Oral Contraceptives Induce Time- and Intestinal Segment-Dependent Shifts in the Gut Microbiota
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
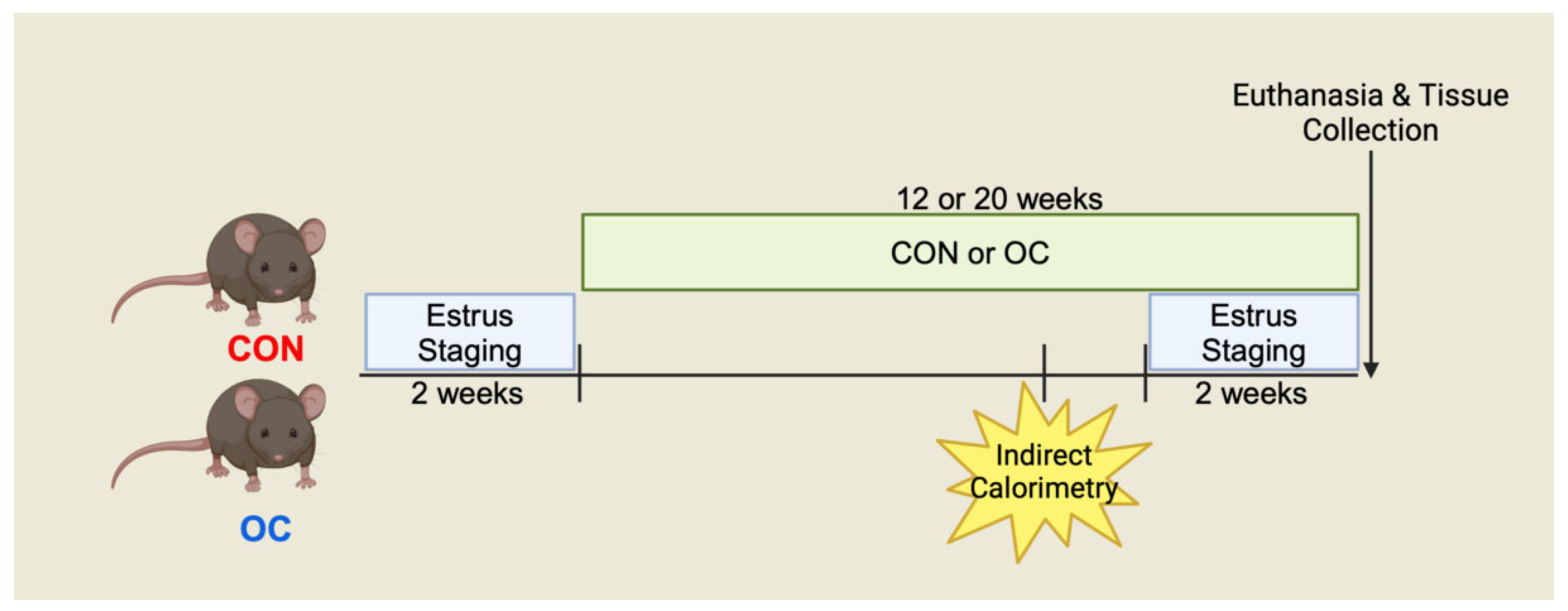
2.2. Experimental Design
2.3. Estrous Cycle Staging
2.4. mRNA Gene Expression
2.5. Cecal Short and Branched-Chain Fatty Acids
2.6. Intestinal Estradiol
2.7. Genomic DNA Extraction
2.8. 16S rRNA Sequencing and Analysis
2.9. Statistical Analysis
3. Results
3.1. Oral Contraceptive Usage Impacted Metabolic State in Mice
3.2. Oral Contraceptive Usage Increased Concentrations of Estradiol in the Colon
3.3. Oral Contraceptive Usage Differentially Impacted Cecal Microbial Short- and Branched-Chain Fatty Acids
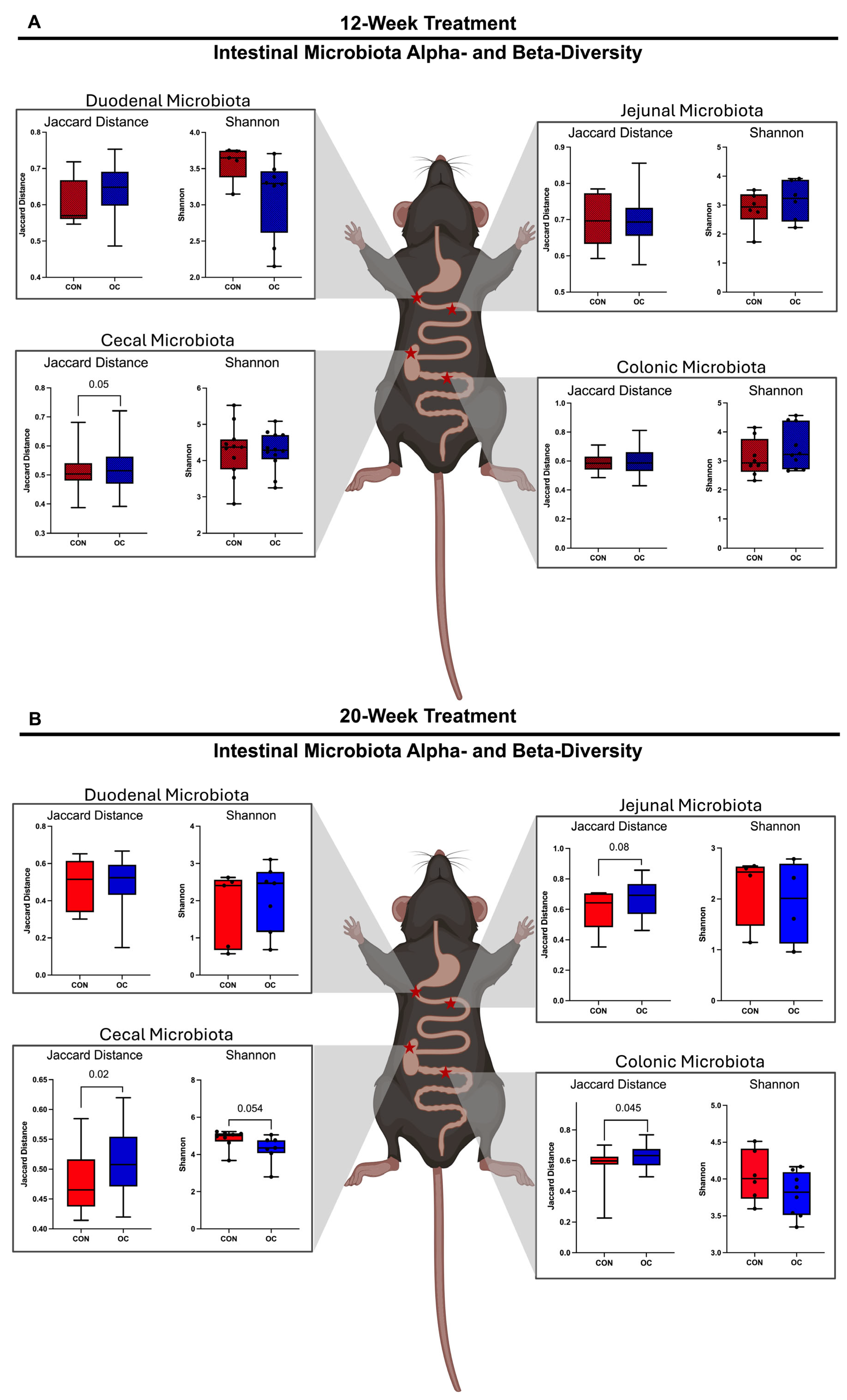
3.4. Oral Contraceptive Usage Altered Intestinal Microbiota Alpha- and Beta-Diversity in a Site-Specific Manner
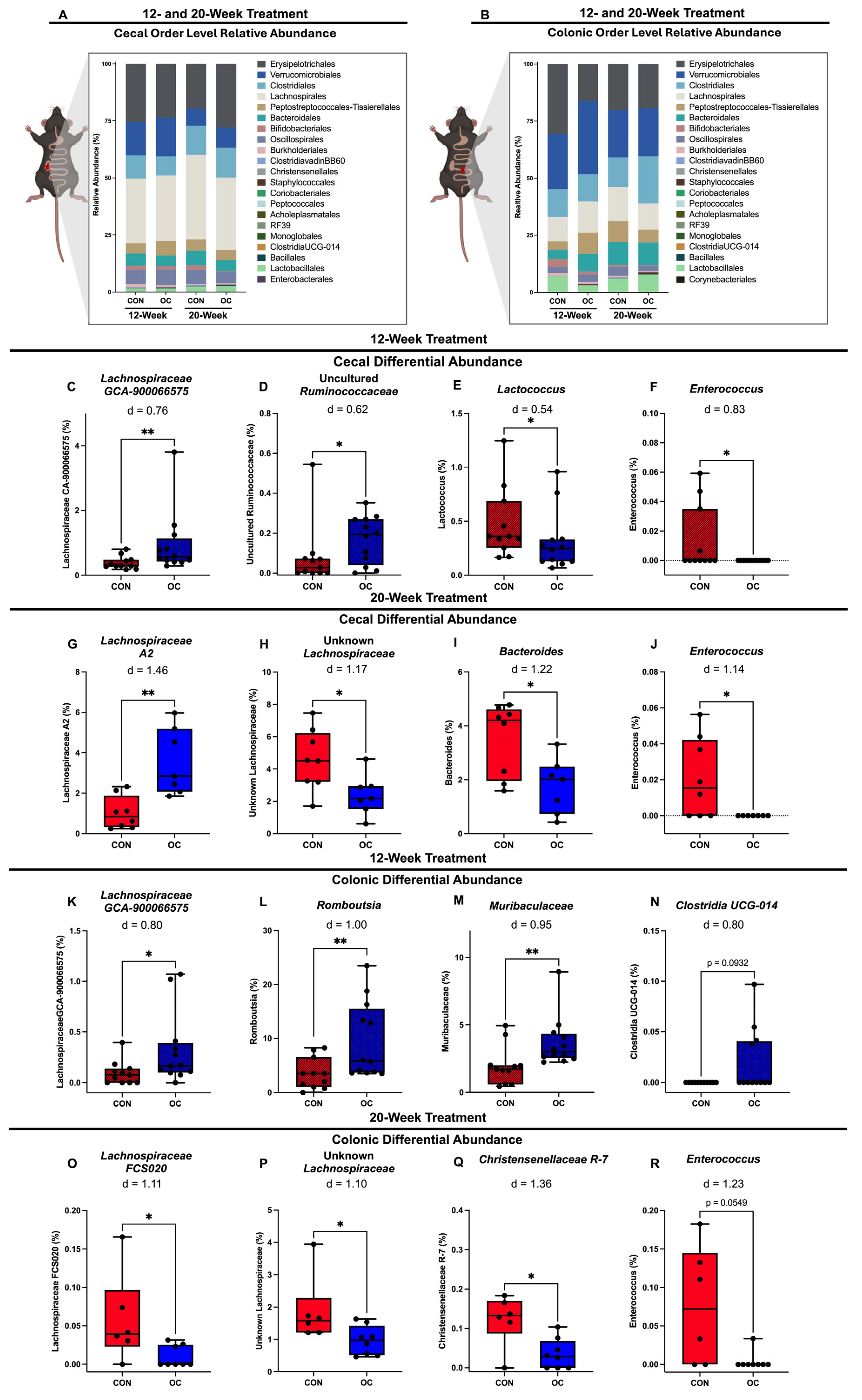
3.5. Oral Contracteptive Usage Significantly Altered Proportions of Taxa in Cecal and Colonic Microbiota
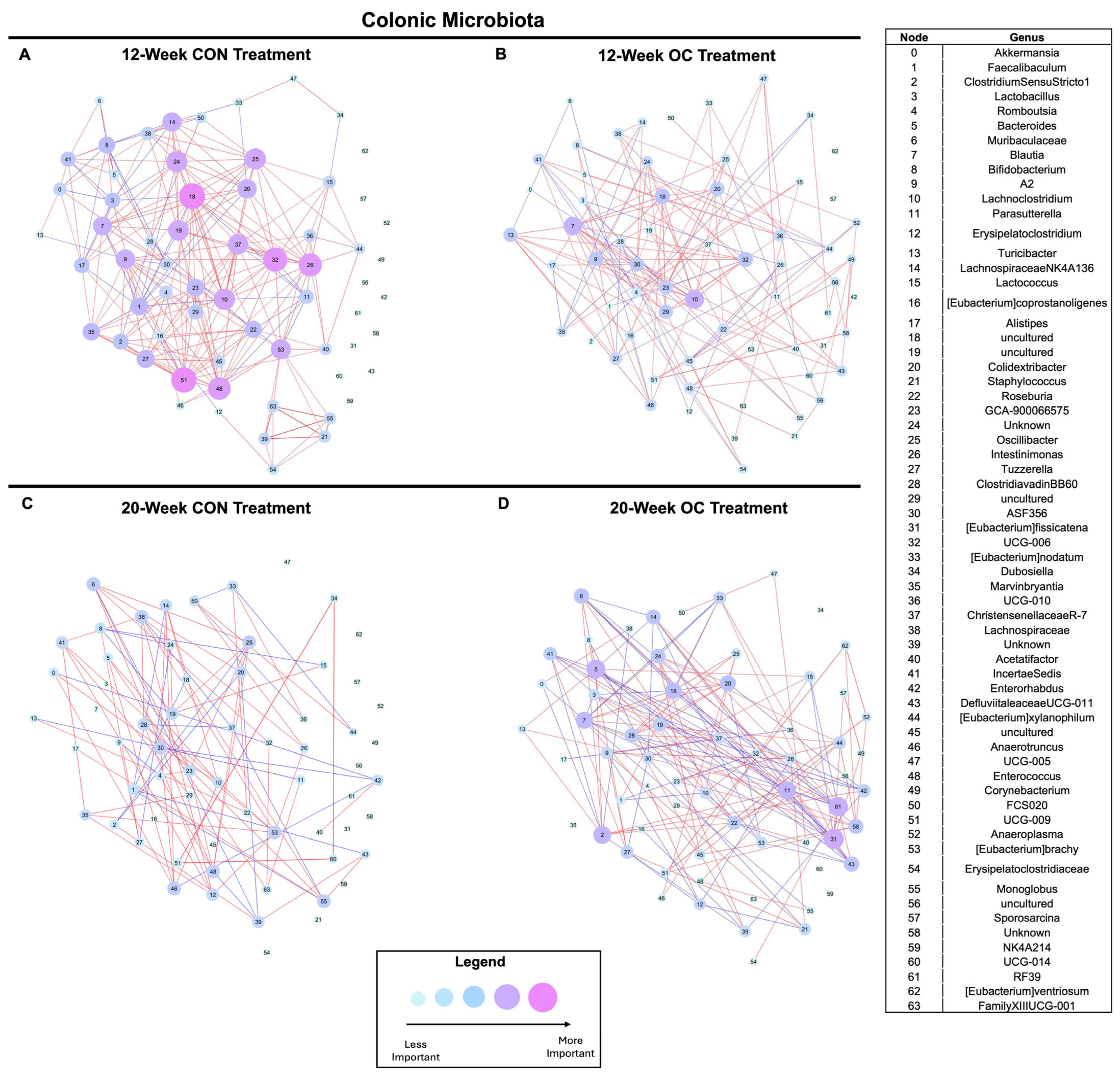
3.6. Oral Contraceptive Usage Impacts Cecal and Colonic Microbiota Community Dynamics in a Time-Dependent Manner
