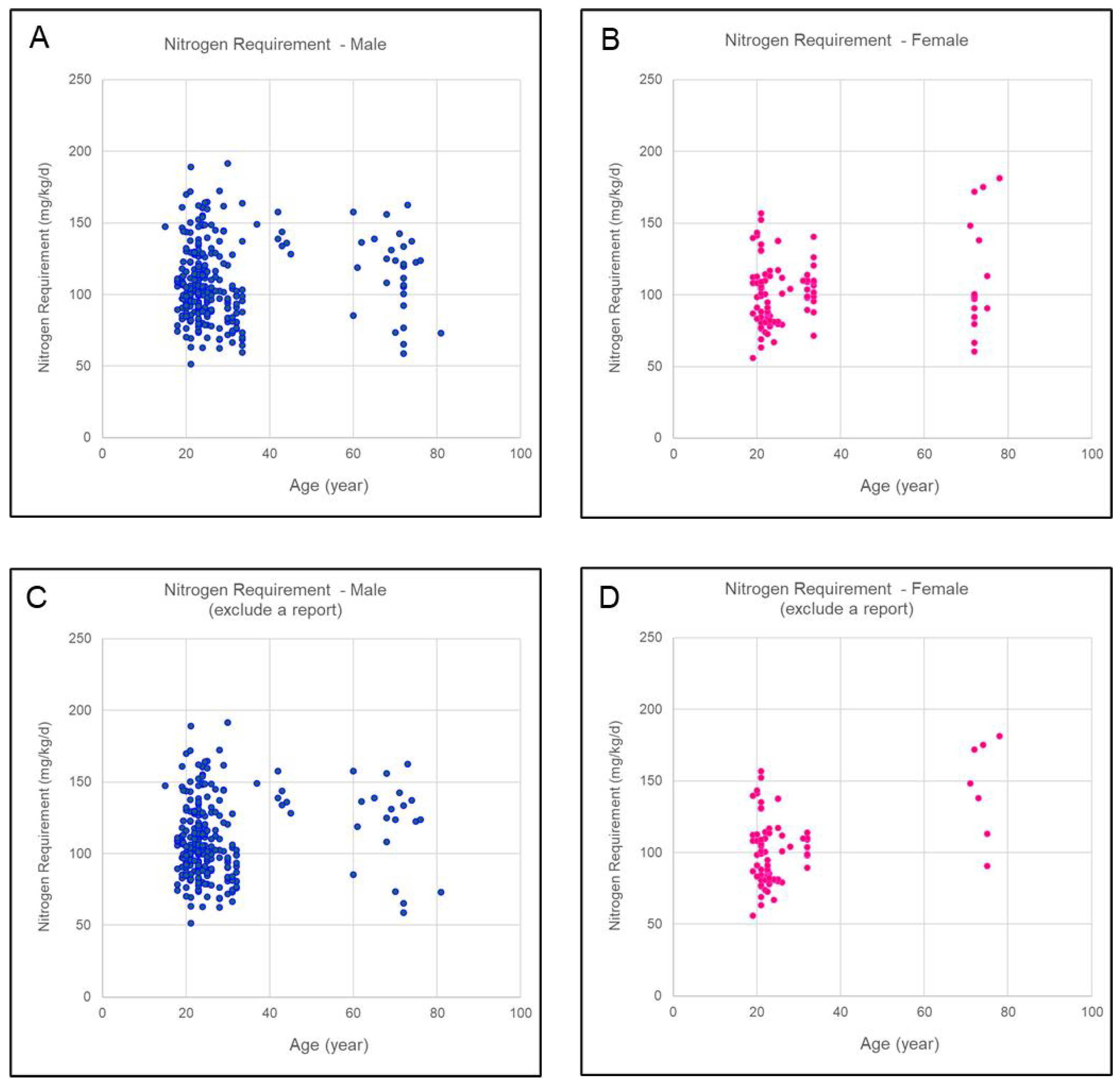
3.3. Nitrogen Requirement
3.4. Overview of Nitrogen Requirements
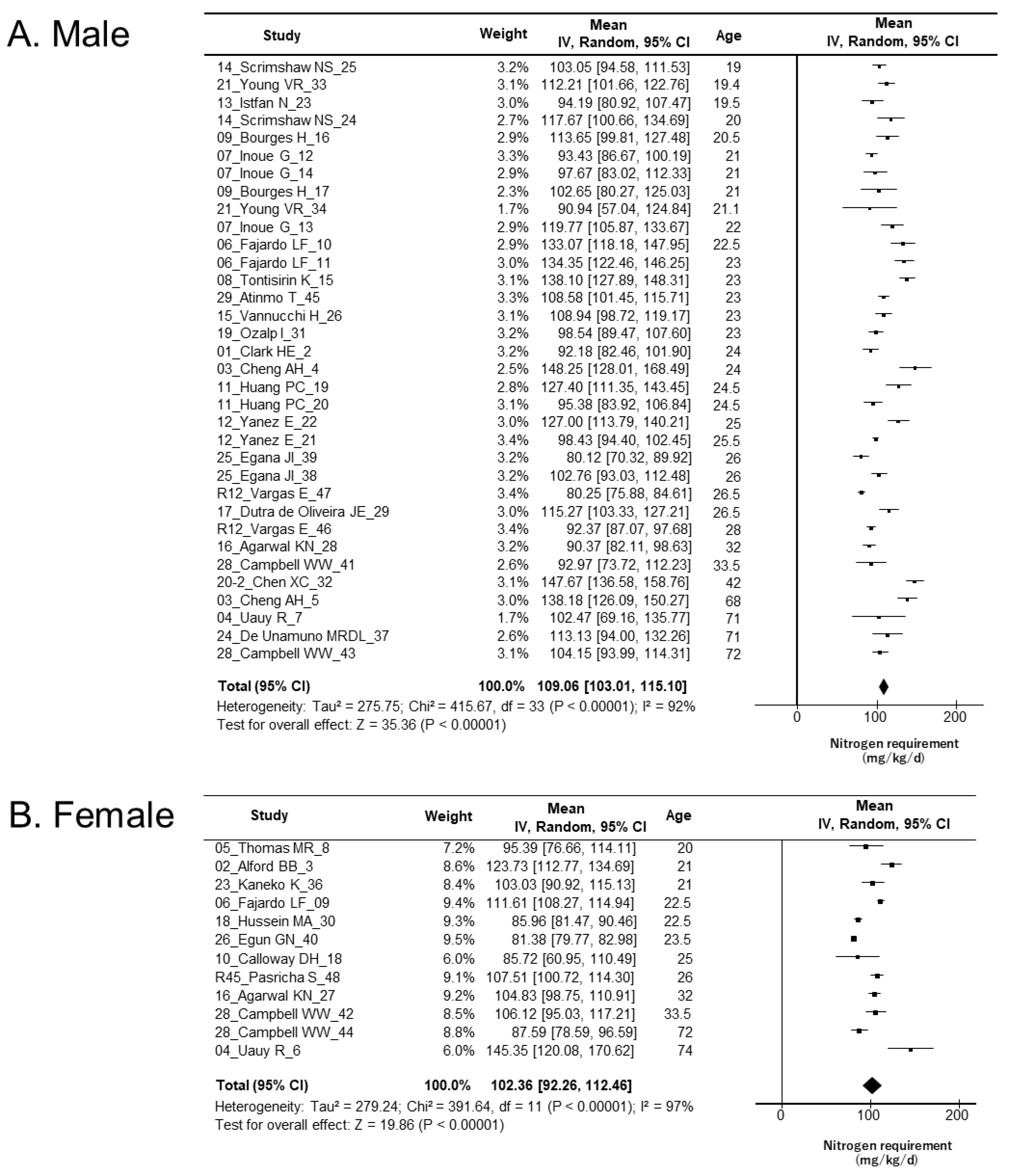
3.5. Meta-Analysis
4. Discussion
4.1. Nitrogen Requirements
4.2. Effects of Climate
4.3. Effects of Sex
4.4. Effects of Age
4.5. Effect of Protein Source
4.6. Effect of Experimental Protocol
4.7. Application of the Nitrogen Requirements Based on the Nitrogen Balance Method to Reference Values
4.8. Usefulness and Limitations of the Dataset
4.9. Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CONSORT | Consolidated Standards of Reporting Trials |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| GRADE | Grading Recommendations Assessment, Development, and Evaluation |
References
- Sia, M.; Akh, T. Bidiak IV PROTEIN REQUIREMENTS. REPORTS OF A JOINT FAO-WHO EXPERT GROUP. World Health Organ. Tech. Rep. Ser. 1965, 301, 1–71. [Google Scholar]
- Joint FAO/WHO Ad Hoc Expert Committee on Energy, and Protein Requirements. Energy and Protein Requirements: Report of a Joint FAO-WHO Ad Hoc Expert Committee. Rome, 22 March–2 April 1971. World Health Organ. Tech. Rep. Ser. 1973, 522, 1–118. [Google Scholar]
- Energy and Protein Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organ. Tech. Rep. Ser. 1985, 724, 1–206.
