Early Gestational Diabetes Mellitus Diagnosis: A Strategy for Mitigating Excessive Maternal Weight Gain—LINDA-Brasil Study
Abstract
1. Introduction
2. Materials and Methods
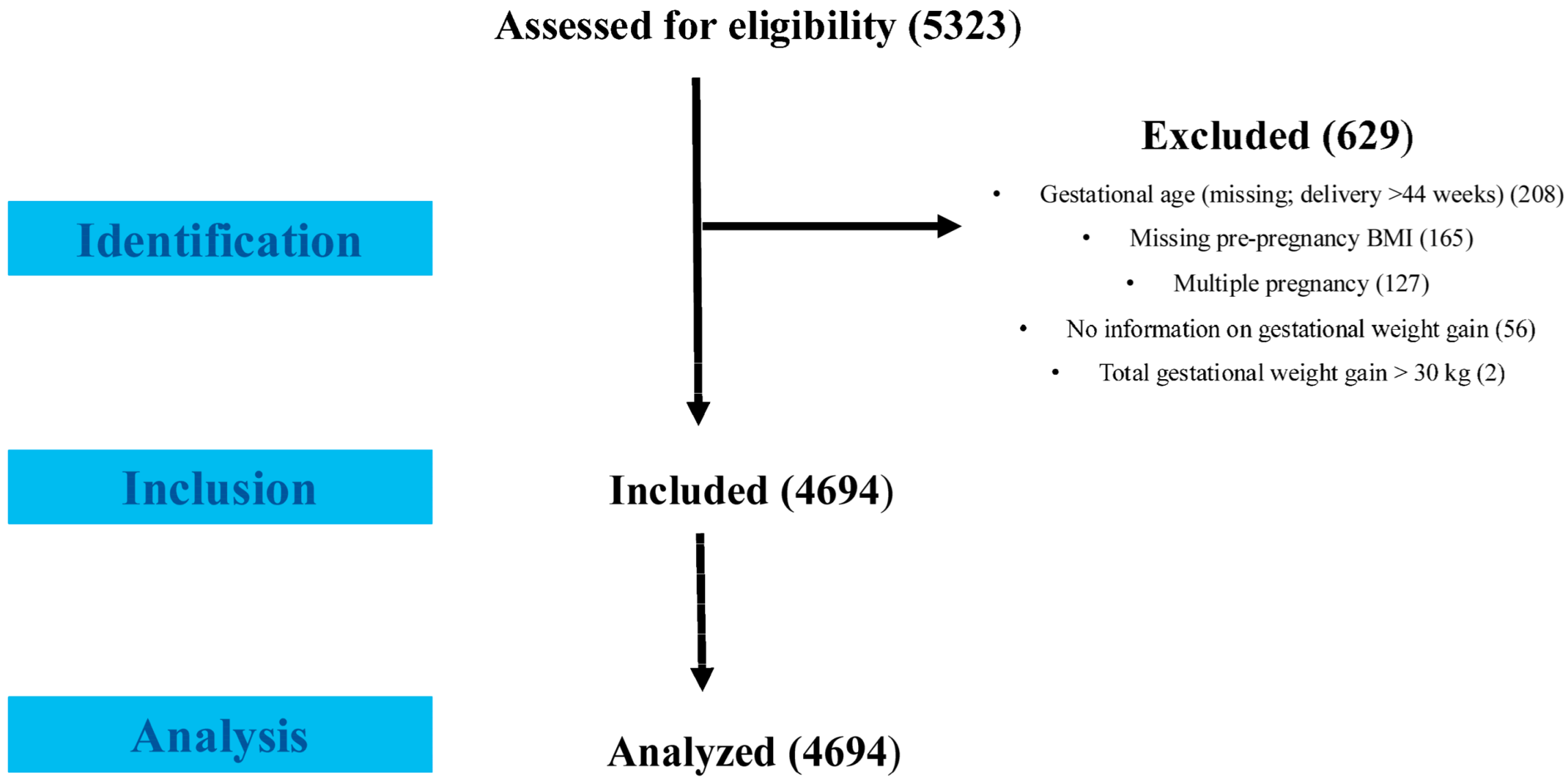
2.1. Study Population and Design
2.2. Data Collection
2.3. Pre-Pregnancy Body Mass Index (BMI)
2.4. Gestational Diabetes Mellitus Diagnostic Criteria
2.5. Gestational Age (GA)
2.6. Total Gestational Weight Gain (GWG) Calculation and Classification
2.7. Postpartum Follow-Up
2.8. Statistical Analysis
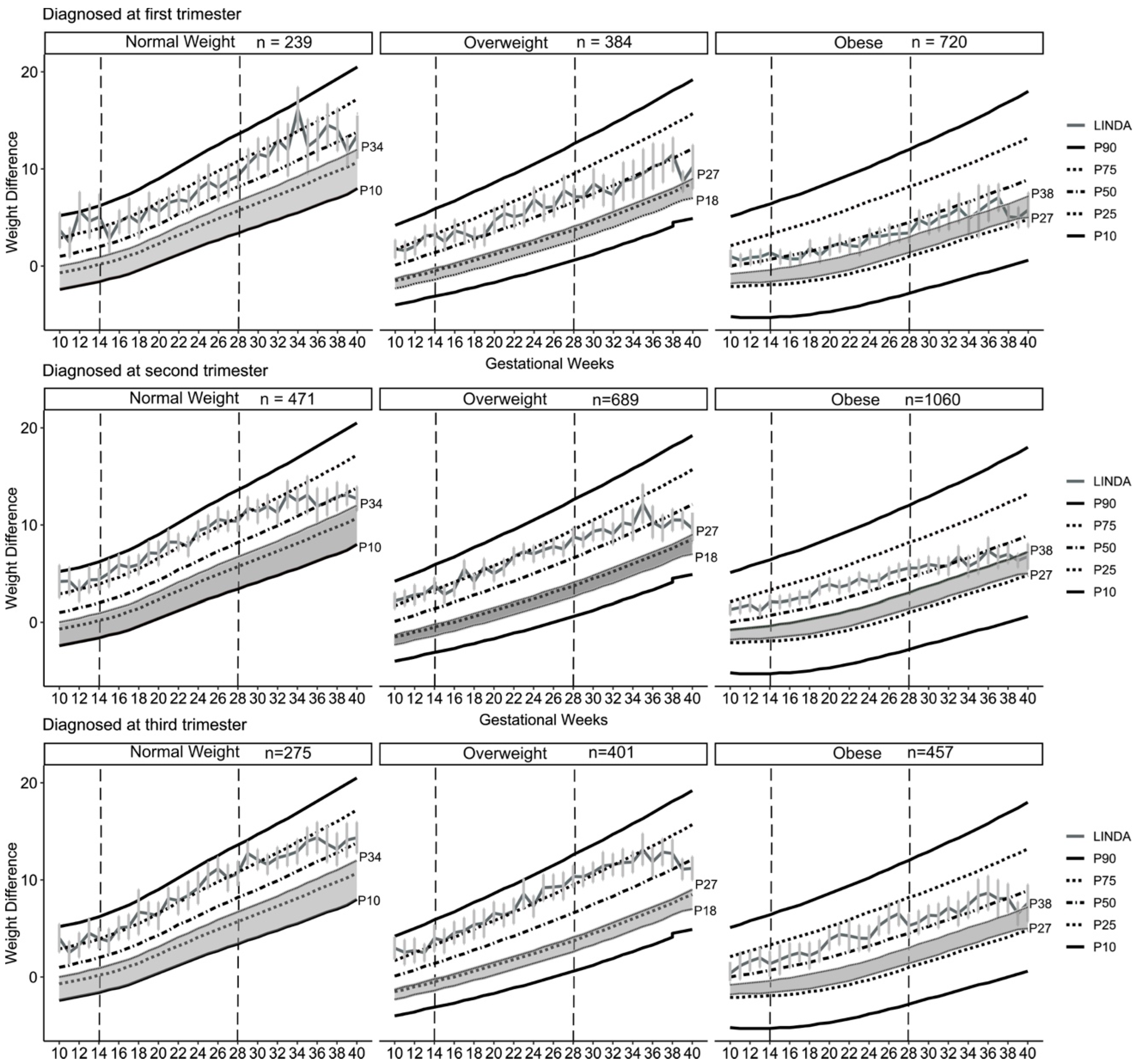
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF diabetes atlas: Estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Dennison, R.A.; Chen, E.S.; Green, M.E.; Legard, C.; Kotecha, D.; Farmer, G.; Sharp, S.J.; Ward, R.J.; Usher-Smith, J.A.; Griffin, S.J. The absolute and relative risk of type 2 diabetes after gestational diabetes: A systematic review and meta-analysis of 129 studies. Diabetes Res. Clin. Pract. 2021, 171, 108625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Stecher, L.; Ziebarth, S.; Nehring, I.; Rifas-Shiman, S.L.; Sommer, C.; Hauner, H.; von Kries, R. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: A meta-analysis. Diabetologia 2015, 58, 2229–2237. [Google Scholar] [CrossRef]
- Muswali, G.; Onyango, R.; Guyah, B.; Otieno, W. Association among Cases of Women with first degree Family History of Diabetes Mellitus, Previous Macrosomia and Preterm Births, and Pregnancy Weight Gain at Mama Lucy Kibaki Hospital, Nairobi City. East Afr. J. Health Sci. 2023, 6, 1–8. [Google Scholar] [CrossRef]
