The Complex Gene–Carbohydrate Interaction in Type 2 Diabetes: Between Current Knowledge and Future Perspectives
Abstract
1. Introduction
2. Genetics of Type 2 Diabetes
| Gene | Acronym | Original Function | Variant | Role in T2D | References |
|---|---|---|---|---|---|
| Transcription factor 7-like | TCF7L2 | Encoding a Wnt signaling-associated transcription factor | rs7903146 | Decrease in insulin secretion; morphological and functional changes in β cells | [48,49,50,51] |
| Proliferator-activated receptor gamma | PPAR-γ | Encoding a ligand-activated superfamily member of ligand-dependent transcription | rs1801282 | Increase in insulin resistance; impairment of anthropometric, glucose, and lipid metabolism biomarkers | [58,59,60] |
| Glucose dependent insulin polypeptide receptor | GIPR | Encoding a G protein-coupled receptor for the GIP hormone | rs10423928 | Increased fasting glucose and proinsulin levels; reduced incretin effects | [69,70] |
| Insulin receptor substrate-1 | IRS-1 | Encoding a cytoplasmic adaptor protein involved in insulin signal transmission | rs1801278 | Increased insulin resistance | [77,78,79] |
3. Carbohydrate–Gene Interactions in Type 2 Diabetes
3.1. Carbohydrates and Their Role in Human Health
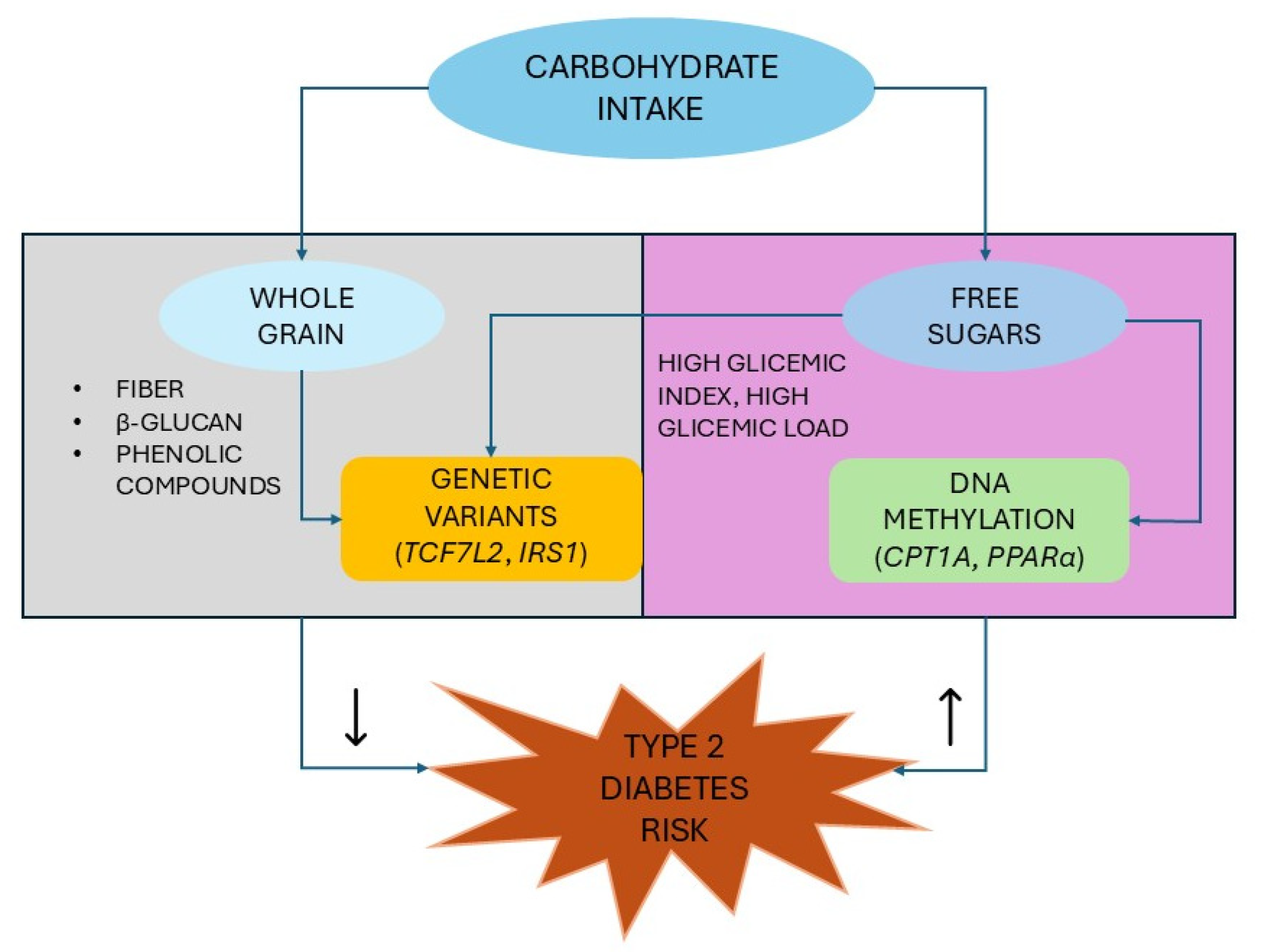
3.2. Whole-Grain Intake and Risk of Type 2 Diabetes
| Clues | Reference | Pitfalls | Reference |
|---|---|---|---|
| WG intake (3–5 servings per day) significantly associated with a reduced risk of T2D | [96,106,111,116] | Potential overestimation due to incomplete adjustment for lifestyle and dietary factors, as well as unmeasured or residual confounding | [26,96,106,111] |
| Significantly inverse associations between WG bread, whole grain cereals, wheat bran, and brown rice and risk of T2D | [96,115] | Most studies conducted among Caucasian populations in the United States | [106,111] |
| No significant association between refined grain consumption and T2D risk | [96] | Small number of cohort studies | [96] |
| Whole grain intake (the highest category vs. the lowest category) significantly inversely associated with T2D occurrence | [26] | High heterogeneity in the dose–response analysis of WGs and T2D | [96] |
| Whole grain cold breakfast cereals and dark bread (≥1 serving per day) significantly associated with a reduced risk of T2D | [26] | No possibility to control publication bias | [96] |
| Possible inadequate reporting of WG consumption from subjects | [106] | ||
| Possible measurement errors and differences between studies in the exposure assessment | [96,111] | ||
