Autonomic and Neuroendocrine Reactivity to VR Game Exposure in Children and Adolescents with Obesity: A Factor Analytic Approach to Physiological Reactivity and Eating Behavior
Abstract
1. Introduction
2. Methods
2.1. Study Participants
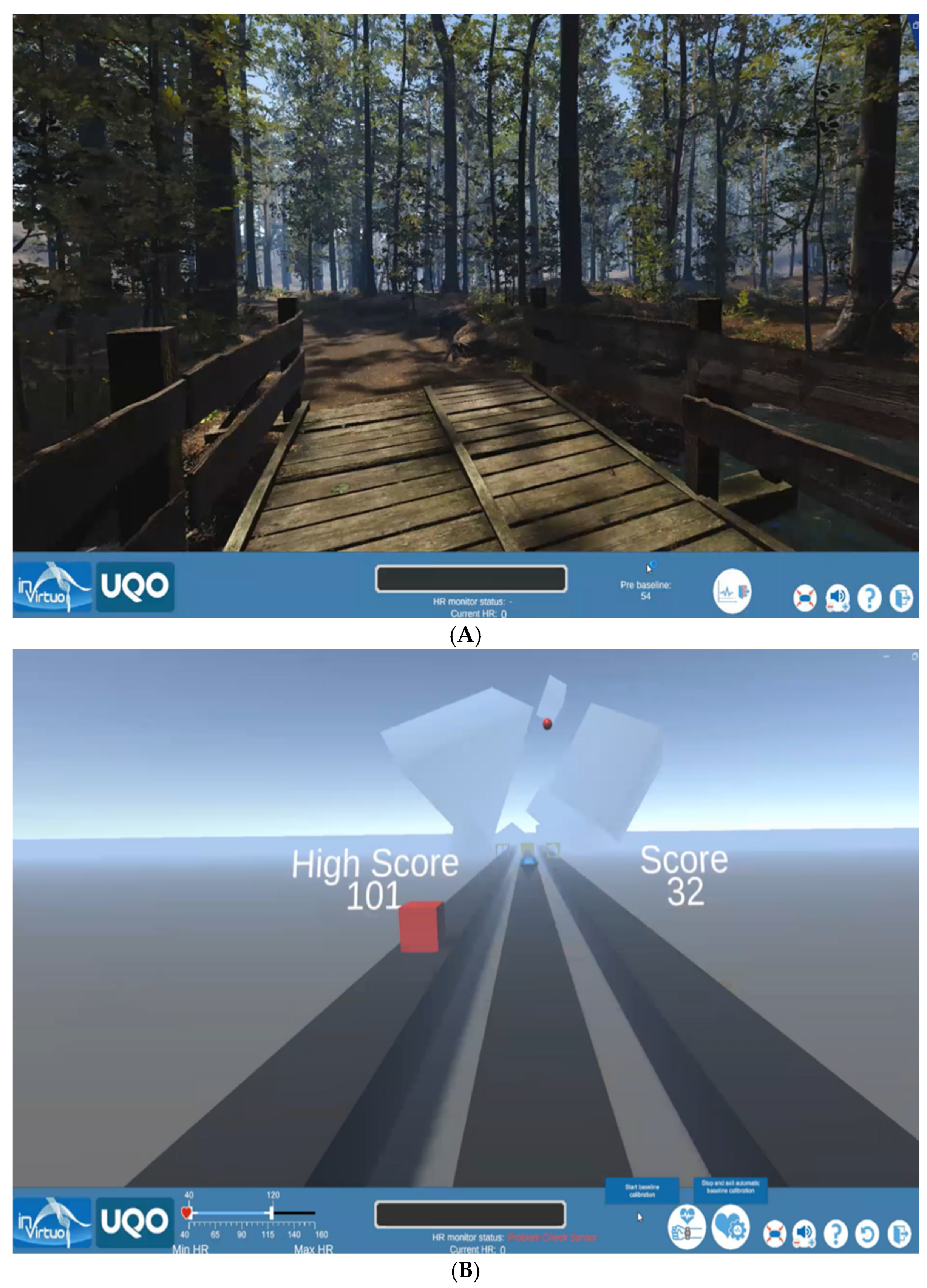
2.2. The VR-Exposure
2.3. Measurements
2.3.1. Heart Rate Variability (HRV) to Assess the Autonomic Nervous System
2.3.2. Psychological Measurements
2.3.3. Saliva Collection and Measurement of Stress Markers
2.3.4. Anthropometric Measurements
2.4. Virtual Reality (VR) Game-Based Experimental Model
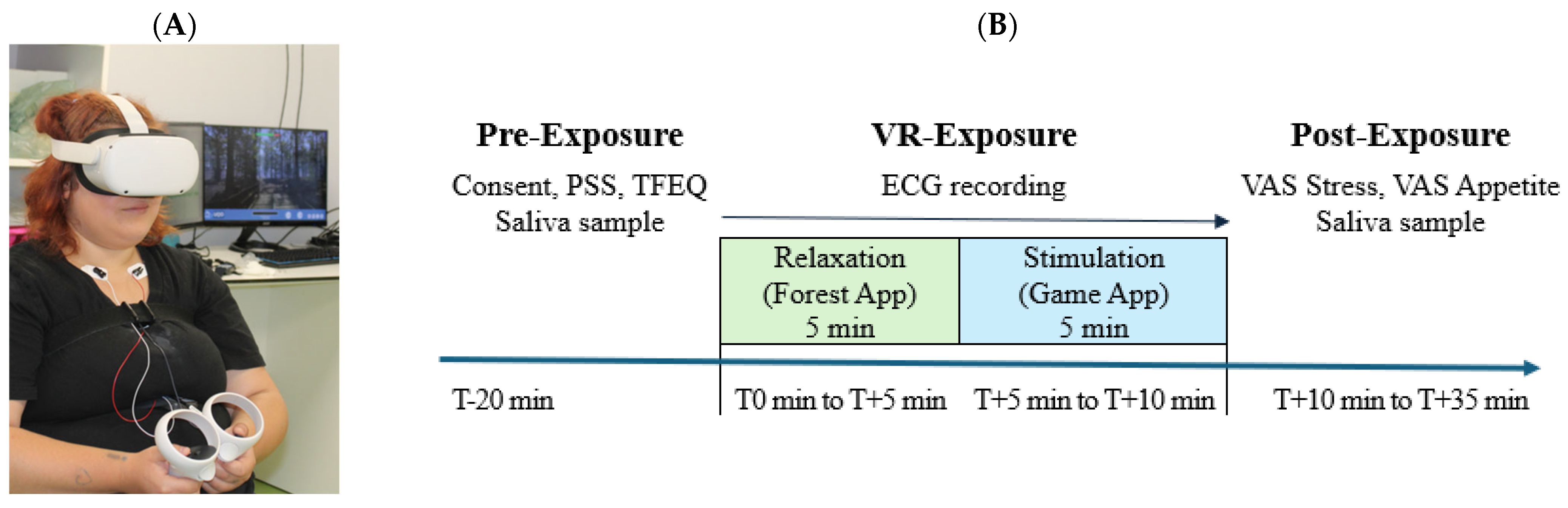
2.4.1. Pre-Exposure Stage (T − 20 min to T0 min)
2.4.2. VR Exposure Stage (T0 min to T + 10 min)
2.4.3. Post-Exposure Stage (T + 10 min to T + 35 min)
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
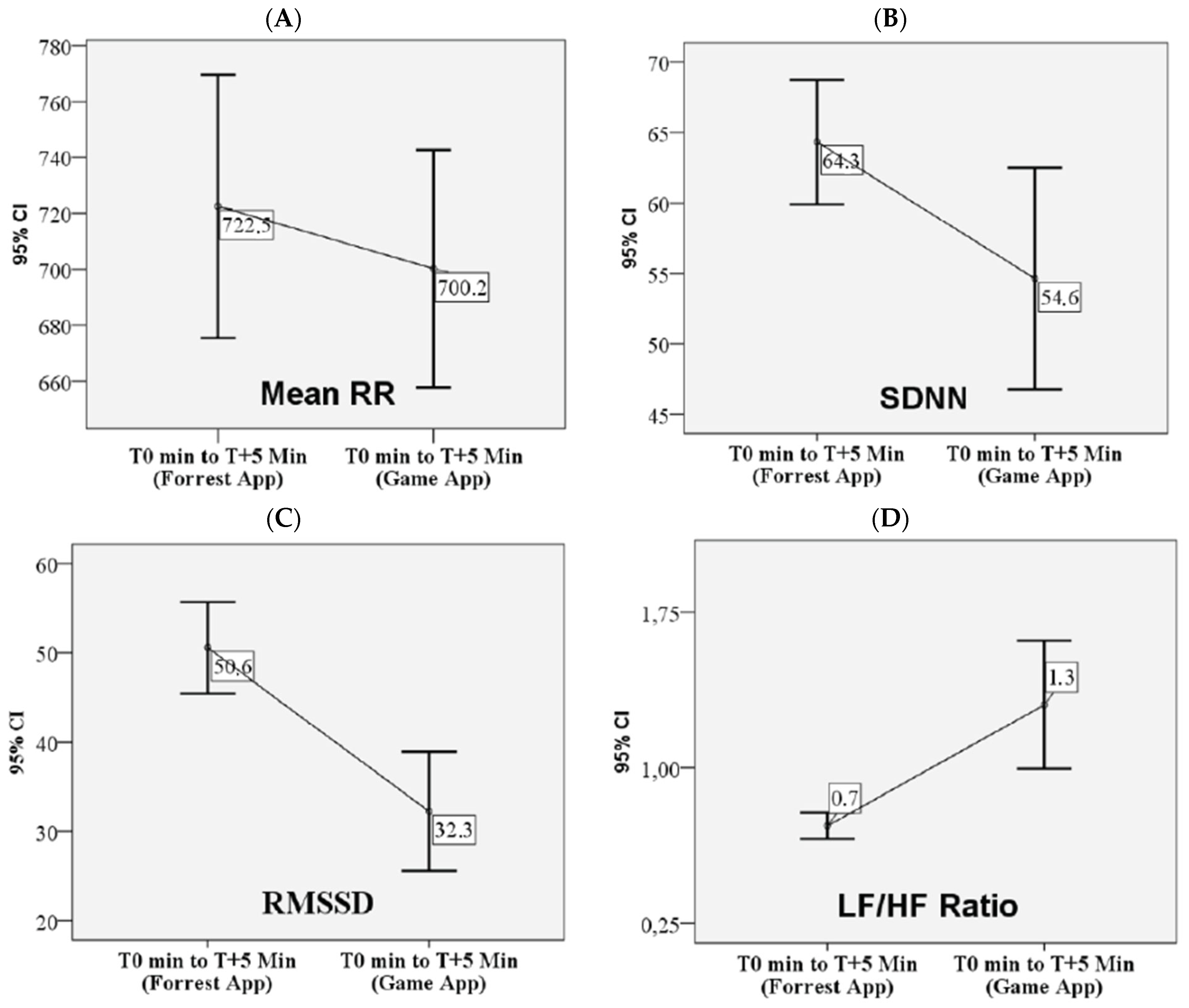
3.2. Autonomic Nervous System Responses to VR Game-Based Experimental Model
3.3. The Salivary Stress Markers’ Responses to VR Game-Based Experimental Model
3.4. Subjective Stress and Eating Responses to VR Game-Based Experimental Model
3.4.1. Perceived Stress Response
3.4.2. Eating Behavior Response
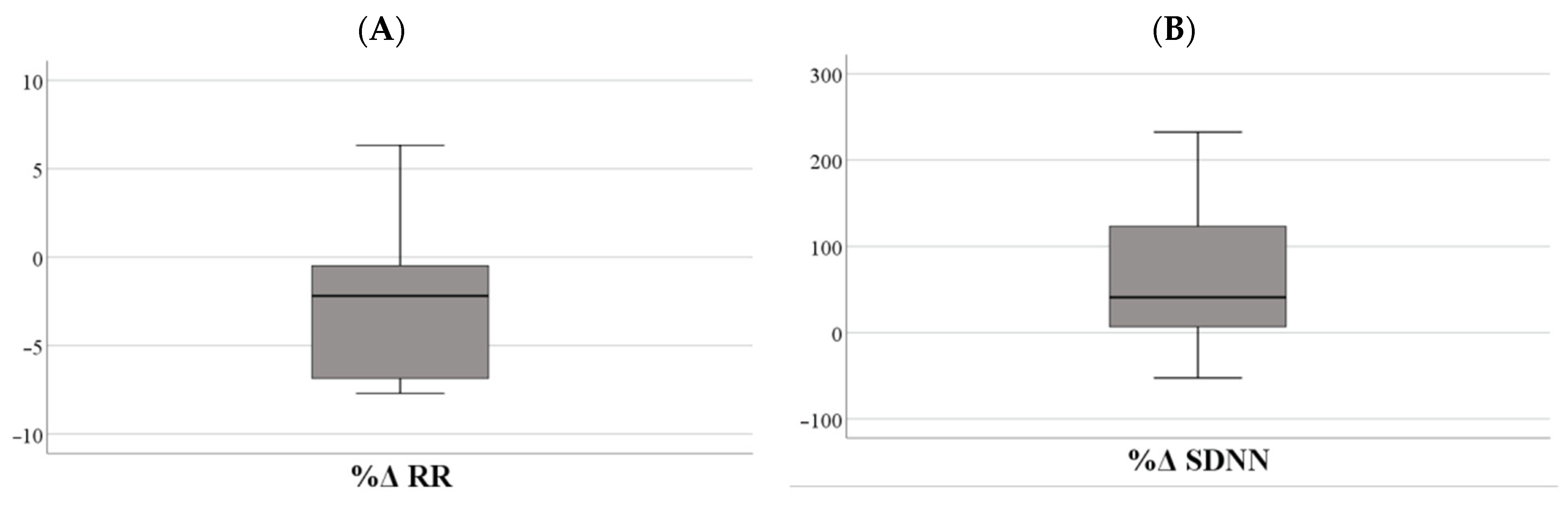
3.5. Physiological Stress Reactivity
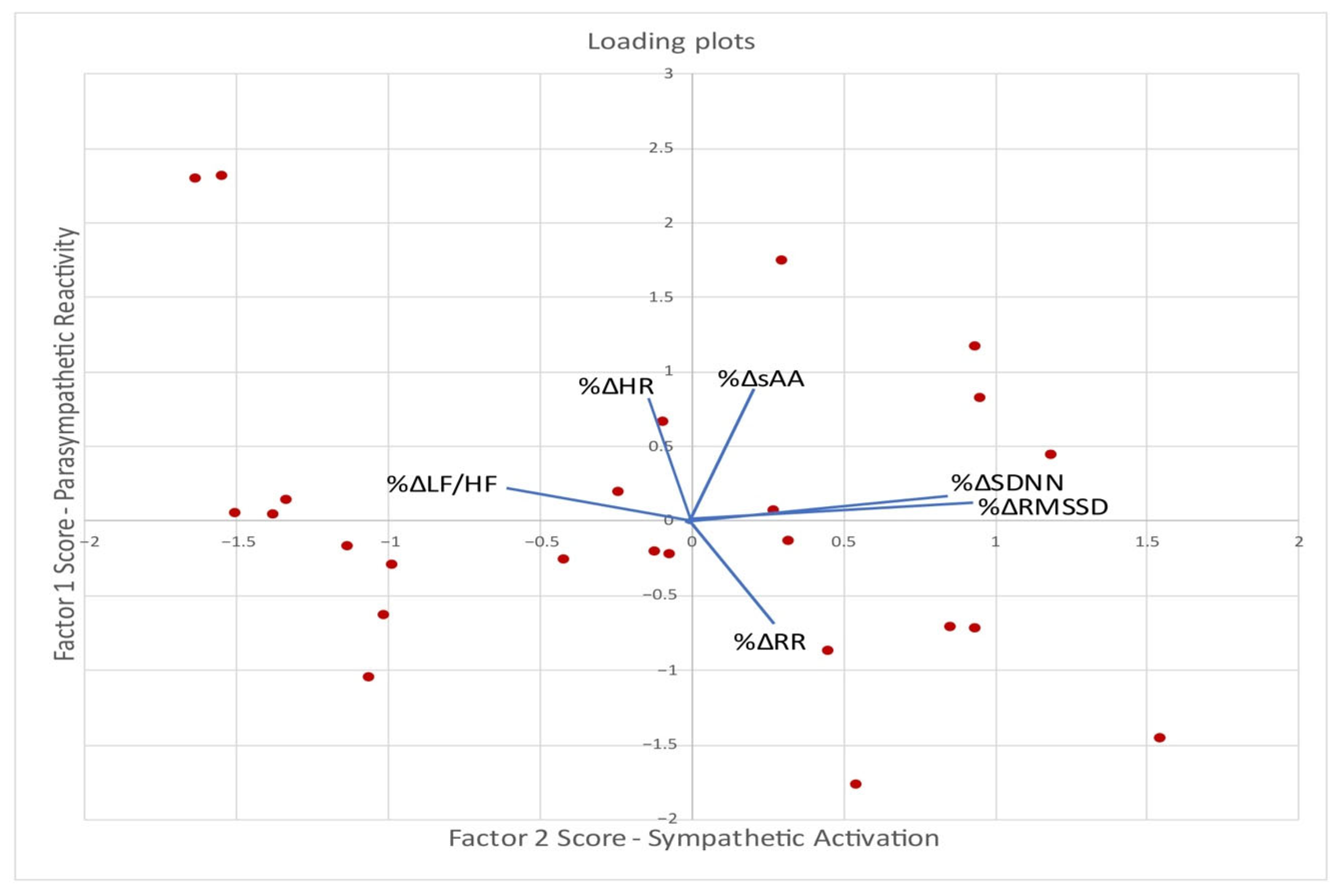
3.6. Factor Analysis and Associations with Eating Patterns
3.7. Spearman Correlations Between Physiological Response Factor Scores and Eating Pattern Measures
4. Discussions
4.1. VR Game-Based Stimulation [35]
