Gut-Microbiota-Derived Metabolites and Probiotic Strategies in Colorectal Cancer: Implications for Disease Modulation and Precision Therapy
Abstract
1. Introduction
| SCFA 1 | Primary Function | Physiological Effects 2 | Reference |
|---|---|---|---|
| Acetate | Substrate for lipogenesis and cholesterol synthesis | Regulates appetite via hypothalamic signaling, contributes to anti-inflammatory effects via GPR43 activation | [34,35] |
| Propionate | Gluconeogenic substrate in the liver | Lowers blood glucose and inhibits cholesterol synthesis via PPAR-γ activation; modulates intestinal gluconeogenesis | [36,37] |
| Butyrate | Primary energy source for colonocytes | Promotes Treg differentiation via HDAC inhibition; enhances epithelial barrier integrity; anti-cancer via gene regulation | [18,19,38,39] |
2. Methodology and Analysis
2.1. Literature Search Strategy
2.2. Literature Overview Approach
2.3. Analytical Approach
3. Gut Microbiota in Health and Disease
3.1. Homeostatic Functions in the Healthy Host
3.2. Microbiota–Metabolite Interactions Across Host Systems
3.2.1. Gastrointestinal and Metabolic Disorders
3.2.2. Neuroimmune and CNS Disorders
3.2.3. Cardiovascular and Renal Systems
3.3. The Pivotal Role of SCFAs
4. Probiotics and Therapeutic Microbial Modulation
4.1. Core Mechanisms of Probiotic Action
4.2. Probiotic Interventions in Disease-Specific Contexts
4.2.1. CRC
4.2.2. Probiotic Applications in Inflammatory and Functional Bowel Disorders
4.2.3. PD
4.2.4. Other Systemic Disorders
5. Butyrate and CRC
5.1. Microbial Metabolite–Host Signaling Pathways in Tumor Suppression
5.2. Dietary and Functional Approaches
6. Discussion
6.1. SCFA and Probiotics in CRC
6.2. Critical Evaluation of Evidence
| Study Context 1 | Intervention | Key Outcomes 2 | Limitations | Disease Focus/Reference | |
|---|---|---|---|---|---|
| DSS-induced colitis (mouse) | Sodium butyrate | ↑ Barrier integrity, ↓ TNF-α, ↑ Treg cells (via HDAC inhibition) | Acute inflammation model; lacks microbiota diversity | IBD/[94] | |
| ApcMin/+ mouse model (CRC) | Clostridium butyricum | ↓ Tumor burden, ↓ IL-6, ↑ Foxp3 expression, HDAC inhibition | No human microbiota or diet variability | CRC/[38] | |
| CRC patients (perioperative RCT) | L. rhamnosus GG/ B. longum | ↓ Postoperative infection, ↑ IL-10, improved epithelial barrier markers | Small cohort; mixed probiotic strains | CRC/[48] | |
| Ulcerative colitis patients (RCT) | VSL#3 probiotic mixture | ↑ Remission maintenance, ↓ IL-6 and TNF-α | Mixed response in Crohn’s vs. UC; strain-specificity unclear | IBD/[75] | |
| IBS patients (RCT) | L. plantarum/B. breve | ↓ Bloating and discomfort, improved quality of life | Placebo response possible; symptom-based endpoints | IBS/[115] | |
| CKD mouse model | Synbiotic (inulin + Bifidobacterium) | ↓ Uremic toxins, ↑ tight junction protein expression | Model does not replicate full human CKD complexity | CKD/[90] | |
| PD patients (pilot trial) | Multi-strain probiotic | ↓ Constipation, ↑ SCFA levels, ↓ systemic inflammation | Short duration; no direct neurofunctional endpoints | PD/[107] | |
| Neuropsychiatric disorder patients (RCT) | L. helveticus/B. longum | ↓ Anxiety/depression scores; modulated cytokines (IL-6, TNF-α) | Small sample; microbiota composition not stratified | CNS/[147] | |
6.3. Methodological and Translational Limitations
6.3.1. Methodological Limitations in the Literature
6.3.2. Inconsistencies and Failures in Probiotic Trials
6.4. Future Directions and Personalized Microbiome Therapeutics
6.4.1. Rigorous Clinical Trials
6.4.2. Integration of Multi-Omics
6.4.3. Diet–Microbiota Interactions
6.4.4. Regulatory and Implementation Frameworks
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCFA | Short-chain fatty acid |
| CRC | Colorectal cancer |
| IBD | Inflammatory bowel disease |
| PD | Parkinson’s disease |
| GABA | gamma-aminobutyric acid |
| IBS | Irritable bowel syndrome |
| LDL | Low-density lipoprotein |
| CKD | Chronic kidney disease |
| HDACs | Histone deacetylases |
| CSE1L | Chromosome segregation 1 Like |
| PLAC8 | Placenta-associated 8 |
| FMT | fecal microbiota transplantation |
| SLC5A8 | solute carrier family 5 member 8 |
| GPRs | G protein-coupled receptors |
References
- Coker, O.O. Non-bacteria microbiome (virus, fungi, and archaea) in gastrointestinal cancer. J. Gastroenterol. Hepatol. 2022, 37, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bonete, M.J.; Rajan, A.; Suriano, F.; Layunta, E. The Underrated Gut Microbiota Helminths, Bacteriophages, Fungi, and Archaea. Life 2023, 13, 1765. [Google Scholar] [CrossRef] [PubMed]
- Golshany, H.; Helmy, S.A.; Morsy, N.F.S.; Kamal, A.; Yu, Q.; Fan, L. The gut microbiome across the lifespan: How diet modulates our microbial ecosystem from infancy to the elderly. Int. J. Food Sci. Nutr. 2025, 76, 95–121. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Dai, L.; Mafra, D.; Shiels, P.G.; Hackeng, T.M.; Stenvinkel, P.; Schurgers, L.J. Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome. Nutrients 2023, 15, 2727. [Google Scholar] [CrossRef]