| Lack of a uniform definition for WG foods | [96,106] | ||
| Wide range of whole grain intake across studies | [106] | ||
| Possibility of false results due to the assessment of associations of WG foods simultaneously | [26] | ||
| Findings mainly related to white health professionals | [26] | ||
| Evidence for the association between dietary fiber and whole grain intake and the risk of T2D rated as low or moderate by the GRADE criteria assessment | [116] |
3.2.1. Whole Grain Intake and the Impact on Glycemic Control
3.2.2. Nutrigenetic Interaction Between Whole Grain and Type 2 Diabetes Genes
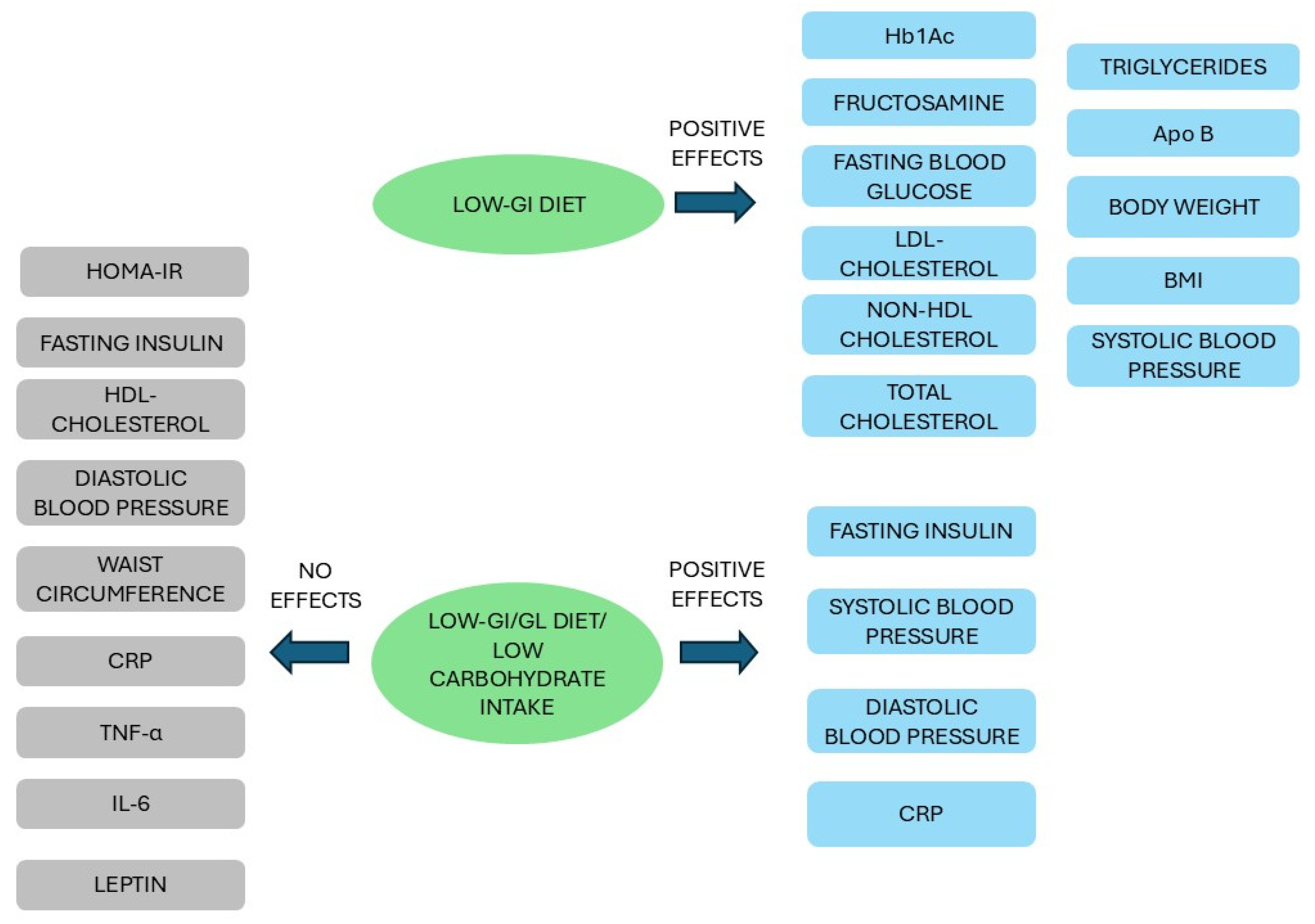
3.3. Glycemic Index, Glycemic Load, and Risk of Type 2 Diabetes
3.3.1. Carbohydrate Intake and the Impact on Glycemic Control
3.3.2. Nutrigenetic Interaction Between Carbohydrates and Type 2 Diabetes Genes
4. The Carbohydrate–Epigenetics Relationship in Type 2 Diabetes
4.1. Epigenetics in Type 2 Diabetes: The Role of DNA Methylation
4.2. Carbohydrate–Epigenetics Interactions in Type 2 Diabetes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CpG | Cytosine–guanine dinucleotides |
| CVD | Cardiovascular disease |
| DNMT | DNA methyltransferase |
| EWASs | Epigenome wide association studies |
| FFQ | Food frequency questionnaire |
| GCKR | Glucokinase regulator gene |
| GI | Glycemic index |
| GIP | Glucose dependent insulin polypeptide |
| GIPR | Glucose dependent insulin polypeptide receptor |
| GL | Glycemic load |
| GLP-1 | Glucagon-like peptide-1 |
| GWAS | Genome-wide association studies |
| HbA1c | Glycosylated hemoglobin A1c |
| HDL | High density lipoprotein |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| HR | Hazard ratio |
| IRS | Insulin receptor substrate |
| LCD | Low-carbohydrate diet |
| LDL | Low-density lipoprotein |
| LncRNA | Long non-coding RNA |
| MetS | Metabolic syndrome |
| miRNA | Micro-RNA |
| PPAR | Peroxisome proliferator-activated receptor |
| RCT | Randomized controlled trial |
| RR | Relative risk |
| SCFA | Short-chain fatty acid |
| SFA | Short fatty acid |
| SNP | Single-nucleotide polymorphisms |
| T2D | Type 2 diabetes |
| TCF7L2 | Transcription factor 7-like 2 |
| WGs | Whole grains |
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, F.; Montaño, D.; Lizcano, F. Diabetes Mellitus Is a Chronic Disease that Can Benefit from Therapy with Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 8685. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas 10th Edition. 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 24 February 2025).