4.2. Autonomic and Endocrine Responses to VR Game-Based Experimental Model
4.3. Correlations Between Physiological Response Factor Scores and Eating Pattern Measures
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becetti, I.; Bwenyi, E.L.; de Araujo, I.E.; Ard, J.; Cryan, J.F.; Farooqi, I.S.; Ferrario, C.R.; Gluck, M.E.; Holsen, L.M.; Kenny, P.J.; et al. The Neurobiology of Eating Behavior in Obesity: Mechanisms and Therapeutic Targets: A Report from the 23rd Annual Harvard Nutrition Obesity Symposium. Am. J. Clin. Nutr. 2023, 118, 314–328. [Google Scholar] [CrossRef]
- Shields, G.S.; Slavich, G.M. Lifetime Stress Exposure and Health: A Review of Contemporary Assessment Methods and Biological Mechanisms. Soc. Personal Psychol. Compass 2017, 11, e12335. [Google Scholar] [CrossRef]
- Jayasinghe, S.U.; Torres, S.J.; Fraser, S.F.; Turner, A.I. Cortisol, blood pressure, and heart rate responses to food intake were independent of physical fitness levels in women. Appl. Physiol. Nutr. Metab. 2015, 40, 1186–1192. [Google Scholar] [CrossRef]
- Matei, D.; Constantinescu, V.; Corciova, C.; Ignat, B.; Popescu, D.D. Autonomic impairment in patients with migraine. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3922–3927. [Google Scholar]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Lee, J.; Kim, C.; Lee, K.C. An Empirical Approach to Analyzing the Effects of Stress on Individual Creativity in Business Problem-Solving: Emphasis on the Electrocardiogram, Electroencephalogram Methodology. Front. Psychol. 2022, 13, 705442. [Google Scholar] [CrossRef]