- Hivert, M.F.; Backman, H.; Benhalima, K.; Catalano, P.; Desoye, G.; Immanuel, J.; McKinlay, C.J.D.; Meek, C.L.; Nolan, C.J.; Ram, U.; et al. Pathophysiology from preconception, during pregnancy, and beyond. Lancet 2024, 404, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.; Bhavadharini, B.; Beks, H.; Deepa, M.; Anjana, R.M.; Uma, R.; Martin, E.; McNamara, K.; Versace, V.; Saravanan, P.; et al. Global burden of early pregnancy gestational diabetes mellitus (eGDM): A systematic review. Acta Diabetol. 2022, 59, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.; Immanuel, J.; Hague, W.M.; Teede, H.; Nolan, C.J.; Peek, M.J.; Flack, J.R.; McLean, M.; Wong, V.; Hibbert, E.; et al. Treatment of Gestational Diabetes Mellitus Diagnosed Early in Pregnancy. N. Engl. J. Med. 2023, 388, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Seifu, C.N.; Immanuel, J.; Hague, W.M.; Teede, H.; Cheung, N.W.; Hibbert, E.J.; Nolan, C.J.; Peek, M.J.; Wong, V.W.; Flack, J.R.; et al. Association Between Immediate Treatment of Early Gestational Diabetes Mellitus and Breastfeeding Outcomes: Findings from the TOBOGM Study. Diabetes Care 2024, 47, e99–e101. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Castilhos, C.; Wendland, E.M.; Hallal, P.C.; Schaan, B.D.; Drehmer, M.; Costa e Forti, A.; Façanha, C.; Nunes, M.A. Lifestyle INtervention for Diabetes prevention After pregnancy (LINDA-Brasil): Study protocol for a multicenter randomized controlled trial. BMC Pregnancy Childbirth 2016, 16, 68. [Google Scholar] [CrossRef]
