Diet to Data: Validation of a Bias-Mitigating Nutritional Screener Using Assembly Theory
Abstract
1. Introduction
1.1. What Is the Guide Against Age Related Disease (GARD)?
1.2. What Is Assembly Theory and How Does It Apply to Human Health?
- Assembly Index (Ai)—the smallest number of physical steps needed to construct an object from basic building blocks.
- Copy Number (Ni)—the number of identical copies of that object in a given environment.
1.3. How Assembly Theory Can Measure Diet Behaviors
2. Materials and Methods
2.1. Data Collection Methods
2.2. Collecting the Food Diary
2.3. Defining a Point
2.4. Grading of High-Complexity Variables
2.4.1. Fresh Plant
2.4.2. Farm-Direct Animal Product
2.4.3. Fermented Food
2.4.4. Fasting (Autophagy)
2.4.5. Social Eating
2.4.6. Outside Eating
2.5. Grading of Low-Complexity Variables
2.5.1. Processed Ingredients
2.5.2. Refined Ingredients
2.5.3. Processed Animal Products
2.5.4. Ultra-Processed Foods
2.5.5. Distracted Eating
2.5.6. Over-Consumptive Eating
2.6. Grading the Food Diary
2.7. Survey Distribution
2.8. Population
2.9. Statistical Analysis
- 1.
- There is no significant positive correlation within a patient’s diet between high-complexity diets and high-complexity behaviors (test of convergent validity).
- 2.
- There is no significant positive correlation within a patient’s diet between low-complexity diets and low-complexity behaviors (test of convergent validity)
- 3.
- There is no significant negative correlation within a patient’s diet between mismatched pairs: high-complexity diets with low-complexity behaviors, and low-complexity diets with high-complexity behaviors (tests of discriminant validity).
- 4.
- We expect to reject the nulls for aligned and mismatched pairs.
3. Results
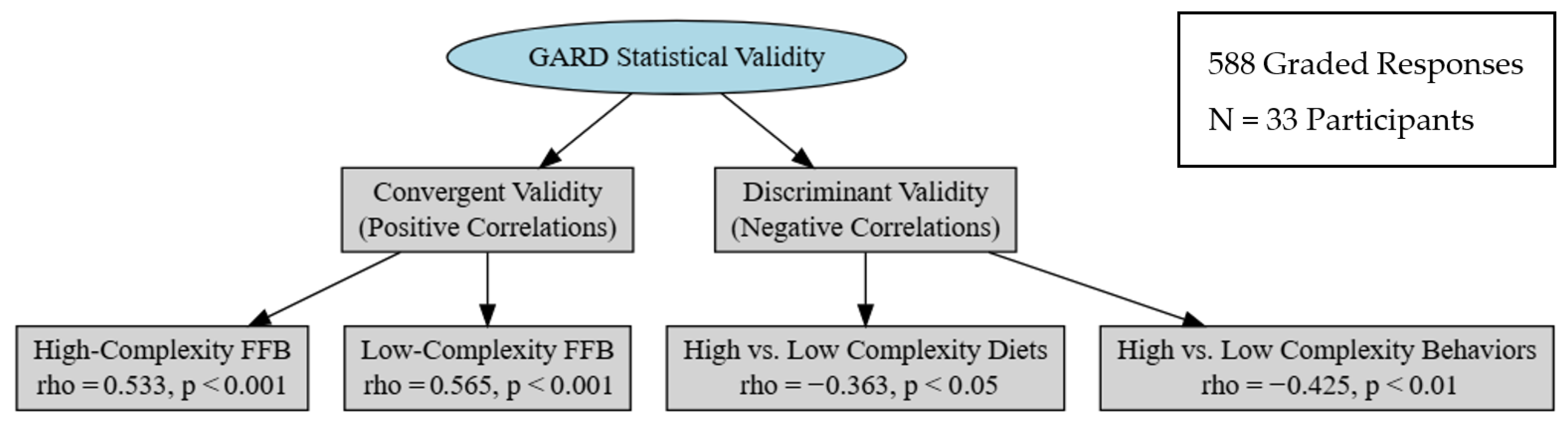
3.1. Internal Validity
3.1.1. Convergent Validity
3.1.2. Discriminant Validity
3.1.3. Face Validity
3.2. Construct Validity
Known Group Validity
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ai | Assembly Index |
| Ni | Copy Number |
| FFB | Food and Food Behavior |
| GARD | Guide Against Age-Related Disease |
| UPF | Ultra-Processed Food |
| MoCA | Montreal Cognitive Assessment |
| NIH | National Institutes of Health |
| PUFA | Polyunsaturated Fatty Acid |
References
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Termannsen, A.-D.; Søndergaard, C.S.; Færch, K.; Andersen, T.H.; Raben, A.; Quist, J.S. Effects of Plant-Based Diets on Markers of Insulin Sensitivity: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2024, 16, 2110. [Google Scholar] [CrossRef] [PubMed]
- Seguias, L.; Tapper, K. The effect of mindful eating on subsequent intake of a high calorie snack. Appetite 2018, 121, 93–100. [Google Scholar] [CrossRef]
- Fernández-Fígares Jiménez, M.D.C. A Whole Plant–Foods Diet in the Prevention and Treatment of Overweight and Obesity: From Empirical Evidence to Potential Mechanisms. J. Am. Nutr. Assoc. 2025, 44, 137–155. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Crimarco, A.; Landry, M.J.; Gardner, C.D. Ultra-processed Foods, Weight Gain, and Co-morbidity Risk. Curr. Obes. Rep. 2022, 11, 80–92. [Google Scholar] [CrossRef]