- Dammen, L.V.; Finseth, T.T.; McCurdy, B.H.; Barnett, N.P.; Conrady, R.A.; Leach, A.G.; Deick, A.F.; Van Steenis, A.L.; Gardner, R.; Smith, B.L.; et al. Evoking stress reactivity in virtual reality: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 138, 104709. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Barrington, W.E.; Beresford, S.A.; McGregor, B.A.; White, E. Perceived stress and eating behaviors by sex, obesity status, and stress vulnerability: Findings from the vitamins and lifestyle (VITAL) study. J. Acad. Nutr. Diet. 2014, 114, 1791–1799. [Google Scholar] [CrossRef]
- Pasquali, R.; Anconetani, B.; Chattat, R.; Biscotti, M.; Spinucci, G.; Casimirri, F.; Vicennati, V.; Carcello, A.; Labate, A.M. Hypothalamic-pituitary-adrenal axis activity and its relationship to the autonomic nervous system in women with visceral and subcutaneous obesity: Effects of the corticotropin-releasing factor/arginine-vasopressin test and of stress. Metabolism 1996, 45, 351–356. [Google Scholar] [CrossRef]
- Therrien, F.; Drapeau, V.; Lalonde, J.; Lupien, S.J.; Beaulieu, S.; Dore, J.; Tremblay, A.; Richard, D. Cortisol response to the Trier Social Stress Test in obese and reduced obese individuals. Biol. Psychol. 2010, 84, 325–329. [Google Scholar] [CrossRef]
- Jeanningros, A.; Baillot, A.; Corno, G.; Rivard, M.C.; Aime, A.; Bouchard, S. Validation of a Virtual Environment to Induce State Social Physique Anxiety in Women with Obesity and Social Physique Anxiety. J. Clin. Med. 2023, 12, 6065. [Google Scholar] [CrossRef]