- Degnan, P.H.; Taga, M.E.; Goodman, A.L. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014, 20, 769–778. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Hsu, T.; Sirota-Madi, A.; Shafquat, A.; Abu-Ali, G.; Morgan, X.C.; Huttenhower, C. Sequencing and beyond: Integrating molecular ‘omics’ for microbial community profiling. Nat. Rev. Microbiol. 2015, 13, 360–372. [Google Scholar] [CrossRef]
- Jo, J.; Oh, J.; Park, C. Microbial community analysis using high-throughput sequencing technology: A beginner’s guide for microbiologists. J. Microbiol. 2020, 58, 176–192. [Google Scholar] [CrossRef]
- Dason, M.S.; Cora, D.; Re, A. Sequence modeling tools to decode the biosynthetic diversity of the human microbiome. mSystems 2025, 10, e0033325. [Google Scholar] [CrossRef]
- Wu, G.; Xu, T.; Zhao, N.; Lam, Y.Y.; Ding, X.; Wei, D.; Fan, J.; Shi, Y.; Li, X.; Li, M.; et al. A core microbiome signature as an indicator of health. Cell 2024, 187, 6550–6565.e11. [Google Scholar] [CrossRef]
- Ojeda, P.; Bobe, A.; Dolan, K.; Leone, V.; Martinez, K. Nutritional modulation of gut microbiota—The impact on metabolic disease pathophysiology. J. Nutr. Biochem. 2016, 28, 191–200. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A.; Rogovsky, V.S. Role of Neurochemicals in the Interaction between the Microbiota and the Immune and the Nervous System of the Host Organism. Probiotics Antimicrob Proteins 2017, 9, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Backhed, F. The gut microbiota–Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Singh, R.; Ro, S.; Ghoshal, U.C. Gut microbiota dysbiosis in functional gastrointestinal disorders: Underpinning the symptoms and pathophysiology. JGH Open 2021, 5, 976–987. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes 2024, 16, 2304900. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Gheorghe, A.S.; Negru, S.M.; Preda, M.; Mihaila, R.I.; Komporaly, I.A.; Dumitrescu, E.A.; Lungulescu, C.V.; Kajanto, L.A.; Georgescu, B.; Radu, E.A.; et al. Biochemical and Metabolical Pathways Associated with Microbiota-Derived Butyrate in Colorectal Cancer and Omega-3 Fatty Acids Implications: A Narrative Review. Nutrients 2022, 14, 1152. [Google Scholar] [CrossRef]
- Lama Tamang, R.; Juritsch, A.F.; Ahmad, R.; Salomon, J.D.; Dhawan, P.; Ramer-Tait, A.E.; Singh, A.B. The diet-microbiota axis: A key regulator of intestinal permeability in human health and disease. Tissue Barriers 2023, 11, 2077069. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Su, R. Interplay of human gastrointestinal microbiota metabolites: Short-chain fatty acids and their correlation with Parkinson’s disease. Medicine 2024, 103, e37960. [Google Scholar] [CrossRef]
- Inamoto, T.; Furuta, K.; Han, C.; Uneme, M.; Kano, T.; Ishikawa, K.; Kaito, C. Short-chain fatty acids stimulate dendrite elongation in dendritic cells by inhibiting histone deacetylase. FEBS J. 2023, 290, 5794–5810. [Google Scholar] [CrossRef]
- Fawad, J.A.; Luzader, D.H.; Hanson, G.F.; Moutinho, T.J., Jr.; McKinney, C.A.; Mitchell, P.G.; Brown-Steinke, K.; Kumar, A.; Park, M.; Lee, S.; et al. Histone Deacetylase Inhibition by Gut Microbe-Generated Short-Chain Fatty Acids Entrains Intestinal Epithelial Circadian Rhythms. Gastroenterology 2022, 163, 1377–1390.e11. [Google Scholar] [CrossRef]
- Song, L.; Sun, Q.; Zheng, H.; Zhang, Y.; Wang, Y.; Liu, S.; Duan, L. Roseburia hominis Alleviates Neuroinflammation via Short-Chain Fatty Acids through Histone Deacetylase Inhibition. Mol. Nutr. Food Res. 2022, 66, e2200164. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, C.; Zhang, Q.; Lv, Q.; Liu, H.; Yuan, H.; Wang, C.; Meng, F.; Guo, Y.; Pei, J.; et al. Gut microbiota modulates depressive-like behaviors induced by chronic ethanol exposure through short-chain fatty acids. J. Neuroinflamm. 2024, 21, 290. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, X.; Liu, M.; Yang, J.; Yuan, M.; Sun, C.; Zhou, Q.; Chen, J.; Liu, B. Bile acid and short chain fatty acid metabolism of gut microbiota mediate high-fat diet induced intestinal barrier damage in Macrobrachium rosenbergii. Fish Shellfish Immunol. 2024, 146, 109376. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.X.; Lv, E.H.; Wen, P.B.; Liu, X.; Wang, Y.T.; Cai, X.C.; Tian, J.Q.; et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Im, S.H. The gut-immune-brain axis in neurodevelopment and neurological disorders. Microbiome Res. Rep. 2022, 1, 23. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Ren, Q.; Zhu, C.; Du, H.; Wang, Z.; Qi, Y.; Xian, X.; Chen, D. Gut microbiota as a biomarker and modulator of anti-tumor immunotherapy outcomes. Front. Immunol. 2024, 15, 1471273. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Ugwu, O.P.; Alum, E.U.; Okon, M.B.; Obeagu, E.I. Mechanisms of microbiota modulation: Implications for health, disease, and therapeutic interventions. Medicine 2024, 103, e38088. [Google Scholar] [CrossRef]
- Schupack, D.A.; Mars, R.A.T.; Voelker, D.H.; Abeykoon, J.P.; Kashyap, P.C. The promise of the gut microbiome as part of individualized treatment strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 7–25. [Google Scholar] [CrossRef]
- Mandaliya, D.K.; Patel, S.; Seshadri, S. The Combinatorial Effect of Acetate and Propionate on High-Fat Diet Induced Diabetic Inflammation or Metaflammation and T Cell Polarization. Inflammation 2021, 44, 68–79. [Google Scholar] [CrossRef]
