The Shuttling of Methyl Groups Between Folate and Choline Pathways
Abstract
1. Introduction
2. Health Effects of Sufficient Folate and Choline Intakes
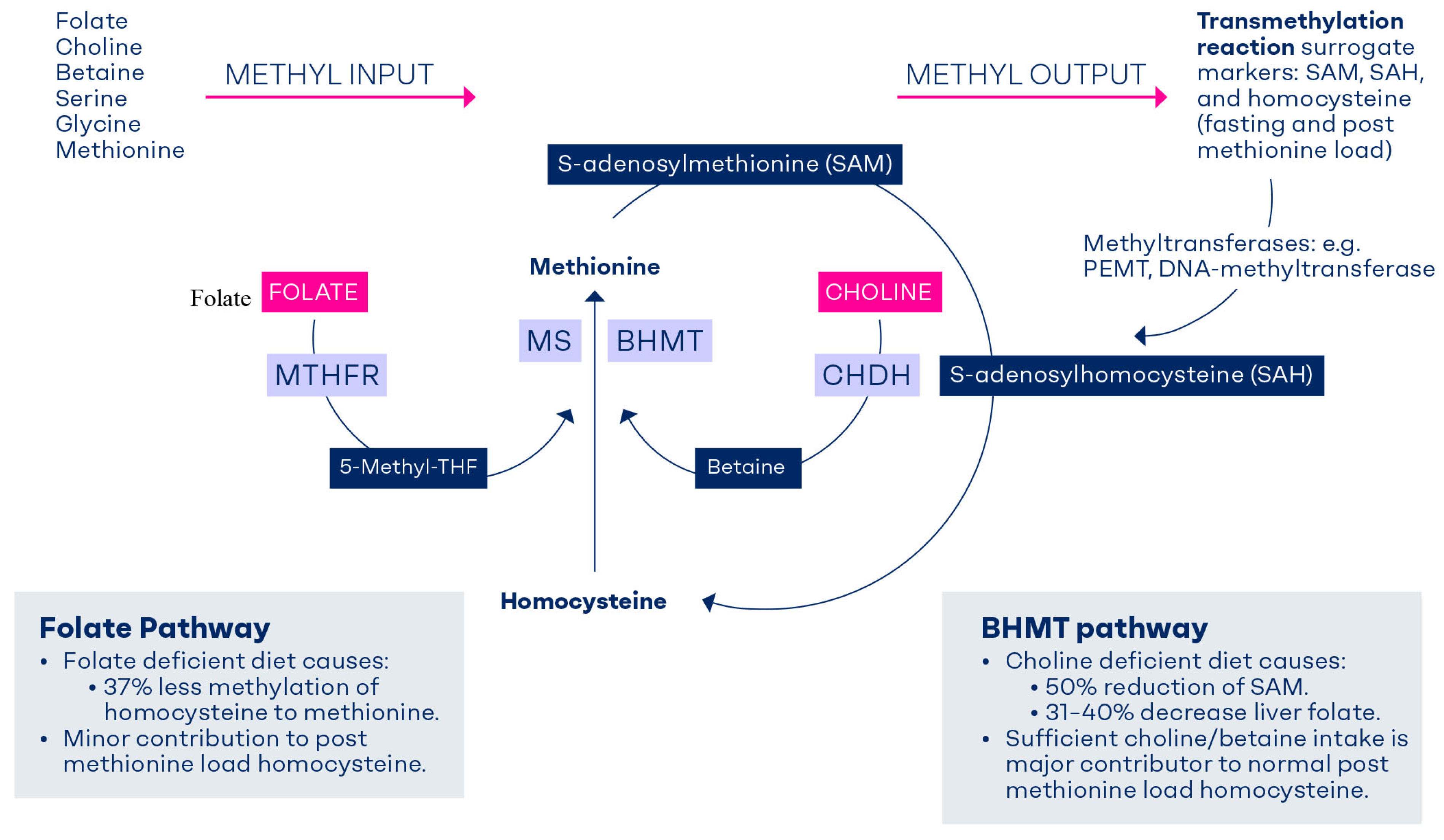
3. Formation of Methyl Groups in C1-Metabolism
Homocysteine Methylation to Methionine
4. Determinants and Indicators of C1-Metabolism
5. The Methionine Load Test: A Functional Test of the BHMT Pathway
6. Safeguarding the Methyl Balance Through Diet or Methylneogenesis
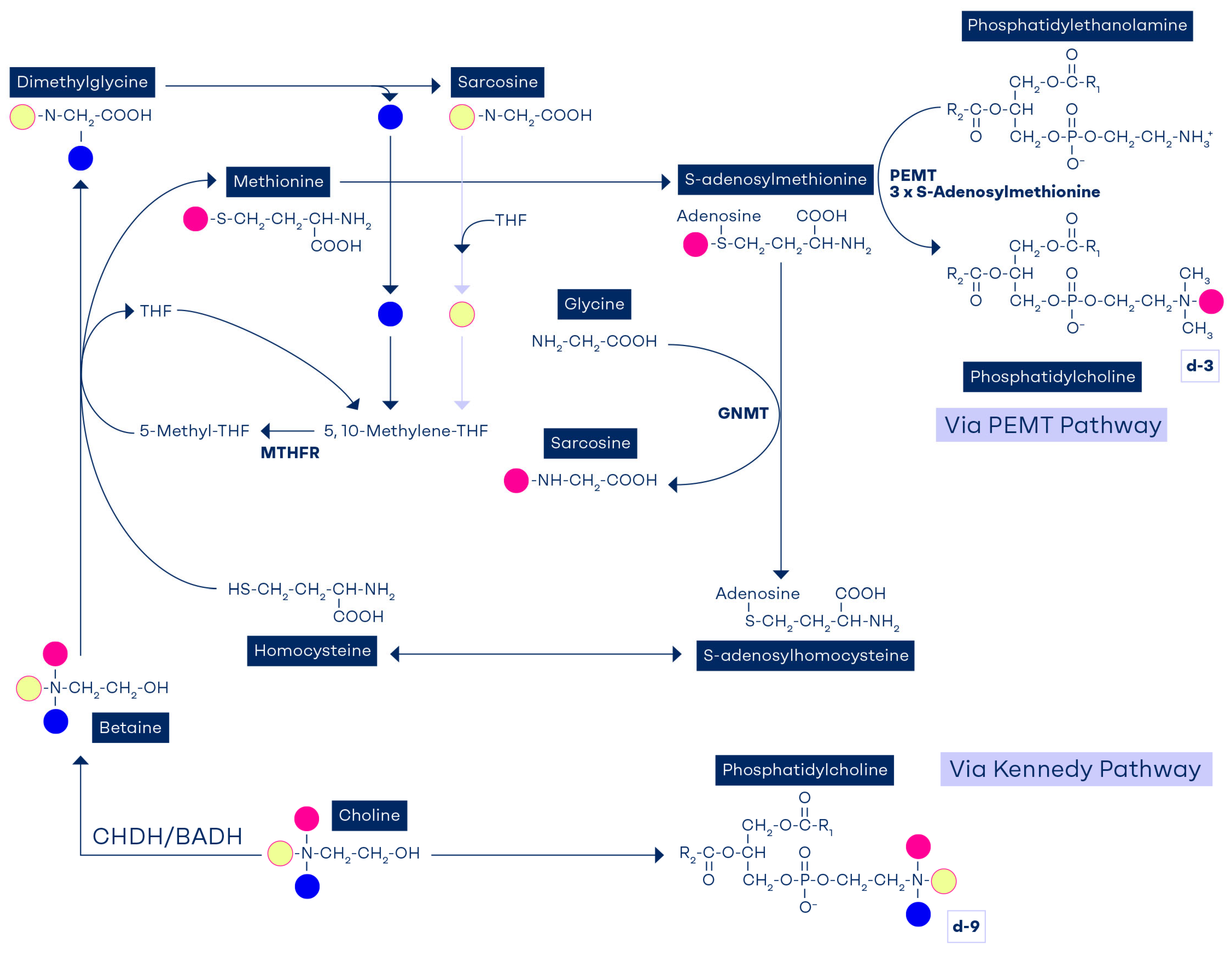
7. Factors Affecting Methylneogenesis
8. The Role of Phosphatidylethanolamine Methyltransferase in Methylneogenesis
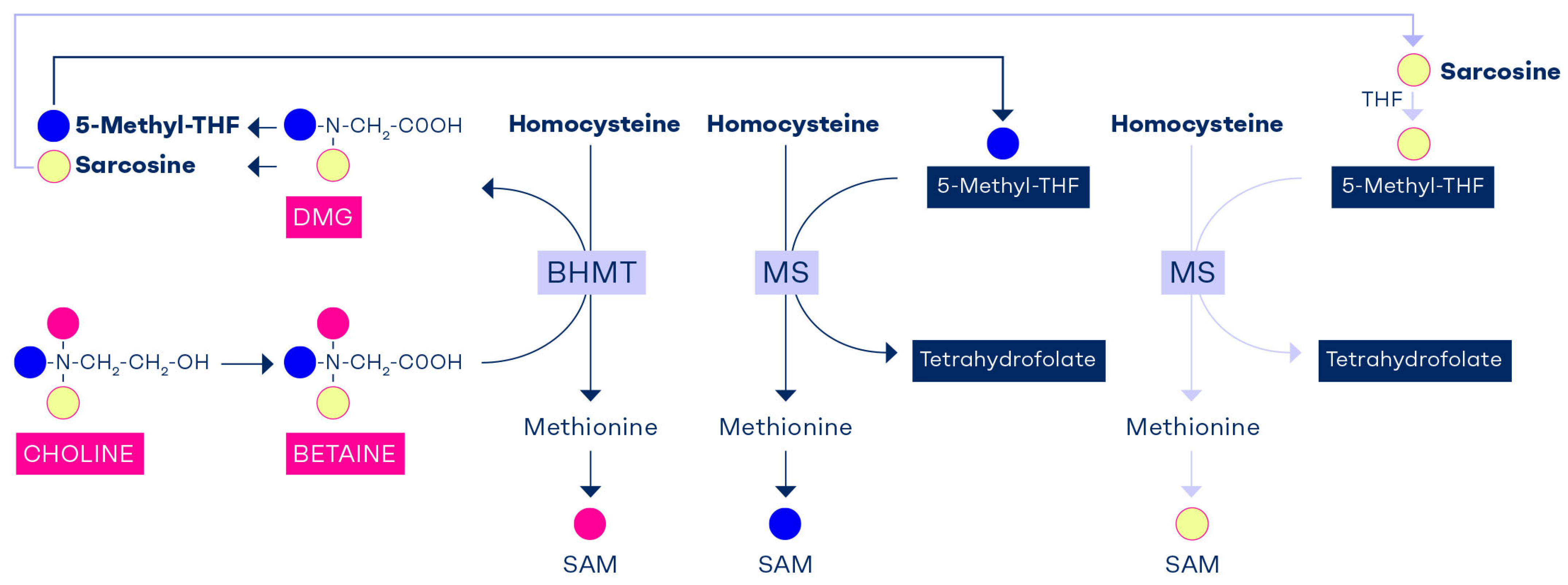
9. Tracking the Methyl Groups of Betaine and Choline
10. Interdependency of Folate and Choline
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Falnes, P.O. Closing in on human methylation-the versatile family of seven-beta-strand (METTL) methyltransferases. Nucleic Acids Res. 2024, 52, 11423–11441. [Google Scholar] [CrossRef] [PubMed]
- da Mota, J.C.N.; Ribeiro, A.A.; Carvalho, L.M.; Esteves, G.P.; Sieczkowska, S.M.; Goessler, K.F.; Gualano, B.; Nicoletti, C.F. Impact of Methyl-Donor Micronutrient Supplementation on DNA Methylation Patterns: A Systematic Review and Meta-Analysis of in vitro, Animal, and Human Studies. Lifestyle Genom. 2023, 16, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Kyle, W.E.; Harris, B.J. Methionine metabolism in mammals: Regulatory effects of S-adenosylhomocysteine. Arch. Biochem. Biophys. 1974, 165, 774–779. [Google Scholar] [CrossRef]
- Teng, Y.W.; Mehedint, M.G.; Garrow, T.A.; Zeisel, S.H. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J. Biol. Chem. 2011, 286, 36258–36267. [Google Scholar] [CrossRef] [PubMed]
- Steenge, G.R.; Verhoef, P.; Katan, M.B. Betaine supplementation lowers plasma homocysteine in healthy men and women. J. Nutr. 2003, 133, 1291–1295. [Google Scholar] [CrossRef]
- Atkinson, W.; Elmslie, J.; Lever, M.; Chambers, S.T.; George, P.M. Dietary and supplementary betaine: Acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am. J. Clin. Nutr. 2008, 87, 577–585. [Google Scholar] [CrossRef]
- Selhub, J.; Seyoum, E.; Pomfret, E.A.; Zeisel, S.H. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991, 51, 16–21. [Google Scholar]
- Horne, D.W.; Cook, R.J.; Wagner, C. Effect of dietary methyl group deficiency on folate metabolism in rats. J. Nutr. 1989, 119, 618–621. [Google Scholar] [CrossRef]
- Cuskelly, G.J.; Stacpoole, P.W.; Williamson, J.; Baumgartner, T.G.; Gregory, J.F., III. Deficiencies of folate and vitamin B(6) exert distinct effects on homocysteine, serine, and methionine kinetics. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1182–E1190. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J. Biol. Chem. 1984, 259, 9508–9513. [Google Scholar]
- US Preventive Services Task Force; Barry, M.J.; Nicholson, W.K.; Silverstein, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; Li, L. Folic Acid Supplementation to Prevent Neural Tube Defects: US Preventive Services Task Force Reaffirmation Recommendation Statement. J. Am. Med. Assoc. 2023, 330, 454–459. [Google Scholar]