- Aliyari, H.; Sahraei, H.; Daliri, M.R.; Minaei-Bidgoli, B.; Kazemi, M.; Agaei, H.; Sahraei, M.; Hosseini, S.; Hadipour, M.M.; Mohammadi, M.; et al. The Beneficial or Harmful Effects of Computer Game Stress on Cognitive Functions of Players. Basic. Clin. Neurosci. 2018, 9, 177–186. [Google Scholar] [CrossRef]
- von Dawans, B.; Strojny, J.; Domes, G. The effects of acute stress and stress hormones on social cognition and behavior: Current state of research and future directions. Neurosci. Biobehav. Rev. 2021, 121, 75–88. [Google Scholar] [CrossRef]
- Martens, M.A.; Antley, A.; Freeman, D.; Slater, M.; Harrison, P.J.; Tunbridge, E.M. It feels real: Physiological responses to a stressful virtual reality environment and its impact on working memory. J. Psychopharmacol. 2019, 33, 1264–1273. [Google Scholar] [CrossRef]
- Chaput, J.P.; Visby, T.; Nyby, S.; Klingenberg, L.; Gregersen, N.T.; Tremblay, A.; Astrup, A.; Sjodin, A. Video game playing increases food intake in adolescents: A randomized crossover study. Am. J. Clin. Nutr. 2011, 93, 1196–1203. [Google Scholar] [CrossRef]
- Ketelhut, S.; Nigg, C.R. Heartbeats and high scores: Esports triggers cardiovascular and autonomic stress response. Front. Sports Act. Living 2024, 6, 1380903. [Google Scholar] [CrossRef]
- Yeo, M.; Lim, S.; Yoon, G. Analysis of Biosignals During Immersion in Computer Games. J. Med. Syst. 2017, 42, 3. [Google Scholar] [CrossRef]