- John, H.S.; Doucet, E.; Power, K.A. Dietary pulses as a means to improve the gut microbiome, inflammation, and appetite control in obesity. Obes. Rev. 2023, 24, e13598. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, J.; Bai, X.; Jiang, Y.; Li, J.; Fan, S.; Cheng, Y.; Jiang, G. Propionate ameliorates diabetes-induced neurological dysfunction through regulating the PI3K/Akt/eNOS signaling pathway. Eur. J Pharmacol. 2022, 925, 174974. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; An, Y.; Du, Y.; Song, Y.; Zhao, Q.; Lu, Y. Effects of short-chain fatty acids on blood glucose and lipid levels in mouse models of diabetes mellitus: A systematic review and network meta-analysis. Pharmacol. Res. 2024, 199, 107041. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Kao, W.Y.; Liu, C.Y.; Su, H.H.; Kan, Y.A.; Lin, P.Y.; Ku, W.C.; Chang, K.W.; Yang, R.N.; Huang, C.J. Butyrate supplementation regulates expression of chromosome segregation 1-like protein to reverse the genetic distortion caused by p53 mutations in colorectal cancer. Int. J. Oncol. 2022, 60, 64. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Barba-Ostria, C.; Simancas-Racines, D.; Guaman, L.P. Protective role of butyrate in obesity and diabetes: New insights. Front. Nutr. 2022, 9, 1067647. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Kitamoto, S.; Kuffa, P.; Kamada, N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest. Res. 2016, 14, 127–138. [Google Scholar] [CrossRef]
- Huang, C.C.; Shen, M.H.; Chen, S.K.; Yang, S.H.; Liu, C.Y.; Guo, J.W.; Chang, K.W.; Huang, C.J. Gut butyrate-producing organisms correlate to Placenta Specific 8 protein: Importance to colorectal cancer progression. J. Adv. Res. 2020, 22, 7–20. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Chang, S.C.; Shen, M.H.; Liu, C.Y.; Pu, C.M.; Hu, J.M.; Huang, C.J. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer. Oncol. Lett. 2020, 20, 327. [Google Scholar] [CrossRef]
- Cheong, Y.J.; Trefely, S. Divergent roles for propionate and butyrate in colorectal cancer epigenetics. Nat. Metab. 2025, 7, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Thulasinathan, B.; Suvilesh, K.N.; Maram, S.; Grossmann, E.; Ghouri, Y.; Teixeiro, E.P.; Chan, J.; Kaif, J.T.; Rachagani, S. The impact of gut microbial short-chain fatty acids on colorectal cancer development and prevention. Gut Microbes 2025, 17, 2483780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 58 (Suppl. S10), S164–S179. [Google Scholar] [CrossRef]
- Luangphiphat, W.; Prombutara, P.; Jamjuree, P.; Chantarangkul, C.; Vitheejongjaroen, P.; Muennarong, C.; Fukfon, K.; Onwan, M.; Taweechotipatr, M. The efficacy of Lacticaseibacillus paracasei MSMC39-1 and Bifidobacterium animalis TA-1 probiotics in modulating gut microbiota and reducing the risk of the characteristics of metabolic syndrome: A randomized, double-blinded, placebo-controlled study. PLoS ONE 2025, 20, e0317202. [Google Scholar] [CrossRef]
- Karam, F.; El Deghel, Y.; Iratni, R.; Dakroub, A.H.; Eid, A.H. The Gut Microbiome and Colorectal Cancer: An Integrative Review of the Underlying Mechanisms. Cell Biochem. Biophys. 2025, 83, 1–14. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Caesar, R. The impact of novel probiotics isolated from the human gut on the gut microbiota and health. Diabetes Obes. Metab. 2025, 27 (Suppl. S1), 3–14. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.K.; Florman, J.T.; Araya, A.; Fox, B.W.; Thackeray, A.; Schroeder, F.C.; Walhout, A.J.M.; Alkema, M.J. Vitamin B12 produced by gut bacteria modulates cholinergic signalling. Nat. Cell Biol. 2024, 26, 72–85. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e10. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Yang, X.; Li, Y.; Wang, Y.; Du, Y.; Wang, M.; Ye, R.; Wang, J.; Zhang, Y.; Chen, Y.; et al. Gut microbiota regulates gut homeostasis, mucosal immunity and influences immune-related diseases. Mol. Cell. Biochem. 2025, 480, 1969–1981. [Google Scholar] [CrossRef]
- Martin, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermudez-Humaran, L.G.; Sokol, H.; Chatel, J.M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Winter, S.E.; Baumler, A.J. Gut dysbiosis: Ecological causes and causative effects on human disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2316579120. [Google Scholar] [CrossRef]
- Singh, G.; Chaudhry, Z.; Boyadzhyan, A.; Sasaninia, K.; Rai, V. Dysbiosis and colorectal cancer: Conducive factors, biological and molecular role, and therapeutic prospectives. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002329. [Google Scholar] [CrossRef]
- Leo, S.; Leonard, M.M.; Valitutti, F.; Fasano, A. Gut dysbiosis: Cause or consequence of intestinal inflammation in celiac disease? Expert Rev. Gastroenterol. Hepatol. 2025, 19, 505–513. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Zgair, A.K. Escherichia coli flagellin stimulates pro-inflammatory immune response. World J. Microbiol. Biotechnol. 2012, 28, 2139–2146. [Google Scholar] [CrossRef]
- Quevrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermudez-Humaran, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Tu, V.; Tanes, C.; Wilson, N.; Quinn, R.; Kachelries, K.; Friedman, E.S.; Bittinger, K.; Baldassano, R.N.; Compher, C.; et al. A pro-inflammatory diet is associated with growth and virulence of Escherichia coli in pediatric Crohn’s disease. J. Crohn’s Colitis 2025, 19, jjaf018. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sun, S.; Fu, Q. The role of short-chain fatty acid in metabolic syndrome and its complications: Focusing on immunity and inflammation. Front. Immunol. 2025, 16, 1519925. [Google Scholar]
- Protasiewicz-Timofticiuc, D.C.; Badescu, D.; Mota, M.; Stefan, A.G.; Mitrea, A.; Clenciu, D.; Efrem, I.C.; Rosu, M.M.; Vladu, B.E.; Gheonea, T.C.; et al. Back to Roots: Dysbiosis, Obesity, Metabolic Syndrome, Type 2 Diabetes Mellitus, and Obstructive Sleep Apnea-Is There an Objective Connection? A Narrative Review. Nutrients 2024, 16, 4057. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; Ramirez-Moreno, E.; Quintero-Lira, A.; Contreras-Lopez, E.; Jaimez-Ordaz, J.; Castaneda-Ovando, A.; Anorve-Morga, J.; Calderon-Ramos, Z.G.; Arias-Rico, J.; et al. Impact of the Gut Microbiota Balance on the Health-Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods 2021, 10, 1261. [Google Scholar] [CrossRef]
- Pham, N.H.T.; Joglekar, M.V.; Wong, W.K.M.; Nassif, N.T.; Simpson, A.M.; Hardikar, A.A. Short-chain fatty acids and insulin sensitivity: A systematic review and meta-analysis. Nutr. Rev. 2024, 82, 193–209. [Google Scholar] [CrossRef]
- De Filippis, A.; Ullah, H.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Gastrointestinal Disorders and Metabolic Syndrome: Dysbiosis as a Key Link and Common Bioactive Dietary Components Useful for their Treatment. Int. J. Mol. Sci. 2020, 21, 4929. [Google Scholar] [CrossRef]
- Perez-Reytor, D.; Puebla, C.; Karahanian, E.; Garcia, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Yang, R.; Hu, X.; Xie, X.; Chen, H.; Fang, H.; Zhu, L.; Li, Z. Propionic Acid Targets the TLR4/NF-kappaB Signaling Pathway and Inhibits LPS-Induced Intestinal Barrier Dysfunction: In Vitro and In Vivo Studies. Front. Pharmacol. 2020, 11, 573475. [Google Scholar]
- Kaltsas, A.; Giannakodimos, I.; Markou, E.; Adamos, K.; Stavropoulos, M.; Kratiras, Z.; Zachariou, A.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M. The Role of Gut Microbiota Dysbiosis in Erectile Dysfunction: From Pathophysiology to Treatment Strategies. Microorganisms 2025, 13, 250. [Google Scholar] [CrossRef]
- Agagunduz, D.; Celik, E.; Cemali, O.; Bingol, F.G.; Ozenir, C.; Ozogul, F.; Capasso, R. Probiotics, Live Biotherapeutic Products (LBPs), and Gut-Brain Axis Related Psychological Conditions: Implications for Research and Dietetics. Probiotics Antimicrob Proteins 2023, 15, 1014–1031. [Google Scholar] [CrossRef]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food. Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Roles of the gut microbiota in human neurodevelopment and adult brain disorders. Front. Neurosci. 2024, 18, 1446700. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef]
- Leng, X.; Wei, X.; Wang, J.; Yao, X.; Zhang, M.; Sun, D.; Liang, J.; Chi, L.; Cheng, Y. Impacts of intestinal microbiota metabolite trimethylamine N-oxide on cardiovascular disease: A bibliometric analysis. Front. Microbiol. 2024, 15, 1491731. [Google Scholar] [CrossRef]
- Ghanbari, F.; Hasani, S.; Aghili, Z.S.; Asgary, S. The potential preventive effect of probiotics, prebiotics, and synbiotics on cardiovascular risk factors through modulation of gut microbiota: A review. Food Sci. Nutr. 2024, 12, 4569–4580. [Google Scholar] [CrossRef]
- Dawson, S.L.; Todd, E.; Ward, A.C. The Interplay of Nutrition, the Gut Microbiota and Immunity and Its Contribution to Human Disease. Biomedicines 2025, 13, 329. [Google Scholar] [CrossRef]
- Forte, N.; Marfella, B.; Nicois, A.; Palomba, L.; Paris, D.; Motta, A.; Pina Mollica, M.; Di Marzo, V.; Cristino, L. The short-chain fatty acid acetate modulates orexin/hypocretin neurons: A novel mechanism in gut-brain axis regulation of energy homeostasis and feeding. Biochem. Pharmacol. 2024, 226, 116383. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, A.; Wang, H.; Ma, J.; Hou, J. Positive Regulation of Acetate in Adipocyte Differentiation and Lipid Deposition in Obese Mice. Nutrients 2023, 15, 3736. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Y.Y.; Li, S.P.; Zhao, H.M.; Jiang, F.J.; Wu, Y.X.; Tong, Y.; Pang, Q.F. Maternal propionate supplementation ameliorates glucose and lipid metabolic disturbance in hypoxia-induced fetal growth restriction. Food Funct. 2022, 13, 10724–10736. [Google Scholar] [CrossRef]
- He, L.; Zhong, Z.; Wen, S.; Li, P.; Jiang, Q.; Liu, F. Gut microbiota-derived butyrate restores impaired regulatory T cells in patients with AChR myasthenia gravis via mTOR-mediated autophagy. Cell Commun. Signal. 2024, 22, 215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel. Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Sun, P.; Liu, J.; Chen, G.; Guo, Y. The Role of G Protein-Coupled Receptors in the Regulation of Orthopaedic Diseases by Gut Microbiota. Nutrients 2025, 17, 1702. [Google Scholar] [CrossRef]