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes-Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, M.; Rosengren, A.; Svensson, A.M.; Kosiborod, M.; Pivodic, A.; Gudbjörnsdottir, S.; Wedel, H.; Clements, M.; Dahlqvist, S.; Lind, M. Excess Mortality among Persons with Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, Y.; Yang, S.; Zhu, D.; Chen, F.; Chen, J.; Ji, X.; Hou, K. The global, regional and national burden of type 2 diabetes mellitus in the past, present and future: A systematic analysis of the Global Burden of Disease Study 2019. Front. Endocrinol. 2023, 14, 1192629. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fu, Y.; Tan, X.; Wang, N.; Qi, L.; Lu, Y. Assessing the impact of type 2 diabetes on mortality and life expectancy according to the number of risk factor targets achieved: An observational study. BMC Med. 2024, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Ali, O. Genetics of type 2 diabetes. World J. Diabetes 2013, 4, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Á.; Berná, G.; Rojas, A.; Martín, F.; Soria, B. Gene-Diet Interactions in Type 2 Diabetes: The Chicken and Egg Debate. Int. J. Mol. Sci. 2017, 18, 1188. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, M.; Keaton, J.M.; Lynch, J.A.; Miller, D.R.; Zhou, J.; Tcheandjieu, C.; Huffman, J.E.; Assimes, T.L.; Lorenz, K.; Zhu, X.; et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020, 52, 680–691. [Google Scholar] [CrossRef] [PubMed]
- DeForest, N.; Majithia, A.R. Genetics of Type 2 Diabetes: Implications from Large-Scale Studies. Curr. Diabetes Rep. 2022, 22, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.M.; Phillips, C.M. Nutrigenetics: Bridging two worlds to understand type 2 diabetes. Curr. Diabetes Rep. 2014, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Berná, G.; Oliveras-López, M.J.; Jurado-Ruíz, E.; Tejedo, J.; Bedoya, F.; Soria, B.; Martín, F. Nutrigenetics and nutrigenomics insights into diabetes etiopathogenesis. Nutrients 2014, 6, 5338–5369. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Ordovas, J.M. Fundamentals of nutrigenetics and nutrigenomics. Prog. Mol. Biol. Transl. Sci. 2012, 108, 1–15. [Google Scholar] [PubMed]
- Marcum, J.A. Nutrigenetics/Nutrigenomics, Personalized Nutrition, and Precision Healthcare. Curr. Nutr. Rep. 2020, 9, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Jacobs, S.; Zheng, J.S.; Meidtner, K.; Schwingshackl, L.; Schulze, M.B. Gene-lifestyle interaction on risk of type 2 diabetes: A systematic review. Obes. Rev. 2019, 20, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, S.J.; VonHandorf, A.; Viel, K.C.M.F.; Weirauch, M.T.; Kottyan, L.C. Gene-environment interactions and their impact on human health. Genes Immun. 2023, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Apio, C.; Chung, W.; Moon, M.K.; Kwon, O.; Park, T. Gene-diet interaction analysis using novel weighted food scores discovers the adipocytokine signaling pathway associated with the development of type 2 diabetes. Front. Endocrinol. 2023, 14, 1165744. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and Diet in the Prevention of Chronic Diseases in Future Generations. Int. J. Mol. Sci. 2020, 21, 2633. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, L.; Spinelli, R.; Nicolò, A.; Longo, M.; Mirra, P.; Raciti, G.A.; Miele, C.; Beguinot, F. Nutritional Factors, DNA Methylation, and Risk of Type 2 Diabetes and Obesity: Perspectives and Challenges. Int. J. Mol. Sci. 2019, 20, 2983. [Google Scholar] [CrossRef] [PubMed]
- Okburan, G.; Gezer, C. Carbohydrates as Nutritional Components for Health and Longevity. In Nutrition, Food and Diet in Ageing and Longevity. Healthy Ageing and Longevity; Rattan, S.I.S., Kaur, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 14. [Google Scholar]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of whole grain foods and risk of type 2 diabetes: Results from three prospective cohort studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.; Stojanovski, E.; McEvoy, M.; Garg, M.L. Macronutrient intakes and development of type 2 diabetes: A systematic review and meta-analysis of cohort studies. J. Am. Coll. Nutr. 2012, 31, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Lillioja, S.; Wilton, A. Agreement among type 2 diabetes linkage studies but a poor correlation with results from genome-wide association studies. Diabetologia 2009, 52, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, X.; Mao, Z.; Huo, W.; Hou, J.; Wang, C.; Li, W.; Yu, S. Heritability Estimation and Environmental Risk Assessment for Type 2 Diabetes Mellitus in a Rural Region in Henan, China: Family-Based and Case-Control Studies. Front. Public Health 2021, 9, 690889. [Google Scholar] [CrossRef] [PubMed]
- Almgren, P.; Lehtovirta, M.; Isomaa, B.; Sarelin, L.; Taskinen, M.R.; Lyssenko, V.; Tuomi, T.; Groop, L.; Botnia Study Group. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 2011, 54, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Cupples, L.A.; Wilson, P.W. Parental transmission of type 2 diabetes: The Framingham Offspring Study. Diabetes 2000, 49, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Florez, J.C.; Hirschhorn, J.; Altshuler, D. The inherited basis of diabetes mellitus: Implications for the genetic analysis of complex traits. Annu. Rev. Genom. Hum. Genet. 2003, 4, 257–291. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, P.; Grunnet, L.G.; Pilgaard, K.; Storgaard, H.; Alibegovic, A.; Sonne, M.P.; Carstensen, B.; Beck-Nielsen, H.; Vaag, A. Increased risk of type 2 diabetes in elderly twins. Diabetes 2009, 58, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Li, X.; Sundquist, K.; Sundquist, J. Familial risks for type 2 diabetes in Sweden. Diabetes Care 2010, 33, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Dawn Teare, M.; Barrett, J.H. Genetic linkage studies. Lancet 2005, 366, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Ott, J.; Wang, J.; Leal, S.M. Genetic linkage analysis in the age of whole-genome sequencing. Nat. Rev. Genet. 2015, 16, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Del Bosque-Plata, L.; Martínez-Martínez, E.; Espinoza-Camacho, M.Á.; Gragnoli, C. The Role of TCF7L2 in Type 2 Diabetes. Diabetes 2021, 70, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.M.; Goate, A.M. The candidate gene approach. Alcohol Res. Health 2000, 24, 164–168. [Google Scholar] [PubMed]