- Pereira, A.; Leclerc-Thérien, C.-É.; Bouchard, S. Predictors of subjective stress in a competitive performance task used to practice stress management with biofeedback in virtual environments: The role of perfectionism. In Proceedings of the 26th Annual CyberPsychology, CyberTherapy & Social Networking Conference, Paris, France, 11–13 July 2023. [Google Scholar]
- Sharma, V.K.; Subramanian, S.K.; Arunachalam, V.; Rajendran, R. Heart Rate Variability in Adolescents—Normative Data Stratified by Sex and Physical Activity. J. Clin. Diagn. Res. 2015, 9, CC08-13. [Google Scholar] [CrossRef]
- Camm, A.J.; Fei, L. Chronotropic incompetence—Part I: Normal regulation of the heart rate. Clin. Cardiol. 1996, 19, 424–428. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Cohen, S.; Williamson, G. Perceived stress in a probability sample of the United States. Soc. Psychol. Health 1988, 5, 31–67. [Google Scholar]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Yabsley, J.L.; Gunnell, K.E.; Bryant, E.J.; Drapeau, V.; Thivel, D.; Adamo, K.B.; Chaput, J.P. Validation of a child version of the Three-Factor Eating Questionnaire in a Canadian sample: A psychometric tool for the evaluation of eating behaviour. Public Health Nutr. 2019, 22, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- World Health Organization. Growth Reference 5–19 years—BMI-for-Age (5–19 years). 2007. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 22 August 2021).