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The Microbiome and Blood Pressure: Can Microbes Regulate Our Blood Pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef]
- Ribeiro, F.P.B.; de Luna Freire, M.O.; de Oliveira Coutinho, D.; de Santana Cirilo, M.A.; de Brito Alves, J.L. Gut Dysbiosis and Probiotic Therapy in Chronic Kidney Disease: A Comprehensive Review. Probiotics Antimicrob Proteins 2024, 16, 1–23. [Google Scholar] [CrossRef]
- Wang, Y.C.; Ku, W.C.; Liu, C.Y.; Cheng, Y.C.; Chien, C.C.; Chang, K.W.; Huang, C.J. Supplementation of Probiotic Butyricicoccus pullicaecorum Mediates Anticancer Effect on Bladder Urothelial Cells by Regulating Butyrate-Responsive Molecular Signatures. Diagnostics 2021, 11, 2270. [Google Scholar] [CrossRef]
- Kopczynska, J.; Kowalczyk, M. The potential of short-chain fatty acid epigenetic regulation in chronic low-grade inflammation and obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef]
- Dang, G.; Wu, W.; Zhang, H.; Everaert, N. A new paradigm for a new simple chemical: Butyrate & immune regulation. Food Funct. 2021, 12, 12181–12193. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, H.; Guo, X.; Zhao, H.; Wang, J.; Li, J.; He, J.; Huang, H.; Huang, C.; Zhao, C.; et al. Pretreatment with an antibiotics cocktail enhances the protective effect of probiotics by regulating SCFA metabolism and Th1/Th2/Th17 cell immune responses. BMC Microbiol. 2024, 24, 91. [Google Scholar] [CrossRef] [PubMed]
- Agbemavor, W.S.K.; Buys, E.M. Dynamic Interactions between Diarrhoeagenic Enteroaggregative Escherichia coli and Presumptive Probiotic Bacteria: Implications for Gastrointestinal Health. Microorganisms 2023, 11, 2942. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, B.A.; Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef]
- Heiss, B.E.; Ehrlich, A.M.; Maldonado-Gomez, M.X.; Taft, D.H.; Larke, J.A.; Goodson, M.L.; Slupsky, C.M.; Tancredi, D.J.; Raybould, H.E.; Mills, D.A. Bifidobacterium catabolism of human milk oligosaccharides overrides endogenous competitive exclusion driving colonization and protection. Gut Microbes. 2021, 13, 1986666. [Google Scholar] [CrossRef]
- Baradaran Ghavami, S.; Asadzadeh Aghdaei, H.; Sorrentino, D.; Shahrokh, S.; Farmani, M.; Ashrafian, F.; Dore, M.P.; Keshavarz Azizi Raftar, S.; Mobin Khoramjoo, S.; Zali, M.R. Probiotic-Induced Tolerogenic Dendritic Cells: A Novel Therapy for Inflammatory Bowel Disease? Int. J. Mol. Sci. 2021, 22, 8274. [Google Scholar] [CrossRef]
- Liu, H.Y.; Li, S.; Ogamune, K.J.; Yuan, P.; Shi, X.; Ennab, W.; Ahmed, A.A.; Kim, I.H.; Hu, P.; Cai, D. Probiotic Lactobacillus johnsonii Reduces Intestinal Inflammation and Rebalances Splenic Treg/Th17 Responses in Dextran Sulfate Sodium-Induced Colitis. Antioxidants 2025, 14, 433. [Google Scholar] [CrossRef]
- Inatomi, T.; Honma, M. Ameliorating effect of probiotics in a rat model of chronic kidney disease. PLoS ONE 2023, 18, e0281745. [Google Scholar] [CrossRef]
- Qiu, X.; Han, X.; Zhang, X.; Teng, L.A.; Sriwastva, M.K.; Zhen, L.; Li, Z.; Liu, M.; Ren, Y.; Wang, S. Lactobacillus rhamnosus GG alleviates colitis caused by chemotherapy via biofilm formation. J. Gastroenterol. Hepatol. 2023, 38, 1158–1169. [Google Scholar] [CrossRef]
- Boehme, M.; Remond-Derbez, N.; Lerond, C.; Lavalle, L.; Keddani, S.; Steinmann, M.; Rytz, A.; Dalile, B.; Verbeke, K.; Van Oudenhove, L.; et al. Bifidobacterium longum subsp. longum Reduces Perceived Psychological Stress in Healthy Adults: An Exploratory Clinical Trial. Nutrients 2023, 15, 3122. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Hu, Y.; Lin, T.; Gao, F.; Xu, Z.; Hou, X.; Yin, Y.; Kan, S.; Zhu, H.; Chen, D. Selenium-enriched Bifidobacterium longum DD98 relieves irritable bowel syndrome induced by chronic unpredictable mild stress in mice. Food Funct. 2023, 14, 5355–5374. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shin, J.; Kim, S.; Kim, S.; Cho, B.; Park, S.J.; Park, G.; Shin, H.; Park, M.S.; Kim, J. Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI promotes neuronal rejuvenation in aged mice. Biochem. Biophys. Res. Commun. 2022, 603, 41–48. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, F.; James, K.; Cottenet, G.; Dominick, M.; Katja, J. Combining Bifidobacterium longum subsp. infantis and human milk oligosaccharides synergistically increases short chain fatty acid production ex vivo. Commun. Biol. 2024, 7, 943. [Google Scholar] [CrossRef]
- Liu, T.; Song, X.; An, Y.; Wu, X.; Zhang, W.; Li, J.; Sun, Y.; Jin, G.; Liu, X.; Guo, Z.; et al. Lactobacillus rhamnosus GG Colonization in Early Life Ameliorates Inflammaging of Offspring by Activating SIRT1/AMPK/PGC-1alpha Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 3328505. [Google Scholar] [CrossRef]