- Clugston, G.; Dewey, K.G.; Fjeld, C.; Millward, J.; Reeds, P.; Scrimshaw, N.S.; Tontisirin, K.; Waterlow, J.C.; Young, V.R. Report of the Working Group on Protein and Amino Acid Requirements. Eur. J. Clin. Nutr. 1996, 50 (Suppl. 1), S193–S195. [Google Scholar] [PubMed]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-Analysis of Nitrogen Balance Studies for Estimating Protein Requirements in Healthy Adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [CrossRef]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition. World Health Organ. Tech. Rep. Ser. 2007, 1–265. [Google Scholar]
- Martin, C.J.; Robison, R. The Minimum Nitrogen Expenditure of Man and the Biological Value of Various Proteins for Human Nutrition. Biochem. J. 1922, 16, 407–447. [Google Scholar] [CrossRef]
- Mimorandams, I.L. Protein and Energy Requirements: A Joint FAO/WHO Memorandum. Bull. World Health Organ. 1979, 57, 65–79. [Google Scholar]
- Millward, D.J. Methodological Considerations. Proc. Nutr. Soc. 2001, 60, 3–5. [Google Scholar] [CrossRef]
- Richter, M.; Baerlocher, K.; Bauer, J.M.; Elmadfa, I.; Heseker, H.; Leschik-Bonnet, E.; Stangl, G.; Volkert, D.; Stehle, P.; on behalf of the German Nutrition Society (DGE). Revised Reference Values for the Intake of Protein. Ann. Nutr. Metab. 2019, 74, 242–250. [Google Scholar] [CrossRef]
- Weiler, M.; Hertzler, S.R.; Dvoretskiy, S. Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake? Nutrients 2023, 15, 838. [Google Scholar] [CrossRef] [PubMed]
- Elango, R. Protein Requirements in Humans: A Need for Reassessment. J. Nutr. 2023, 153, 3355–3356. [Google Scholar] [CrossRef]
- Matsumoto, M.; Narumi-Hyakutake, A.; Kakutani, Y.; Tsuji, M.; Hatamoto, Y.; Higaki, Y.; Sasaki, S. Evaluation of Protein Requirements Using the Indicator Amino Acid Oxidation Method: A Scoping Review. J. Nutr. 2023, 153, 3472–3489. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, F.; Piao, J.H.; Yang, X.G. Protein Requirements in Healthy Adults: A Meta-Analysis of Nitrogen Balance Studies. Biomed. Environ. Sci. 2014, 27, 606–613. [Google Scholar] [CrossRef]
- Morse, M.H.; Haub, M.D.; Evans, W.J.; Campbell, W.W. Protein Requirement of Elderly Women: Nitrogen Balance Responses to Three Levels of Protein Intake. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M724–M730. [Google Scholar] [CrossRef]
- Campbell, W.W.; Johnson, C.A.; McCabe, G.P.; Carnell, N.S. Dietary Protein Requirements of Younger and Older Adults. Am. J. Clin. Nutr. 2008, 88, 1322–1329. [Google Scholar] [CrossRef]
- Atinmo, T.; Elemo, G.; Mbofung, C.M.F.; Oguntona, T.; Erukainure, O.L. Assessment of Protein Needs of Nigeria Adult Males Using Short-Term Nitrogen Balance Technique. Pak. J. Nutr. 2010, 9, 128–133. [Google Scholar] [CrossRef]
- Begg, C.; Cho, M.; Eastwood, S.; Horton, R.; Moher, D.; Olkin, I.; Pitkin, R.; Rennie, D.; Schulz, K.F.; Simel, D.; et al. Improving the Quality of Reporting of Randomized Controlled Trials. The CONSORT Statement. JAMA 1996, 276, 637–639. [Google Scholar] [CrossRef]
- Moher, D.; Cook, D.J.; Eastwood, S.; Olkin, I.; Rennie, D.; Stroup, D.F. Improving the Quality of Reports of Meta-Analyses of Randomised Controlled Trials: The QUOROM Statement. Quality of Reporting of Meta-Analyses. Lancet 1999, 354, 1896–1900. [Google Scholar] [CrossRef]
- Yin, T.; Yang, H.; Bai, J.; Huang, Z.; Chen, X. Protein Requirements of Chinese Male Adults. Acta Nutr. Sin. 1984, 6, 199–207. [Google Scholar]
