Honey as a Neuroprotective Agent: Molecular Perspectives on Its Role in Alzheimer’s Disease
Abstract
1. Introduction
2. Phytochemical Characterization of Honeys
3. Effects of Honey on AD Features
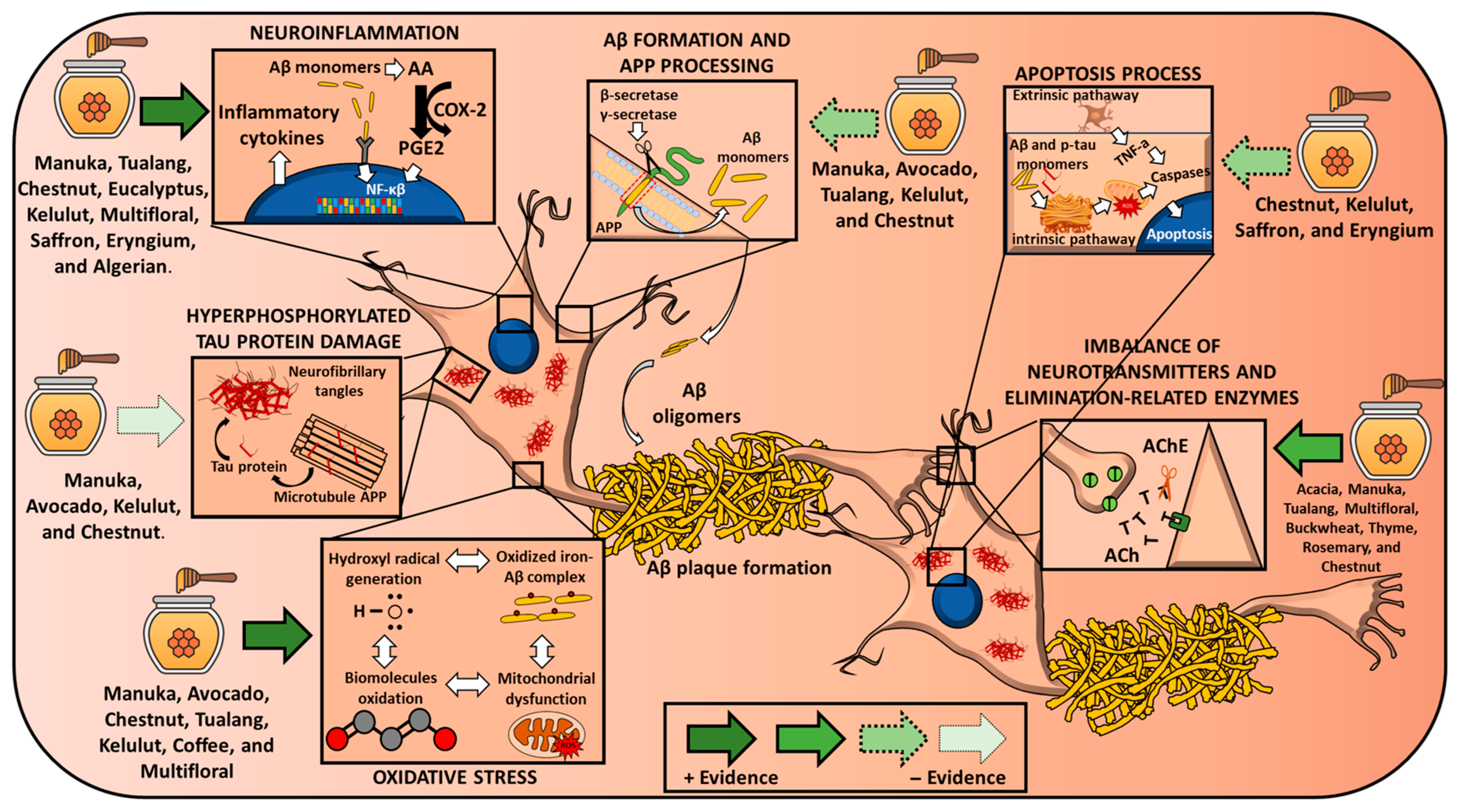
3.1. Oxidative Stress
3.2. Mitochondrial Dysfunction
3.3. Neuroinflammation
3.4. Apoptosis
3.5. Aβ Plaque Damage and APP Processing
3.6. Hyperphosphorylated Tau Protein Damage
3.7. Imbalance of Neurotransmitters and Elimination-Related Enzymes
4. Limitations and Final Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gaiteri, C.; Mostafavi, S.; Honey, C.J.; De Jager, P.L.; Bennett, D.A. Genetic Variants in Alzheimer Disease—Molecular and Brain Network Approaches. Nat. Rev. Neurol. 2016, 12, 413–427. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 10 December 2022).
- World Health Organization Alzheimer’s Disease Fact Sheet. Available online: http://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 21 December 2021).