- Grinshpan, L.S.; Eilat-Adar, S.; Ivancovsky-Wajcman, D.; Kariv, R.; Gillon-Keren, M.; Zelber-Sagi, S. Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: A systematic review. JHEP Rep. 2024, 6, 100964. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef]
- Song, Z.; Song, R.; Liu, Y.; Wu, Z.; Zhang, X. Effects of ultra-processed foods on the microbiota-gut-brain axis: The bread-and-butter issue. Food Res. Int. 2023, 167, 112730. [Google Scholar] [CrossRef]
- Yaroch, A.L.; Tooze, J.; Thompson, F.E.; Blanck, H.M.; Thompson, O.M.; Colón-Ramos, U.; Shaikh, A.R.; McNutt, S.; Nebeling, L.C. Evaluation of Three Short Dietary Instruments to Assess Fruit and Vegetable Intake: The National Cancer Institute’s Food Attitudes and Behaviors Survey. J. Acad. Nutr. Diet. 2012, 112, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Green, S.M.; James, E.P. Barriers and Facilitators to Undertaking Nutritional Screening of Patients: A systematic review. J. Hum. Nutr. Diet. 2013, 26, 211–221. [Google Scholar] [CrossRef]
- Tanweer, A.; Khan, S.; Mustafa, F.N.; Imran, S.; Humayun, A.; Hussain, Z. Improving dietary data collection tools for better nutritional assessment—A systematic review. Comput. Methods Programs Biomed. Update 2022, 2, 100067. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, Y.; Zhang, Q.; Yu, S.; Wu, S. Ultra-processed foods and risk of all-cause mortality: An updated systematic review and dose-response meta-analysis of prospective cohort studies. Syst. Rev. 2025, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.; Ju, Y.J.; Shin, J.; Jang, S.-I.; Park, E.-C. Association between eating behaviour and diet quality: Eating alone vs. eating with others. Nutr. J. 2018, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Wada, T.; Okumiya, K.; Ishimoto, Y.; Fukutomi, E.; Kasahara, Y.; Chen, W.; Sakamoto, R.; Fujisawa, M.; Otsuka, K.; et al. Eating alone among community-dwelling Japanese elderly: Association with depression and food diversity. J. Nutr. Health Aging 2012, 16, 728–731. [Google Scholar] [CrossRef]
- Matos, J.V.; Lopes, R.J. Food System Sustainability Metrics: Policies, Quantification, and the Role of Complexity Sciences. Sustainability 2021, 13, 12408. [Google Scholar] [CrossRef]
- Bleich, S.N.; Jones-Smith, J.; Wolfson, J.A.; Zhu, X.; Story, M. The Complex Relationship Between Diet and Health. Health Aff. Proj. Hope 2015, 34, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.W.; Quinn, R.; Burke, F.; Piccinin, D.; Sartor, L.; Lewis, J.D.; Wu, G.D. Development of the Penn Healthy Diet screener with reference to adult dietary intake data from the National Health and Nutrition Examination Survey. Nutr. J. 2022, 21, 70. [Google Scholar] [CrossRef]
- Sharma, A.; Czégel, D.; Lachmann, M.; Kempes, C.P.; Walker, S.I.; Cronin, L. Assembly theory explains and quantifies selection and evolution. Nature 2023, 622, 321–328. [Google Scholar] [CrossRef]
- Walker, S.I.; Mathis, C.; Marshall, S.; Cronin, L. Experimental Measurement of Assembly Indices are Required to Determine the Threshold for Life. arXiv 2024, arXiv:2406.06826. [Google Scholar] [CrossRef]
- Jirasek, M.; Sharma, A.; Bame, J.R.; Mehr, S.H.M.; Bell, N.; Marshall, S.M.; Mathis, C.; MacLeod, A.; Cooper, G.J.T.; Swart, M.; et al. Investigating and Quantifying Molecular Complexity Using Assembly Theory and Spectroscopy. ACS Cent. Sci. 2024, 10, 1054–1064. [Google Scholar] [CrossRef]