- Zhang, C.; Chen, Y.; Hou, F.; Li, Y.; Wang, W.; Guo, L.; Zhang, C.; Li, L.; Lu, C. Safety and Efficacy of High-Dose Folinic Acid in Children with Autism: The Impact of Folate Metabolism Gene Polymorphisms. Nutrients 2025, 17, 1602. [Google Scholar] [CrossRef]
- Maruf, A.A.; Poweleit, E.A.; Brown, L.C.; Strawn, J.R.; Bousman, C.A. Systematic Review and Meta-Analysis of L-Methylfolate Augmentation in Depressive Disorders. Pharmacopsychiatry 2022, 55, 139–147. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef]
- Buchman, A.L.; Dubin, M.; Jenden, D.; Moukarzel, A.; Roch, M.H.; Rice, K.; Gornbein, J.; Ament, M.E.; Eckhert, C.D. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology 1992, 102, 1363–1370. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food allergens). Choline and contribution to normal liver function of the foetus and exclusively breastfed infants: Evaluation of a health claim pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2023, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Derbyshire, E.; Schon, C. Association between maternal choline, foetal brain development and child neurocognition; systematic review and meta-analysis of human studies. Adv. Nutr. 2022, 13, 2445–2457. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Miller, J.W.; da Costa, K.-A.; Nadeau, M.; Smith, D.; Selhub, J.; Zeisel, S.H.; Mason, J.B. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J. Nutr. 1994, 124, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.C.; James, M.F. Choline deficiency in the baby pig. J. Nutr. 1948, 36, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Varela-Moreiras, G.; Ragel, C.; de Perez, M.J. Choline deficiency and methotrexate treatment induces marked but reversible changes in hepatic folate concentrations, serum homocysteine and DNA methylation rates in rats. J. Am. Coll. Nutr. 1995, 14, 480–485. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Seremak-Mrozikiewicz, A.; Malinowska, A.M.; Różycka, A.; Radziejewska, A.; KurzawiŃska, G.; Barlik, M.; Wolski, H.; Drews, K. Associations between folate and choline intake, homocysteine metabolism, and genetic polymorphism of MTHFR, BHMT and PEMT in healthy pregnant Polish women. Nutr. Diet. 2020, 77, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Poole, J.R. Labile methyl balances for normal humans on various dietary regimens. Metabolism 1975, 24, 721–735. [Google Scholar] [CrossRef]
- Chen, L.H.; Liu, M.L.; Hwang, H.Y.; Chen, L.S.; Korenberg, J.; Shane, B. Human methionine synthase. cDNA cloning, gene localization, and expression. J. Biol. Chem. 1997, 272, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Pathways and regulation of homocysteine metabolism in mammals. Semin. Thromb. Hemost. 2000, 26, 219–225. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Metabolism of sulfur-containing amino acids. Annu. Rev. Nutr. 1986, 6, 179–209. [Google Scholar] [CrossRef]
- Wilcken, D.E.; Wilcken, B.; Dudman, N.P.; Tyrrell, P.A. Homocystinuria--the effects of betaine in the treatment of patients not responsive to pyridoxine. N. Engl. J. Med. 1983, 309, 448–453. [Google Scholar] [CrossRef]