- Laakso, M.; Fernandes Silva, L. Genetics of Type 2 Diabetes: Past, Present, and Future. Nutrients 2022, 14, 3201. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.B.; Groop, L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes 2015, 6, 87–123. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M. Genome-wide association studies go green: Novel and cost-effective opportunities for identifying genetic associations. Mayo Clin. Proc. 2011, 86, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hatzikotoulas, K.; Southam, L.; Taylor, H.J.; Yin, X.; Lorenz, K.M.; Mandla, R.; Huerta-Chagoya, A.; Melloni, G.E.M.; Kanoni, S. Genetic drivers of heterogeneity in type 2 diabetes pathophysiology. Nature 2024, 627, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Bahaaeldin, A.M.; Seif, A.A.; Hamed, A.I.; Kabiel, W.A.Y. Transcription Factor 7-Like-2 (TCF7L2) rs7903146 (C/T) Polymorphism in Patients with Type 2 Diabetes Mellitus. Dubai Diabetes Endocrinol. J. 2020, 26, 112–118. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Y.; Hu, X.; Ma, M.; Wang, X.; Liang, H.; Liu, D. Effect of Wnt Signaling on the Differentiation of Islet β-Cells from Adipose-Derived Stem Cells. BioMed Res. Int. 2017, 2017, 2501578. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Lin, Y.; Zhang, Y.; Yang, J.; Zhang, Y.; Liu, H.; Zhang, B. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: A large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med. Genet. 2009, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xu, L.; Zhang, L.; Han, Z.; Jiang, Q.; Wang, Z.; Jin, S. Meta-analysis of association between TCF7L2 polymorphism rs7903146 and type 2 diabetes mellitus. BMC Med. Genet. 2018, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Le Bacquer, O.; Kerr-Conte, J.; Gargani, S.; Delalleau, N.; Huyvaert, M.; Gmyr, V.; Froguel, P.; Neve, B.; Pattou, F. TCF7L2 rs7903146 impairs islet function and morphology in non-diabetic individuals. Diabetologia 2012, 55, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Cropano, C.; Santoro, N.; Groop, L.; Dalla Man, C.; Cobelli, C.; Galderisi, A.; Kursawe, R.; Pierpont, B.; Goffredo, M.; Caprio, S. The rs7903146 Variant in the TCF7L2 Gene Increases the Risk of Prediabetes/Type 2 Diabetes in Obese Adolescents by Impairing β-Cell Function and Hepatic Insulin Sensitivity. Diabetes Care 2017, 40, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, G.; Simcox, J.; Seldin, M.M.; Parnell, T.J.; Stubben, C.; Just, S.; Begaye, L.; Lusis, A.J.; Villanueva, C.J. Targeted deletion of Tcf7l2 in adipocytes promotes adipocyte hypertrophy and impaired glucose metabolism. Mol. Metab. 2019, 24, 44–63. [Google Scholar] [CrossRef] [PubMed]
- da Silva Xavier, G.; Loder, M.K.; McDonald, A.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; Barg, S.; Rutter, G.A. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes 2009, 58, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Patial, V. Peroxisome proliferator-activated receptor gamma and its natural agonists in the treatment of kidney diseases. Front. Pharmacol. 2022, 13, 991059. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Di Marzio, D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: Current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [PubMed]
- Basak, S.; Murmu, A.; Matore, B.W.; Roy, P.P.; Singh, J. Thiazolidinedione an auspicious scaffold as PPAR-γ agonist: Its possible mechanism to Manoeuvre against insulin resistant diabetes mellitus. Eur. J. Med. Chem. Rep. 2024, 11, 100160. [Google Scholar] [CrossRef]
- Bakhashab, S.; Filimban, N.; Altall, R.M.; Nassir, R.; Qusti, S.Y.; Alqahtani, M.H.; Abuzenadah, A.M.; Dallol, A. The Effect Sizes of PPARγ rs1801282, FTO rs9939609, and MC4R rs2229616 Variants on Type 2 Diabetes Mellitus Risk among the Western Saudi Population: A Cross-Sectional Prospective Study. Genes 2020, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Sarhangi, N.; Sharifi, F.; Hashemian, L.; Hassani Doabsari, M.; Heshmatzad, K.; Rahbaran, M.; Jamaldini, S.H.; Aghaei Meybodi, H.R.; Hasanzad, M. PPARG (Pro12Ala) genetic variant and risk of T2DM: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12764. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, X.; Li, J.; Wang, Q. Association of PPARG Gene Polymorphisms Pro12Ala with Type 2 Diabetes Mellitus: A Meta-analysis. Curr. Diabetes Rev. 2019, 15, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Vergotine, Z.; Yako, Y.Y.; Kengne, A.P.; Erasmus, R.T.; Matsha, T.E. Proliferator-activated receptor gamma Pro12Ala interacts with the insulin receptor substrate 1 Gly972Arg and increase the risk of insulin resistance and diabetes in the mixed ancestry population from South Africa. BMC Genet. 2014, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Stryjecki, C.; Peralta-Romero, J.; Alyass, A.; Karam-Araujo, R.; Suarez, F.; Gomez-Zamudio, J.; Burguete-Garcia, A.; Cruz, M.; Meyre, D. Association between PPAR-γ2 Pro12Ala genotype and insulin resistance is modified by circulating lipids in Mexican children. Sci. Rep. 2016, 6, 24472. [Google Scholar] [CrossRef] [PubMed]
- Reza-López, S.A.; González-Gurrola, S.; Morales-Morales, O.O.; Moreno-González, J.G.; Rivas-Gómez, A.M.; González-Rodríguez, E.; Moreno-Brito, V.; Licón-Trillo, A.; Leal-Berumen, I. Metabolic Biomarkers in Adults with Type 2 Diabetes: The Role of PPAR-γ2 and PPAR-β/δ Polymorphisms. Biomolecules 2023, 13, 1791. [Google Scholar] [CrossRef] [PubMed]
- Winzell, M.S.; Wulff, E.M.; Olsen, G.S.; Sauerberg, P.; Gotfredsen, C.F.; Ahrén, B. Improved insulin sensitivity and islet function after PPARdelta activation in diabetic db/db mice. Eur. J. Pharmacol. 2010, 626, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights 2016, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Namghi, S.M. Association of GIPR gene variant on the risk of type 2 diabetes mellitus: A case-control study. Endocunre Metab. Sci. 2023, 13, 100140. [Google Scholar] [CrossRef]