- Marshall, S.M.; Mathis, C.; Carrick, E.; Keenan, G.; Cooper, G.J.T.; Graham, H.; Craven, M.; Gromski, P.S.; Moore, D.G.; Walker, S.I.; et al. Identifying molecules as biosignatures with assembly theory and mass spectrometry. Nat. Commun. 2021, 12, 3033. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.I. Life as No One Knows It; Riverhead Books: New York, NY, USA, 2024. [Google Scholar]
- National Cancer Institute. DNA Synthesis. Qeios. 2020. Available online: https://www.qeios.com/read/Y1GUWN (accessed on 15 February 2025).
- Bianconi, E.; Piovesan, A.; Facchin, F.; Beraudi, A.; Casadei, R.; Frabetti, F.; Vitale, L.; Pelleri, M.C.; Tassani, S.; Piva, F.; et al. An estimation of the number of cells in the human body. Ann. Hum. Biol. 2013, 40, 463–471. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, Y.C.; Yin, Q.-Z.; Ng, C.Y.; Jackson, W.M. Evidence for direct molecular oxygen production in CO2 photodissociation. Science 2014, 346, 61–64. [Google Scholar] [CrossRef]
- Musacchio, F.-M. The Far Ultraviolet Aurora of Ganymede. Ph.D. Thesis, Universität zu Köln, Cologne, Germany, 2016. Available online: http://www.uni-koeln.de/ (accessed on 23 March 2025).
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Astawan, M.; Prayudani, A.P.G. The Overview of Food Technology to Process Soy Protein Isolate and Its Application toward Food Industry. World Nutr. J. 2020, 4, 12–17. [Google Scholar] [CrossRef]
- Louzada, M.L.d.C.; Martins, A.P.B.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.-C.; Cannon, G.; Monteiro, C.A. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev. Saúde Pública 2015, 49, 38. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Escalante-Araiza, F.; Rivera-Monroy, G.; Loza-López, C.E.; Gutiérrez-Salmeán, G. The effect of plant-based diets on meta-inflammation and associated cardiometabolic disorders: A review. Nutr. Rev. 2022, 80, 2017–2028. [Google Scholar] [CrossRef]
- Sidhu, S.R.K.; Kok, C.W.; Kunasegaran, T.; Ramadas, A. Effect of Plant-Based Diets on Gut Microbiota: A Systematic Review of Interventional Studies. Nutrients 2023, 15, 1510. [Google Scholar] [CrossRef] [PubMed]
- Peña-Jorquera, H.; Cid-Jofré, V.; Landaeta-Díaz, L.; Petermann-Rocha, F.; Martorell, M.; Zbinden-Foncea, H.; Ferrari, G.; Jorquera-Aguilera, C.; Cristi-Montero, C. Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome—A Comprehensive Review. Nutrients 2023, 15, 3244. [Google Scholar] [CrossRef]
- Marshall, S.M.; Moore, D.G.; Murray, A.R.; Walker, S.I.; Cronin, L. Formalising the Pathways to Life Using Assembly Spaces. Entropy 2022, 24, 884. [Google Scholar] [CrossRef]
- Deutsch, D. Constructor theory. Synthese 2013, 190, 4331–4359. [Google Scholar] [CrossRef]
- Marletto, C. Constructor theory of life. J. R. Soc. Interface 2015, 12, 20141226. [Google Scholar] [CrossRef]
- Crerar, L.D. Review: Aquagenesis: The Origin and Evolution of Life in the Sea, by Richard Ellis. Am. Biol. Teach 2004, 66, 579–580. [Google Scholar] [CrossRef]
- Bard, K.A. Social Cognition: Evolutionary History of Emotional Engagements with Infants. Curr. Biol. 2009, 19, R941–R943. [Google Scholar] [CrossRef]
- Mechanisms of Social Cognition. Annual Reviews. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev-psych-120710-100449 (accessed on 24 March 2025).