4. Colonic Estradiol Levels Are Associated with Local Bacterial Taxa Relevant to Health
4.1. Cecal and Colonic Microbial Taxa Are Associated with Physical Activity and Metabolism
4.2. Cecal and Colonic Microbial Taxa Are Associated with Hepatic Markers of Oxidative Stress and Liver Steatosis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansour, S.R.; Moustafa, M.A.A.; Saad, B.M.; Hamed, R.; Moustafa, A.-R.A. Impact of diet on human gut microbiome and disease risk. New Microbes New Infect. 2021, 41, 100845. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lü, M. The Gut Microbiome and Sex Hormone-Related Diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Muranaka, Y.; Fujimura, R.; Ishida, H.; Tazume, S.; Shimamura, T. Normalization of Reproductive Function in Germfree Mice Following Bacterial Contamination. Exp. Anim. 1998, 47, 151–158. [Google Scholar] [CrossRef]
- Hsiao, T.-H.; Chou, C.-H.; Chen, Y.-L.; Wang, P.-H.; Brandon-Mong, G.-J.; Lee, T.-H.; Wu, T.-Y.; Li, P.-T.; Li, C.-W.; Lai, Y.-L.; et al. Circulating androgen regulation by androgen-catabolizing gut bacteria in male mouse gut. Gut Microbes 2023, 15, 2183685. [Google Scholar] [CrossRef]