- Chen, X.-C.; Yin, T.-A.; Bai, J.-G.; Yang, X.-J.; Huang, Z.-S. Protein Requirements of Chinese Male Adults on Ordinary Chinese Mixed Diet with Ordinary Energy Intake. Nutr. Res. 1985, 5, 227–238. [Google Scholar] [CrossRef]
- Campbell, W.W.; Trappe, T.A.; Wolfe, R.R.; Evans, W.J. The Recommended Dietary Allowance for Protein May Not Be Adequate for Older People to Maintain Skeletal Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M373–M380. [Google Scholar] [CrossRef]
- Gersovitz, M.; Motil, K.; Munro, H.N.; Scrimshaw, N.S.; Young, V.R. Human Protein Requirements: Assessment of the Adequacy of the Current Recommended Dietary Allowance for Dietary Protein in Elderly Men and Women. Am. J. Clin. Nutr. 1982, 35, 6–14. [Google Scholar] [CrossRef]
- Consolazio, C.F.; Nelson, R.A.; Matoush, L.O.; Harding, R.S.; Canham, J.E. The sweat excretion of nitrogen in relation to balance, environment and physical activity. Rep. US Army Med. Res. Nutr. Lab. Denver 1962, 1, 22. [Google Scholar]
- Drozdova, T.M. Nekotorye pokazateli belkovogo obmena u molodykh zdorovykh muzhchin v usloviiakh reguliarnogo normirovanogo pitaniia [Protein metabolic indices in young healthy men with regular standardized nutrition]. Vopr. Pitan. 1979, 2–5. [Google Scholar]
- Gonţea, I.; Suţescu, P.; Dumitrache, S.; Cocora, D.; Suma, S. Nevoia de proteine a omului în activitatea musculară. Influenţa cantităţii de azot ingerat anteefort asupra solicitării organismului [Protein requirements in man during muscular activity. Effect of the amount of nitrogen intake before exertion on stress of the organism]. Fiziol. Norm. Patol. 1965, 11, 133–142. [Google Scholar]
- Gontzea, I.; Sutzesco, P.; Dumitrache, S. Recherches concernant l’influence de l’activité musculaire sur le métabolisme azoté et sur le besoin en protéines de l’homme [Reasearch on the influence of muscular activity on nitrogen metabolism and on the protein requirement of man]. Ann. Nutr. Aliment. 1968, 22, 183–236. [Google Scholar] [PubMed]
- Gontzea, I.; Sutzesco, P.; Dumitrache, S. Influence of adaptation to effort on nitrogen balance in man. Arch. Sci. Physiol. 1962, 16, 127–138. [Google Scholar]
- Gontzea, I.; Sutzescou, P.; Dumitrache, S. Protein requirements of the man at work; influence of physical exertion on nitrogen balance. Arch. Sci. Physiol. 1959, 13, 99–108. [Google Scholar]
- Gontzea, J.; Sutescu, P.; Dimitrakis, S. Effect of high environmental temperature on human protein requirement (according to nitrogen balance) during muscular activity. Vopr. Pitan. 1960, 19, 12–17. [Google Scholar]
- Grigorov, I.G.; Solomko, G.I. Vliianie ratsionov pitaniia na balanc i usvoiaemost’ belkov u pozhilykh liudeĭ [Balance and assimilation of proteins in elderly people under various dietary protein rations]. Vrach. Delo. 1972, 3, 35–37. [Google Scholar]
- Hartig, W.; Czarnetzki, H.D.; Keitel, R. Die Beziehungen zwischen Eiweibbzufuhr und Stickstoffbilanz [Relationship between protein intake and nitrogen equilibrium]. Z. Exp. Chir. 1971, 4, 121–128. [Google Scholar]
- Iatsyshina, T.A.; Brents, M.; Mamaeva, E.M. Cliniko-éksperimental’noe izuchenie alimentarnogo statusa cheloveka pri razlichnykh urovniakh potrebleniia soevogo belka [Clinico-experimental study on the nutritional status of humans during different levels of consumption of soy bean protein]. Vopr. Pitan. 1982, 27–33. [Google Scholar]
- Iatsyshina, T.A.; Vysotskiĭ, V.G.; Safronova, A.M.; Eganian, R.A. Eksperimental’naia ostenka biologicheskoĭ éffektivnosti chastichno gidrolizovannykh belkov soi i kazeina [Experimental evaluation of the biological effectiveness of partially hydrolyzed soy and casein proteins]. Vopr. Pitan. 1983, 22–24. [Google Scholar]