- Therrien, F.; Drapeau, V.; Lalonde, J.; Lupien, S.J.; Beaulieu, S.; Tremblay, A.; Richard, D. Awakening cortisol response in lean, obese, and reduced obese individuals: Effect of gender and fat distribution. Obesity 2007, 15, 377–385. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef]
- Pham, T.; Lau, Z.J.; Chen, S.H.A.; Makowski, D. Heart Rate Variability in Psychology: A Review of HRV Indices and an Analysis Tutorial. Sensors 2021, 21, 3998. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Brytek-Matera, A.; Czepczor-Bernat, K.; Modrzejewska, A. The relationship between eating patterns, body image and emotional dysregulation: Similarities between an excessive and normal body weight sample. Psychiatr. Pol. 2021, 55, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef]
- Herle, M.; Fildes, A.; Llewellyn, C.H. Emotional eating is learned not inherited in children, regardless of obesity risk. Pediatr. Obes. 2018, 13, 628–631. [Google Scholar] [CrossRef]
- Reddy, S.; Molleti, M.; Li, L. Impacts of Acute Psychological Stress on the Emotions of Individuals with Early Life Stress. Alpha Psychiatry 2024, 25, 513–518. [Google Scholar]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Lopez-Duran, N.L.; Mayer, S.E.; Abelson, J.L. Modeling neuroendocrine stress reactivity in salivary cortisol: Adjusting for peak latency variability. Stress 2014, 17, 285–295. [Google Scholar] [CrossRef]
- Jonsson, P.; Wallergard, M.; Osterberg, K.; Hansen, A.M.; Johansson, G.; Karlson, B. Cardiovascular and cortisol reactivity and habituation to a virtual reality version of the Trier Social Stress Test: A pilot study. Psychoneuroendocrinology 2010, 35, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Helminen, E.C.; Morton, M.L.; Wang, Q.; Felver, J.C. Stress Reactivity to the Trier Social Stress Test in Traditional and Virtual Environments: A Meta-Analytic Comparison. Psychosom. Med. 2021, 83, 200–211. [Google Scholar] [CrossRef]
- Shiban, Y.; Diemer, J.; Brandl, S.; Zack, R.; Muhlberger, A.; Wust, S. Trier Social Stress Test in vivo and in virtual reality: Dissociation of response domains. Int. J. Psychophysiol. 2016, 110, 47–55. [Google Scholar] [CrossRef]
- Kothgassner, O.D.; Goreis, A.; Glenk, L.M.; Kafka, J.X.; Pfeffer, B.; Beutl, L.; Kryspin-Exner, I.; Hlavacs, H.; Palme, R.; Felnhofer, A. Habituation of salivary cortisol and cardiovascular reactivity to a repeated real-life and virtual reality Trier Social Stress Test. Physiol. Behav. 2021, 242, 113618. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Buttlar, B.; Halbeisen, G.; Walther, E.; Domes, G. Virtually stressed? A refined virtual reality adaptation of the Trier Social Stress Test (TSST) induces robust endocrine responses. Psychoneuroendocrinology 2019, 101, 186–192. [Google Scholar] [CrossRef]
- Kelly, O.; Matheson, K.; Martinez, A.; Merali, Z.; Anisman, H. Psychosocial stress evoked by a virtual audience: Relation to neuroendocrine activity. Cyberpsychol Behav. 2007, 10, 655–662. [Google Scholar] [CrossRef]
- Gotca, I.; Druica, A.; Timofte, D.V.; Preda, C.; Anton-Paduraru, D.-T.; Ghiciuc, C.M.; Ungureanu, M.C.; Leustean, L.; Mocanu, V. Cortisol Reactivity to a Digital Version of Trier Social Stress Test and Eating Behavior in Non-Overweight and Overweight Adolescents: A Pilot Study. Appl. Sci. 2021, 11, 9683. [Google Scholar] [CrossRef]
- Michels, N.; Sioen, I.; Clays, E.; De Buyzere, M.; Ahrens, W.; Huybrechts, I.; Vanaelst, B.; De Henauw, S. Children’s heart rate variability as stress indicator: Association with reported stress and cortisol. Biol. Psychol. 2013, 94, 433–440. [Google Scholar] [CrossRef]