- Ropero, A.B.; Eghbali, M.; Minosyan, T.Y.; Tang, G.; Toro, L.; Stefani, E. Heart estrogen receptor alpha: Distinct membrane and nuclear distribution patterns and regulation by estrogen. J. Mol. Cell. Cardiol. 2006, 41, 496–510. [Google Scholar] [CrossRef]
- Babiker, F.A.; De Windt, L.J.; Van Eickels, M.; Thijssen, V.; Bronsaer, R.J.P.; Grohé, C.; Van Bilsen, M.; Doevendans, P.A. 17β-Estradiol Antagonizes Cardiomyocyte Hypertrophy by Autocrine/Paracrine Stimulation of a Guanylyl Cyclase A Receptor-Cyclic Guanosine Monophosphate-Dependent Protein Kinase Pathway. Circulation 2004, 109, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, A.; Kratz, M.; Bolego, C. Emerging role of estrogen in the control of cardiometabolic disease. Trends Pharmacol. Sci. 2010, 31, 183–189. [Google Scholar] [CrossRef]
- Khader, Y.S.; Rice, J.; John, L.; Abueita, O. Oral contraceptives use and the risk of myocardial infarction: A meta-analysis. Contraception 2003, 68, 11–17. [Google Scholar] [CrossRef]
- Lidegaard, O.; Nielsen, L.H.; Skovlund, C.W.; Skjeldestad, F.E.; Lokkegaard, E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study. BMJ 2011, 343, d6423. [Google Scholar] [CrossRef] [PubMed]