- Wilcken, D.E.; Dudman, N.P.; Tyrrell, P.A. Homocystinuria due to cystathionine beta-synthase deficiency—The effects of betaine treatment in pyridoxine-responsive patients. Metabolism 1985, 34, 1115–1121. [Google Scholar] [CrossRef]
- Teng, Y.W.; Cerdena, I.; Zeisel, S.H. Homocysteinemia in mice with genetic betaine homocysteine S-methyltransferase deficiency is independent of dietary folate intake. J. Nutr. 2012, 142, 1964–1967. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Jacques, P.F.; Wilson, P.W.; Rush, D.; Rosenberg, I.H. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. J. Am. Med. Assoc. 1993, 270, 2693–2698. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Giovannucci, E.L.; Hankinson, S.E.; Zeisel, S.H.; Dougherty, L.W.; Willett, W.C.; Rimm, E.B. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr. 2007, 86, 1073–1081. [Google Scholar] [CrossRef]
- Olthof, M.R.; Brink, E.J.; Katan, M.B.; Verhoef, P. Choline supplemented as phosphatidylcholine decreases fasting and postmethionine-loading plasma homocysteine concentrations in healthy men. Am. J. Clin. Nutr. 2005, 82, 111–117. [Google Scholar] [CrossRef][Green Version]
- Olthof, M.R.; van Vliet, T.; Boelsma, E.; Verhoef, P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J. Nutr. 2003, 133, 4135–4138. [Google Scholar] [CrossRef]
- Ubbink, J.B.; Becker, P.J.; Delport, R.; Bester, M.; Riezler, R.; Vermaak, W.J. Variability of post-methionine load plasma homocysteine assays. Clin. Chim. Acta 2003, 330, 111–119. [Google Scholar] [CrossRef]
- van der Griend, R.; Haas, F.J.; Duran, M.; Biesma, D.H.; Meuwissen, O.J.; Banga, J.D. Methionine loading test is necessary for detection of hyperhomocysteinemia. J. Lab. Clin. Med. 1998, 132, 67–72. [Google Scholar] [CrossRef]
- van der Griend, R.; Biesma, D.H.; Banga, J.D. Postmethionine-load homocysteine determination for the diagnosis hyperhomocysteinaemia and efficacy of homocysteine lowering treatment regimens. Vasc. Med. 2002, 7, 29–33. [Google Scholar] [CrossRef]
- Bostom, A.G.; Jacques, P.F.; Nadeau, M.R.; Williams, R.R.; Ellison, R.C.; Selhub, J. Post-methionine load hyperhomocysteinemia in persons with normal fasting total plasma homocysteine: Initial results from the NHLBI Family Heart Study. Atherosclerosis 1995, 116, 147–151. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S.; McGregor, D.O.; Dellow, W.J.; George, P.M.; Chambers, S.T. Variability of plasma and urine betaine in diabetes mellitus and its relationship to methionine load test responses: An observational study. Cardiovasc. Diabetol. 2012, 11, 34. [Google Scholar] [CrossRef]
- Holm, P.I.; Bleie, O.; Ueland, P.M.; Lien, E.A.; Refsum, H.; Nordrehaug, J.E.; NygarD, O. Betaine as a determinant of postmethionine load total plasma homocysteine before and after B-vitamin supplementation. Arter. Thromb. Vasc. Biol. 2004, 24, 301–307. [Google Scholar] [CrossRef]
- Holm, P.I.; Ueland, P.M.; Vollset, S.E.; Midttun, Ø.; Blom, H.J.; Keijzer, M.B.; Heijer, M.D. Betaine and folate status as cooperative determinants of plasma homocysteine in humans. Arter. Thromb. Vasc. Biol. 2005, 25, 379–385. [Google Scholar] [CrossRef]