- Lin, T.; Chen, M.L.; Chen, J.S. Observation on dietary protein utilization in vegetarians. Chin. J. Physiol. 1973, 21, 143–150. [Google Scholar]
- Soenke, M.L.; Horning, M.G.; Watson, E.H. Maintaining nitrogen balance with a partially hydrolyzed protein; three adults maintained by the oral administration of partially hydrolyzed lactalbumin. Am. Pract. Dig. Treat. 1947, 1, 489–492. [Google Scholar]
- Soenke, M.L.; Horning, M.G.; Watson, E.H. Maintaining nitrogen balance with amino acids; four adults maintained by acid hydrolyzed casein fortified with one per cent dl-tryptophane given orally. Am. Pract. Dig. Treat. 1947, 1, 276–282. [Google Scholar]
- Vankhanen, V.D.; Chistiakova, A.M. O potrebnosti v belke u shakhterov [Protein requirements in miners]. Vopr. Pitan. 1969, 28, 33–39. [Google Scholar] [PubMed]
- Vysotskiĭ, V.G.; Kochetkova, A.N.; Iatsyshina, T.A. Metodicheskie aspekty opredeleniia biologicheskoĭ tsennosti belka v issledovaniiakh s uchastiem cheloveka [Methodologic aspects of determining the biological value of protein in studies with human participation]. Vopr. Pitan. 1977, 3–9. [Google Scholar]
- Vysotskiĭ, V.G. K otsenke potrebnosti cheloveka v belke [Assessment of the human requirement for protein]. Vopr. Pitan. 1978, 8–17. [Google Scholar]
- Pasricha, S.; Rao, N.; Mohanram, K.; Gopalan, C. Nitrogen Balance Studies on Women in India. J. Am. Diet. Assoc. 1965, 47, 269–273. [Google Scholar] [CrossRef]
- Vargas, E.; Bressani, R.; Navarrete, D.; Braham, J.E.; Elías, L.G. Protein Digestibility and Energy in Diets Based on Rice and Beans in Human Adults. Arch. Latinoam. Nutr. 1984, 34, 109–129. [Google Scholar] [PubMed]
- Vargas, E.; Bressani, R.; Navarrete, D.A.; Braham, J.E.; Elías, L.G. Effect of Animal Protein Supplementation and Energy on the Protein Quality of Diets Based on Rice and Beans in Adult Males. Arch. Latinoam. Nutr. 1984, 34, 46–68. [Google Scholar]
- Fajardo, L.F.; Bolanos, O.; Acciarri, G.; Victoria, F.; Restrepo, J.; Ramirez, A.B.; Angel, L.M. Protein Requirements for Young Colombian Adults Consuming Local Diets Containing Primarily Animal or Vegetable Protein. In Protein-Energy Requirements of Developing Countries: Evaluations of New Data; Torún, B., Young, V.R., Rand, W.M., Eds.; United Nations University: Tokyo, Japan, 1981; pp. 54–62. [Google Scholar]
- Dutra de Oliveira, J.; Vannucchi, H. The Protein Requirements of Brazilian Rural Workers: Studies with a Rice and Bean Diet. In Protein-Energy-Requirement Studies in Developing Countries: Results of International Research; Rand, W.M., Uauy, R., Scrimshaw, N.S., Eds.; United Nations University: Tokyo, Japan, 1984; pp. 111–118. [Google Scholar]
- Clark, H.E.; Howe, J.M.; Magee, J.L.; Malzer, J.L. Nitrogen Balances of Adult Human Subjects Who Consumed Four Levels of Nitrogen from a Combination of Rice, Milk and Wheat. J. Nutr. 1972, 102, 1647–1654. [Google Scholar] [CrossRef]
- Uauy, R.; Scrimshaw, N.S.; Young, V.R. Human Protein Requirements: Nitrogen Balance Response to Graded Levels of Egg Protein in Elderly Men and Women. Am. J. Clin. Nutr. 1978, 31, 779–785. [Google Scholar] [CrossRef]
- Vannucchi, H.; Duarte, R.M.; Dutra de Oliveira, J.E. Studies on the Protein Requirement of Brazilian Rural Workers (“boias Frias”) given a Rice and Bean Diet. Int. J. Vitam. Nutr. Res. 1983, 53, 338–344. [Google Scholar]