- van Hylckama Vlieg, A.; Helmerhorst, F.M.; Vandenbroucke, J.P.; Doggen, C.J.M.; Rosendaal, F.R. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: Results of the MEGA case-control study. BMJ 2009, 339, b2921. [Google Scholar] [CrossRef]
- de Oliveira, C.A.R.; Araujo, T.D.R.; de Souza Aguiar, G.; da Silva Junior, J.A.; Vettorazzi, J.; Freitas, I.N.; Oliveira, K.; Boschero, A.C.; Bonfleur, M.L.; Clarke, J.R.; et al. Combined oral contraceptive in female mice causes hyperinsulinemia due to β-cell hypersecretion and reduction in insulin clearance. J. Steroid Biochem. Mol. Biol. 2019, 190, 54–63. [Google Scholar] [CrossRef]
- Fuller, K.; Mccoin, C.S.; Stierwalt, H.; Thyfault, J.P. 213-LB: Combined Oral Contraceptives Drive Inactivity and Hepatic Mitochondrial Oxidative Stress in Female Mice. Diabetes 2022, 71, 213-LB. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Caputi, V.; Bastiaanssen, T.F.S.; Peterson, V.; Sajjad, J.; Murphy, A.; Stanton, C.; McNamara, B.; Shorten, G.D.; Cryan, J.F.; O’Mahony, S.M. Sex, pain, and the microbiome: The relationship between baseline gut microbiota composition, gender and somatic pain in healthy individuals. Brain Behav. Immun. 2022, 104, 191–204. [Google Scholar] [CrossRef]
- Hua, X.; Cao, Y.; Morgan, D.M.; Miller, K.; Chin, S.M.; Bellavance, D.; Khalili, H. Longitudinal analysis of the impact of oral contraceptive use on the gut microbiome. J. Med. Microbiol. 2022, 71. Available online: https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.001512 (accessed on 20 January 2025). [CrossRef]
- Kimberly, D.; Abma, J.C. Current Contraceptive Status Among Women Aged 15–49: United States, 2017–2019. Available online: https://www.cdc.gov/nchs/data/databriefs/db388-H.pdf (accessed on 28 July 2025).
- Maguire, K.; Westhoff, C. The state of hormonal contraception today: Established and emerging noncontraceptive health benefits. Am. J. Obstet. Gynecol. 2011, 205, S4–S8. [Google Scholar] [CrossRef]
- Wong, C.L.; Farquhar, C.; Roberts, H. Oral contraceptive pills for primary dysmenorrhoea. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2001; p. CD002120. Available online: https://doi.wiley.com/10.1002/14651858.CD002120 (accessed on 6 August 2024).