- Lee, J.E.; Jacques, P.F.; Dougherty, L.; Selhub, J.; Giovannucci, E.; Zeisel, S.H.; Cho, E. Are dietary choline and betaine intakes determinants of total homocysteine concentration? Am. J. Clin. Nutr. 2010, 91, 1303–1310. [Google Scholar] [CrossRef]
- da Costa, K.A.; Gaffney, C.E.; Fischer, L.M.; Zeisel, S.H. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am. J. Clin. Nutr. 2005, 81, 440–444. [Google Scholar] [CrossRef]
- Verhoef, P.; Steenge, G.R.; Boelsma, E.; van Vliet, T.; Olthof, M.R.; Katan, M.B. Dietary serine and cystine attenuate the homocysteine-raising effect of dietary methionine: A randomized crossover trial in humans. Am. J. Clin. Nutr. 2004, 80, 674–679. [Google Scholar] [CrossRef]
- Figueroa-Torres, A.G.; Matias-Aguilar, L.O.; Coria-Ramirez, E.; Bonilla-Gonzalez, E.; Gonzalez-Marquez, H.; Ibarra-Gonzalez, I.; Hernandez-Lopez, J.R.; Hernandez-Juarez, J.; Dominguez-Reyes, V.M.; Isordia-Salas, I.; et al. Cystathionine beta-synthase and methylenetetrahydrofolate reductase mutations in Mexican individuals with hyperhomocysteinemia. SAGE Open Med. 2020, 8, 2050312120974193. [Google Scholar] [CrossRef]
- Lievers, K.J.; Kluijtmans, L.A.; Heil, S.G.; Boers, G.H.; Verhoef, P.; Heijer, M.D.; Trijbels, F.J.M.; Blom, H.J. Cystathionine beta-synthase polymorphisms and hyperhomocysteinaemia: An association study. Eur. J. Hum. Genet. 2003, 11, 23–29. [Google Scholar] [CrossRef]
- Bhat, D.S.; Gruca, L.L.; Bennett, C.D.; Katre, P.; Kurpad, A.V.; Yajnik, C.S.; Kalhan, S.C.; Loor, J.J. Evaluation of tracer labelled methionine load test in vitamin B-12 deficient adolescent women. PLoS ONE 2018, 13, e0196970. [Google Scholar] [CrossRef]
- Chiang, E.P.; Selhub, J.; Bagley, P.J.; Dallal, G.; Roubenoff, R. Pyridoxine supplementation corrects vitamin B6 deficiency but does not improve inflammation in patients with rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R1404–R1411. [Google Scholar] [CrossRef]
- de Jonge, R.; Griffioen, P.H.; van Zelst, B.; Brouns, R.M.; Visser, W.; Lindemans, J. Evaluation of a shorter methionine loading test. Clin. Chem. Lab. Med. 2004, 42, 1027–1031. [Google Scholar] [CrossRef]
- Sadre-Marandi, F.; Dahdoul, T.; Reed, M.C.; Nijhout, H.F. Sex differences in hepatic one-carbon metabolism. BMC Syst. Biol. 2018, 12, 89. [Google Scholar] [CrossRef]
- Nelen, W.L.; Blom, H.J.; Thomas, C.M.; Steegers, E.A.; Boers, G.H.; Eskes, T.K. Methylenetetrahydrofolate reductase polymorphism affects the change in homocysteine and folate concentrations resulting from low dose folic acid supplementation in women with unexplained recurrent miscarriages. J. Nutr. 1998, 128, 1336–1341. [Google Scholar] [CrossRef]
- Mudd, S.H.; Brosnan, J.T.; Brosnan, M.E.; Jacobs, R.L.; Stabler, S.P.; Allen, R.H.; Vance, D.E.; Wagner, C. Methyl balance and transmethylation fluxes in humans. Am. J. Clin. Nutr. 2007, 85, 19–25. [Google Scholar] [CrossRef]
- Mudd, S.H.; Ebert, M.H.; Scriver, C.R. Labile methyl group balances in the human: The role of sarcosine. Metabolism 1980, 29, 707–720. [Google Scholar] [CrossRef]
- Storch, K.J.; Wagner, D.A.; Burke, J.F.; Young, V.R. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am. J. Physiol. 1988, 255, E322–E331. [Google Scholar] [CrossRef]