- Hillman, J.B.; Dorn, L.D.; Loucks, T.L.; Berga, S.L. Obesity and the hypothalamic-pituitary-adrenal axis in adolescent girls. Metabolism 2012, 61, 341–348. [Google Scholar] [CrossRef][Green Version]
- Herhaus, B.; Ullmann, E.; Chrousos, G.; Petrowski, K. High/low cortisol reactivity and food intake in people with obesity and healthy weight. Transl. Psychiatry 2020, 10, 40. [Google Scholar] [CrossRef]
- Gonzalez-Velazquez, V.E.; Pedraza-Rodriguez, E.M.; Carrazana-Escalona, R.; Moreno-Padilla, M.; Munoz-Bustos, G.A.; Sanchez-Hechavarria, M.E. Cardiac vagal imbalance to the isometric sustained weight test in adolescents with emotional eating behavior. Physiol. Behav. 2020, 223, 112994. [Google Scholar] [CrossRef]
- Karlsson, J.; Persson, L.O.; Sjostrom, L.; Sullivan, M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1715–1725. [Google Scholar] [CrossRef]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Newman, E.; O’Connor, D.B.; Conner, M. Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology 2007, 32, 125–132. [Google Scholar] [CrossRef]
- Meule, A.; Vogele, C.; Kubler, A. Restrained eating is related to accelerated reaction to high caloric foods and cardiac autonomic dysregulation. Appetite 2012, 58, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pierart, C.; Chaplin, T.M.; Hommer, R.E.; Mayes, L.C.; Crowley, M.J. Getting to the heart of food craving with resting heart rate variability in adolescents. Appetite 2020, 155, 104816. [Google Scholar] [CrossRef]
- Azevedo, E.P.; Ivan, V.J.; Friedman, J.M.; Stern, S.A. Higher-Order Inputs Involved in Appetite Control. Biol. Psychiatry 2022, 91, 869–878. [Google Scholar] [CrossRef]
- Stern, S.A.; Azevedo, E.P.; Pomeranz, L.E.; Doerig, K.R.; Ivan, V.J.; Friedman, J.M. Top-down control of conditioned overconsumption is mediated by insular cortex Nos1 neurons. Cell Metab. 2021, 33, 1418–1432.e1416. [Google Scholar] [CrossRef]
- Loffler, A.; Luck, T.; Then, F.S.; Sikorski, C.; Kovacs, P.; Bottcher, Y.; Breitfeld, J.; Tonjes, A.; Horstmann, A.; Loffler, M.; et al. Eating Behaviour in the General Population: An Analysis of the Factor Structure of the German Version of the Three-Factor-Eating-Questionnaire (TFEQ) and Its Association with the Body Mass Index. PLoS ONE 2015, 10, e0133977. [Google Scholar] [CrossRef]


| Characteristics | Participants (N = 30) |
|---|---|
| Age | |
| Age (years) | 10 (9–14) |
| 8–11 years (%) | 56.7 (17) |
| 12–17 years (%) | 43.3 (13) |
| Anthropometric parameters | |
| Body Mass Index (BMI) (kg/m2) | 27.6 (26.1–32.9) |
| Central Obesity Index | 0.62 (0.58–0.65) |
| Severe obesity 1 (>3 SD) (%, N) | 60 (18) |
| Parameters | VR Game-Based Experimental Model | ||
|---|---|---|---|
| Heart rate variability | T0 min to T + 5 min Relaxation Forest App | T + 5 min to T + 10 min Stimulation Game App | p-value |
| ECG real-time monitoring | |||
| Mean heart rate (min−1) | 89 (80.5–92.3) | 90 (85.3–97.3) | 0.002 |
| Mean RR (ms) | 679 (632–814.3) | 673 (616–785) | 0.003 |
| SDNN (ms) | 65 (55.8–69) | 60 (39.8–69.5) | 0.026 |
| RMSSD (ms) | 47.5 (41–61) | 25 (18.5–47.5) | 0.000 |
| PNN50 (%) | 22 (14–31) | 19 (14–28) | 0.213 |
| LF/HF ratio | 0.8 (0.6–0.9) | 1.3 (0.7–1.5) | 0.001 |
| Salivary stress markers | T + 10 min (Post VR) | T + 35 min (Peak-time) | p-value |
| Saliva collection | Saliva collection | ||
| Salivary cortisol (ng/mL) | 22.4 (16.1–33.5) | 20.6 (17.8–24.8) | 0.6 |