- Ju, Y.; Tam, K.Y. Pathological Mechanisms and Therapeutic Strategies for Alzheimer’s Disease. Neural Regen. Res. 2021, 17, 543–549. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, X. Alzheimer’s Disease: Insights into Pathology, Molecular Mechanisms, and Therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef]
- Alzheimer’s Association Alzheimer’s Disease Facts and Figures. Available online: https://www.alz.org/alzheimers-dementia/facts-figures (accessed on 10 December 2022).
- Wolters, F.J.; Chibnik, L.B.; Waziry, R.; Anderson, R.; Berr, C.; Beiser, A.; Bis, J.C.; Blacker, D.; Bos, D.; Brayne, C.; et al. Twenty-Seven-Year Time Trends in Dementia Incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 2020, 95, e519–e531. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Osta, S.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Varela-López, A. Natural Bioactive Products and Alzheimer’s Disease Pathology: Lessons from Caenorhabditis Elegans Transgenic Models. Diseases 2022, 10, 28. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Bee Honey Protects Astrocytes against Oxidative Stress: A Preliminary in Vitro Investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Calvi, P.; Nuzzo, D.; Picone, P.; Allegra, M.; Mulè, F.; Amato, A. Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice. Int. J. Mol. Sci. 2023, 24, 3467. [Google Scholar] [CrossRef] [PubMed]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Eddie Tan, T.T. Antioxidant Activity of Three Honey Samples in Relation with Their Biochemical Components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Muñoz-Ollero, P.; Jiménez-Trigo, V.; Esteban-Muñoz, A.; Tutusaus, K.; Giampieri, F.; Battino, M.; Sánchez-González, C.; Rivas-García, L.; et al. Amyloid β-but Not Tau-Induced Neurotoxicity Is Suppressed by Manuka Honey via HSP-16.2 and SKN-1/Nrf2 Pathways in an in Vivo Model of Alzheimer’s Disease. Food Funct. 2022, 13, 11185–11199. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Orantes, F.J.; Esteban-Muñoz, A.; Pérez-Oleaga, C.M.; Battino, M.; Sánchez-González, C.; Rivas-García, L.; Giampieri, F.; Quiles, J.L.; et al. In Vivo Anti-Alzheimer and Antioxidant Properties of Avocado (Persea Americana Mill.) Honey from Southern Spain. Antioxidants 2023, 12, 404. [Google Scholar] [CrossRef]
- Candiracci, M.; Piatti, E.; Dominguez-Barragán, M.; García-Antrás, D.; Morgado, B.; Ruano, D.; Gutiérrez, J.F.; Parrado, J.; Castaño, A. Anti-Inflammatory Activity of a Honey Flavonoid Extract on Lipopolysaccharide-Activated N13 Microglial Cells. J. Agric. Food Chem. 2012, 60, 12304–12311. [Google Scholar] [CrossRef]
- Petretto, G.L.; Cossu, M.; Alamanni, M.C. Phenolic Content, Antioxidant and Physico-Chemical Properties of Sardinian Monofloral Honeys. Int. J. Food Sci. Technol. 2015, 50, 482–491. [Google Scholar] [CrossRef]
- Trinh, N.T.N.; Tuan, N.N.; Thang, T.D.; Kuo, P.-C.; Thanh, N.B.; Tam, L.N.; Tuoi, L.H.; Nguyen, T.H.D.; Vu, D.C.; Ho, T.L.; et al. Chemical Composition Analysis and Antioxidant Activity of Coffea Robusta Monofloral Honeys from Vietnam. Foods 2022, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Nguyen, T.V.; Le, D.T.; Diep, L.M.N.; Nguyen, K.N.; To, T.H.N.; Le, T.H.; Nguyen, Q.V. Phenolic Profiles, Antioxidant, Antibacterial Activities and Nutritional Value of Vietnamese Honey from Different Botanical and Geographical Sources. AgriEngineering 2022, 4, 1116–1138. [Google Scholar] [CrossRef]
- Castro, E.; Quicazán, M.; Mojica, A.; Zuluaga-Domínguez, C. Bioactive and Physicochemical Profile of Honey Collected from Colombian Organic and Conventional Coffee Growing Areas. J. Apic. Res. 2023, 62, 518–529. [Google Scholar] [CrossRef]