- Qu, S.; Yu, Z.; Zhou, Y.; Wang, S.; Jia, M.; Chen, T.; Zhang, X. Gut microbiota modulates neurotransmitter and gut-brain signaling. Microbiol. Res. 2024, 287, 127858. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Ramesh, A.; Srinivasan, D.; Subbarayan, R.; Chauhan, A.; Krishnamoorthy, L.; Kumar, J.; Krishnan, M.; Shrestha, R. Enhancing Colorectal Cancer Treatment: The Role of Bifidobacterium in Modulating Gut Immunity and Mitigating Capecitabine-Induced Toxicity. Mol. Nutr. Food Res. 2025, 69, e70023. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Mankowska-Wierzbicka, D.; Mardas, M.; Stelmach-Mardas, M. Role of Probiotics in Modulating Human Gut Microbiota Populations and Activities in Patients with Colorectal Cancer-A Systematic Review of Clinical Trials. Nutrients 2021, 13, 1160. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Liu, C.Y.; Su, I.C.; Lee, Y.J.; Yeh, H.J.; Chen, W.C.; Yu, C.J.; Kao, W.Y.; Liu, Y.C.; Huang, C.J. Functional Plasmon-Activated Water Increases Akkermansia muciniphila Abundance in Gut Microbiota to Ameliorate Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 11422. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zheng, Q.; Li, L. The Efficacy of Probiotics, Prebiotics, Synbiotics, and Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Nutrients 2024, 16, 2114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Zhang, J.; Sun, F.; Duan, L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 859967. [Google Scholar] [CrossRef]
- Chang, Y.H.; Choi, Y.J.; Shin, C.M.; Moon, J.S.; Kim, T.Y.; Yoon, H.; Park, Y.S.; Kim, N.; Lee, D.H. Efficacy of Quadruple-coated Probiotics in Patients With Irritable Bowel Syndrome: A Randomized, Double-blind, Placebo-controlled, Parallel-group Study. J. Neurogastroenterol. Motil. 2024, 30, 73–86. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, D.; Huang, J.; Liu, K.; Liu, H.; Wu, H.; Bao, C. Probiotics for inflammatory bowel disease: Is there sufficient evidence? Open Life Sci. 2024, 19, 20220821. [Google Scholar] [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef]
- Vakadaris, G.; Stefanis, C.; Giorgi, E.; Brouvalis, M.; Voidarou, C.C.; Kourkoutas, Y.; Tsigalou, C.; Bezirtzoglou, E. The Role of Probiotics in Inducing and Maintaining Remission in Crohn’s Disease and Ulcerative Colitis: A Systematic Review of the Literature. Biomedicines 2023, 11, 494. [Google Scholar] [CrossRef]
- Anwar, L.; Ahmad, E.; Imtiaz, M.; Ahmad, M.; Faisal Aziz, M.; Ibad, T. The Impact of Diet on Parkinson’s Disease: A Systematic Review. Cureus 2024, 16, e70337. [Google Scholar] [CrossRef]
- Hirayama, M.; Ohno, K. Parkinson’s Disease and Gut Microbiota. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 28–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Qian, Y.; Mo, C.; Ai, P.; Yang, X.; Xiao, Q. Sodium butyrate ameliorates gut dysfunction and motor deficits in a mouse model of Parkinson’s disease by regulating gut microbiota. Front. Aging Neurosci. 2023, 15, 1099018. [Google Scholar] [CrossRef]
- Wu, S.; Chen, N.; Wang, C.; So, K.F. Research focus and trends of the association between gut microbiota and neuroinflammation. Front. Microbiol. 2025, 16, 1564717. [Google Scholar] [CrossRef]
- Hattori, N.; Yamashiro, Y. The Gut-Brain Axis. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 1–3. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review). Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Siedlecka, K.; Marano, L.; Merola, E.; Roviello, F.; Polom, K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front. Cell. Infect. Microbiol. 2022, 12, 1023806. [Google Scholar] [CrossRef]
- Daroqui, M.C.; Augenlicht, L.H. Transcriptional attenuation in colon carcinoma cells in response to butyrate. Cancer Prev. Res. 2010, 3, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Modoux, M.; Rolhion, N.; Lefevre, J.H.; Oeuvray, C.; Nadvornik, P.; Illes, P.; Emond, P.; Parc, Y.; Mani, S.; Dvorak, Z.; et al. Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut Microbes. 2022, 14, 2105637. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Laerke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Jasbi, P.; Mohr, A.E.; Murthy, M.H.S.; Klein-Seetharaman, J. Understanding metabolic resilience by unraveling temporal dynamics of cellular responses. Trends Endocrinol. Metab. 2025, article in press. [Google Scholar] [CrossRef] [PubMed]
- Leysen, H.; Walter, D.; Christiaenssen, B.; Vandoren, R.; Harputluoglu, I.; Van Loon, N.; Maudsley, S. GPCRs Are Optimal Regulators of Complex Biological Systems and Orchestrate the Interface between Health and Disease. Int. J. Mol. Sci. 2021, 22, 13387. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.R.; Belt, S.C.; Bakker, B.M.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N. Beyond butyrate: Microbial fiber metabolism supporting colonic epithelial homeostasis. Trends Microbiol. 2024, 32, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef]
- Gu, B.H.; Kim, M.; Yun, C.H. Regulation of Gastrointestinal Immunity by Metabolites. Nutrients 2021, 13, 167. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef]
- Kromann, E.H.; Cearra, A.P.; Neves, J.F. Organoids as a tool to study homeostatic and pathological immune-epithelial interactions in the gut. Clin. Exp. Immunol. 2024, 218, 28–39. [Google Scholar] [CrossRef]
- Saxena, A.; Mitchell, C.; Bogdon, R.; Roark, K.; Wilson, K.; Staley, S.; Hailey, M.; Williams, M.C.; Rutkovsky, A.; Nagarkatti, P.; et al. Aryl Hydrocarbon Receptor Regulates Muc2 Production Independently of IL-22 during Colitis. Int. J. Mol. Sci. 2024, 25, 2404. [Google Scholar] [CrossRef]