- Reséndiz-Benhumea, G.M.; Froese, T. Enhanced Neural Complexity is Achieved by Mutually Coordinated Embodied Social Interaction: A State-Space Analysis. 2020. Available online: https://www.semanticscholar.org/paper/Enhanced-Neural-Complexity-is-Achieved-by-Mutually-Res%C3%A9ndiz-Benhumea-Froese/398560644ae1a4f3da7bbbcb12eb856184c72834 (accessed on 27 February 2025).
- Bangsgaard, P.; Yeomans, L.; Darabi, H.; Gregersen, K.M.; Olsen, J.; Richter, T.; Mortensen, P. Feasting on Wild Boar in the Early Neolithic. Evidence from an 11,400-year-old Placed Deposit at Tappeh Asiab, Central Zagros. Camb. Archaeol. J. 2019, 29, 443–463. [Google Scholar] [CrossRef]
- Agam, A.; Barkai, R. Elephant and Mammoth Hunting during the Paleolithic: A Review of the Relevant Archaeological, Ethnographic and Ethno-Historical Records. Quaternary 2018, 1, 3. [Google Scholar] [CrossRef]
- Ollivier, M. Thousands of years of human-dog relationship. Biologie aujourd’hui 2024, 218, 115–127. [Google Scholar] [CrossRef]
- Tromop-van Dalen, C.; Thorne, K.; Common, K.; Edge, G.; Woods, L. Audit to investigate junior doctors’ knowledge of how to administer and score the Montreal Cognitive Assessment (MoCA). N. Z. Med. J. 2018, 131, 91–108. [Google Scholar] [PubMed]
- Laska, M.N.; Hearst, M.O.; Lust, K.; Lytle, L.A.; Story, M. How we eat what we eat: Identifying meal routines and practices most strongly associated with healthy and unhealthy dietary factors among young adults. Public Health Nutr. 2015, 18, 2135–2145. [Google Scholar] [CrossRef]
- 24-Hour Dietary Recall (24HR) At a Glance | Dietary Assessment Primer. Available online: https://dietassessmentprimer.cancer.gov/profiles/recall/index.html (accessed on 20 July 2025).
- Mahal, S.; Kucha, C.; Kwofie, E.M.; Ngadi, M. A systematic review of dietary data collection methodologies for diet diversity indicators. Front. Nutr. 2024, 11, 1195799. [Google Scholar] [CrossRef]
- McDonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American Gut: An Open Platform for Citizen Science Microbiome Research. mSystems 2018, 3, e00031-18. [Google Scholar] [CrossRef]
- ur Rahman, U.; Sahar, A.; Ishaq, A.; Aadil, R.M.; Zahoor, T.; Ahmad, M.H. Advanced Meat Preservation Methods: A Mini Review. J. Food Saf. 2018, 38, e12467. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial Community Analysis of Sauerkraut Fermentation Reveals a Stable and Rapidly Established Community. Foods 2018, 7, 77. [Google Scholar] [CrossRef]
- Basavarajaiah, D.M.; Narasimhamurhy, B. Predictive Modeling of Autophagy Interrelation with Fasting. J. Biom. Biostat. 2020, 5, 102. [Google Scholar]
- Bagherniya, M.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. 2018, 47, 183–197. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. Breaking Bread: The Functions of Social Eating. Adapt. Hum. Behav. Physiol 2017, 3, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Tremblay, M.S.; Katzmarzyk, P.T.; Fogelholm, M.; Mikkilä, V.; Hu, G.; Lambert, E.V.; Maher, C.; Maia, J.; Olds, T.; et al. Outdoor Time and Dietary Patterns in Children Around the World. J. Public Health 2018, 40, e493–e501. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. Refined carbohydrates—A cause of suboptimal nutrient intake. Med. Hypotheses 1983, 10, 411–424. [Google Scholar] [CrossRef]
- Khalid, W.; Maggiolino, A.; Kour, J.; Arshad, M.S.; Aslam, N.; Afzal, M.F.; Meghwar, P.; Zafar, K.-U.; De Palo, P.; Korma, S.A. Dynamic alterations in protein, sensory, chemical, and oxidative properties occurring in meat during thermal and non-thermal processing techniques: A comprehensive review. Front. Nutr. 2023, 9, 1057457. [Google Scholar] [CrossRef]