- Ismail, N.I.; Abdul Kadir, M.R.; Mahmood, N.H.; Singh, O.P.; Iqbal, N.; Zulkifli, R.M. Apini and Meliponini Foraging Activities Influence the Phenolic Content of Different Types of Malaysian Honey. J. Apic. Res. 2016, 55, 137–150. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A.M.; Hamid, H.A.; Khazaai, H. Malaysian Stingless Bee and Tualang Honeys: A Comparative Characterization of Total Antioxidant Capacity and Phenolic Profile Using Liquid Chromatography-Mass Spectrometry. LWT 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geană, E.-I. Phenolic Compounds Profile and Biochemical Properties of Honeys in Relationship to the Honey Floral Sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Jiménez-Trigo, V.; Xiao, J.; Giampieri, F.; Forbes-Hernández, T.Y.; Grosso, G.; Battino, M.; Sánchez-González, C.; Quiles, J.L. Molecular Bases for the Use of Functional Foods in the Management of Healthy Aging: Berries, Curcumin, Virgin Olive Oil and Honey; Three Realities and a Promise. Crit. Rev. Food Sci. Nutr. 2022, 63, 11967–11986. [Google Scholar] [CrossRef]
- Gasparrini, M.; Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective Effects of Manuka Honey on LPS-Treated RAW 264.7 Macrophages. Part 2: Control of Oxidative Stress Induced Damage, Increase of Antioxidant Enzyme Activities and Attenuation of Inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Alvarez-Suarez, J.M.; Ansary, J.; Quinzi, D.; Amici, A.; Navarro-Hortal, M.D.; Esteban-Muñoz, A.; Quiles, J.L.; Battino, M.; et al. Anti-Inflammatory Activities of Italian Chestnut and Eucalyptus Honeys on Murine RAW 264.7 Macrophages. J. Funct. Foods 2021, 87, 104752. [Google Scholar] [CrossRef]
- Phokasem, P.; Jantrapirom, S.; Karinchai, J.; Yoshida, H.; Yamaguchi, M.; Chantawannakul, P. Honeybee Products and Edible Insect Powders Improve Locomotive and Learning Abilities of Ubiquilin-Knockdown Drosophila. BMC Complement. Med. Ther. 2020, 20, 267. [Google Scholar] [CrossRef]
- Shati, A.A.; Elsaid, F.G.; Hafez, E.E. Biochemical and Molecular Aspects of Aluminium Chloride-Induced Neurotoxicity in Mice and the Protective Role of Crocus sativus L. Extraction and Honey Syrup. Neuroscience 2011, 175, 66–74. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; KNS, S.; Asari, M.A.; Mummedy, S.; Muzaimi, M.; Sulaiman, S.A. Effect of Tualang Honey against KA-Induced Oxidative Stress and Neurodegeneration in the Cortex of Rats. BMC Complement. Altern. Med. 2017, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Hasim, H.; Salam, S.K.N.M.; Rao, P.V.; Muthuraju, S.; Asari, M.A. Tualang Honey-Mediated Silver Nanoparticles Attenuate Hippocampal Oxidative Stress in Kainic Acid-Induced Male Rats. Biomed. Res. Ther. 2022, 9, 5465–5475. [Google Scholar] [CrossRef]
- Adeniyi, I.A.; Babalola, K.T.; Adekoya, V.A.; Oyebanjo, O.; Ajayi, A.M.; Onasanwo, S.A. Neuropharmacological Effects of Honey in Lipopolysaccharide-Induced Neuroinflammation, Cognitive Impairment, Anxiety and Motor Impairment. Nutr. Neurosci. 2022, 26, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, W.I.; Sulieman, H.B.; Zubayr, M.O.; Imam, A.; Amin, A.; Biliaminu, S.A.; Oyewole, L.A.; Owoyele, B.V. Honey Prevents Neurobehavioural Deficit and Oxidative Stress Induced by Lead Acetate Exposure in Male Wistar Rats- a Preliminary Study. Metab. Brain Dis. 2016, 31, 37–44. [Google Scholar] [CrossRef]
- Yaacob, W.M.H.W.; Long, I.; Zakaria, R.; Othman, Z. Tualang Honey and Its Methanolic Fraction Ameliorate Lipopolysaccharide-Induced Oxidative Stress, Amyloid Deposition and Neuronal Loss of the Rat Hippocampus. Adv. Tradit. Med. 2021, 21, 121–129. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R.; Abdul Aziz, C.B.; Othman, Z. Tualang Honey Attenuates Noise Stress-Induced Memory Deficits in Aged Rats. Oxid. Med. Cell. Longev. 2016, 2016, 1549158. [Google Scholar] [CrossRef]
- Asari, M.A.; Sirajudeen, K.N.S.; Mohd Yusof, N.A.; Mohd Amin, M.S.I. DHA-Rich Fish Oil and Tualang Honey Reduce Chronic Stress-Induced Oxidative Damage in the Brain of Rat Model. J. Tradit. Complement. Med. 2021, 12, 361–366. [Google Scholar] [CrossRef]