- Atinmo, T.; Mbofung, C.M.F.; Egun, G.; Osotimehin, B. Nitrogen Balance Study in Young Nigerian Adult Males Using Four Levels of Protein Intake. Br. J. Nutr. 1988, 60, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Alford, B.B.; Onley, K. The Minimum Cottonseed Protein Required for Nitrogen Balance in Women. J. Nutr. 1978, 108, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.H.; Gomez, A.; Bergan, J.G.; Lee, T.C.; Monckeberg, F.; Chichester, C.O. Comparative Nitrogen Balance Study between Young and Aged Adults Using Three Levels of Protein Intake from a Combination Wheat-Soy-Milk Mixture. Am. J. Clin. Nutr. 1978, 31, 12–22. [Google Scholar] [CrossRef]
- Thomas, M.R.; Ashby, J.; Sneed, S.M.; O’Rear, L.M. Minimum Nitrogen Requirement from Glandless Cottonseed Protein for Nitrogen Balance in College Women. J. Nutr. 1979, 109, 397–405. [Google Scholar] [CrossRef]
- Inoue, G.; Takahashi, T.; Kishi, K.; Komatsu, T.; Niiyama, Y. The Evaluation of Soy Protein Isolate Alone and in Combination with Fish in Adult Japanese Men. In Protein-Energy Requirements of Developing Countries: Evaluations of New Data; Torún, B., Young, V.R., Rand, W.M., Eds.; United Nations University: Tokyo, Japan, 1981; pp. 77–87. [Google Scholar]
- Tontisirin, K.; Sirichakawal, P.P.; Valyasevi, A. Protein Requirements of Adult Thai Males. In Protein-Energy Requirements of Developing Countries: Evaluations of New Data; Torún, B., Young, V.R., Rand, W.M., Eds.; United Nations University: Tokyo, Japan, 1981; pp. 88–97. [Google Scholar]
- Bourges, H.; López-Castro, B.R. Protein Requirements of Young Adult Men Fed a Mexican Rural Diet. Arch. Latinoam. Nutr. 1982, 32, 630–649. [Google Scholar]
- Calloway, D.H.; Kurzer, M.S. Menstrual Cycle and Protein Requirements of Women. J. Nutr. 1982, 112, 356–366. [Google Scholar] [CrossRef]
- Huang, P.C.; Lin, C.P. Protein Requirements of Young Chinese Male Adults on Ordinary Chinese Mixed Diet and Egg Diet at Ordinary Levels of Energy Intake. J. Nutr. 1982, 112, 897–907. [Google Scholar] [CrossRef]
- Yáñez, E.; Uauy, R.; Ballester, D.; Barrera, G.; Chávez, N.; Guzmán, E.; Saitúa, M.T.; Zacarías, I. Capacity of the Chilean Mixed Diet to Meet the Protein and Energy Requirements of Young Adult Males. Br. J. Nutr. 1982, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Istfan, N.; Murray, E.; Janghorbani, M.; Young, V.R. An Evaluation of the Nutritional Value of a Soy Protein Concentrate in Young Adult Men Using the Short-Term N-Balance Method. J. Nutr. 1983, 113, 2516–2523. [Google Scholar] [CrossRef]
- Scrimshaw, N.S.; Wayler, A.H.; Murray, E.; Steinke, F.H.; Rand, W.M.; Young, V.R. Nitrogen Balance Response in Young Men given One of Two Isolated Soy Proteins or Milk Proteins. J. Nutr. 1983, 113, 2492–2497. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Bhatia, B.; Agarwal, D.; Shanker, R. Assessment of Protein Energy Needs of Indian Adults Using Short-Term Nitrogen Balance Methodology. In Protein-Energy-Requirement Studies in Developing Countries: Results of International Research; Rand, W.M., Uauy, R., Scrimshaw, N.S., Eds.; United Nations University: Tokyo, Japan, 1984; pp. 89–95. [Google Scholar]
- Hussein, M. Protein Requirements of Egyptian Women. In Protein-Energy-Requirement Studies in Developing Countries: Results of International Research; Rand, W.M., Uauy, R., Scrimshaw, N.S., Eds.; United Nations University: Tokyo, Japan, 1984; pp. 102–106. [Google Scholar]