- Cross, T.-W.L.; Simpson, A.M.R.; Lin, C.-Y.; Hottmann, N.M.; Bhatt, A.P.; Pellock, S.J.; Nelson, E.R.; Loman, B.R.; Wallig, M.A.; Vivas, E.I.; et al. Gut microbiome responds to alteration in female sex hormone status and exacerbates metabolic dysfunction. Gut Microbes 2024, 16, 2295429. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.N.Z.; Thyfault, J.P. Barriers in translating preclinical rodent exercise metabolism findings to human health. J. Appl. Physiol. 2021, 130, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Mosher, W.D.; Daniels, K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl. Health Stat. Rep. 2012, 60, 1–25. [Google Scholar]
- Fuller, K.N.Z.; McCoin, C.S.; Stierwalt, H.; Allen, J.; Gandhi, S.; Perry, C.G.R.; Jambal, P.; Shankar, K.; Thyfault, J.P. Oral combined contraceptives induce liver mitochondrial reactive oxygen species and whole-body metabolic adaptations in female mice. J. Physiol. 2022, 600, 5215–5245. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, T.; Fu, W.; Kennett, M.; Cox, A.D.; Lee, D.; Vanamala, J.K.P.; Reddivari, L. Role of Gut Microbiota in the Anti-Colitic Effects of Anthocyanin-Containing Potatoes. Mol. Nutr. Food Res. 2021, 65, 2100152. [Google Scholar] [CrossRef] [PubMed]
- Orczyk, G.P.; Behrman, H.R. Ovulation blockade by aspirin or indomethacin—In vivo evidence for a role of prostaglandin in gonadotrophin secretion. Prostaglandins 1972, 1, 3–20. [Google Scholar] [CrossRef]
- Baltes, M.R.H.; Dubois, J.G.; Hanocq, M. Ethyl acetate extraction procedure and isocratic high-performance liquid chromatographic assay for testosterone metabolites in cell microsomes. J. Chromatogr. B Biomed. Sci. Appl. 1998, 706, 201–207. [Google Scholar] [CrossRef]
- Kreznar, J.H.; Keller, M.P.; Traeger, L.L.; Rabaglia, M.E.; Schueler, K.L.; Stapleton, D.S.; Zhao, W.; Vivas, E.I.; Yandell, B.S.; Broman, A.T.; et al. Host Genotype and Gut Microbiome Modulate Insulin Secretion and Diet-Induced Metabolic Phenotypes. Cell Rep. 2017, 18, 1739–1750. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- van Rossum, G. Python Tutorial; CWI: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Ofosu-Boateng, M.; Shaik, F.; Choi, S.; Ekuban, F.A.; Gebreyesus, L.H.; Twum, E.; Nnamani, D.O.; Yeyeodu, S.T.; Yadak, N.; Collier, D.M.; et al. High-fat diet induced obesity promotes inflammation, oxidative stress, and hepatotoxicity in female styleFVB/N mice. BioFactors 2024, 50, 572–591. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, X.; Xu, L.; Yan, N.; Sun, Z.; Duan, X.; Meng, L.; Qi, R.; Ren, F.; Wang, Z. Sodium butyrate attenuates oxidative stress, apoptosis, and excessive mitophagy in sodium fluoride-induced hepatotoxicity in rats. Ecotoxicol. Environ. Saf. 2025, 291, 117821. [Google Scholar] [CrossRef]
- Gart, E.; van Duyvenvoorde, W.; Toet, K.; Caspers, M.P.M.; Verschuren, L.; Nielsen, M.J.; Leeming, D.J.; Souto Lima, E.; Menke, A.; Hanemaaijer, R.; et al. Butyrate Protects against Diet-Induced NASH and Liver Fibrosis and Suppresses Specific Non-Canonical TGF-β Signaling Pathways in Human Hepatic Stellate Cells. Biomedicines 2021, 9, 1954. [Google Scholar] [CrossRef] [PubMed]
- Luerce, T.D.; Gomes-Santos, A.C.; Rocha, C.S.; Moreira, T.G.; Cruz, D.N.; Lemos, L.; Sousa, A.L.; Pereira, V.B.; De Azevedo, M.; Moraes, K.; et al. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog. 2014, 6, 33. [Google Scholar] [CrossRef]
- Shin, J.-H.; Park, Y.-H.; Sim, M.; Kim, S.-A.; Joung, H.; Shin, D.-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef]
- Barrat, A.; Barthélemy, M.; Pastor-Satorras, R.; Vespignani, A. The architecture of complex weighted networks. Proc. Natl. Acad. Sci. USA 2004, 101, 3747–3752. [Google Scholar] [CrossRef]
