Balancing the Cellular Inflammatory-Homeostatic Axis Through Natural Ingredient Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation and Preparation of the Multi-Ingredient Supplementation Used in This Study
- The dietary supplement object of this study is composed by coenzyme Q10 (CoQ10), spermidine, D-ribose, resveratrol, phosphatidylserine, griffonia dry extract [Griffonia simplicifolia (Vahl ex DC) Baill.] seed, bacopa dry extract [Bacopa monnieri (L.) Wettst.] grass, vitamins B1, B2, B3, B5, B6, B9, B12, C, E, selenium, and zinc. In Table S1 there is the quantity for each ingredient relative to the recommended dose.
- For cell experiments we dissolved the daily recommended dose in the minimum necessary volume (30 mL) of distilled water for the hydrophilic component and of DMSO for the lipophilic one. Before each experiment, the dietary supplement was prepared by adding two parts of the hydrophilic components to one part of the lipophilic one (to reproduce the correct proportions present in the capsules) and then we performed the dilutions.
2.2. Cells Culture
2.3. Measurement of Cell Viability
2.4. Cell Motility Assay (Boyden Chamber Assay)
2.5. Western Blot Analyses
2.6. RNA Isolation and Quantitative Real-Time PCR (RT-qPCR)
2.7. Measurement of Cytokines Release
2.8. Pyroptosis Assay
2.9. Adhesion Assay
2.10. Measurement of Cellular Oxidative Stress
2.11. Chemical Analysis
2.11.1. Chemicals
2.11.2. Sample Preparation
2.11.3. HPLC-PDA-MS/MS Analysis
2.11.4. GC-MS Analysis
2.12. Data Analysis
3. Results
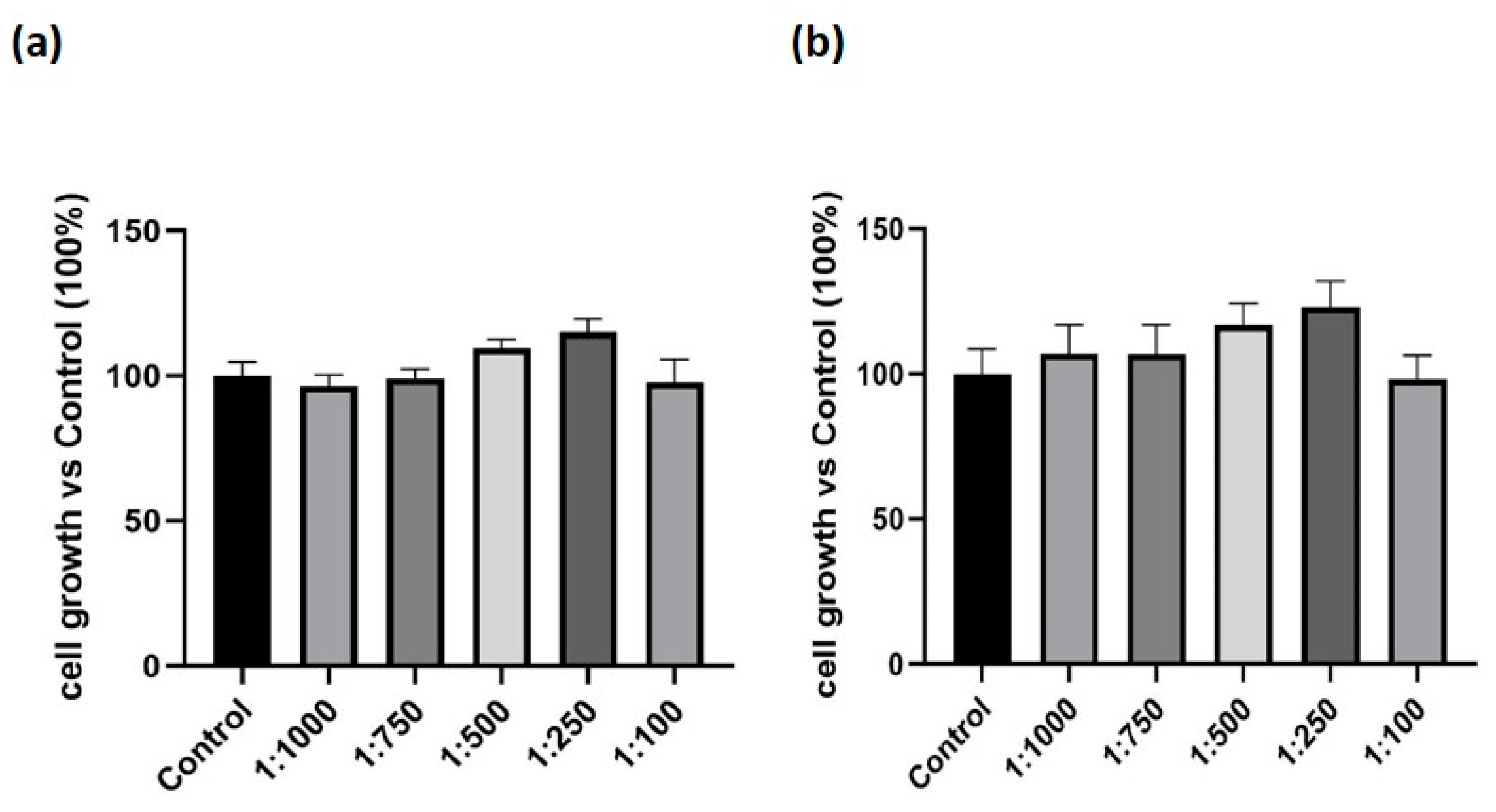
3.1. Effect of the Dietary Supplement on Cell Growth in Macrophage-like Cells
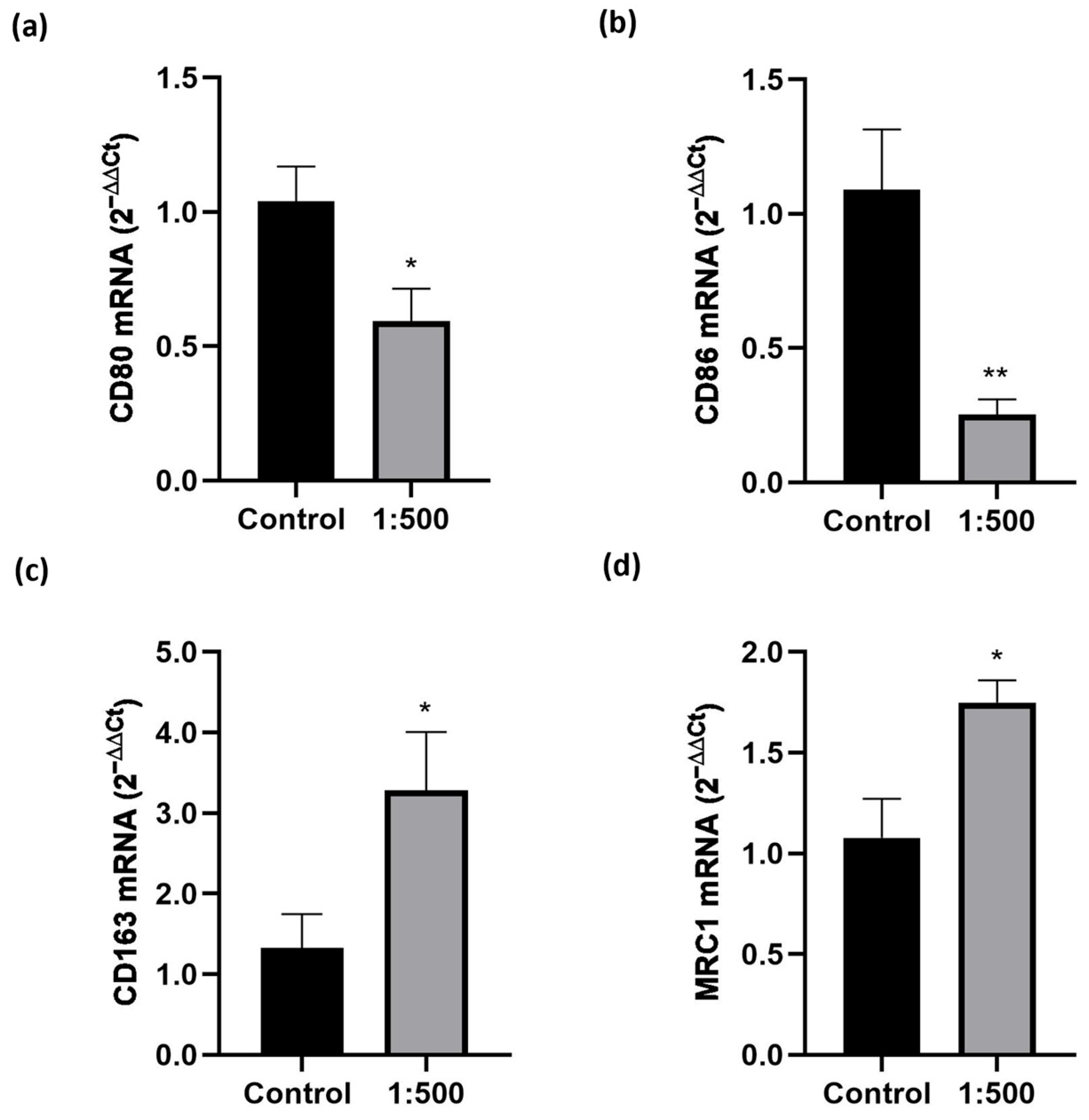
3.2. Effect of the Dietary Supplement on Macrophage Migration and Polarization
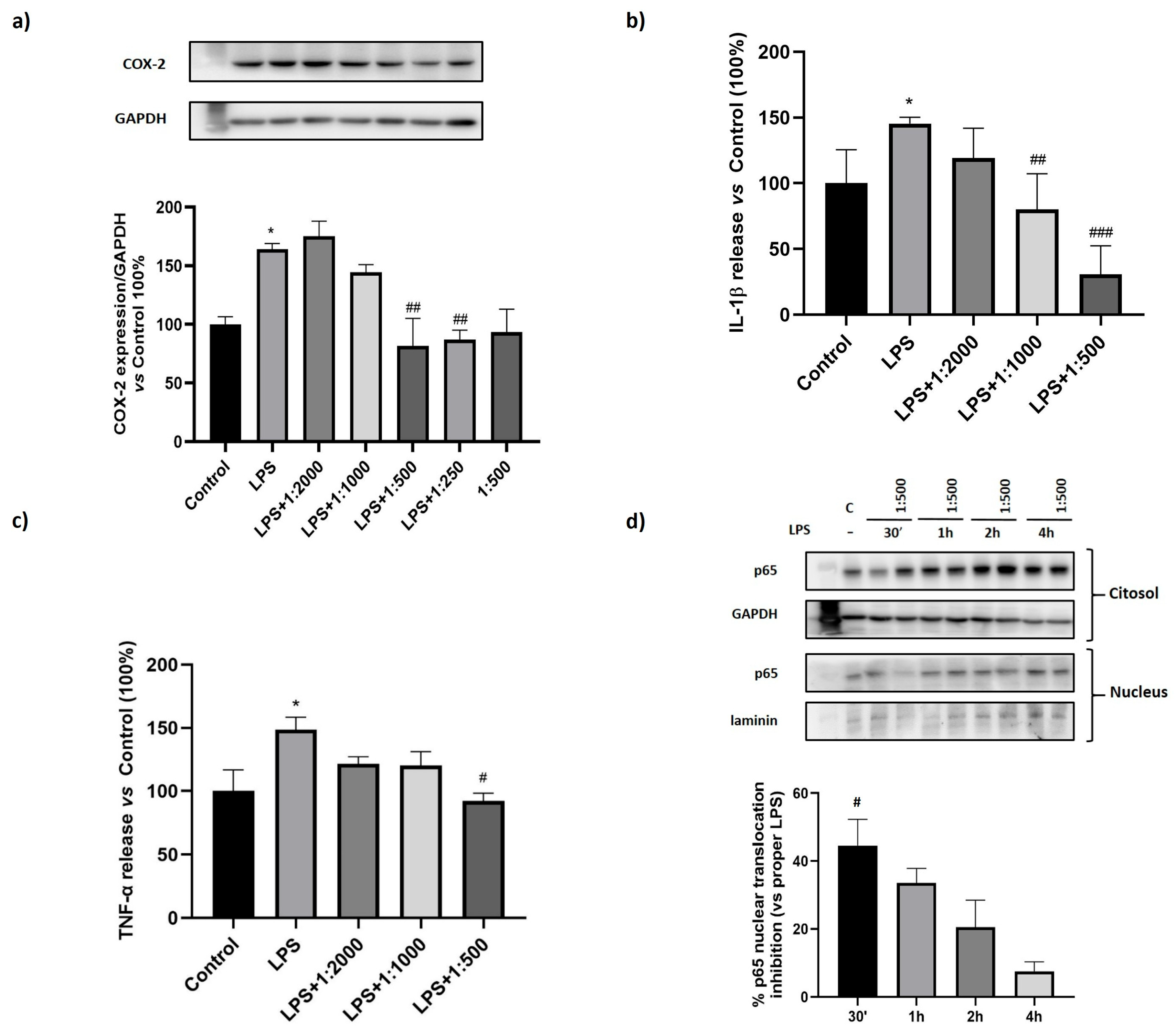
3.3. Effect of the Dietary Supplement on LPS-Induced Inflammation
3.4. Effect of the Dietary Supplement on Pyroptosis
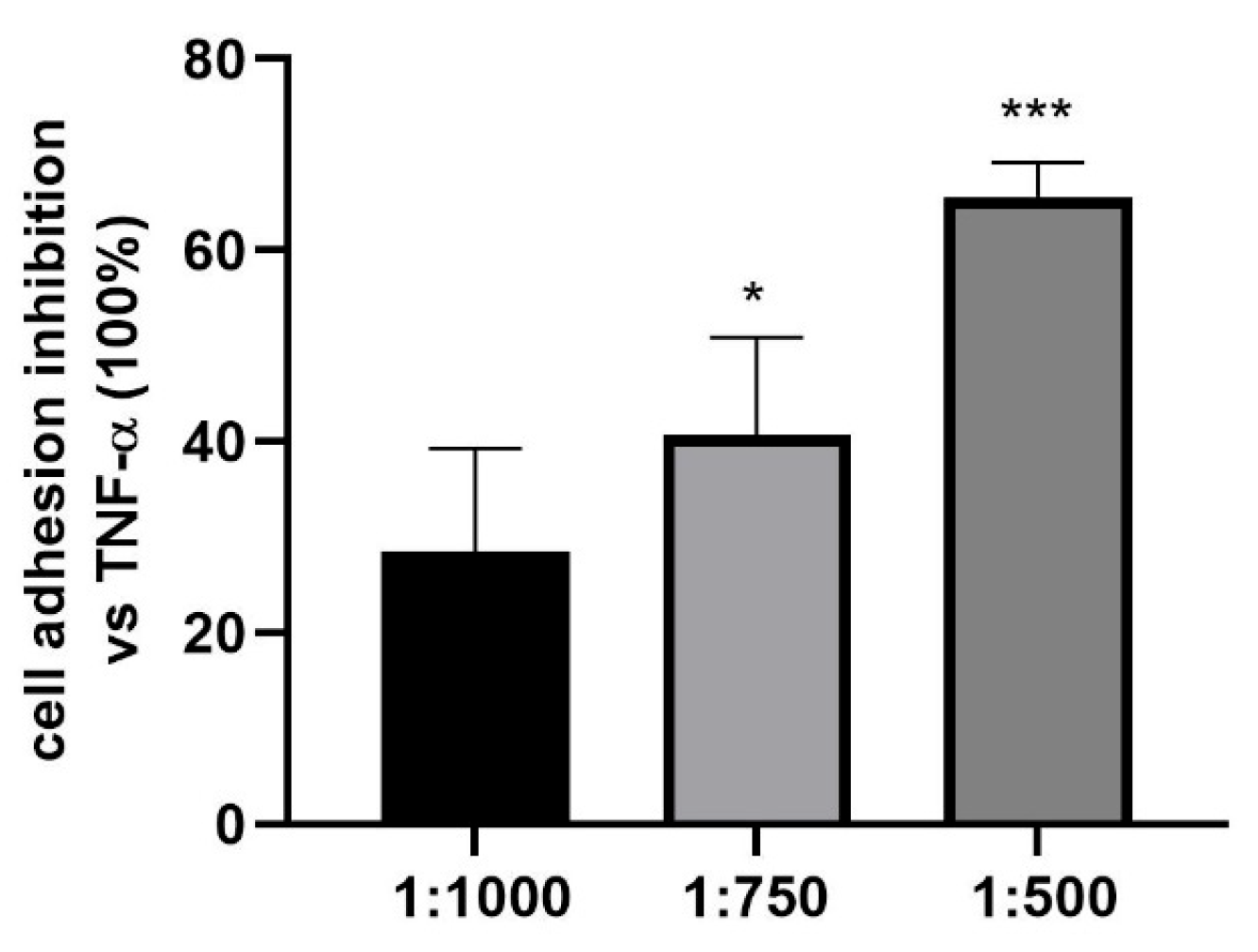