- World Heath Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Carrilho, T.R.B.; Hutcheon, J.A.; Rasmussen, K.M.; Reichenheim, M.E.; Farias, D.R.; Freitas-Costa, N.C.; Kac, G.; Brazilian Maternal and Child Nutrition Consortium. Gestational weight gain according to the Brazilian charts and its association with maternal and infant adverse outcomes. Am. J. Clin. Nutr. 2023, 117, 414–425. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013; Available online: http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf (accessed on 7 July 2025).
- O’Sullivan, J.B. Establishing criteria for gestational diabetes. Diabetes Care 1980, 3, 437–439. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [Google Scholar] [CrossRef]
- Kac, G.; Carilho, T.R.B.; Rasmussen, K.M.; Reichenheim, M.E.; Farias, D.R.; Hutcheon, J.A.; Brazilian Maternal and Child Nutrition Consortium. Gestational weight gain charts: Results from the Brazilian Maternal and Child Nutrition Consortium. Am. J. Clin. Nutr. 2021, 113, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Guia para a Organização da Vigilância Alimentar e Nutricional na Atenção Primária à Saúde; Ministério da Saúde, Universidade Federal de Sergipe: Brasília, Brazil, 2022; 51p. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_organizacao_vigilancia_alimentar_nutricional.pdf (accessed on 7 July 2025).