- Shaikh, A.; Ahmad, F.; Teoh, S.L.; Kumar, J.; Yahaya, M.F. Unveiling the Therapeutic Potential of Kelulut (Stingless Bee) Honey in Alzheimer’s Disease: Findings from a Rat Model Study. Antioxidants 2024, 13, 926. [Google Scholar] [CrossRef]
- Zaidi, H.; Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Debbache, N.; Pacheco, R.; Luisa Serralheiro, M.; Eduarda Araujo, M. Biological Properties of Phenolic Compound Extracts in Selected Algerian Honeys-The Inhibition of Acetylcholinesterase and Alpha-Glucosidase Activities. Eur. J. Integr. Med. 2019, 25, 77–84. [Google Scholar] [CrossRef]
- Ooi, T.C.; Yaacob, M.; Rajab, N.F.; Shahar, S.; Sharif, R. The Stingless Bee Honey Protects against Hydrogen Peroxide-Induced Oxidative Damage and Lipopolysaccharide-Induced Inflammation in Vitro. Saudi J. Biol. Sci. 2021, 28, 2987–2994. [Google Scholar] [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Comparable Benefits of Stingless Bee Honey and Caffeic Acid in Mitigating the Negative Effects of Metabolic Syndrome on the Brain. Antioxidants 2022, 11, 2154. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S.; Muzaimi, M.; Mummedy, S.; Asari, M.A.; Sulaiman, S.A. Tualang Honey Reduced Neuroinflammation and Caspase-3 Activity in Rat Brain after Kainic Acid-Induced Status Epilepticus. Evid.-Based Complement. Alternat. Med. 2018, 2018, 7287820. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. Honey as the Potential Natural Source of Cholinesterase Inhibitors in Alzheimer’s Disease. Plant Foods Hum. Nutr. 2020, 75, 30–32. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Gajowniczek-Ałasa, D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients 2022, 14, 2969. [Google Scholar] [CrossRef]
- Muhammad, A.; Odunola, O.A.; Gbadegesin, M.A.; Sallau, A.B.; Ndidi, U.S.; Ibrahim, M.A. Inhibitory Effects of Sodium Arsenite and Acacia Honey on Acetylcholinesterase in Rats. Int. J. Alzheimer’s Dis. 2015, 2015, 903603. [Google Scholar] [CrossRef]
- Yildiz, O.; Karahalil, F.; Can, Z.; Sahin, H.; Kolayli, S. Total Monoamine Oxidase (MAO) Inhibition by Chestnut Honey, Pollen and Propolis. J. Enzym. Inhib. Med. Chem. 2014, 29, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Li, W.; Yang, H.J.; Kim, S.-G.; Choi, H.M.; Choi, J.-G.; Oh, Y.-C. Ethyl Acetate Fraction of Chestnut Honey Attenuates Scopolamine-Induced Cognitive Impairment in Mice and Glutamate-Induced Neurotoxicity in HT22 Cells. Antioxidants 2024, 13, 1346. [Google Scholar] [CrossRef] [PubMed]
- Teimury, A.; Khaledi, E.M.; Hosseini, E.S. Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities of Saffron and Eryngium Honey Extracts. BMC Complement. Med. Ther. 2025, 25, 131. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.G.; Marfil, V.; Li, C. Use of Caenorhabditis Elegans as a Model to Study Alzheimer’s Disease and Other Neurodegenerative Diseases. Front. Genet. 2014, 5, 279. [Google Scholar] [CrossRef]
- Prüßing, K.; Voigt, A.; Schulz, J.B. Drosophila Melanogaster as a Model Organism for Alzheimer’s Disease. Mol. Neurodegener. 2013, 8, 35. [Google Scholar] [CrossRef]
- Azman, K.F.; Aziz, C.B.A.; Zakaria, R.; Ahmad, A.H.; Shafin, N.; Ismail, C.A.N. Tualang Honey: A Decade of Neurological Research. Molecules 2021, 26, 5424. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The Role of Mitochondrial Dysfunction in Alzheimer’s Disease Pathogenesis. Alzheimer’s Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, W.M.H.W.; Long, I.; Zakaria, R.; Othman, Z. Tualang Honey and Its Methanolic Fraction Improve LPS-Induced Learning and Memory Impairment in Male Rats: Comparison with Memantine. Curr. Nutr. Food Sci. 2020, 16, 333–342. [Google Scholar] [CrossRef]
- Muhammad, A.; Odunola, O.A.; Ibrahim, M.A.; Sallau, A.B.; Erukainure, O.L.; Aimola, I.A.; Malami, I. Potential Biological Activity of Acacia Honey. FBE 2016, 8, 351–357. [Google Scholar] [CrossRef]