3.5. Effect of the Dietary Supplement on Granulocyte Cell Adhesion to HUVEC
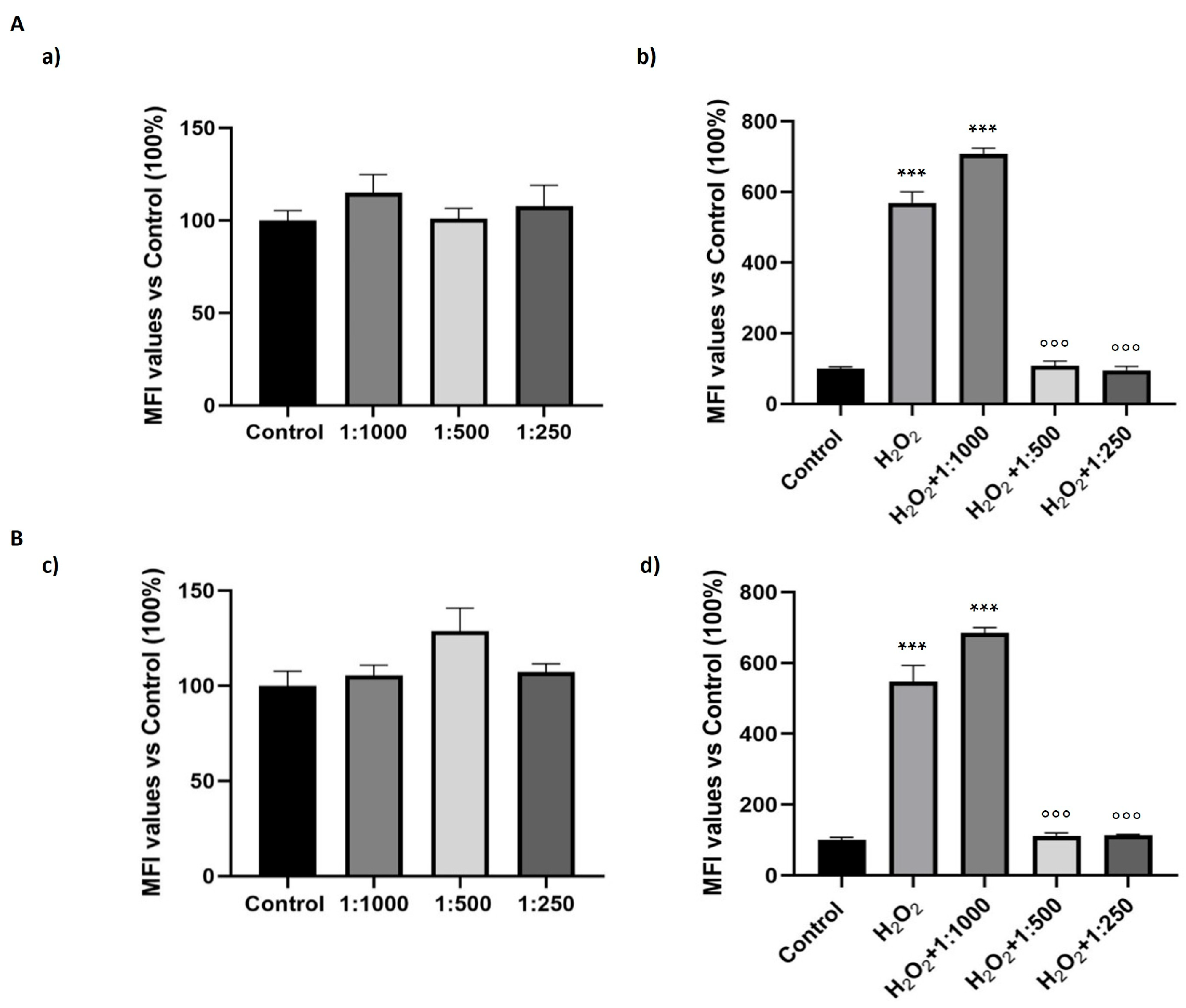
3.6. Effect of the Dietary Supplement on Oxidative Stress
3.7. Evaluation of the Presence of Dietary Supplement Ingredients in Cell Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority. Food Supplements. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 1 June 2025).
- Meizlish, M.L.; Franklin, R.A.; Zhou, X.; Medzhitov, R. Tissue Homeostasis and Inflammation. Annu. Rev. Immunol. 2021, 39, 557–581. [Google Scholar] [CrossRef]
- Chiba, T.; Tanemura, N. Differences in the Perception of Dietary Supplements between Dietary Supplement/Medicine Users and Non-Users. Nutrients 2022, 14, 4114. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Rodriguez-Morales, P.; Franklin, R.A. Macrophage phenotypes and functions: Resolving inflammation and restoring homeostasis. Trends Immunol. 2023, 44, 986–998. [Google Scholar] [CrossRef]
- Colaco, H.G.; Moita, L.F. Initiation of innate immune responses by surveillance of homeostasis perturbations. FEBS J. 2016, 283, 2448–2457. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Bordano, V.; Kinsella, G.K.; Cannito, S.; Dianzani, C.; Gigliotti, C.L.; Stephens, J.C.; Monge, C.; Bocca, C.; Rosa, A.C.; Miglio, G.; et al. G protein-coupled receptor 21 in macrophages: An in vitro study. Eur. J. Pharmacol. 2022, 926, 175018. [Google Scholar] [CrossRef]
- Freytes, D.O.; Kang, J.W.; Marcos-Campos, I.; Vunjak-Novakovic, G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J. Cell Biochem. 2013, 114, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L.; Argenziano, M.; Cucci, M.A.; Grattarola, M.; de Graaf, I.A.M.; Dianzani, C.; Barrera, G.; Sanchez Nieves, J.; Gomez, R.; Cavalli, R.; et al. Carbosilane Dendrimers Loaded with siRNA Targeting Nrf2 as a Tool to Overcome Cisplatin Chemoresistance in Bladder Cancer Cells. Antioxidants 2020, 9, 993. [Google Scholar] [CrossRef] [PubMed]