- Ozalp, I.; Ozgüç, M.; Tokol, S.; Koksal, G.; Tasci, N.; Soysal, G. Nitrogen Balances of Young Turkish Adults on Graded Levels of Protein Intake. In Protein-Energy-Requirement Studies in Developing Countries: Results of International Research; Rand, W.M., Uauy, R., Scrimshaw, N.S., Eds.; United Nations University: Tokyo, Japan, 1984; pp. 107–110. [Google Scholar]
- Young, V.R.; Puig, M.; Queiroz, E.; Scrimshaw, N.S.; Rand, W.M. Evaluation of the Protein Quality of an Isolated Soy Protein in Young Men: Relative Nitrogen Requirements and Effect of Methionine Supplementation. Am. J. Clin. Nutr. 1984, 39, 16–24. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishikawa, K.; Setoguchi, K.; Koike, G. Utilization and Requirement of Dietary Protein Taking into Account the Dermal and Miscellaneous Nitrogen Losses in Japanese Women. J. Nutr. Sci. Vitaminol. 1988, 34, 459–467. [Google Scholar] [CrossRef] [PubMed]
- De Unamuno, M.R.D.L.; Dutra De Oliveira, J.E.; Vannucchi, H.; S6rgio Marchini, J. PROTEIN REQUIREMENT ASSESSMENT OF ELDERLY MEN ON A RICE AND BEANS DIET. Nutr. Res. 1991, 11, 149–157. [Google Scholar] [CrossRef]
- Egaña, J.I.; Uauy, R.; Cassorla, X.; Barrera, G.; Yañez, E. Sweet Lupin Protein Quality in Young Men. J. Nutr. 1992, 122, 2341–2347. [Google Scholar] [CrossRef]
- Egun, G.N.; Atinmo, T. Protein Requirement of Young Adult Nigerian Females on Habitual Nigerian Diet at the Usual Level of Energy Intake. Br. J. Nutr. 1993, 70, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Inayama, T.; Koike, G. Utilization of Soy Protein Isolate Mixed with Rice Protein in Japanese Women. J. Nutr. Sci. Vitaminol. 1985, 31, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.F.; Kishi, K.; Takahashi, T.; Komatsu, T.; Ohnaka, M.; Inoue, G. Efficiency of Utilization of Soy Protein Isolate in Japanese Young Men. J. Nutr. Sci. Vitaminol. 1983, 29, 201–216. [Google Scholar] [CrossRef]
- Rand, W.M.; Uauy, R.; Scrimshaw Nevin, S. (Eds.) Methodology. In Protein-Energy-Requirement Studies in Developing Countries: Results of International Research; The United Nations University: Tokyo, Japan, 1984. [Google Scholar]
- Scrimshaw, N.S.; Hussein, M.A.; Murray, E.; Rand, W.M.; Young, V.R. Protein Requirements of Man: Variations in Obligatory Urinary and Fecal Nitrogen Losses in Young Men. J. Nutr. 1972, 102, 1595–1604. [Google Scholar] [CrossRef]
- Rand, W.M.; Young, V.R.; Scrimshaw, N.S. Change of Urinary Nitrogen Excretion in Response to Low-Protein Diets in Adults. Am. J. Clin. Nutr. 1976, 29, 639–644. [Google Scholar] [CrossRef]
- Brooks, G.A.; Butte, N.F.; Rand, W.M.; Flatt, J.-P.; Caballero, B. Chronicle of the Institute of Medicine Physical Activity Recommendation: How a Physical Activity Recommendation Came to Be among Dietary Recommendations. Am. J. Clin. Nutr. 2004, 79, 921S–930S. [Google Scholar] [CrossRef]
- McMurray, R.G.; Soares, J.; Caspersen, C.J.; McCurdy, T. Examining Variations of Resting Metabolic Rate of Adults: A Public Health Perspective. Med. Sci. Sports Exerc. 2014, 46, 1352–1358. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare of Japan. Dietary Reference Intakes (DRIs) for Japanese (2025); Ministry of Health, Labour and Welfare of Japan: Tokyo, Japan, 2025. [Google Scholar]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Burke, L.M.; Castell, L.M.; Casa, D.J.; Close, G.L.; Costa, R.J.S.; Desbrow, B.; Halson, S.L.; Lis, D.M.; Melin, A.K.; Peeling, P.; et al. International Association of Athletics Federations Consensus Statement 2019: Nutrition for Athletics. Int. J. Sport. Nutr. Exerc. Metab. 