- Descalzo, A.; Rossetti, L.; Grigioni, G.; Irurueta, M.; Sancho, A.; Carrete, J.; Pensel, N. Antioxidant status and odour profile in fresh beef from pasture or grain-fed cattle. Meat Sci. 2007, 75, 299–307. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.A.; Nickols-Richardson, S.M. The Effects of Distraction on Consumption, Food Preference, and Satiety: A Proposal of Methods. FASEB J. 2017, 31, 957.30. [Google Scholar] [CrossRef]
- Rolls, B.J.; Cunningham, P.M.; Diktas, H.E. Properties of Ultraprocessed Foods That Can Drive Excess Intake. Nutr. Today 2020, 55, 109. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Small, D.M.; DiFeliceantonio, A.G. Processed foods and food reward. Science 2019, 363, 346–347. [Google Scholar] [CrossRef]
- Bureau, U.C. Census.gov | U.S. Census Bureau Homepage. Census.gov. Available online: https://www.census.gov/en.html (accessed on 22 July 2025).
- McDonald, R.P. Test Theory: A Unified Treatment; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; p. xi, 485. [Google Scholar]
- Healthy Meal Planning: Tips for Older Adults. National Institute on Aging. Available online: https://www.nia.nih.gov/health/healthy-eating-nutrition-and-diet/healthy-meal-planning-tips-older-adults (accessed on 26 March 2025).
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The Mediterranean Diet and Nutritional Adequacy: A Review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Hess, J.M.; Comeau, M.E.; Casperson, S.; Slavin, J.L.; Johnson, G.H.; Messina, M.; Raatz, S.; Scheett, A.J.; Bodensteiner, A.; Palmer, D.G. Dietary Guidelines Meet NOVA: Developing a Menu for A Healthy Dietary Pattern Using Ultra-Processed Foods. J. Nutr. 2023, 153, 2472–2481. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Keast, D.R.; Fulgoni, V.L.; Nicklas, T.A. Food Sources of Energy and Nutrients among Adults in the US: NHANES 2003–2006. Nutrients 2012, 4, 2097–2120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Continuity of Identity: The Sameness of Self from Childhood to Adulthood. Commun. Humanit. Res. 2023, 18, 140–143. [Google Scholar] [CrossRef]
- Lalitha, A. Phytochemicals and health benefits. Ann. Geriatr. Educ. Med. Sci. 2024, 11, 29–31. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Ma, Z.; Chen, H.; Gao, H. Toward an understanding of potato starch structure, function, biosynthesis, and applications. Food Front. 2023, 4, 980–1000. [Google Scholar] [CrossRef]
- Thomson, J.L.; Landry, A.S.; Walls, T.I. Can United States Adults Accurately Assess Their Diet Quality? Am. J. Health Promot. AJHP 2023, 37, 499–506. [Google Scholar] [CrossRef]
- Fulkerson, J.A.; Larson, N.; Horning, M.; Neumark-Sztainer, D. A Review of Associations Between Family or Shared Meal Frequency and Dietary and Weight Status Outcomes Across the Lifespan. J. Nutr. Educ. Behav 2014, 46, 2–19. [Google Scholar] [CrossRef]
- van Meer, F.; de Vos, F.; Hermans, R.C.J.; Peeters, P.A.; van Dillen, L.F. Daily distracted consumption patterns and their relationship with BMI. Appetite 2022, 176, 106136. [Google Scholar] [CrossRef]
- Jaeger, J. Assembly Theory: What It Does and What It Does Not Do. J. Mol. Evol. 2024, 92, 87–92. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Policy and Global Affairs; Committee on Women in Science, Engineering, and Medicine; Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research; Bibbins-Domingo, K.; Helman, A. Why Diverse Representation in Clinical Research Matters and the Current State of Representation within the Clinical Research Ecosystem. In Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups; National Academies Press: Washington, DC, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK584396/ (accessed on 22 July 2025).