- Lamers, Y.; Williamson, J.; Gilbert, L.R.; Stacpoole, P.W.; Gregory, J.F., III. Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1,2-(13)C2]glycine and [(2)H3]leucine. J. Nutr. 2007, 137, 2647–2652. [Google Scholar] [CrossRef]
- Im, Y.S.; Chiang, P.K.; Cantoni, G.L. Guanidoacetate methyltransferase. Purification and molecular properties. J. Biol. Chem. 1979, 254, 11047–11050. [Google Scholar]
- Resseguie, M.; Song, J.; Niculescu, M.D.; da Costa, K.A.; Randall, T.A.; Zeisel, S.H. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007, 21, 2622–2632. [Google Scholar] [CrossRef]
- Resseguie, M.E.; da Costa, K.A.; Galanko, J.A.; Patel, M.; Davis, I.J.; Zeisel, S.H. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J. Biol. Chem. 2011, 286, 1649–1658. [Google Scholar] [CrossRef]
- da Costa, K.A.; Kozyreva, O.G.; Song, J.; Galanko, J.A.; Fischer, L.M.; Zeisel, S.H. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006, 20, 1336–1344. [Google Scholar] [CrossRef]
- Fischer, L.M.; Dacosta, K.A.; Kwock, L.; Stewart, P.W.; Lu, T.-S.; Stabler, S.P.; Allen, R.H.; Zeisel, S.H. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007, 85, 1275–1285. [Google Scholar] [CrossRef]
- Schwahn, B.C.; Laryea, M.D.; Chen, Z.; Melnyk, S.; Pogribny, I.; Garrow, T.; James, S.J.; Rozen, R. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem. J. 2004, 382, 831–840. [Google Scholar] [CrossRef]
- Chen, Z.; Karaplis, A.C.; Ackerman, S.L.; Pogribny, I.P.; Melnyk, S.; Lussier-Cacan, S.; Chen, M.F.; Pai, A.; John, S.W.; Smith, R.S.; et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum. Mol. Genet. 2001, 10, 433–443. [Google Scholar] [CrossRef]
- Colson, N.J.; Naug, H.L.; Nikbakht, E.; Zhang, P.; McCormack, J. The impact of MTHFR 677 C/T genotypes on folate status markers: A meta-analysis of folic acid intervention studies. Eur. J. Nutr. 2017, 56, 247–260. [Google Scholar] [CrossRef]
- Yan, J.; Wang, W.; Gregory, J.F.; Malysheva, O.; Brenna, J.T.; Stabler, S.P.; Allen, R.H.; Caudill, M.A. MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming d9-choline. Am. J. Clin. Nutr. 2011, 93, 348–355. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies NDA. Scientific opinion on dietary reference values for folate. EFSA J. 2014, 12, 3893.
- EFSA Panel on Dietetic Products, Nutrition and Allergies NDA. Dietary reference values for choline. EFSA J. 2016, 14, 4484. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998; pp. 390–422. [Google Scholar]
- Vance, D.E.; Ridgway, N.D. The methylation of phosphatidylethanolamine. Prog. Lipid Res. 1988, 27, 61–79. [Google Scholar] [CrossRef]
- DeLong, C.J.; Hicks, A.M.; Cui, Z. Disruption of choline methyl group donation for phosphatidylethanolamine methylation in hepatocarcinoma cells. J. Biol. Chem. 2002, 277, 17217–17225. [Google Scholar] [CrossRef]
- Vance, D.E.; Walkey, C.J.; Cui, Z. Phosphatidylethanolamine N-methyltransferase from liver. Biochim. Biophys. Acta 1997, 1348, 142–150. [Google Scholar] [CrossRef]
- Stead, L.M.; Brosnan, J.T.; Brosnan, M.E.; Vance, D.E.; Jacobs, R.L. Is it time to reevaluate methyl balance in humans? Am. J. Clin. Nutr. 2006, 83, 5–10. [Google Scholar] [CrossRef]