- Miglio, G.; Vitarelli, G.; Klein, T.; Benetti, E. Effects of linagliptin on human immortalized podocytes: A cellular system to study dipeptidyl-peptidase 4 inhibition. Br. J. Pharmacol. 2017, 174, 809–821. [Google Scholar] [CrossRef]
- Kinsella, G.K.; Cannito, S.; Bordano, V.; Stephens, J.C.; Rosa, A.C.; Miglio, G.; Guaschino, V.; Iannaccone, V.; Findlay, J.B.C.; Benetti, E. GPR21 Inhibition Increases Glucose-Uptake in HepG2 Cells. Int. J. Mol. Sci. 2021, 22, 10784. [Google Scholar] [CrossRef]
- Di Maira, G.; Foglia, B.; Napione, L.; Turato, C.; Maggiora, M.; Sutti, S.; Novo, E.; Alvaro, M.; Autelli, R.; Colombatto, S.; et al. Oncostatin M is overexpressed in NASH-related hepatocellular carcinoma and promotes cancer cell invasiveness and angiogenesis. J. Pathol. 2022, 257, 82–95. [Google Scholar] [CrossRef]
- Dianzani, C.; Collino, M.; Lombardi, G.; Garbarino, G.; Fantozzi, R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br. J. Pharmacol. 2003, 139, 1103–1110. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S. Vitamin and Mineral Supplements: What Clinicians Need to Know. JAMA 2018, 319, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Dietary Supplements Market Size, Share & Trends Analysis Report by Ingredient, by Form, by Application, by End-User, by Distribution Channel, by Region, and Segment Forecasts, 2024–2030; Grand View Research: San Francisco, CA, USA, 2024. [Google Scholar]
- Grand View Research. Europe Nutrition and Supplements Market Size, Share & Trends Analysis Report, by Product (Sports Nutrition, Fat Burners, Dietary Supplements), by Consumer Group, by Formulation, by Sales Channel, and Segment Forecasts, 2023–2030; Grand View Research: San Francisco, CA, USA, 2024. [Google Scholar]