- Carrilho, T.R.B.; Farias, D.R.; Batalha, M.A.; Costa, N.C.F.; Rasmussen, K.M.; Reichenheim, M.E.; Ohuma, E.O.; Hutcheon, J.A.; Kac, G.; Brazilian Maternal and Child Nutrition Consortium. Brazilian Maternal and Child Nutrition Consortium: Establishment, data harmonization and basic characteristics. Sci. Rep. 2020, 10, 14869. [Google Scholar] [CrossRef]
- Hillier, T.A.; Ogasawara, K.K.; Pedula, K.L.; Vesco, K.K.; Oshiro, C.E.S.; Van Marter, J.L. Timing of Gestational Diabetes Diagnosis by Maternal Obesity Status: Impact on Gestational Weight Gain in a Diverse Population. J. Women’s Health 2020, 29, 1068–1076. [Google Scholar] [CrossRef]
- Immanuel, J.; Cheung, N.W.; Mohajeri, M.; Simmons, D.J.; Hague, W.M.; Teede, H.; Nolan, C.J.; Peek, M.J.; Flack, J.R.; McLean, M.; et al. Association Between Glycemia, Glycemic Variability, and Pregnancy Complications in Early GDM. Diabetes Care 2025, 48, 285–291. [Google Scholar] [CrossRef]
- Galdikaitė, G.; Simanauskaitė, A.; Ramonienė, G.; Savukynė, E.; Malakauskienė, L.; Tarasevičienė, V. The Effect of Timing and Methods for the Diagnosis of Gestational Diabetes Mellitus on Obstetric Complications. Medicina 2023, 59, 854. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Zajdenverg, L.; Façanha, C.; Dualib, P.; Goldbert, A.; Negrato, C.; Bertoluci, M. Planejamento, metas e monitorização do diabetes durante a gestação. In Diretriz Oficial da Sociedade Brasileira de Diabetes; Conectando Pessoas: Brasília, Brazil, 2023; ISBN 978-85-5722-906-8. [Google Scholar] [CrossRef]
- Simmons, D.; Gupta, Y.; Hernandez, T.L.; Levitt, N.; van Poppel, M.; Yang, X.; Zarowsky, C.; Backman, H.; Feghali, M.; Nielsen, K.K. Call to action for a life course approach. Lancet 2024, 404, 193–214. [Google Scholar] [CrossRef]
- Hinkle, S.N.; Li, M.; Grewal, J.; Yisahak, S.F.; Grobman, W.A.; Newman, R.B.; Wing, D.A.; Grantz, K.L.; Zhang, C. Changes in Diet and Exercise in Pregnant Women after Diagnosis with Gestational Diabetes: Findings from a Longitudinal Prospective Cohort Study. J. Acad. Nutr. Diet. 2021, 121, 2419–2428.e4. [Google Scholar] [CrossRef] [PubMed]
- García-Patterson, A.; Balsells, M.; Solà, I.; Corcoy, R. Detection and treatment of early gestational diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, W.; Liu, C.; Yan, Q.; Zhang, L.; Tian, Z.; Yuan, X.; Li, G. Weight gain after diagnosis of gestational diabetes mellitus and its association with adverse pregnancy outcomes: A cohort study. BMC Pregnancy Childbirth 2021, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Castrejon, M.; Powell, T.L. Placental Nutrient Transport in Gestational Diabetic Pregnancies. Front. Endocrinol. 2017, 8, 306, Erratum in Front. Endocrinol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Carrilho, T.R.B.; Rasmussen, K.M.; Rodrigues Farias, D.; Freitas Costa, N.C.; Araújo Batalha, M.; EReichenheim, M.; OOhuma, E.; Hutcheon, J.A.; Kac, G.; Brazilian Maternal and Child Nutrition Consortium. Agreement between self-reported pre-pregnancy weight and measured first-trimester weight in Brazilian women. BMC Pregnancy Childbirth 2020, 20, 734. [Google Scholar] [CrossRef]