- Shields, D.J.; Lingrell, S.; Agellon, L.B.; Brosnan, J.T.; Vance, D.E. Localization-independent regulation of homocysteine secretion by phosphatidylethanolamine N-methyltransferase. J. Biol. Chem. 2005, 280, 27339–27344. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Stead, L.M.; Devlin, C.; Tabas, I.; Brosnan, M.E.; Brosnan, J.T.; Vance, D.E. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J. Biol. Chem. 2005, 280, 28299–28305. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Devlin, C.; Tabas, I.; Vance, D.E. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase alpha in mice decreases plasma high density and very low density lipoproteins. J. Biol. Chem. 2004, 279, 47402–47410. [Google Scholar] [CrossRef]
- Schwahn, B.C.; Wendel, U.; Lussier-Cacan, S.; Mar, M.-H.; Zeisel, S.H.; Leclerc, D.; Castro, C.; Garrow, T.A.; Rozen, R. Effects of betaine in a murine model of mild cystathionine-beta-synthase deficiency. Metabolism 2004, 53, 594–599. [Google Scholar] [CrossRef]
- Schwahn, B.C.; Chen, Z.; Laryea, M.D.; Wendel, U.; Lussier-Cacan, S.; Genest, J., Jr.; Mar, M.-H.; Zeisel, S.H.; Castro, C.; Garrow, T. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003, 17, 512–514. [Google Scholar] [CrossRef]
- Watkins, S.M.; Zhu, X.; Zeisel, S.H. Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J. Nutr. 2003, 133, 3386–3391. [Google Scholar] [CrossRef]
- DeLong, C.J.; Shen, Y.J.; Thomas, M.J.; Cui, Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 1999, 274, 29683–29688. [Google Scholar] [CrossRef]
- Augustin, P.; Hromic, A.; Pavkov-Keller, T.; Gruber, K.; Macheroux, P. Structure and biochemical properties of recombinant human dimethylglycine dehydrogenase and comparison to the disease-related H109R variant. FEBS J. 2016, 283, 3587–3603. [Google Scholar] [CrossRef]
- Robinson, J.L.; McBreairty, L.E.; Randell, E.W.; Harding, S.V.; Bartlett, R.K.; Brunton, J.A.; Bertolo, R.F. Betaine or folate can equally furnish remethylation to methionine and increase transmethylation in methionine-restricted neonates. J. Nutr. Biochem. 2018, 59, 129–135. [Google Scholar] [CrossRef]
- Barak, A.J.; Kemmy, R.J. Methotrexate effects on hepatic betaine levels in choline-supplemented and choline-deficient rats. Drug Nutr. Interact. 1982, 1, 275–278. [Google Scholar]
- Barak, A.J.; Tuma, D.J.; Beckenhauer, H.C. Methotrexate hepatotoxicity. J. Am. Coll. Nutr. 1984, 3, 93–96. [Google Scholar] [CrossRef]
- Freeman-Narrod, M.; Narrod, S.A.; Custer, R.P. Chronic toxicity of methotrexate in rats: Partial to complete projection of the liver by choline: Brief communication. J. Natl. Cancer Inst. 1977, 59, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Pomfret, E.A.; daCosta, K.A.; Zeisel, S.H. Effects of choline deficiency and methotrexate treatment upon rat liver. J. Nutr. Biochem. 1990, 1, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Berge, R.K.; Aarsland, A.; Aarsaether, N.; Refsum, H.; Svardal, A.M.; Lonning, P.E. Effect of methotrexate on homocysteine and other sulfur compounds in tissues of rats fed a normal or a defined, choline-deficient diet. Cancer Chemother. Pharmacol. 1988, 21, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Poirier, L.A.; Grantham, P.H.; Rogers, A.E. The effects of a marginally lipotrope-deficient diet on the hepatic levels of S-adenosylmethionine and on the urinary metabolites of 2-acetylaminofluorene in rats. Cancer Res. 1977, 37, 744–748. [Google Scholar]