- Erfanian, S.; Mir, H.; Abdoli, A.; Roustazadeh, A. Association of gastric inhibitory polypeptide receptor (GIPR) gene polymorphism with type 2 diabetes mellitus in iranian patients. BMC Med. Genom. 2023, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, L.S.; Gabe, M.B.N.; Hartmann, B.; Christensen, M.B.; Knop, F.K.; Holst, J.J.; Rosenkilde, M.M. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents. Peptides 2018, 100, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Fisman, E.Z.; Tenenbaum, A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: A novel cardiometabolic therapeutic prospect. Cardiovasc. Diabetol. 2021, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Vedtofte, L.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011, 60, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Asmar, M.; Simonsen, L.; Madsbad, S.; Stallknecht, B.; Holst, J.J.; Bulow, J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes 2010, 59, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Asmar, M.; Simonsen, L.; Arngrim, N.; Holst, J.J.; Dela, F.; Bülow, J. Glucose-dependent insulinotropic polypeptide has impaired effect on abdominal, subcutaneous adipose tissue metabolism in obese subjects. Int. J. Obes. 2014, 38, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Yañez, R.L.; Markova, M.; Dambeck, U.; Honsek, C.; Machann, J.; Schüler, R.; Kabisch, S.; Pfeiffer, A.F.H. Predictive effect of GIPR SNP rs10423928 on glucose metabolism liver fat and adiposity in prediabetic and diabetic subjects. Peptides 2020, 125, 170237. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Windeløv, J.A.; Boer, G.A.; Pedersen, J.; Svendsen, B.; Christensen, M.; Torekov, S.; Asmar, M.; Hartmann, B.; Nissen, A. Searching for the physiological role of glucose-dependent insulinotropic polypeptide. J. Diabetes Investig. 2016, 7 (Suppl. 1), 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bagger, J.I.; Knop, F.K.; Lund, A.; Vestergaard, H.; Holst, J.J.; Vilsbøll, T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Lavin, D.P.; White, M.F.; Brazil, D.P. IRS proteins and diabetic complications. Diabetologia 2016, 59, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Boura-Halfon, S.; Zick, Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E581–E591. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Federici, M.; Hribal, M.L.; Lauro, D.; Sbraccia, P.; Lauro, R. Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J. 2001, 15, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.A.; Behiry, E.G.; Allah, W.M.A.; Hussien, A.M.; Abdelmoneam, A.A.; Imam, M.H.; Hikal, D.M. IRS-1 genetic polymorphism (r.2963G>A) in type 2 diabetes mellitus patients associated with insulin resistance. Appl. Clin. Genet. 2018, 11, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Albegali, A.A.; Shahzad, M.; Mahmood, S.; Ullah, M.I. Genetic association of insulin receptor substrate-1 (IRS-1, rs1801278) gene with insulin resistant of type 2 diabetes mellitus in a Pakistani population. Mol. Biol. Rep. 2019, 46, 6065–6070. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, B.; Cong, R.; Hao, M.; Peng, Y.; Yang, H.; Song, J.; Feng, D.; Zhang, N.; Li, D. RING-finger E3 ligases regulatory network in PI3K/AKT-mediated glucose metabolism. Cell Death Discov. 2022, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Alsalman, H.A.; Kaabi, Y.A. Lack of association between the insulin receptor substrates-1 Gly972Arg polymorphism and type-2 diabetes mellitus among Saudis from Eastern Saudi Arabia. Saudi Med. J. 2015, 36, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Arikoglu, H.; Aksoy Hepdogru, M.; Erkoc Kaya, D.; Asik, A.; Ipekci, S.H.; Iscioglu, F. IRS1 gene polymorphisms Gly972Arg and Ala513Pro are not associated with insulin resistance and type 2 diabetes risk in non-obese Turkish population. Meta Gene 2014, 2, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Maeda, S. Perspectives on genetic studies of type 2 diabetes from the genome-wide association studies era to precision medicine. J. Diabetes Investig. 2024, 15, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Winkelmann, B.R.; Dietrich, J.W.; Boehm, B.O. Whole-exome sequencing in familial type 2 diabetes identifies an atypical missense variant in the RyR2 gene. Front. Endocrinol. 2024, 15, 1258982. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.; Wu, Y.; Zhu, Z.; Zhang, F.; Kemper, K.E.; Zheng, Z.; Yengo, L.; Lloyd-Jones, L.R.; Sidorenko, J.; Wu, Y.; et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018, 9, 2941. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Chagoya, A.; Schroeder, P.; Mandla, R.; Li, J.; Morris, L.; Vora, M.; Alkanaq, A.; Nagy, D.; Szczerbinski, L.; Madsen, J.G.S.; et al. Rare variant analyses in 51,256 type 2 diabetes cases and 370,487 controls reveal the pathogenicity spectrum of monogenic diabetes genes. Nat. Genet. 2024, 56, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Flannick, J.; Mercader, J.M.; Fuchsberger, C.; Udler, M.S.; Mahajan, A.; Wessel, J.; Teslovich, T.M.; Caulkins, L.; Koesterer, R.; Barajas-Olmos, F.; et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature 2019, 570, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Kwak, S.H.; Park, K.S. Update on Monogenic Diabetes in Korea. Diabetes Metab. J. 2020, 44, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Jakiel, P.; Gadzalska, K.; Juścińska, E.; Gorządek, M.; Płoszaj, T.; Skoczylas, S.; Borowiec, M.; Zmysłowska, A. Identification of rare variants in candidate genes associated with monogenic diabetes in polish mody-x patients. J. Diabetes Metab. Disord. 2023, 23, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Wainschtein, P.; Jain, D.; Zheng, Z.; TOPMed Anthropometry Working Group; NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium; Cupples, L.A.; Shadyab, A.H.; McKnight, B.; Shoemaker, B.M.; Mitchell, B.D.; et al. Assessing the contribution of rare variants to complex trait heritability from whole-genome sequence data. Nat. Genet. 2022, 54, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Zeggini, E.; Ioannidis, J.P. Meta-analysis in genome-wide association studies. Pharmacogenomics 2009, 10, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.W.; Pearson, E.; Florez, J.C. Gene-environment and gene-treatment interactions in type 2 diabetes: Progress, pitfalls, and prospects. Diabetes Care 2013, 36, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.W.; Mesa, J.L.; Harding, A.H.; Wareham, N.J. Gene-lifestyle interaction on risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; USDA and HHS: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/ (accessed on 8 May 2025).