| Sample Characteristics | Total Sample n = 4694 | Timing of Gestational Diabetes Diagnosis | ||
|---|---|---|---|---|
| 1st Trimester n = 1342 | 2nd Trimester n = 2220 | 3rd Trimester n = 1132 | ||
| Brazilian Region/Center, n (%) | ||||
| Northeast | 1120 (23.9%) | 175 (13.0%) | 575 (25.9%) | 370 (32.7%) |
| Southeast | 539 (11.5%) | 178 (13.3%) | 199 (9.0%) | 162 (14.3%) |
| South | 3035 (64.6%) | 989 (73.7%) | 1446 (65.1%) | 600 (53.0%) |
| Age (years), mean ± SD | 31.7 ± 6.3 | 31.1 ± 6.3 | 32.0 ± 6.2 | 31.7 ± 6.4 |
| 18 to 29, n (%) | 1679 (36.2) | 518 (39.1) | 748 (34.1) | 413 (37.2) |
| 30 to 39, n (%) | 2430 (52.5) | 686 (51.8) | 1178 (53.7) | 566 (50.9) |
| ≥40 n (%) | 522 (11.3) | 121 (9.1) | 269 (12.2) | 132 (11.9) |
| Skin color, n (%) | ||||
| White | 2167 (46.2) | 689 (51.3) | 1010 (45.5) | 468 (41.3) |
| Non-White | 2527 (53.8) | 653 (48.7) | 1210 (54.5) | 664 (58.7) |
| Education (years), mean ± SD | ||||
| <8, n (%) | 1365 (29.1) | 389 (29.0) | 656 (29.5) | 320 (28.3) |
| 8 to 12, n (%) | 2556 (54.4) | 725 (54.0) | 1218 (54.9) | 613 (54.1) |
| ≥13, n (%) | 773 (16.5) | 228 (17.0) | 346 (15.6) | 199 (17.6) |
| Parity | ||||
| 0, n (%) | 1478 (31.5) | 444 (33.1) | 666 (30.0) | 368 (32.5) |
| 1 to 2, n (%) | 2535 (54.0) | 716 (53.3) | 1212 (54.6) | 607 (53.6) |
| ≥3, n (%) | 680 (14.5) | 182 (13.6) | 341 (15.4) | 157 (13.9) |
| Pre-pregnancy BMI (kg/m2), mean ± SD | 30.4 ± 6.5 | 31.2 ± 6.8 | 30.3 ± 6.5 | 29.4 ± 6.2 |
| <25, n (%) | 984 (21.0) | 239 (17.8) | 471 (21.2) | 274 (24.2) |
| 25 to 30, n (%) | 1474 (31.4) | 384 (28.6) | 689 (31.0) | 401 (35.4) |
| ≥30, n (%) | 2236 (47.6) | 719 (53.6) | 1060 (47.8) | 457 (40.4) |
| Smoking before pregnancy | ||||
| No, n (%) | 3685 (78.5) | 1033 (77.0) | 1763 (79.5) | 889 (78.5) |
| Yes, n (%) | 1007 (21.5) | 308 (23.0) | 456 (20.5) | 243 (21.5) |
| GA at GDM diagnosis (weeks), mean ± SD | 19.9 ± 8.2 | 8.9 ± 2.5 | 21.7 ± 3.7 | 29.3 ± 2.3 |
| Insulin use during pregnancy, n (%) | ||||
| No | 2766 (58.9) | 705 (52.5) | 1270 (57.2) | 791 (69.9) |
| Yes | 1928 (41.1) | 637 (47.5) | 950 (42.8) | 341 (30.1) |
| Total gestational weight gain (kg) mean ± SD | 9.1 ± 7.7 | 8.0 ± 7.8 | 9.0 ± 7.5 | 10.5 ± 7.5 |
| Gestational age at delivery (weeks) mean ± SD | 38.3 ± 2.1 | 38.2 ± 1.9 | 38.2 ± 2.2 | 38.5 ± 1.9 |
| Mode of delivery | ||||
| Vaginal, n (%) | 1654 (37.1) | 484 (38.0) | 770 (36.7) | 400 (37.0) |
| Cesarean, n (%) | 2799 (62.9) | 790 (62.0) | 1329 (63.3) | 680 (63.0) |
| Birth weight (g) mean ± SD | 3295.2 ± 712.9 | 3297.5 ± 1010.8 | 3275.8 ± 566.9 | 3330.4 ± 508.5 |
| Preterm birth (GA < 37 weeks), n (%) | 504 (10.7) | 141 (10.5) | 258 (11.7) | 105 (9.3) |
| Extreme preterm birth (<34 weeks), n (%) | 94 (2.0) | 28 (2.1) | 58 (2.6) | 8 (0.7) |
| Low birth weight (<2500 g), n (%) | 301 (6.9) | 85 (6.7) | 162 (7.8) | 54 (5.1) |