- Nisa, N.; Rasmita, B.; Arati, C.; Uditraj, C.; Siddhartha, R.; Dinata, R.; Bhanushree, B.; Bidanchi, R.M.; Manikandan, B.; Laskar, S.A.; et al. Repurposing of Phyto-Ligand Molecules from the Honey Bee Products for Alzheimer’s Disease as Novel Inhibitors of BACE-1: Small Molecule Bioinformatics Strategies as Amyloid-Based Therapy. Environ. Sci. Pollut. Res. 2023, 30, 51143–51169. [Google Scholar] [CrossRef]
- Rosli, N.H.M.; Yahya, H.M.; Ibrahim, F.W.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Rajab, N.F. Serum Metabolomics Profiling of Commercially Mixed Functional Foods-Effects in Beta-Amyloid Induced Rats Measured Using 1H NMR Spectroscopy. Nutrients 2020, 12, 3812. [Google Scholar] [CrossRef]
- Navarro Hortal, M.D. Evaluation of Redox-Based Bioactive Compounds Obtained from Products and by-Products of Agri-Food Industry and Beekeeping of Interest in Aging and Aging-Related Diseases. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2023. [Google Scholar]
| Compound/Honey Type | AH | AVH | CH | COH | EH | KH | LH | MH | MFH | RH | THH | TH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavonoids | ||||||||||||
| Apigenin | X | X | X | X | X | X | X | X | X | |||
| Catechin | X | X | X | X | X | X | ||||||
| Chrysin | X | X | X | X | X | X | X | X | ||||
| Epicatechin | X | X | ||||||||||
| Galangin | X | X | X | X | X | |||||||
| Genistein | X | X | ||||||||||
| Isorhamnetin | X | X | X | X | X | |||||||
| Kaempferol | X | X | X | X | X | X | X | X | X | X | ||
| Luteolin | X | X | X | X | X | X | X | X | X | X | X | |
| Myricetin | X | X | X | X | ||||||||
| Naringenin | X | X | X | X | ||||||||
| Pinobanksin | X | X | X | X | X | X | X | X | X | |||
| Pinocembrin | X | X | X | X | X | X | X | X | ||||
| Quercetin | X | X | X | X | X | X | X | X | X | X | X | |
| Rutin | X | X | X | X | X | |||||||
| Phenolic acids | ||||||||||||
| 2-Hydroxycinnamic acid | X | X | X | |||||||||
| Caffeic acid | X | X | X | X | X | X | X | X | X | X | ||
| Chlorogenic acid | X | X | X | X | X | X | X | |||||
| Cinnamic acid | X | X | X | X | X | X | X | X | X | |||
| Ellagic acid | X | X | X | |||||||||
| Ferulic acid | X | X | X | X | X | X | X | X | X | X | ||
| Gallic acid | X | X | X | X | X | X | X | |||||
| p-Coumaric acid | X | X | X | X | X | X | X | X | X | X | X | |
| p-Hydroxybenzoic acid | X | X | X | X | X | |||||||
| Rosmarinic acid | X | X | ||||||||||
| Syringic acid | X | X | X | X | X | X | X | X | X | |||
| Vanillic acid | X | X | ||||||||||
| Model | Treatment | Dosage and Duration | Effects | Reference |
|---|---|---|---|---|
| Oxidative stress | ||||
| In vitro. Astrocyte cell culture injured with H2O2 | Honey | 1% (v/v) for 24 h followed by 100 μM H2O2 for 3 h | ↑ Cell viability | [10] |
| In vitro. RAW 264.7 macrophages injured with LPS | Manuka honey | 8 mg/mL honey with 1 μg/mL for 24 h | ↓ ROS content ↓ TBARS and carbonyl protein levels ↓ Protein expression of OGG1 (DNA damage) ↑ GSH levels ↑ GR and GST activity ↑ Protein expression of Nrf-2, Keap-1, CAT and SOD | [30] |
| In vitro. RAW 264.7 macrophages injured with LPS | Chestnut and Eucalyptus honey | 1 mg/mL Honey for 24 h followed by 1 µg/mL LPS for 24 h | = ROS content ↓ NO levels ↑ GSH levels ↑ GPx activity = GR and GST activity = Nrf-2 gene expression | [31] |
| In vivo. C. elegans worms injured with AAPH | Manuka honey | 100 mg/mL for 48 h followed by 2.5 mM AAPH for 15 min | ↓ ROS content | [17] |
| In vivo. C. elegans worms injured with AAPH | Avocado honey | 100 mg/mL for 48 h followed by 2.5 mM AAPH for 15 min | ↓ ROS content | [18] |
| In vivo. C. elegans transgenic strains | Manuka honey | 100 mg/mL for 48 h | ↓ SOD3 and HSP-16.2 expression = SKN-1/NRF2, DAF-16/FOXO, GST-4, and HSF-1 expression | [17] |
| In vivo. C. elegans transgenic strains | Avocado honey | 100 mg/mL for 48 h | ↑ DAF-16/FOXO expression ↓ SKN-1/NRF2, HSF-1, SOD-3 and HSP-16.2 expression = GST-4 expression | [18] |