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gan, L.; Graubard, B.I.; Männistö, S.; Fang, F.; Weinstein, S.J.; Liao, L.M.; Sinha, R.; Chen, X.; Albanes, D.; et al. Plant and Animal Fat Intake and Overall and Cardiovascular Disease Mortality. JAMA Intern. Med. 2024, 184, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F. Animal and Plant Protein Sources and Cardiometabolic Health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef] [PubMed]
- Mathers, J.C. Dietary fibre and health: The story so far. Proc. Nutr. Soc. 2023, 82, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C. Whole-Grain Intake in the Mediterranean Diet and a Low Protein to Carbohydrates Ratio Can Help to Reduce Mortality from Cardiovascular Disease, Slow Down the Progression of Aging, and to Improve Lifespan: A Review. Nutrients 2021, 13, 2540. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Dunford, E.K.; Miles, D.R.; Popkin, B.; Ng, S.W. Whole Grain and Refined Grains: An Examination of US Household Grocery Store Purchases. J. Nutr. 2022, 152, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Chanson-Rolle, A.; Meynier, A.; Aubin, F.; Lappi, J.; Poutanen, K.; Vinoy, S.; Braesco, V. Systematic Review and Meta-Analysis of Human Studies to Support a Quantitative Recommendation for Whole Grain Intake in Relation to Type 2 Diabetes. PLoS ONE 2015, 10, e0131377. [Google Scholar] [CrossRef] [PubMed]
- Ramne, S.; Alves Dias, J.; González-Padilla, E.; Olsson, K.; Lindahl, B.; Engström, G.; Ericson, U.; Johansson, I.; Sonestedt, E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population-based prospective cohorts. Am. J. Clin. Nutr. 2019, 109, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Gray, S.R.; Welsh, P.; Petermann-Rocha, F.; Foster, H.; Waddell, H.; Anderson, J.; Lyall, D.; Sattar, N.; Gill, J.M.R.; et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: Prospective cohort study of UK Biobank participants. BMJ 2020, 368, m688. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Jebb, S.A.; Aveyard, P.; Ambrosini, G.L.; Perez-Cornago, A.; Carter, J.; Sun, X.; Piernas, C. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK Biobank: A prospective cohort study. BMC Med. 2021, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Reducing Free Sugars Intake in Adults to Reduce the Risk of Noncommunicable Diseases. 2023. Available online: https://www.who.int/tools/elena/interventions/free-sugars-adults-ncds (accessed on 8 May 2025).
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Frølich, W.; Aman, P.; Tetens, I. Whole grain foods and health—A Scandinavian perspective. Food Nutr. Res. 2013, 57, 18503. [Google Scholar] [CrossRef] [PubMed]
- Özer, M.S.; Yazici, G.N. Phytochemicals of Whole Grains and Effects on Health. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Prasadi, N.P.V.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef] [PubMed]
- Kyrø, C.; Tjønneland, A.; Overvad, K.; Olsen, A.; Landberg, R. Higher Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes among Middle-Aged Men and Women: The Danish Diet, Cancer, and Health Cohort. J. Nutr. 2018, 148, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Vetrani, C.; Vitale, M.; Godos, J.; Riccardi, G.; Grosso, G. Whole Grain Intake and Glycaemic Control in Healthy Subjects: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.; Zhu, Y.; Wilcox, M.L.; Koecher, K.; Maki, K.C. Whole grain intake, compared to refined grain, improves postprandial glycemia and insulinemia: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 5339–5357. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yan, H.; Chen, L.; Wang, Y.; Liang, J.; Feng, X.; Hui, S.; Wang, K. Effects of whole grain intake on glycemic control: A meta-analysis of randomized controlled trials. J. Diabetes Investig. 2022, 13, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883.e31. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Zheng, J.; Kan, J.; Li, W.; Xue, K.; Du, J.; Liu, Y.; He, G. Effects of whole grains on glycemic control: A systematic review and dose-response meta-analysis of prospective cohort studies and randomized controlled trials. Nutr. J. 2024, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Khayyat, R.; Zurbau, A.; Komishon, A.; Mazhar, N.; Sievenpiper, J.L.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Jenkins, A.L.; et al. Should Viscous Fiber Supplements Be Considered in Diabetes Control? Results From a Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2019, 42, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Gelevam, D.; Thomas, W.; Gannon, M.C.; Keenan, J.M. A solubilized cellulose fiber decreases peak postprandial cholecystokinin concentrations after a liquid mixed meal in hypercholesterolemic men and women. J. Nutr. 2003, 133, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.M.; Zhu, Y.; Wilcox, M.L.; Koecher, K.; Maki, K.C. Effects of Whole Grain Intake, Compared with Refined Grain, on Appetite and Energy Intake: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1177–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Mio, K.; Goto, Y.; Matsuoka, T.; Komatsu, M.; Ishii, C.; Yang, J.; Kobayashi, T.; Aoe, S.; Fukuda, S. Barley β-glucan consumption improves glucose tolerance by increasing intestinal succinate concentrations. NPJ Sci. Food 2024, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [PubMed]