- Xiong, L.; Diwakarla, S.; Chatzis, R.; Artaiz, O.; Macowan, M.; Zhang, S.; Garnham, A.; Morgan, P.K.; Mellett, N.A.; Meikle, P.J.; et al. Acute exposure to high-fat diet impairs ILC3 functions and gut homeostasis. Immunity 2025, 58, 1185–1200.e8. [Google Scholar] [CrossRef]
- Zahedifard, Z.; Mahmoodi, S.; Ghasemian, A. Genetically Engineered Bacteria as a Promising Therapeutic Strategy Against Cancer: A Comprehensive Review. Biotechnol. Appl. Biochem. 2025. ahead of print. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The association of diet, gut microbiota and colorectal cancer: What we eat may imply what we get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G.; Garg, N.; Dhiman, S.; Gupta, S.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Dysbiosis and Alzheimer’s Disease: A Role for Chronic Stress? Biomolecules 2021, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Feng, X.; Hu, Y.; Su, S. The human gut microbiota is associated with host lifestyle: A comprehensive narrative review. Front. Microbiol. 2025, 16, 1549160. [Google Scholar] [CrossRef]
- Nogal, B.; Blumberg, J.B.; Blander, G.; Jorge, M. Gut Microbiota-Informed Precision Nutrition in the Generally Healthy Individual: Are We There Yet? Curr. Dev. Nutr. 2021, 5, nzab107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bates, K.A.; Hoang, K.L.; Hector, T.E.; Knowles, S.C.L.; King, K.C. Experimental temperatures shape host microbiome diversity and composition. Glob. Change Biol. 2023, 29, 41–56. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Skowron, K.; Budzynska, A.; Wiktorczyk-Kapischke, N.; Chomacka, K.; Grudlewska-Buda, K.; Wilk, M.; Walecka-Zacharska, E.; Andrzejewska, M.; Gospodarek-Komkowska, E. The Role of Psychobiotics in Supporting the Treatment of Disturbances in the Functioning of the Nervous System-A Systematic Review. Int. J. Mol. Sci. 2022, 23, 7820. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Wei, H.; Huang, Y. Precision Microbiome: A New Era of Targeted Therapy with Core Probiotics. Research 2025, 8, 0658. [Google Scholar] [CrossRef]
- Zeilstra, D.; Younes, J.A.; Brummer, R.J.; Kleerebezem, M. Perspective: Fundamental Limitations of the Randomized Controlled Trial Method in Nutritional Research: The Example of Probiotics. Adv. Nutr. 2018, 9, 561–571. [Google Scholar] [CrossRef]
- An, S.; Kim, K.; Kim, M.H.; Jung, J.H.; Kim, Y. Perioperative Probiotics Application for Preventing Postoperative Complications in Patients with Colorectal Cancer: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1644. [Google Scholar] [CrossRef]
- Ceccherini, C.; Daniotti, S.; Bearzi, C.; Re, I. Evaluating the Efficacy of Probiotics in IBS Treatment Using a Systematic Review of Clinical Trials and Multi-Criteria Decision Analysis. Nutrients 2022, 14, 2689. [Google Scholar] [CrossRef]
- Shang, X.; E, F.F.; Guo, K.L.; Li, Y.F.; Zhao, H.L.; Wang, Y.; Chen, N.; Nian, T.; Yang, C.Q.; Yang, K.H.; et al. Effectiveness and Safety of Probiotics for Patients with Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials. Nutrients 2022, 14, 2482. [Google Scholar] [CrossRef]
- Groenewegen, B.; van Lingen, E.; Kovynev, A.; van den Berg, A.J.; Berssenbrugge, E.K.L.; Sanders, I.; van Prehn, J.; van Nood, E.; Goorhuis, A.; Kuijper, E.J.; et al. The presence of Clostridioides difficile in faeces before and after faecal microbiota transplantation and its relation with recurrent C. difficile infection and the gut microbiota in a Dutch cohort. Clin. Microbiol. Infect. 2025, 31, 568–574. [Google Scholar] [CrossRef]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef]
- da Silva, T.F.; Gloria, R.A.; Americo, M.F.; Freitas, A.D.S.; de Jesus, L.C.L.; Barroso, F.A.L.; Laguna, J.G.; Coelho-Rocha, N.D.; Tavares, L.M.; le Loir, Y.; et al. Unlocking the Potential of Probiotics: A Comprehensive Review on Research, Production, and Regulation of Probiotics. Probiotics Antimicrob Proteins 2024, 16, 1687–1723. [Google Scholar] [CrossRef]
- Fang, H.; Rodrigues, E.L.R.; Barra, N.G.; Kukje Zada, D.; Robin, N.; Mehra, A.; Schertzer, J.D. Postbiotic Impact on Host Metabolism and Immunity Provides Therapeutic Potential in Metabolic Disease. Endocr. Rev. 2025, 46, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Lupuliasa, D.; Neacsu, S.M.; Olteanu, G.; Busnatu, S.S.; Mihai, A.; Popovici, V.; Maru, N.; Boroghina, S.C.; Mihai, S.; et al. Polyunsaturated Fatty Acids and Human Health: A Key to Modern Nutritional Balance in Association with Polyphenolic Compounds from Food Sources. Foods 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Cuciniello, R.; Di Meo, F.; Filosa, S.; Crispi, S.; Bergamo, P. The Antioxidant Effect of Dietary Bioactives Arises from the Interplay between the Physiology of the Host and the Gut Microbiota: Involvement of Short-Chain Fatty Acids. Antioxidants 2023, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Pal, N.; Sharma, P.; Kumawat, M.; Sarma, D.K.; Nabi, B.; Verma, V.; Tiwari, R.R.; Shubham, S.; Arjmandi, B.; et al. Omega-3 Fatty Acids and Their Interaction with the Gut Microbiome in the Prevention and Amelioration of Type-2 Diabetes. Nutrients 2022, 14, 1723. [Google Scholar] [CrossRef]
- Mkilima, T. Engineering artificial microbial consortia for personalized gut microbiome modulation and disease treatment. Ann. N. Y. Acad. Sci. 2025, 1548, 29–55. [Google Scholar] [CrossRef]