| High Assembly Index (Ai) and High Copy Number (Ni) | |
| DNA polymerase | Enzyme with a complex sequence and local geometry (high Ai) Replicated billions of times in biology (high Ni) |
| Apples | Biological structure: cells, tissues, proteins, pigments (high A i) Harvested globally (high Ni) |
| The English language | Evolved over thousands of years and formed by thousands of words (high Ai) Recreated with little variation globally (high Ni) |
| High Assembly Index (Ai) and Low Copy Number (Ni) | |
| Experimental protein designs | Complex sequence and local geometry (high Ai) Novel Molecule after first synthesis (low Ni) |
| Hand-crafted pastry | Complex structure involving multiple layers, fillings, and precise techniques (high Ai) Produced in small batches by artisanal bakers (low Ni) |
| The word “alacrity” | Thousands of years of culture to create the word (high Ai) Infrequently used (Low Ai) |
| Low Assembly Index (Ai) and High Copy Number (Ni) | |
| Water (H2O) | Often a biproduct on single step organic reactions (Low Ai) Found universally in high abundance (high Ni) |
| High fructose corn syrup | Fructose molecules refined from a source which initially required <15 steps to assemble the carbohydrate (Low Ai); * Produced globally for sweeteners (high Ni) |
| The sound of a rock falling on impact | Created by a single step process (low Ai) Occurs universally (high Ni) |
| Low Assembly Index (Ai) and Low Copy Number (Ni) | |
| Nitric oxide radical (NO•) | Simple molecule often a biproduct (Low Ai) Reactive and short-lived (Low Ni) |
| Snowball | Formed by aggregation of ice crystals via simple mechanical action (Low Ai) Individually formed and short-lived (Low Ni) |
| Examples of Behavioral Complexity | |||
|---|---|---|---|
| Social Eating * | Value | Distracted Eating | Value |
| Eating a meal with family and/or friends | (+1) | Any eating while watching television | (−1) |
| Eating a meal while at an indoor soccer game | (+1) | Eating during a lecture | (−1) |
| Eating a meal while periodically petting your dog | (+1) | Eating while diving | (−1) |
| Outside Eating | Value | Over-Consumptive Eating | Value |
| Eating while on a park bench alone | (+1) | Eating more servings than on the nutrition label | (−1) |
| Eating while at an outdoor soccer game | (+1) | Eating to the point of discomfort | (−1) |
| Eating strawberries while harvesting | (+1) | Drinking more than 4 standard drinks in an evening | (−1) |
| Quantifiable Food and Food Behavior Categories | |||
|---|---|---|---|
| High-Complexity Diet | Point Value | Low-Complexity Diet | Point Value |
| Farm-Direct Animal Product | (+1) | Processed Animal Product | (−1) |
| Fresh Plants | (+1) | Processed Ingredient | (−1) |
| Fermented Foods | (+1) | Refined Ingredient | (−1) |
| Fasting (Autophagy) | (+7) | Ultra-Processed Food | (−1) |
| High-Complexity Behavior | Point Value | Low-Complexity Behavior | Point Value |
| Social Eating | (+1) | Distracted Eating | (−1) |
| Outside Eating | (+1) | Over-consumptive Eating | (−1) |
| Complexity Based Off of Degrees of Separation | |||
|---|---|---|---|
| 2 or less degrees of separation | Value | 3 or more degrees of separation | Value |
| Deer meat the patient hunted | (+1) | Packaged meat from a store | (−1) |
| Chicken eggs from a friend | (+1) | Deli meat from the supermarket | (−1) |
| Meat from the farmer’s market | (+1) | Barbeque from a restaurant | (−1) |
| Complexity Grade for a Generic Ham Sandwich | |||
|---|---|---|---|
| Ingredient | Variable | Complexity Grade | Point Value |
| White Bread | Refined Ingredient | Low | (−1) |
| Deli Ham | Processed Animal Product | Low | (−1) |
| Mustard | Processed Ingredient (Food Dye) | Low | (−1) |
| Tomato | Fresh Plant | High | (+1) |