| Macrosomia (>4000 g), n (%) | 331 (7.6) | 86 (6.83) | 155 (7.5) | 90 (8.5) |
| GDM Diagnosis | n (%) | Total Gestational Weight Gain (kg) |
|---|---|---|
| Adjusted Mean * (95% CI) | ||
| Total Sample | 4597 (100) | |
| Diagnosis in the 1st trimester | 1315 (28.6%) | 8.37 (7.88 to 8.87) a |
| Diagnosis in the 2nd trimester | 2178 (47.4%) | 9.43 (9.00 to 9.86) b |
| Diagnosis in the 3rd trimester | 1104 (24.0%) | 10.66 (10.16 to 11.17) c |
| Diagnosis before 20 weeks | 1925 (41.9%) | 8.56 (8.12 to 8.99) a |
| Diagnosis after 20 weeks | 2672 (58.1%) | 10.14 (9.73 to 10.55) b |
| Diagnosis before 24 weeks | 2563 (55.8%) | 8.81 (8.40 to 9.22) a |
| Diagnosis after 24 weeks | 2034 (44.2%) | 10.25 (9.82 to 10.68) b |
| n (%) | Total Gestational Weight Gain Adjusted Model * | |
|---|---|---|
| β 95% CI | ||
| Total Sample | 4597 (100) | |
| Diagnosis in the 1st trimester | 1315 (28.6%) | −2.29 (−2.87 to −1.71) |
| Diagnosis in the 2nd trimester | 2178 (47.4%) | −1.23 (−1.75 to −0.72) |
| Diagnosis in the 3rd trimester | 1104 (24.0%) | ref |
| Diagnosis before 20 weeks | 1925 (41.9%) | −1.58 (−2.01 to −1.16) |
| Diagnosis after 20 weeks | 2672 (58.1%) | ref |
| Diagnosis before 24 weeks | 2563 (55.8%) | −1.44 (−1.86 to −1.02) |
| Diagnosis after 24 weeks | 2034 (44.2%) | ref |
| n (%) | Excessive Gestational Weight Gain Adjusted Model * | |
|---|---|---|
| OR 95% CI | ||
| Total Sample | 3942 (100) | |
| Diagnosis in the 1st trimester | 1178 (29.9%) | 0.78 (0.72 to 0.86) |
| Diagnosis in the 2nd trimester | 1868 (47.4%) | 0.90 (0.83 to 0.97) |
| Diagnosis in the 3rd trimester | 896 (22.7%) | ref |
| Diagnosis before 20 weeks | 1705 (43.3%) | 0.82 (0.77 to 0.88) |
| Diagnosis after 20 weeks | 2237 (56.7%) | ref |
| Diagnosis before 24 weeks | 2246 (57.0%) | 0.81 (0.76 to 0.87) |
| Diagnosis after 24 weeks | 1696 (43.0%) | ref |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silveira, L.R.P.; Schmidt, M.I.; Bracco, P.; Mattiello, R.; Drehmer, M. Early Gestational Diabetes Mellitus Diagnosis: A Strategy for Mitigating Excessive Maternal Weight Gain—LINDA-Brasil Study. Nutrients 2025, 17, 2600. https://doi.org/10.3390/nu17162600
da Silveira LRP, Schmidt MI, Bracco P, Mattiello R, Drehmer M. Early Gestational Diabetes Mellitus Diagnosis: A Strategy for Mitigating Excessive Maternal Weight Gain—LINDA-Brasil Study. Nutrients. 2025; 17(16):2600. https://doi.org/10.3390/nu17162600
Chicago/Turabian Styleda Silveira, Letícia Ribeiro Pavão, Maria Inês Schmidt, Paula Bracco, Rita Mattiello, and Michele Drehmer. 2025. "Early Gestational Diabetes Mellitus Diagnosis: A Strategy for Mitigating Excessive Maternal Weight Gain—LINDA-Brasil Study" Nutrients 17, no. 16: 2600. https://doi.org/10.3390/nu17162600
APA Styleda Silveira, L. R. P., Schmidt, M. I., Bracco, P., Mattiello, R., & Drehmer, M. (2025). Early Gestational Diabetes Mellitus Diagnosis: A Strategy for Mitigating Excessive Maternal Weight Gain—LINDA-Brasil Study. Nutrients, 17(16), 2600. https://doi.org/10.3390/nu17162600