2019, 29, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Hector, A.J.; Phillips, S.M. Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 170–177. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Metabolic Demands for Amino Acids and the Human Dietary Requirement: Millward and RRvers (1988) Revisited. J. Nutr. 1998, 128, 2563S–2576S. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J.; Forrester, T.; Ah-Sing, E.; Yeboah, N.; Gibson, N.; Badaloo, A.; Boyne, M.; Reade, M.; Persaud, C.; Jackson, A. The Transfer of 15N from Urea to Lysine in the Human Infant. Br. J. Nutr. 2000, 83, 505–512. [Google Scholar] [CrossRef] [PubMed]
| n | Nitrogen Requirement (mg/kg/Day) | |||||
|---|---|---|---|---|---|---|
| Mean 1 | 95% CI 1 | |||||
| Sex | Male | 285 | 105.2 | 102.2 | – | 108.2 |
| Female | 96 | 99.6 | 94.9 | – | 104.5 | |
| Unknown | 14 | 118.1 | 107.1 | – | 130.2 | |
| Climate | Temperate | 276 | 103.1 | 100.0 | – | 106.2 |
| Tropical | 119 | 106.9 | 102.8 | – | 111.1 | |
| Protein source | Animal | 116 | 101.4 | 96.4 | – | 106.6 |
| Plant | 130 | 106.7 | 103.1 | – | 110.5 | |
| Mixed | 149 | 104.3 | 100.2 | – | 108.5 | |
| Total | 395 | 104.2 | 101.7 | – | 106.7 | |
| Sex | Protein Source | Study No. | Nitrogen Requirement (mg N/kg/day) | 95% CI | I2 |
|---|---|---|---|---|---|
| Male | All | 34 | 109.1 | 103.0–115.1 | 92% |
| Animal-based | 12 | 103.3 | 94.3–112.4 | 88% | |
| Plant-based | 12 | 108.9 | 100.9–117.0 | 85% | |
| Mixed | 10 | 116.0 | 100.0–132.0 | 96% | |
| Female | All | 12 | 102.4 | 92.3–112.5 | 97% |
| Animal-based | 5 | 105.8 | 91.6–120.0 | 89% | |
| Plant-based | 3 | 109.0 | 94.2–123.8 | 81% | |
| Mixed | 4 | 93.7 | 82.4–105.0 | 95% |
| Sex | Climate | Study No. | Nitrogen Requirement (mg N/kg/Day) | 95% CI | I2 |
|---|---|---|---|---|---|
| Male | Temperate | 25 | 107.7 | 101.0–114.5 | 91% |
| Tropical | 9 | 112.5 | 99.3–125.7 | 93% | |
| Female | Temperate | 7 | 106.0 | 92.7–119.3 | 84% |
| Tropical | 5 | 98.2 | 83.4–113.0 | 99% |
| Sex | Nitrogen Requirement (mg N/kg/Day) | Estimate | SE | p |
|---|---|---|---|---|
| Male | Untransformed values | 0.1815 | 0.2161 | 0.407 |
| Log transformed values | 0.001394 | 0.001974 | 0.485 | |
| Female | Untransformed values | 0.2255 | 0.2867 | 0.450 |
| Log transformed values | 0.001559 | 0.00271 | 0.578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, D.; Hayamizu, K.; Uno, C.; Hasegawa, Y.; Kuwahata, M.; Kido, Y.; Suzuki, Y. Nitrogen Requirements in Healthy Adults: A Systematic Review and Meta-Analysis of Nitrogen Balance Studies. Nutrients 2025, 17, 2615. https://doi.org/10.3390/nu17162615
Suzuki D, Hayamizu K, Uno C, Hasegawa Y, Kuwahata M, Kido Y, Suzuki Y. Nitrogen Requirements in Healthy Adults: A Systematic Review and Meta-Analysis of Nitrogen Balance Studies. Nutrients. 2025; 17(16):2615. https://doi.org/10.3390/nu17162615
Chicago/Turabian StyleSuzuki, Daisuke, Kohsuke Hayamizu, Chiharu Uno, Yoko Hasegawa, Masashi Kuwahata, Yasuhiro Kido, and Yoshio Suzuki. 2025. "Nitrogen Requirements in Healthy Adults: A Systematic Review and Meta-Analysis of Nitrogen Balance Studies" Nutrients 17, no. 16: 2615. https://doi.org/10.3390/nu17162615
APA StyleSuzuki, D., Hayamizu, K., Uno, C., Hasegawa, Y., Kuwahata, M., Kido, Y., & Suzuki, Y. (2025). Nitrogen Requirements in Healthy Adults: A Systematic Review and Meta-Analysis of Nitrogen Balance Studies. Nutrients, 17(16), 2615. https://doi.org/10.3390/nu17162615