| Salivary alpha-amylase (ng/mL) | 2.9 (2.2–6.1) | 5.6 (3.8–6.8) | 0.001 |
| Pre VR-exposure | Post VR-exposure | ||
| Perceived stress | Questionnaires | ||
| Perceived Stress Scale (PSS) | 17.5 (13–23) | ||
| Stress and Anxiety Assessment (VAS Method) | |||
| How stressed are you feeling right now? | - | 1 (1–2.3) | |
| How anxious are you feeling right now? | - | 1 (1–4) | |
| Eating behavior parameters 1 | |||
| CR-factor | 13 (11.8–18.2) | - | |
| UE-factor | 21.5 (16–28.3) | - | |
| EE-factor | 8 (6–10.2) | - | |
| Appetite and Craving Ratings (VAS Method) | |||
| How strong is your desire to eat right now? | - | 4.5 (2–6.3) | |
| How strong is your desire to eat sweets right now? | - | 1 (1–3) | |
| HRV Parameter/Salivary Marker/ | Normoreactive (N, %) | Hyporeactive/No Response (N, %) |
|---|---|---|
| ↓ RR ≥ 10% | 30 (100%) | 0 (0%) |
| ↓ RMSSD ≥ 10% | 26 (86.7%) | 4 (13.3%) |
| ↑ LF/HF ≥ 10% | 21 (70%) | 9 (30%) |
| ↑ sCortisol ≥ 20% | 4 (13.3%) | 26 (86.7%) |
| ↑ sAA ≥ 20% | 28 (93.3%) | 2 (6.7%) |
| Variable | Component | |
|---|---|---|
| 1 | 2 | |
| %ΔRMSSD | +0.931 | |
| %ΔSDNN | +0.869 | |
| %ΔLF/HF | −0.583 | |
| %ΔsAA | +0.839 | |
| %ΔHR | +0.800 | |
| %ΔRR | −0.645 | |
| Variable | Parasympathetic Reactivity | Sympathetic Activation |
|---|---|---|
| (Factor 1 *) | (Factor 2 *) | |
| PSS (pre-exposure) | −0.365 (p = 0.05) | −0.135 (p = 0.48) |
| VAS Stress | −0.194 (p = 0.30) | 0.322 (p = 0.08) |
| VAS Anxiety | −0.430 (p = 0.02) | 0.470 (p < 0.01) |
| VAS Appetite | 0.249 (p = 0.19) | −0.184 (p = 0.33) |
| VAS Sweet Craving | 0.289 (p = 0.12) | −0.172 (p = 0.36) |
| Cognitive Restraint (CR) | −0.174 (p = 0.37) | 0.422 (p = 0.02) |
| Uncontrolled Eating (UE) | 0.553 (p < 0.01) | −0.225 (p = 0.23) |
| Emotional Eating (EE) | 0.082 (p = 0.67) | 0.185 (p = 0.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onita, C.A.; Matei, D.-V.; Trandafir, L.-M.; Petrescu-Miron, D.; Corciova, C.; Fuior, R.; Manole, L.-M.; Mihai, B.-M.; Dascalu, C.-G.; Tarcea, M.; et al. Autonomic and Neuroendocrine Reactivity to VR Game Exposure in Children and Adolescents with Obesity: A Factor Analytic Approach to Physiological Reactivity and Eating Behavior. Nutrients 2025, 17, 2492. https://doi.org/10.3390/nu17152492
Onita CA, Matei D-V, Trandafir L-M, Petrescu-Miron D, Corciova C, Fuior R, Manole L-M, Mihai B-M, Dascalu C-G, Tarcea M, et al. Autonomic and Neuroendocrine Reactivity to VR Game Exposure in Children and Adolescents with Obesity: A Factor Analytic Approach to Physiological Reactivity and Eating Behavior. Nutrients. 2025; 17(15):2492. https://doi.org/10.3390/nu17152492
Chicago/Turabian StyleOnita, Cristiana Amalia, Daniela-Viorelia Matei, Laura-Mihaela Trandafir, Diana Petrescu-Miron, Calin Corciova, Robert Fuior, Lorena-Mihaela Manole, Bogdan-Mircea Mihai, Cristina-Gena Dascalu, Monica Tarcea, and et al. 2025. "Autonomic and Neuroendocrine Reactivity to VR Game Exposure in Children and Adolescents with Obesity: A Factor Analytic Approach to Physiological Reactivity and Eating Behavior" Nutrients 17, no. 15: 2492. https://doi.org/10.3390/nu17152492
APA StyleOnita, C. A., Matei, D.-V., Trandafir, L.-M., Petrescu-Miron, D., Corciova, C., Fuior, R., Manole, L.-M., Mihai, B.-M., Dascalu, C.-G., Tarcea, M., Bouchard, S., & Mocanu, V. (2025). Autonomic and Neuroendocrine Reactivity to VR Game Exposure in Children and Adolescents with Obesity: A Factor Analytic Approach to Physiological Reactivity and Eating Behavior. Nutrients, 17(15), 2492. https://doi.org/10.3390/nu17152492