- Barak, A.J.; Kemmy, R.J.; Tuma, D.J. The effect of methotrexate on homocysteine methylating agents in rat liver. Drug Nutr. Interact. 1982, 1, 303–306. [Google Scholar]
- Shivapurkar, N.; Poirier, L.A. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis 1983, 4, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Zola, T.; daCosta, K.A.; Pomfret, E.A. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem. J. 1989, 259, 725–729. [Google Scholar] [CrossRef] [PubMed]
| Determinants of Homocysteine Concentrations After Methionine Load | Direction |
|---|---|
| Elevated fasting plasma homocysteine [37,38]. | ↑↑ |
| Higher plasma betaine [37,38,39]; betaine intake (diet or supplements) [5,6,40]; or choline intake (diet or supplements) [6,40,41]. | ↓ ↓ ↓ |
| Acute intake of choline/betaine (single dose studies or after a meal) [31,32]. | ↓ ↓ ↓ |
| Higher intake of serine or cysteine [42]; higher folate status [39]; or folate intake [5]. | ↓ |
| Polymorphisms in the transsulfuration pathway (e.g., cystathionine β-synthase) [43,44]. | (↓↑) |
| Low vitamin B12 status [45]; vitamin B6 supplementation [38,46]. | (↑); (↓) |
| Conditions Where the Post-Methionine Load Test May Be Used to Detect Hyperhomocysteinemia | |
| Insufficient choline intake or status. | |
| Carriers of polymorphisms in methylenetetrahydrofolate reductase (MTHFR) [47], phosphatidylethanolamine methyl transferase (PEMT) or BHMT genes. | |
| Anti-folate drugs (e.g., methotrexate, antimalarial) or drugs interfering with folate absorption/metabolism. | |
| Pregnant and lactating women and children with high choline requirements not met through diet. | |
| B12 deficiency (e.g., vegan, elderly). | |
| Mild to moderate fasting hyperhomocysteinemia not explained by low folate, B6 or B12 concentrations. | |
| PML-Homocysteine | F-Homocysteine | PML-Minus F-Homocysteine | %Change (PML vs. F-Homocysteine) | Prediction of PML-Homocysteine From F-Homocysteine Under Different Intake Conditions |
|---|---|---|---|---|
| 1: Native Condition—No Supplement (Olthof et al. [31] and Steenge et al. [5]) | ||||
| 32.6 µmol/L | 15.6 µmol/L | 17.0 µmol/L | +109.0% | PML-homocysteine = F-homocysteine × 2.09. |
| 34.8 µmol/L | 12.2 µmol/L | 22.6 µmol/L | +185.2% | PML-homocysteine = F-homocysteine × 2.85. |
| 31.6 µmol/L | 13.0 µmol/L | 18.6 µmol/L | +143.1% | PML-homocysteine = F-homocysteine × 2.43. |
| Mean = 33.0 µmol/L1 | 13.6 µmol/L | 19.4 µmol/L | +145.8% | PML-homocysteine = F-homocysteine × 2.46. |
| 2: Supplemented with 2.6 g/d Choline for 2 Weeks (Olthof et al. [31]) | ||||
| 22.3 µmol/L | 13.6 µmol/L | 8.7 µmol/L | +64.0% | PML-homocysteine = F-homocysteine × 1.64. Methionine load test in choline intake-optimized persons led to roughly 55% lower PML-homocysteine compared to non-supplemented people (8.7 vs. 19.4 µmol/L) |