| In vivo. Drosophila melanogaster dUbqn knockdown | Coffee honey | 1% (v/v) | ↓ Brain ROS content | [32] |
| In vivo. Balb/c mice injured with AlCl3 (model of AD) | Honey syrup | 40 mg/kg/d (i.p.) AlCl3 followed by 500 mg/kg honey syrup (i.p.) for 45 days | ↑ TAC ↓ TBARS = SOD activity ↑ CAT and GSH-Px activity | [33] |
| In vivo. C57BL/6 mice injured with AlCl3 (model of AD) | Honey syrup | 40 mg/kg/d (i.p.) AlCl3 followed by 500 mg/kg honey syrup (i.p.) for 45 days | ↑ TAC ↓ TBARS ↑ SOD, CAT and GSH-Px activity | [33] |
| In vivo. Male C57BL/6 mice injured with HFD | Chestnut honey | 45 mg/d (orally) for 10 weeks | ↓ ROS levels in brain | [13] |
| In vivo. Male Sprague Dawley rats injured with KA | Tualang Honey | 1.0 g/kg (i.g.) five times every 12 h and 15 mg/kg KA (s.c.) 30 min after last dose of treatment | ↑ Viable cells in cortex ↑ TAC in cortex ↓ TBARS levels in cortex | [34] |
| In vivo. Male Sprague Dawley rats injured with KA | Tualang Honey | 10 or 50 mg/kg honey (p.o.) and 15 mg/kg KA (s.c.) 30 min after last dose of treatment | ↑ TAC ↓ MDA and NOx levels in brain = Protein carbonyl levels in brain ↑ CAT activity ↑ GSH levels | [35] |
| In vivo. Male Wistar rats injured with LPS | Honey | 250 µg/kg LPS (i.p.) for 7 days followed by 0.31 and 0.36 g/kg honey for other 7 days | ↓ MDA levels in brain ↑ GSH levels in brain | [36] |
| In vivo. Male Wistar rats injured with lead acetate | Honey | 1.5 mL/kg of honey concomitantly with 0.2% lead for 28 days (orally) | ↑ Memory function = MDA levels ↑ SOD levels ↑ GST activity = CAT levels ↑ GSH levels | [37] |
| In vivo. Male Sprague Dawley rats injured with LPS | Tualang honey | 200 mg/kg tualang honey (i.p.) for 14 days, 5 mg/kg LPS (i.p.) applied on day 4 | ↑ CAT and GPx levels ↑ SOD and GR levels | [38] |
| In vivo. Aged male Sprague Dawley rats injured with noise stress | Tualang honey | 200 mg/kg bw/day (i.g.) 14 days prior to the stress procedure and continued for an additional 14 days during the stress exposure | ↑ Short- and long-term memory ↓ MDA levels in brain ↑ SOD activity in brain = GPx, GR and CAT activity in brain | [39] |
| In vivo. Male Sprague Dawley rats subjected to stresses | Tualang honey | 1 g/kg bw/twice daily (i.g.) and exposed to stress for 28 consecutive days | ↑ TAC in brain ↓ TBARS levels in brain ↑ GSH:GSSG ratio in brain ↓ GSSG levels in brain | [40] |
| In vivo. Male Sprague Dawley rats injured with Aβ1-42 (AD model) | Kelulut honey | 2.5 µg/µL Aβ1-42 (icv) 1 week before 1 g/kg bw/d honey (i.g.) for 28 days | = SOD1 and MDA levels in hippocampus | [41] |
| Neuroinflammation | ||||
| In vitro | Algerian honeys | IC50 = 1.72–7.43 mg/mL | ↓ Activity of BSA denaturation induced by heat | [42] |
| In vitro. RAW 264.7 macrophages injured with LPS | Manuka honey | 8 mg/mL honey with 1 μg/mL LPS for 24 h | ↓ Nitrite levels ↓ NF-κB, iNOS, TNF-α and IL-1β gene expression ↓ TLR4, NF-κB, p-Iκ-Bα, iNOS protein expression ↓ TNF-α, IL-1β, IL-6 protein expression ↑ IL-10 protein expression | [30] |
| In vitro. RAW 264.7 macrophages injured with LPS | Chestnut and Eucalyptus honey | 1 mg/mL honey for 24 h followed by 1 µg/mL LPS for 24 h | ↓ Nitrite levels | [31] |
| In vitro. RAW 264.7 cell line injured with LPS | Kelulut honey | 0.5 and 1% (v/v) honey for 2 h followed by the addition of 1 µg/mL LPS for another 22 h | ↓ iNOS expression ↓ NO levels = COX-2 expression | [43] |
| In vivo. Male C57BL/6 mice injured with HFD | Chestnut honey | 45 mg/d (orally) for 10 weeks | ↓ Nitrite levels in brain ↓ TNF-α gene expression in brain ↓ COX-2 and iNOS protein levels in brain | [13] |
| In vivo. Male Wistar rats injured with HFD | Stingless Bee Honey | HFD for 8 weeks, followed by HFD with 10 mg/kg bw/d honey (i.g.) for another 8 weeks | ↓ TNF-α levels in brain | [44] |