- Siro, I.; Kapolna, E.; Kapolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Okabe, Y.; Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 2016, 17, 9–17. [Google Scholar] [CrossRef]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Cui, K.; Ardell, C.L.; Podolnikova, N.P.; Yakubenko, V.P. Distinct Migratory Properties of M1, M2, and Resident Macrophages Are Regulated by alpha(D)beta(2) and alpha(M)beta(2) Integrin-Mediated Adhesion. Front. Immunol. 2018, 9, 2650. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Heijnen, P.D.; Breur, M.; de Vries, H.E.; Tool, A.T.; Amor, S.; Dijkstra, C.D. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J. Neuroinflamm. 2014, 11, 23. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-kappaB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Cirilli, I.; Damiani, E.; Dludla, P.V.; Hargreaves, I.; Marcheggiani, F.; Millichap, L.E.; Orlando, P.; Silvestri, S.; Tiano, L. Role of Coenzyme Q(10) in Health and Disease: An Update on the Last 10 Years (2010–2020). Antioxidants 2021, 10, 1325. [Google Scholar] [CrossRef]
- Hernandez-Camacho, J.D.; Bernier, M.; Lopez-Lluch, G.; Navas, P. Coenzyme Q(10) Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef]
- Bessler, H.; Bergman, M.; Blumberger, N.; Djaldetti, M.; Salman, H. Coenzyme Q10 decreases TNF-alpha and IL-2 secretion by human peripheral blood mononuclear cells. J. Nutr. Sci. Vitaminol. 2010, 56, 77–81. [Google Scholar] [CrossRef][Green Version]
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.L.; Chen, L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef]
- Zhai, J.; Bo, Y.; Lu, Y.; Liu, C.; Zhang, L. Effects of Coenzyme Q10 on Markers of Inflammation: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0170172. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzad, H.; Aghdashi, M.A.; Asghari Jafarabadi, M.; Alipour, B. Effects of Coenzyme Q10 Supplementation on Inflammatory Cytokines (TNF-alpha, IL-6) and Oxidative Stress in Rheumatoid Arthritis Patients: A Randomized Controlled Trial. Arch. Med. Res. 2015, 46, 527–533. [Google Scholar] [CrossRef]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Doring, F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 2008, 32, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Cha, H.J.; Han, M.H.; Hwang, S.J.; Lee, D.S.; Yoo, J.S.; Choi, I.W.; Kim, S.; Kim, H.S.; Kim, G.Y.; et al. Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomol. Ther. 2018, 26, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Ma, H.; Yang, Q.; Shang, Q.; Song, L.; Zheng, Z.; Zhang, S.; Pan, Y.; Huang, P.; et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1alpha upregulation and autophagy. Free Radic. Biol. Med. 2020, 161, 339–350. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Guo, X.; Feng, X.; Yang, Y.; Zhang, H.; Bai, L. Spermidine attenuates chondrocyte inflammation and cellular pyroptosis through the AhR/NF-kappaB axis and the NLRP3/caspase-1/GSDMD pathway. Front. Immunol. 2024, 15, 1462777. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Adarshan, S.; Shin, H.; Ramesh, M. Assessing the Anti-inflammatory Effects of Bacopa-Derived Bioactive Compounds Using Network Pharmacology and In Vitro Studies. ACS Omega 2022, 7, 40344–40354. [Google Scholar] [CrossRef] [PubMed]
- Channa, S.; Dar, A.; Anjum, S.; Yaqoob, M.; Atta Ur, R. Anti-inflammatory activity of Bacopa monniera in rodents. J. Ethnopharmacol. 2006, 104, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Shyni, G.L.; Helen, A. Efficacy of Bacopa monniera (L.) Wettst in alleviating lysosomal instability in adjuvant-induced arthritis in rats. Inflammation 2011, 34, 630–638. [Google Scholar] [CrossRef]
- Viji, V.; Helen, A. Inhibition of lipoxygenases and cyclooxygenase-2 enzymes by extracts isolated from Bacopa monniera (L.) Wettst. J. Ethnopharmacol. 2008, 118, 305–311. [Google Scholar] [CrossRef]
- Nemetchek, M.D.; Stierle, A.A.; Stierle, D.B.; Lurie, D.I. The Ayurvedic plant Bacopa monnieri inhibits inflammatory pathways in the brain. J. Ethnopharmacol. 2017, 197, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Singh, D.; Sandhir, R. Bacopa monnieri prevents colchicine-induced dementia by anti-inflammatory action. Metab. Brain Dis. 2019, 34, 505–518. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Verdrengh, M.; Tarkowski, A. Riboflavin in innate and acquired immune responses. Inflamm. Res. 2005, 54, 390–393. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Buchala, B.; Plytycz, B. Riboflavin deprivation inhibits macrophage viability and activity—A study on the RAW 264.7 cell line. Br. J. Nutr. 2013, 110, 509–514. [Google Scholar] [CrossRef]
- Toyosawa, T.; Suzuki, M.; Kodama, K.; Araki, S. Highly purified vitamin B2 presents a promising therapeutic strategy for sepsis and septic shock. Infect. Immun. 2004, 72, 1820–1823. [Google Scholar] [CrossRef]
- Burbank, A.J.; Duran, C.G.; Almond, M.; Wells, H.; Jenkins, S.; Jiang, Q.; Yang, C.; Wang, T.; Zhou, H.; Hernandez, M.L.; et al. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. J. Allergy Clin. Immunol. 2017, 140, 1179–1181. [Google Scholar] [CrossRef]
- Tan, P.H.; Sagoo, P.; Chan, C.; Yates, J.B.; Campbell, J.; Beutelspacher, S.C.; Foxwell, B.M.; Lombardi, G.; George, A.J. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J. Immunol. 2005, 174, 7633–7644. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. Antioxidants and vitamins to reduce cardiovascular disease. Curr. Atheroscler. Rep. 2000, 2, 342–351. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.; Soehnlein, O.; Viola, J.R. Organ-Specific Mechanisms of Transendothelial Neutrophil Migration in the Lung, Liver, Kidney, and Aorta. Front. Immunol. 2018, 9, 2739. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef]
- Franklin, R.A. Fibroblasts and macrophages: Collaborators in tissue homeostasis. Immunol. Rev. 2021, 302, 86–103. [Google Scholar] [CrossRef]
- Miki, H.; Manresa, M.C. Novel fibroblast phenotypes in homeostasis and chronic inflammation: From functions to potential regulators. J. Physiol. 2023, 601, 2273–2291. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Zagotta, I.; Dimova, E.Y.; Debatin, K.M.; Wabitsch, M.; Kietzmann, T.; Fischer-Posovszky, P. Obesity and inflammation: Reduced cytokine expression due to resveratrol in a human in vitro model of inflamed adipose tissue. Front. Pharmacol. 2015, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Hsu, P.Y.; Meng, M.; Su, C.C. Supplement of 5-hydroxytryptophan before induction suppresses inflammation and collagen-induced arthritis. Arthritis Res. Ther. 2015, 17, 364. [Google Scholar] [CrossRef]
- Bae, S.J.; Lee, J.S.; Kim, J.M.; Lee, E.K.; Han, Y.K.; Kim, H.J.; Choi, J.; Ha, Y.M.; No, J.K.; Kim, Y.H.; et al. 5-Hydroxytrytophan inhibits tert-butylhydroperoxide (t-BHP)-induced oxidative damage via the suppression of reactive species (RS) and nuclear factor-kappaB (NF-kappaB) activation on human fibroblast. J. Agric. Food Chem. 2010, 58, 6387–6394. [Google Scholar] [CrossRef]
- Ohgi, Y.; Futamura, T.; Kikuchi, T.; Hashimoto, K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2013, 103, 853–859. [Google Scholar] [CrossRef]


| Primer | Sense | Reverse |
|---|---|---|
| Human CD80 | 5′-CCTACTGCTTTGCCCCAAGA-3′ | 5′-CAGGGCGTACACTTTCCCTT-3′ |
| Human CD86 | 5′-TGGAAACTGACAAGACGCGG-3′ | 5′-CAAGGAATGTGGTCTGGGGG-3′ |
| Human MRC1 | 5′-CAGCGCTTGTGATCTTCATT-3′ | 5′-TACCCCTGCTCCTGGTTTTT-3′ |
| Human CD163 | 5′-GCAGTTTCCTCAAGAGGAGAGAA-3′ | 5′-GCTCAGAATGGCCTCCTTTTC-3′ |
| Human GAPDH | 5′-TGGTATCGTGGAAGGACTCATGAC-3′ | 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′ |
| Sample Preparation | Analytical Platform | Stationary Phase | Gradient Program | Detection Mode * | Component |
|---|---|---|---|---|---|
| Extraction in MeOH 20% | HPLC-MS | Ascentis Express C18 column (15 cm × 2.1 mm, 2.7 μm, Supelco, Bellefonte, PA, USA) | 5% B for 3 min, 5–15% B in 17 min, 15–25% B in 10 min, 25–70% B in 12 min, 70–100% B in 10 min, 100% B for 1 min | SRM+ (265→122) | Vitamin B1 |
| SRM+ (123→80) | Vitamin B3 | ||||
| SRM+ (146→72) | Spermidine | ||||
| SRM+ (221→134) | 5-hydroxytryptophan | ||||
| SRM+ (220→70) | Vitamin B5 | ||||
| SRM+ (337→172) | Vitamin B2 | ||||