| 3: Supplemented with 3 × 2 g/d Betaine for 6 Weeks (Steenge et al. [5]) | ||||
| 17.6 µmol/L | 10.9 µmol/L | 6.7 µmol/L | +61.5% | PML-homocysteine = F-homocysteine × 1.62. Methionine load test in betaine intake optimized persons led to roughly 65% lower PML-homocysteine compared to non-supplemented people (6.7 vs. 19.4 µmol/L) |
| 4: Supplemented with 400 µg × 2/d Folic Acid for 6 Weeks (Steenge et al. [5]) | ||||
| 33.0 µmol/L | 10.7 µmol/L | 22.3 µmol/L | +208.4% | PML-homocysteine = F-homocysteine × 3.1. Optimization of folate status has no lowering effect on PML-homocysteine compared to non-supplemented people (22.3 vs. 19.4 µmol/L) |
| Question | Elaboration |
|---|---|
| Sex differences: Is the methylation flux higher in men than in women? | The expression of several enzymes in C1-metabolism show sex differences [48]. For example, men have higher plasma homocysteine and betaine than premenopausal women because the PEMT gene is upregulated by estrogen. |
| Is there a dose–response relationship between choline intake and PML-homocysteine? | A dose–response relationship between betaine intake and PML-homocysteine has been demonstrated [32]. Does the same apply for choline and what is the intake level of choline to achieve a maximal reduction of PML-homocysteine? |
| Could high dose choline or betaine compensate for folate deficiency in terms of lowering PML-homocysteine? | Addressing metabolic capacity to upregulate methyl group flow via betaine/choline in individuals with folate deficiency or MTHFRC677T TT genotype. |
| Can PML-homocysteine be used to define the optimal intake of choline or betaine in pregnant and lactating women? | Homocysteine concentrations after a methionine load test can be measured before and after correcting the ‘gap’ of choline or betaine intake. |
| Can the PML-homocysteine test be used to identify women at high risk of neural tube defects or other pregnancy complications such as recurrent pregnancy loss, gestational diabetes, or preeclampsia? | In one study among women with a history of recurrent pregnancy loss, folic acid supplementation (0.5 mg/d for 2 months) did not lower PML-homocysteine in 53% of the women [49]. In theory, the PML-homocysteine test may identify women who could benefit from choline/betaine supplements by increasing methyl group flux via the BHMT pathway and normalizing PML-homocysteine. This may influence disease risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortz, J.; Obeid, R. The Shuttling of Methyl Groups Between Folate and Choline Pathways. Nutrients 2025, 17, 2495. https://doi.org/10.3390/nu17152495
Bortz J, Obeid R. The Shuttling of Methyl Groups Between Folate and Choline Pathways. Nutrients. 2025; 17(15):2495. https://doi.org/10.3390/nu17152495
Chicago/Turabian StyleBortz, Jonathan, and Rima Obeid. 2025. "The Shuttling of Methyl Groups Between Folate and Choline Pathways" Nutrients 17, no. 15: 2495. https://doi.org/10.3390/nu17152495
APA StyleBortz, J., & Obeid, R. (2025). The Shuttling of Methyl Groups Between Folate and Choline Pathways. Nutrients, 17(15), 2495. https://doi.org/10.3390/nu17152495