| In vivo. Male Sprague Dawley rats injured with KA | Tualang Honey | 1.0 g/kg (i.g.) five times every 12 h and 15 mg/kg KA (s.c.) 30 min after the last dose of treatment) | ↓ TNF-α and IL-1β levels ↓ GFAP and AIF-1 levels ↓ COX-2 and 5-LOX levels | [45] |
| In vivo. Male Wistar rats injured with LPS | Honey | 250 µg/kg LPS (i.p) for 7 days followed by 0.31 and 0.36 g/kg honey for other 7 days | ↓ Memory and motor impairment ↓ Nitrite levels in brain ↓ TNF-α and IL-6 levels | [36] |
| In vivo. Male Sprague Dawley rats injured with Aβ1-42 (AD model) | Kelulut honey | 2.5 µg/µL Aβ1-42 (icv) 1 week before 1 g/kg bw/d honey (i.g.) for 28 days | = p-NF-κB levels in hippocampus | [41] |
| Apoptosis | ||||
| In vivo. Male C57BL/6 mice injured with HFD | Chestnut honey | 45 mg/d (orally) for 10 weeks | ↓ Number of apoptotic cells in cortex ↓ FAS-L, P27, and BIM gene expression ↑ BCL2 gene expression | [13] |
| In vivo. Male Wistar rats injured with HFD | Stingless Bee Honey | HFD for 8 weeks, followed by HFD with 10 mg/kg bw/d honey (i.g.) for another 8 weeks | = Number of apoptotic cells | [44] |
| In vivo. Male Sprague Dawley rats injured with Aβ1-42 (AD model) | Kelulut honey | 2.5 µg/µL Aβ1-42 (icv) 1 week before 1 g/kg bw/d honey (i.g.) for 28 days | ↓ Number of apoptotic cells in dentate gyrus, CA1 and CA3 areas | [41] |
| Aβ plaque damage and APP processing | ||||
| In vivo. C. elegans transgenic strain (CL4176, Aβ model) | Manuka honey | 100 mg/mL for 72 h | ↓ Aβ-induced paralysis phenotype ↓ Aβ deposits | [17] |
| In vivo. C. elegans transgenic strain (CL4176, Aβ model) | Avocado honey | 100 mg/mL for 72 h | ↓ Aβ-induced paralysis phenotype | [18] |
| In vivo. Male C57BL/6 mice injured with HFD | Chestnut honey | 45 mg/d (orally) for 10 weeks | ↓ Expression of genes related to APP generation and processing | [13] |
| In vivo. Male Sprague Dawley rats injured with LPS | Tualang honey | 200 mg/kg tualang honey (i.p.) for 14 days, 5 mg/kg LPS (i.p.) applied on day 4 | ↓ Aβ1−40 levels ↑ Aβ1−42 levels | [38] |
| In vivo. Male Sprague Dawley rats injured with Aβ1-42 (AD model) | Kelulut honey | 2.5 µg/µL Aβ1-42 (icv) 1 week before 1 g/kg bw/d honey (i.g.) for 28 days | ↓ Aβ1−42 depositions in dentate gyrus area = Aβ1−42 depositions in CA1 and CA3 areas | [41] |
| Hyperphosphorylated tau protein damage | ||||
| In vivo. C. elegans transgenic strain (BR5706, tauopathy model) | Manuka honey | 100 mg/mL for 72 h | ↓ Mobility parameters | [17] |
| In vivo. C. elegans transgenic strain (BR5706, tauopathy model) | Avocado honey | 100 mg/mL for 72 h | ↓ Mobility parameters | [18] |
| In vivo. Male C57BL/6 mice injured with HFD | Chestnut honey | 45 mg/d (orally) for 10 weeks | ↓ Expression of genes related to tau protein | [13] |
| In vivo. Male Sprague Dawley rats injured with Aβ1-42 (AD model) | Kelulut honey | 2.5 µg/µL Aβ1-42 (icv) 1 week before 1 g/kg bw/d honey (i.g.) for 28 days | ↓ p-Tau levels in hippocampus | [41] |
| Imbalance of neurotransmitters and elimination-related enzymes | ||||
| In vitro | Polish honeys | Buckwheat honey (AChE inhibition 39.51%) Multi-floral honey (BChE inhibition 39.76%) | ↓ AChE and BChE activity | [46] |
| In vitro | Acacia, raspberry, lavender, bean, buckwheat, aloe, heather, linden, eucalyptus, sunflower, goldenrod, linden, thyme, rape, rosemary, hawthorn, orange blossom honeys | ↓ AChE and BChE activity | [47] | |
| In vitro | Acacia honey | IC50 = 0.26% (v/v) | ↓ AChE activity | [48] |
| In vitro: Enzyme isolated from rat liver microsomes | Chestnut honey | IC50 = 41.60 µg/mL | ↓ MAO activity | [49] |
| In vivo. Male Wistar rats | Acacia honey | 20% v/v (orally) for 1 week | ↓ AChE activity in brain and in serum | [48] |
| In vivo. Male Wistar rats injured with LPS | Honey | 250 µg/kg LPS (i.p) for 7 days followed by 0.31 and 0.36 g/kg honey for other 7 days | ↓ AChE activity | [36] |