- Zurbau, A.; Noronha, J.C.; Khan, T.A.; Sievenpiper, J.L.; Wolever, T.M.S. The effect of oat β-glucan on postprandial blood glucose and insulin responses: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Palmnäs-Bédard, M.S.A.; Costabile, G.; Vetrani, C.; Åberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Covasa, M.; Stephens, R.W.; Toderean, R.; Cobuz, C. Intestinal Sensing by Gut Microbiota: Targeting Gut Peptides. Front. Endocrinol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Khan, M.Z.; Ma, Y.; Meng, Y.; Mushtaq, A.; Shen, Q.; Xue, Y. Overview of the Composition of Whole Grains’ Phenolic Acids and Dietary Fibre and Their Effect on Chronic Non-Communicable Diseases. Int. J. Environ. Res. Public Health 2022, 19, 3042. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Choudhury, A.; Dey, B.K. An Overview on Plant Derived Phenolic Compounds and Their Role in Treatment and Management of Diabetes. J. Pharmacopunct. 2022, 25, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Imamura, F.; Ye, Z.; Schulze, M.B.; Zheng, J.; Ardanaz, E.; Arriola, L.; Boeing, H.; Dow, C.; Fagherazzi, G.; et al. Interaction between genes and macronutrient intake on the risk of developing type 2 diabetes: Systematic review and findings from European Prospective Investigation into Cancer (EPIC)-InterAct. Am. J. Clin. Nutr. 2017, 106, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, S.; Weickert, M.O.; Pfeiffer, A.F.H. The role of cereal soluble fiber in the beneficial modulation of glycometabolic gastrointestinal hormones. Crit. Rev. Food Sci. Nutr. 2024, 64, 4331–4347. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Sharma, A.; Vinas, A.; Chilton, R.J. GLP-1 receptor agonists and delayed gastric emptying: Implications for invasive cardiac interventions and surgery. Cardiovasc. Endocrinol. Metab. 2024, 14, e00321. [Google Scholar] [CrossRef] [PubMed]
- Wirström, T.; Hilding, A.; Gu, H.F.; Östenson, C.G.; Björklund, A. Consumption of whole grain reduces risk of deteriorating glucose tolerance, including progression to prediabetes. Am. J. Clin. Nutr. 2013, 97, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Sonestedt, E.; Ericson, U.; Jing, X.J.; Zhou, Y.; Hansson, O.; Renström, E.; Wirfält, E.; Orho-Melander, M. Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia 2012, 55, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Mollet, I.G.; Rukh, G.; Ericson, U.; Orho-Melander, M. Several type 2 diabetes-associated variants in genes annotated to WNT signaling interact with dietary fiber in relation to incidence of type 2 diabetes. Genes Nutr. 2016, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- InterAct Consortium. Investigation of gene-diet interactions in the incretin system and risk of type 2 diabetes: The EPIC-InterAct study. Diabetologia 2016, 59, 2613–2621. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Wu, X.; Chen, Y.; Ji, W.; Wang, J.; Zhang, Y.; Xia, Y.; Tang, Y.; Yuan, J. The Wnt Signaling Pathway in Diabetic Nephropathy. Front. Cell Dev. Biol. 2022, 9, 701547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Habener, J.F. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J. Biol. Chem. 2008, 283, 8723–8735. [Google Scholar] [CrossRef] [PubMed]
- Al-Awaida, W.J.; Hameed, W.S.; Al Hassany, H.J.; Al-Dabet, M.M.; Al-Bawareed, O.; Hadi, N.R. Evaluation of the Genetic Association and Expressions of Notch-2 /Jagged-1 in Patients with Type 2 Diabetes Mellitus. Med. Arch. 2021, 75, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.; Cheng, P.; King, I.N.; Andersen, P.; Shenje, L.; Nigam, V.; Srivastava, D. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat. Cell Biol. 2011, 13, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, M.; Ding, Y.; Zhang, L.S.; Xi, Y.; Pan, W.J.; Tao, D.L.; Wang, J.Y.; Li, L. Identification of zinc-finger BED domain-containing 3 (Zbed3) as a novel Axin-interacting protein that activates Wnt/beta-catenin signaling. J. Biol. Chem. 2009, 284, 6683–6699. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yuan, L.; Hu, W.; Luo, Y.; Suo, L.; Yang, M.; Chen, S.; Wang, Y.; Liu, H.; Yang, G.; et al. Zinc-finger BED domain-containing 3 (Zbed3) is a novel secreted protein associated with insulin resistance in humans. J. Intern. Med. 2014, 275, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Ruan, C.S.; Zhao, F.Z.; Yang, M.; Cui, W.; Cheng, X.; Luo, X.H.; Zhang, X.X.; Zhang, C. ZBED3 exacerbates hyperglycemia by promoting hepatic gluconeogenesis through CREB signaling. Metabolism 2025, 162, 156049. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; McKeown, N.M.; Kanoni, S.; Lemaitre, R.N.; Hivert, M.F.; Ngwa, J.; Van Rooij, F.J.; Sonestedt, E.; Wojczynski, M.K.; Ye, Z.; et al. Interactions of dietary whole-grain intake with fasting glucose- and insulin-related genetic loci in individuals of European descent: A meta-analysis of 14 cohort studies. Diabetes Care 2010, 33, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.S.; Akbarzadeh, M.; Sedaghati-Khayat, B.; Seyedhamzehzadeh, A.; Daneshpour, M.S. GCKR common functional polymorphisms are associated with metabolic syndrome and its components: A 10-year retrospective cohort study in Iranian adults. Diabetol. Metab. Syndr. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Kwak, S.Y.; Lim, H.; Shin, M.J. Genotype effects of glucokinase regulator on lipid profiles and glycemic status are modified by circulating calcium levels: Results from the Korean Genome and Epidemiology Study. Nutr. Res. 2018, 60, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Kao, W.H.; Boerwinkle, E.; Hoogeveen, R.C.; Rasmussen-Torvik, L.J.; Astor, B.C.; North, K.E.; Coresh, J.; Köttgen, A. Association of rs780094 in GCKR with metabolic traits and incident diabetes and cardiovascular disease: The ARIC Study. PLoS ONE 2010, 5, e11690. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Dietary carbohydrate and the risk of type 2 diabetes: An updated systematic review and dose-response meta-analysis of prospective cohort studies. Sci. Rep. 2022, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.O. The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. Afr. Health Sci. 2016, 16, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 274S–280S. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rosenberg, L.; Singer, M.; Hu, F.B.; Djoussé, L.; Cupples, L.A.; Palmer, J.R. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch. Intern. Med. 2007, 167, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Zhang, L.; Zhang, Y.H.; Qin, L.Q. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Br. J. Nutr. 2011, 106, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef] [PubMed]