| Lettuce | Fresh Plant | High | (+1) |
| Category | Variable | Ai (Assembly Index) of Average Molecule | Ni (Copy Number) of Average Molecule | Total | GARD Score |
|---|---|---|---|---|---|
| High Complexity | Fresh Plant | 9 (Extremely High) | 9 (Extremely High) | 18 | (+1) |
| Farm-DirectProduct | 9 (Extremely High) | 9 (Extremely High) | 18 | (+1) | |
| Fermented Food | 9 (Extremely High) | 9 (Extremely High) | 18 | (+1) | |
| Autophagy (Fasting) | 9 (Extremely High) | 9 (Extremely High) | 18 | (+1) | |
| Low Complexity | Processed Ingredient | 3 (Low) | 8 (Very High) | 11 | (−1) |
| Refined Ingredient | 2 (Very Low) | 9 (Extremely High) | 11 | (−1) | |
| Processed Animal Product | 7 (High) | 8 (Very High) | 15 | (−1) | |
| Ultra-Processed Food | 1 (Extremely Low) | 9 (Extremely High) | 10 | (−1) |
| Category | Variable | Internal Validity in Assembly Theory |
|---|---|---|
| High Complexity | Fresh Plant | Fresh plants contain diverse, complex biomolecules (e.g., polyphenols, fibers) requiring many synthetic steps (extremely high Ai), with widespread repetition in plant tissue (extremely high Ni). |
| Farm-Direct Animal Product | Whole animal products (meat, seafood) have structured proteins, fats, carbohydrates, and nucleic acids (extremely high Ai) with considerable repetition within a given tissue (extremely high Ni). | |
| Fermented Food | Fermentation increases biochemical complexity via the presence of microbiotic life (extremely high Ai) with high molecular repetition within individual bacteria (extremely high Ni). | |
| Autophagy (Fasting) | Autophagy recycles highly structured biomolecules, organelles, glycogen, fatty acid chains (extremely high Ai), which exist in repeating cell types with in a tissue (extremely high Ni). | |
| Low Complexity | Processed Ingredient | Processed ingredients are designed for easy manufacturing (low Ai) at large volume (very high Ni) |
| Refined Ingredient | Industrial processing simplifies molecular structure taking complex biomolecules (i.e., Amylopectin) and turning them into simplified molecules (i.e., Glucose, Fructose) (low Ai) while increasing uniformity and repetition (very high Ni). | |
| Processed Animal Product | Research shows measurable differences in nutritional complexity between processed meat and farm-direct, pasture-raised meat. Specifically, meat from farmers’ markets—typically pasture-raised and minimally handled—contains significantly higher levels of omega-3 fatty acids, conjugated linoleic acid (CLA), and fat-soluble vitamins like A and E [62,63]. These nutrients result from the animals’ forage-based diets and shorter storage times, which help preserve delicate compounds and vitamins [64,65]. In contrast, processed meat, while chemically dense and uniform, lacks this diversity and freshness. As a result, processed meat exhibits contain less complex molecules (high Ai) compared to farm-direct meat, which contains a more varied and functionally rich molecular structure. However, processed meat still retains a high number of repeated molecules throughout the tissues (Very High Ni). | |
| Ultra-Processed Food | Ultra-processed foods by definition contain manufactured ingredients (extremely low Ai) and refined ingredients that are mass-produced and highly repetitive (extremely high Ni). |
| Category | Variable | Ai (Assembly Index) of Average Molecule | Ni (Copy Number) of Average Molecule | Total | GARD Score |
|---|---|---|---|---|---|
| High-Complexity Behavior | Social Eating | 8 (High) | 8 (High) | 16 | (+1) |
| Outside Eating | 8 (High) | 8 (High) | 16 | (+1) | |
| Low-Complexity Behavior | Distracted Eating | 4 (Low) | 9 (Extremely High) | 13 | (−1) |
| Over-Indulgent Eating | 3 (Very Low) | 9 (Extremely High) | 12 | (−1) |
| Category | Internal Validity in Assembly Theory |