| Extraction in MeOH 100% | HPLC-MS | Ascentis Express C18 column (15 cm × 2.1 mm, 2.7 μm, Supelco, Bellefonte, PA, USA) | 5% B for 3 min, 5–15% B in 17 min, 15–25% B in 10 min, 25–70% B in 12 min, 70–100% B in 10 min, 100% B for 1 min | SIM+ (943, 973) | Bacosides |
| Dilution in EtOH 100% | HPLC-PDA | Ascentis Express RP-Amide column (10 cm × 2.1 mm × 2.7 μm, Supelco, Bellefonte, PA, USA) | 25% B for 3 min, 25–70% B in 12 min, 70–100% B in 10 min, 100% for 25 min | UV (λ = 305 nm) | Resveratrol |
| UV (λ =274 nm) | Coenzyme Q10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordano, V.; Gerbino, C.; Boscaro, V.; Rubiolo, P.; Marengo, A.; Pizzimenti, S.; Cucci, M.A.; Cannito, S.; Nurcis, J.; Gallicchio, M.; et al. Balancing the Cellular Inflammatory-Homeostatic Axis Through Natural Ingredient Supplementation. Nutrients 2025, 17, 2587. https://doi.org/10.3390/nu17162587
Bordano V, Gerbino C, Boscaro V, Rubiolo P, Marengo A, Pizzimenti S, Cucci MA, Cannito S, Nurcis J, Gallicchio M, et al. Balancing the Cellular Inflammatory-Homeostatic Axis Through Natural Ingredient Supplementation. Nutrients. 2025; 17(16):2587. https://doi.org/10.3390/nu17162587
Chicago/Turabian StyleBordano, Valentina, Chiara Gerbino, Valentina Boscaro, Patrizia Rubiolo, Arianna Marengo, Stefania Pizzimenti, Marie Angèle Cucci, Stefania Cannito, Jessica Nurcis, Margherita Gallicchio, and et al. 2025. "Balancing the Cellular Inflammatory-Homeostatic Axis Through Natural Ingredient Supplementation" Nutrients 17, no. 16: 2587. https://doi.org/10.3390/nu17162587
APA StyleBordano, V., Gerbino, C., Boscaro, V., Rubiolo, P., Marengo, A., Pizzimenti, S., Cucci, M. A., Cannito, S., Nurcis, J., Gallicchio, M., Spampinato, S. F., Cangemi, L., Bocca, C., Dianzani, C., Rosa, A. C., & Benetti, E. (2025). Balancing the Cellular Inflammatory-Homeostatic Axis Through Natural Ingredient Supplementation. Nutrients, 17(16), 2587. https://doi.org/10.3390/nu17162587