- Young, R.B.; Marcelino, V.R.; Chonwerawong, M.; Gulliver, E.L.; Forster, S.C. Key Technologies for Progressing Discovery of Microbiome-Based Medicines. Front. Microbiol. 2021, 12, 685935. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Xue, G.; Han, L.; Jia, H.; Wang, Z.; Zhang, J.; Liu, P.; Yang, C.; Zhou, Y. Role of short-chain fatty acids in host physiology. Anim. Models Exp. Med. 2024, 7, 641–652. [Google Scholar] [CrossRef]
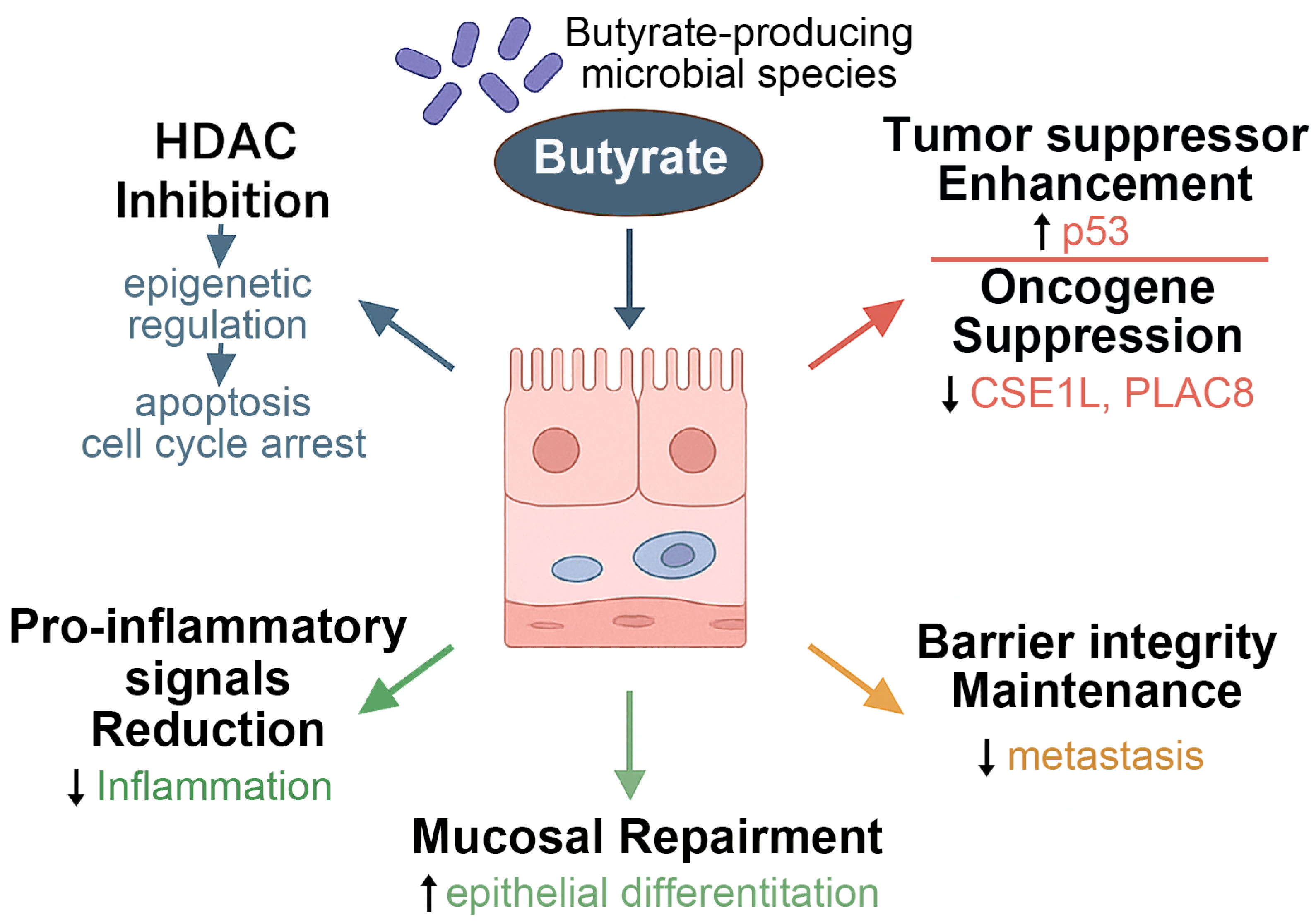
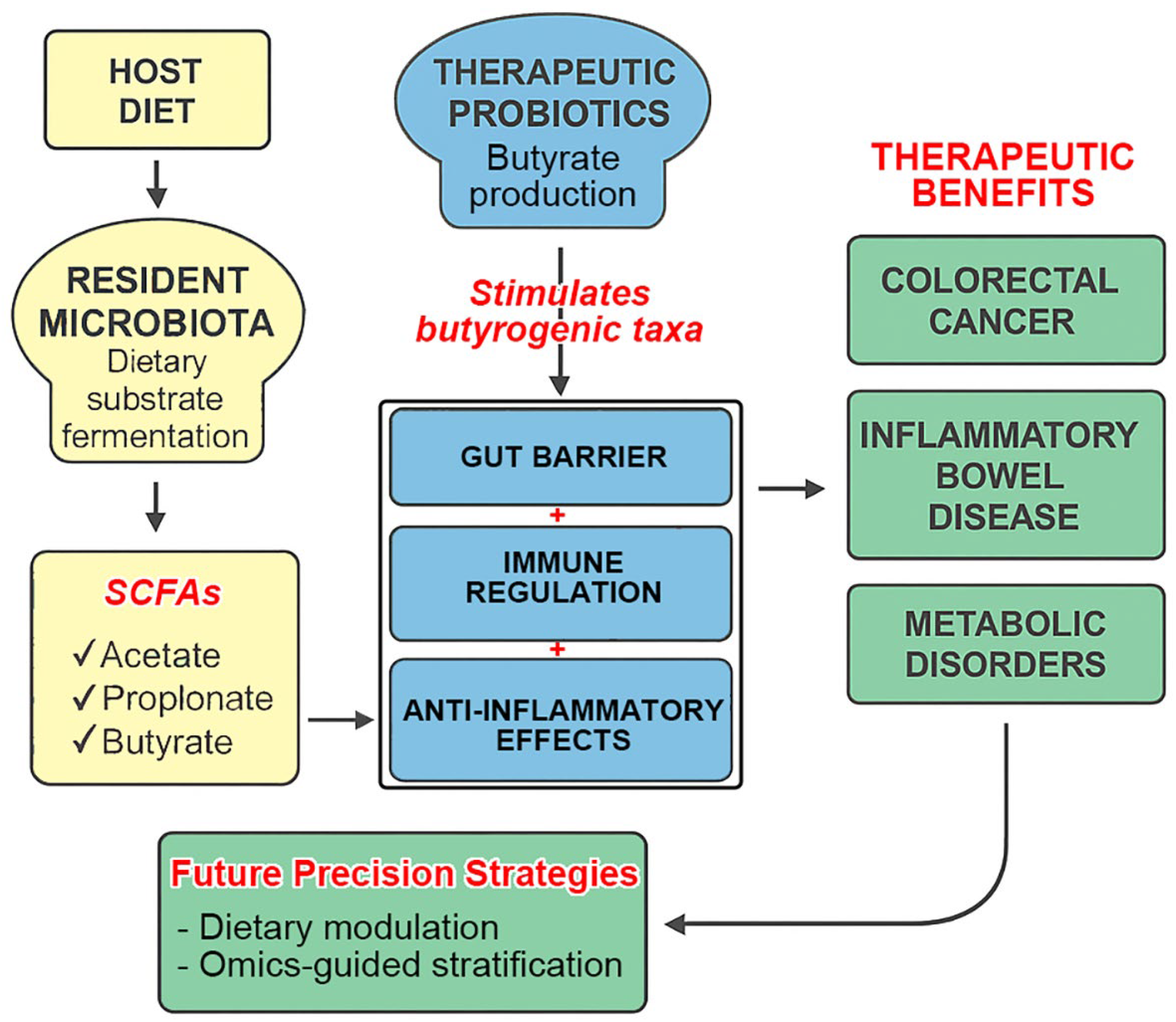
| Disease | Effects of Probiotics 1 | Reference |
|---|---|---|
| Gastrointestinal disorders | Reduces inflammation, improves bowel habits, shortens diarrhea duration | [46] |
| Metabolic disorders | Lowers LDL cholesterol, body weight, waist circumference | [47] |
| Colorectal cancer | Restores microbial balance, enhances immune response | [48] |
| Neuropsychiatric conditions | Alleviates depression, anxiety, modulates neurotransmitters | [42] |
| General immunity | Boosts SCFA production, reduces systemic inflammation | [49] |
| Disease 2 | Probiotic Effect 3 | SCFA Role 4 | Outcome 5 | Reference |
|---|---|---|---|---|
| CKD | Restores gut integrity; reduces uremic toxins | Enhances barrier function; reduces inflammation via GPR109A | Improved renal and GI function | [90] |
| PD | Modulates gut–brain axis; alters microbial composition | Regulates neuroinflammation via SCFA-GPR signaling | Reduced neurodegeneration | [21] |
| IBD | Reduces inflammation, strengthens mucosal barrier | Butyrate promotes epithelial healing and Treg differentiation via HDAC inhibition | Disease symptom alleviation | [94] |
| CVDs | Modulates lipid metabolism; lowers systemic inflammation | Supports vascular homeostasis and lipid regulation; reduces LPS translocation | Reduced cardiometabolic risk | [80] |
| MetS | Lowers weight and LDL; modulates microbiota | Regulates metabolism through PPAR-γ and GPR43 activation | Improved metabolic profile | [47] |
| CRC | Enhances immune function; restores microbial balance | Butyrate induces apoptosis and Foxp3 expression via HDAC inhibition; suppresses oncogenes (e.g., CSE1L, PLAC8) | Tumor suppression and barrier protection | [48] |
| Gut Microbes | Mechanism 1 | Health Effects 2 | Reference |
|---|---|---|---|
| Butyricicoccus pullicaecorum | Upregulates SLC5A8, GPR43 Downregulates CSE1L expression | Reduces CRC progression Reverses p53-related genetic distortion | [43] [38] |
| Clostridium butyricum | Enhances mucosal immunity | Alleviates inflammation, supports colon health | [108] |
| Roseburia spp. | Butyrate production affected by antibiotics | Influences intestinal barrier and CRC risk | [18] |
| Domain | Limitation 1 | Implication |
|---|---|---|
| Sample size | Predominantly small cohorts in clinical trials | Reduces statistical power and limits the generalizability of findings |
| Follow-up duration | Short intervention and monitoring periods | Hinders evaluation of long-term efficacy, durability, and safety |
| Mechanistic insight | Lack of mechanistic endpoints (e.g., SCFA quantification, gene expression) | Obstructs pathway-level understanding and weakens translational value |
| Outcome heterogeneity | Inconsistent endpoints across studies (e.g., microbiota composition vs. symptoms) | Limits cross-study comparability and reduces feasibility of reviews or meta-analyses |
| Personalization | Limited use of host microbiome or metabolomic stratification | Impedes development of targeted or precision interventions |
| Intervention standardization | Variability in probiotic strains, dosages, formulations, and delivery systems | Undermines reproducibility and clinical reliability |
| Regulatory ambiguity | Unclear classification of probiotics (e.g., supplement vs. drug); lack of harmonized international standards | Delays clinical translation, complicates product approval, scalability, and quality control; creates uncertainty for clinicians and policymakers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-C.; Chang, S.-C.; Hung, C.-S.; Shen, M.-H.; Lai, C.-L.; Huang, C.-J. Gut-Microbiota-Derived Metabolites and Probiotic Strategies in Colorectal Cancer: Implications for Disease Modulation and Precision Therapy. Nutrients 2025, 17, 2501. https://doi.org/10.3390/nu17152501
Yang Y-C, Chang S-C, Hung C-S, Shen M-H, Lai C-L, Huang C-J. Gut-Microbiota-Derived Metabolites and Probiotic Strategies in Colorectal Cancer: Implications for Disease Modulation and Precision Therapy. Nutrients. 2025; 17(15):2501. https://doi.org/10.3390/nu17152501
Chicago/Turabian StyleYang, Yi-Chu, Shih-Chang Chang, Chih-Sheng Hung, Ming-Hung Shen, Ching-Long Lai, and Chi-Jung Huang. 2025. "Gut-Microbiota-Derived Metabolites and Probiotic Strategies in Colorectal Cancer: Implications for Disease Modulation and Precision Therapy" Nutrients 17, no. 15: 2501. https://doi.org/10.3390/nu17152501
APA StyleYang, Y.-C., Chang, S.-C., Hung, C.-S., Shen, M.-H., Lai, C.-L., & Huang, C.-J. (2025). Gut-Microbiota-Derived Metabolites and Probiotic Strategies in Colorectal Cancer: Implications for Disease Modulation and Precision Therapy. Nutrients, 17(15), 2501. https://doi.org/10.3390/nu17152501