|---|---|
| High-Complexity Behavior | Social eating requires complex social structures, communication, and shared rituals (high Ai). It has been a fundamental aspect of human evolution across cultures and time (high Ni). |
| Eating in dynamic outdoor environments requires physiological adaptation to variable conditions (high Ai). This behavior was the norm for most of human history (high Ni). | |
| Low-Complexity Behavior | Eating while distracted lacks engagement with environmental and social cues, reducing behavioral complexity (low Ai). It is a modern behavior that has become widespread (extremely high Ni). |
| Overeating prioritizes quantity over adaptive responses to hunger and social context, diminishing behavioral complexity (very low Ai). Engineered food environments have made it exceedingly common (extremely high Ni). |
| NIH: Healthy Meal Planning: Tips for Older Adults | ||
|---|---|---|
| GARD Score | ||
| Daily Diet (Total GARD score for 3 Meals) | Store Bought | Farm Bought All Homemade |
| Daily Meal Plan 1 | 16 | 27 |
| Daily Meal Plan 2 | 14 | 22 |
| Daily Meal Plan 3 | 12 | 25 |
| Average | 14 | 23 |
| GARD Score for Daily Mediterranean Diets | ||
|---|---|---|
| GARD Score | ||
| Daily Diet (Total GARD score for 3 Meals) | Store Bought | Farm Bought All Homemade |
| Daily Meal Plan 1 | 19 | 27 |
| Daily Meal Plan 2 | 17 | 22 |
| Daily Meal Plan 3 | 7 | 13 |
| Average | 14 | 21 |
| 80% Ultra-Processed Food Diet | ||
|---|---|---|
| GARD Score | ||
| Daily Diet (Total GARD score for 3 Meals) | Store Bought | Farm Bought All Homemade |
| Daily Meal Plan 1 | 1 | N/A |
| Daily Meal Plan 2 | −10 | |
| Daily Meal Plan 3 | 1 | |
| Total | −1 | |
| Standard American Diet | ||
|---|---|---|
| GARD Score | ||
| Daily Diet (Total GARD score for 3 Meals) | Store Bought | Farm Bought All Homemade |
| Daily Meal Plan 1 | −12 | N/A |
| Daily Meal Plan 2 | −8 | |
| Daily Meal Plan 3 | −9 | |
| Average | −10 | |
| Proposed GARD Score Correlations for Future Study | |
|---|---|
| Proposed High GARD Score Biomarker Correlations | Proposed Low GARD Score Biomarker Correlations |
| Lower fasting insulin levels | Lower levels of Vitamin D |
| Lower average blood sugar (Hemoglobin A1c) | Lower levels of Cobalamin |
| Lower Apolipoprotein B (marker of serum lipid levels) | Higher C-reactive protein (CRP) |
| Proposed High GARD Score Patient Outcome Correlations | Proposed Low GARD Score Patient Outcome Correlations |
| Lower rates of 30-day hospital readmission | Higher rates of Colon Cancer |
| Lower quantity of diagnosed chronic conditions | Higher rates of End Stage Renal Disease |
| Lower patient Body Mass Index (BMI) | Higher rates of Fragility Fractures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penrose, O.C.; Gross, P.J.; Singh, H.; Rynarzewska, A.I.; Ayazo, C.; Jones, L. Diet to Data: Validation of a Bias-Mitigating Nutritional Screener Using Assembly Theory. Nutrients 2025, 17, 2459. https://doi.org/10.3390/nu17152459
Penrose OC, Gross PJ, Singh H, Rynarzewska AI, Ayazo C, Jones L. Diet to Data: Validation of a Bias-Mitigating Nutritional Screener Using Assembly Theory. Nutrients. 2025; 17(15):2459. https://doi.org/10.3390/nu17152459
Chicago/Turabian StylePenrose, O’Connell C., Phillip J. Gross, Hardeep Singh, Ania Izabela Rynarzewska, Crystal Ayazo, and Louise Jones. 2025. "Diet to Data: Validation of a Bias-Mitigating Nutritional Screener Using Assembly Theory" Nutrients 17, no. 15: 2459. https://doi.org/10.3390/nu17152459
APA StylePenrose, O. C., Gross, P. J., Singh, H., Rynarzewska, A. I., Ayazo, C., & Jones, L. (2025). Diet to Data: Validation of a Bias-Mitigating Nutritional Screener Using Assembly Theory. Nutrients, 17(15), 2459. https://doi.org/10.3390/nu17152459