- Yang, C.-J.; Chang, H.-C.; Sung, P.-C.; Ge, M.-C.; Tang, H.-Y.; Cheng, M.-L.; Cheng, H.-T.; Chou, H.-H.; Lin, C.-Y.; Lin, W.-R.; et al. Oral fecal transplantation enriches Lachnospiraceae and butyrate to mitigate acute liver injury. Cell Rep. 2024, 43, 113591. [Google Scholar] [CrossRef]
- Wang, M.; Ma, W.; Wang, C.; Li, D. Lactococcus G423 improve growth performance and lipid metabolism of broilers through modulating the gut microbiota and metabolites. Front. Microbiol. 2024, 15, 1381756. [Google Scholar] [CrossRef]
- Yoon, S.J.; Yu, J.S.; Min, B.H.; Gupta, H.; Won, S.-M.; Park, H.J.; Han, S.H.; Kim, B.-Y.; Kim, K.H.; Kim, B.K.; et al. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front. Microbiol. 2023, 14, 1129904. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Khattab, R.A.; Ragab, Y.M.; Hassan, M. Safety assessment of Enterococcus lactis strains complemented with comparative genomics analysis reveals probiotic and safety characteristics of the entire species. BMC Genom. 2023, 24, 667. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Hlivak, P.; Odraska, J.; Ferencik, M.; Ebringer, L.; Jahnova, E.; Mikes, Z. One-year application of probiotic strain Enterococcus faecium M-74 decreases serum cholesterol levels. Bratisl. Lek. Listy 2005, 106, 67–72. [Google Scholar]
- Agerbaek, M.; Gerdes, L.U.; Richelsen, B. Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur. J. Clin. Nutr. 1995, 49, 346–352. [Google Scholar] [PubMed]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Al-kassab-Córdova, A.; Cabrera-Guzmán, J.C.; Herrera-Añazco, P.; Benites-Zapata, V.A. Vitamin B12, folate, and homocysteine in metabolic syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1221259. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gu, Q.; Wang, Y.; Yu, Y.; Yang, L.; Chen, J.V. Novel vitamin B12-producing Enterococcus spp. and preliminary in vitro evaluation of probiotic potentials. Appl. Microbiol. Biotechnol. 2017, 101, 6155–6164. [Google Scholar] [CrossRef] [PubMed]
- Fabia, R.; Ar’rajab, A.; Johansson, M.L.; Willén, R.; Andersson, R.; Molin, G.; Bengmark, S. The Effect of Exogenous Administration of Lactobacillus reuteri R2LC and Oat Fiber on Acetic Acid-Induced Colitis in the Rat. Scand. J. Gastroenterol. 1993, 28, 155–162. [Google Scholar] [CrossRef]
- Mao, Y.; Nobaek, S.; Kasravi, B.; Adawi, D.; Stenram, U.; Molin, G.; Jeppsson, B. The effects of Lactobacillus strains and oat fiber on methotrexate- induced enterocolitis in rats. Gastroenterology 1996, 111, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Venegas, C.S.; Martínez-Hernández, S.L.; Cervantes-García, D.; Montes de Oca-Luna, R.; de Jesús Loera-Arias, M.; Mata-Martínez, M.G.; Ventura-Juárez, J.; Muñoz-Ortega, M.H. Modulating effects of the probiotic Lactococcus lactis on the hepatic fibrotic process induced by CCl4 in Wistar rats. Exp. Ther. Med. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-F.; Wang, N.M.; Chu, Y.-W.; Wu, C.-S.; Lin, W.-W. The Anti-Inflammatory Effect of Lactococcus lactis-Ling-Zhi 8 on Ameliorating Atherosclerosis and Nonalcoholic Fatty Liver in High-Fat Diet Rabbits. Int. J. Mol. Sci. 2024, 25, 11278. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.; Olsson, R. Liver damage from low-dose oral contraceptives. J. Intern. Med. 1993, 234, 287–292. [Google Scholar] [CrossRef]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; De Los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related with Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Frankowska, N.; Lisowska, K.; Witkowski, J.M. Proteolysis dysfunction in the process of aging and age-related diseases. Front. Aging 2022, 3, 927630. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.; Wang, H.; Mei, J.; Wei, X.; Qin, X.; Lin, Q.; Huang, Z.; Tang, W.; Luo, T. Isobutyric acid promotes colorectal cancer metastasis through activating RACK1. Cancer Sci. 2023, 114, 3900–3913. [Google Scholar] [CrossRef]