| In vivo. Aged male Sprague Dawley rats | Tualang honey | 200 mg/kg bw/day (i.g.) 28 days | ↓ AChE activity | [39] |
| Model | Treatment | Dosage and Duration | Effects | Reference |
|---|---|---|---|---|
| Oxidative stress | ||||
| In vitro. N13 microglial cells injured with LPS | Flavonoid extract of multifloral honey | 0.5 and 1 μg/mL for 30 min before 2.5 ng/mL LPS for 1 h | ↓ ROS content | [19] |
| In vitro. HT22 cells injured with glutamate | Ethyl acetate Fraction of chestnut honey | 250, 500, or 750 μg/mL for 24 h followed by 5 mM glutamate for 6 h | ↓ ROS content ↑ HO-1 and GCLC protein expression | [50] |
| In vivo. Male Sprague Dawley rats injured with LPS | Phenolic extract of tualang honey | 150 mg/kg extract (i.p.) for 14 days, 5 mg/kg LPS (i.p.) applied on day 4 | ↑ CAT and GPx levels | [38] |
| Mitochondrial dysfunction | ||||
| In vitro. HT22 cells injured with glutamate | Ethyl acetate Fraction of chestnut honey | 500, or 750 μg/mL for 24 h followed by 5 mM glutamate for 6 h | ↑ Mitochondrial membrane potential | [50] |
| Neuroinflammation | ||||
| In vitro. N13 cell culture injured with LPS | Flavonoid extract of multifloral honey | 0.5 and 1 μg/mL, 30 min/6 h | ↓ TNF-α, IL-1β, iNOS mRNA levels ↓ iNOS protein levels | [19] |
| In vitro. RAW 264.7 macrophages injured with LPS | Methanolic extract of saffron honey | 3.4 or 6.8 µg/mL for 2 h, followed by 1 µg/mL LPS for 22 h | ↓ NO production ↓ NF-κB, IL-6 and TNFSF9 gene expression | [51] |
| In vitro. RAW 264.7 macrophages injured with LPS | Methanolic extract of eryngium honeys | 5.5 or 11 µg/mL for 2 h, followed by 1 µg/mL LPS for 22 h | ↓ NO production ↓ NF-κB and TNFSF9 gene expression ↑ Nrf-2 gene expression | [51] |
| Apoptosis | ||||
| In vitro. HT22 cells injured with glutamate | Ethyl acetate Fraction of chestnut honey | 500, or 750 μg/mL for 24 h followed by 5 mM glutamate for 24 h | ↓ Cell death ↓ AIF protein expression ↑ Bcl-2 protein expression | [50] |
| In vitro. RAW 264.7 macrophages injured with LPS | Methanolic extract of saffron and eryngium honeys | 3.4 or 6.8 µg/mL (saffron) or 5.5 or 11 µg/mL (eryngium) for 2 h, followed by 1 µg/mL LPS for 22 h | ↓ Bax gene expression ↑ Bcl-2 gene expression | [51] |
| Aβ plaque damage and APP processing | ||||
| In vivo. Male Sprague Dawley rats injured with LPS | Phenolic extract of tualang honey | 150 mg/kg extract (i.p.) for 14 days, 5 mg/kg LPS (i.p.) applied on day 4 | ↓ Aβ1−40 levels ↑ Aβ1−42 levels | [38] |
| Imbalance of neurotransmitters and elimination-related enzymes | ||||
| In vitro | Phenolic extract of Algerian honeys | IC50 = 0.367–0.629 mg/mL | ↓ AChE activity | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Ansary, J.; Hinojosa-Nogueira, D.; Montalbán-Hernández, C.; Varela-López, A.; Quiles, J.L. Honey as a Neuroprotective Agent: Molecular Perspectives on Its Role in Alzheimer’s Disease. Nutrients 2025, 17, 2577. https://doi.org/10.3390/nu17162577
Navarro-Hortal MD, Romero-Márquez JM, Ansary J, Hinojosa-Nogueira D, Montalbán-Hernández C, Varela-López A, Quiles JL. Honey as a Neuroprotective Agent: Molecular Perspectives on Its Role in Alzheimer’s Disease. Nutrients. 2025; 17(16):2577. https://doi.org/10.3390/nu17162577
Chicago/Turabian StyleNavarro-Hortal, María D., Jose M. Romero-Márquez, Johura Ansary, Daniel Hinojosa-Nogueira, Cristina Montalbán-Hernández, Alfonso Varela-López, and José L. Quiles. 2025. "Honey as a Neuroprotective Agent: Molecular Perspectives on Its Role in Alzheimer’s Disease" Nutrients 17, no. 16: 2577. https://doi.org/10.3390/nu17162577
APA StyleNavarro-Hortal, M. D., Romero-Márquez, J. M., Ansary, J., Hinojosa-Nogueira, D., Montalbán-Hernández, C., Varela-López, A., & Quiles, J. L. (2025). Honey as a Neuroprotective Agent: Molecular Perspectives on Its Role in Alzheimer’s Disease. Nutrients, 17(16), 2577. https://doi.org/10.3390/nu17162577