- Whitehead, M.I.; Townsend, P.T.; Gill, D.K.; Collins, W.P.; Campbell, S. Absorption and metabolism of oral progesterone. BMJ 1980, 280, 825–827. [Google Scholar] [CrossRef]
- Edelman, A.B.; Cherala, G.; Stanczyk, F.Z. Metabolism and pharmacokinetics of contraceptive steroids in obese women: A review. Contraception 2010, 82, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Orme, M.L.; Back, D.J. Factors affecting the enterohepatic circulation of oral contraceptive steroids. Am. J. Obstet. Gynecol. 1990, 163, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Kisiela, M.; Skarka, A.; Ebert, B.; Maser, E. Hydroxysteroid dehydrogenases (HSDs) in bacteria—A bioinformatic perspective. J. Steroid Biochem. Mol. Biol. 2012, 129, 31–46. [Google Scholar] [CrossRef]
- Eriksson, H.; Gustafsson, J.-A. Excretion of Steroid Hormones in Adults. Steroids in Faeces from Adults. Eur. J. Biochem. 1971, 18, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.-Å.; Sjövall, J. Steroids in Germfree and Conventional Rats. Eur. J. Biochem. 1968, 6, 227–235. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.M.P.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium scindens: A human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef]
- Lombardi, P.; Goldin, B.; Boutin, E.; Gorbach, S.L. Metabolism of androgens and estrogens by human fecal microorganisms. J. Steroid Biochem. 1978, 9, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Roach, R.E.J.; Helmerhorst, F.M.; Lijfering, W.M.; Stijnen, T.; Algra, A.; Dekkers, O.M. Combined oral contraceptives: The risk of myocardial infarction and ischemic stroke. Cochrane Database Syst. Rev. 2015, CD011054. Available online: http://doi.wiley.com/10.1002/14651858.CD011054.pub2 (accessed on 17 April 2025). [CrossRef] [PubMed]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Cat | CTCCATCAGGTTTCTTTCTTG | CAACAGGCAAGTTTTTGATG |
| Col1a1 | AGCACGTCTGGTTTGGAGAG | ACATTAGGCGCAGGAAGGTC |
| Ppargc1a | TCACCATATTCCAGGTCAAG | TCATAGGCTTCATAGCTGTC |
| Ppib | TGGAGATGAATCTGTAGGAC | CAAATCCTTTCTCTCCTGTAG |
| Sod2 | CCATTTTCTGGACAAACCTG | GACCTTGCTCCTTATTGAAG |
| Esr1 | CAAGGTAAATGTGTGGAAGG | GTGTACACTCCGGAATTAAG |
| Gpx1 | GGAGAATGGCAAGAATGAAG | TTCGCACTTCTCAAACAATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clapp Organski, A.; Reddivari, A.; Reddivari, L.; Brubaker, D.K.; Fuller, K.N.Z.; Thyfault, J.P.; Cross, T.-W.L. Oral Contraceptives Induce Time- and Intestinal Segment-Dependent Shifts in the Gut Microbiota. Nutrients 2025, 17, 2591. https://doi.org/10.3390/nu17162591
Clapp Organski A, Reddivari A, Reddivari L, Brubaker DK, Fuller KNZ, Thyfault JP, Cross T-WL. Oral Contraceptives Induce Time- and Intestinal Segment-Dependent Shifts in the Gut Microbiota. Nutrients. 2025; 17(16):2591. https://doi.org/10.3390/nu17162591
Chicago/Turabian StyleClapp Organski, Anna, Anjali Reddivari, Lavanya Reddivari, Douglas K. Brubaker, Kelly N. Z. Fuller, John P. Thyfault, and Tzu-Wen L. Cross. 2025. "Oral Contraceptives Induce Time- and Intestinal Segment-Dependent Shifts in the Gut Microbiota" Nutrients 17, no. 16: 2591. https://doi.org/10.3390/nu17162591
APA StyleClapp Organski, A., Reddivari, A., Reddivari, L., Brubaker, D. K., Fuller, K. N. Z., Thyfault, J. P., & Cross, T.-W. L. (2025). Oral Contraceptives Induce Time- and Intestinal Segment-Dependent Shifts in the Gut Microbiota. Nutrients, 17(16), 2591. https://doi.org/10.3390/nu17162591