- Sluijs, I.; Beulens, J.W.; Van der Schouw, Y.T.; Van der, A.D.L.; Buckland, G.; Kuijsten, A.; Schulze, M.B.; Amiano, P.; Ardanaz, E.; Balkau, B.; et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J. Nutr. 2013, 143, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations. Nutrients 2019, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, A.; Sunohara, S.; Kimura, T.; Hao, W.; Moriguchi, T.; Tamakoshi, A. Association between dietary carbohydrate intake and risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetol. Int. 2023, 14, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Yang, Y.; Zhao, B.; Yu, K.; Guo, K.; Fang, F.; Zhou, Z.; Albanes, D.; Huang, J. Dietary carbohydrate intake and risk of type 2 diabetes: A 16-year prospective cohort study. Sci. China Life Sci. 2025, 68, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xia, W.; Zhao, Z.; Zhang, H. Effects comparison between low glycemic index diets and high glycemic index diets on HbA1c and fructosamine for patients with diabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2015, 9, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Mejia, S.B.; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; Wolever, T.M.S.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Elliott, E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009, 2009, CD006296. [Google Scholar] [CrossRef] [PubMed]
- Korsmo-Haugen, H.K.; Brurberg, K.G.; Mann, J.; Aas, A.M. Carbohydrate quantity in the dietary management of type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.M.; Cowland, D.E.; Hooper, L.; Frost, G.S. Low glycaemic index diets and blood lipids: A systematic review and meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. The effect of dietary glycemic index and glycemic load on inflammatory biomarkers: A systematic review and meta-analysis of randomized clinical trials. Am. J. Clin. Nutr. 2018, 107, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Greenwood, D.C.; Threapleton, D.E.; Gale, C.P.; Cleghorn, C.L.; Burley, V.J. Glycemic index, glycemic load, and blood pressure: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 105, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Care in Diabetes. 2025. Available online: https://professional.diabetes.org/standards-of-care (accessed on 1 May 2025).
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Parnell, L.D.; Smith, C.E.; Lee, Y.C.; Jamal-Allial, A.; Ma, Y.; Li, D.; Tucker, K.L.; Ordovás, J.M.; Lai, C.Q. Circulating 25-hydroxyvitamin D, IRS1 variant rs2943641, and insulin resistance: Replication of a gene-nutrient interaction in 4 populations of different ancestries. Clin. Chem. 2014, 60, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Ericson, U.; Rukh, G.; Stojkovic, I.; Sonestedt, E.; Gullberg, B.; Wirfält, E.; Wallström, P.; Orho-Melander, M. Sex-specific interactions between the IRS1 polymorphism and intakes of carbohydrates and fat on incident type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Bray, G.A.; Smith, S.R.; Hu, F.B.; Sacks, F.M.; Qi, L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011, 124, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Arnett, D.K.; Parnell, L.D.; Smith, C.E.; Li, D.; Borecki, I.B.; Tucker, K.L.; Ordovás, J.M.; Lai, C.Q. Modulation by dietary fat and carbohydrate of IRS1 association with type 2 diabetes traits in two populations of different ancestries. Diabetes Care 2013, 36, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.; Máté, B.; Csép, K.; Benedek, T. Epigenetic Modifications Linked to T2D, the Heritability Gap, and Potential Therapeutic Targets. Biochem. Genet. 2018, 56, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mohammad, G. Epigenetic modifications in diabetes. Metabolism 2022, 126, 154920. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, E.; Yu, H.; Yuan, C.; Abbas, M.N.; Cui, H. Methylation across the central dogma in health and diseases: New therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of Histone Modification. In Histone Mutations and Cancer. Advances in Experimental Medicine and Biology; Fang, D., Han, J., Eds.; Springer: Singapore, 2021; Volume 123. [Google Scholar]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Kaimala, S.; Kumar, C.A.; Allouh, M.Z.; Ansari, S.A.; Emerald, B.S. Epigenetic modifications in pancreas development, diabetes, and therapeutics. Med. Res. Rev. 2022, 42, 1343–1371. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qiu, T.; Wei, G.; Que, Y.; Wang, W.; Kong, Y.; Xie, T.; Chen, X. Role of Histone Post-Translational Modifications in Inflammatory Diseases. Front. Immunol. 2022, 13, 852272. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Srijyothi, L.; Ponne, S.; Prathama, T.; Ashok, C.; Baluchamy, S. Roles of Non-Coding RNAs in Transcriptional Regulation. In Transcriptional and Post-Transcriptional Regulation; InTech: London, UK, 2018. [Google Scholar]
- Nalbant, E.; Akkaya-Ulum, Y.Z. Exploring regulatory mechanisms on miRNAs and their implications in inflammation-related diseases. Clin. Exp. Med. 2024, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Niu, M.; Wang, Y.; Xu, R.; Guo, Y.; Zhang, C. Roles of long noncoding RNAs in human inflammatory diseases. Cell Death Discov. 2024, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Walaszczyk, E.; Luijten, M.; Spijkerman, A.M.W.; Bonder, M.J.; Lutgers, H.L.; Snieder, H.; Wolffenbuttel, B.H.R.; Van Vliet-Ostaptchouk, J.V. DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA1c levels: A systematic review and replication in a case-control sample of the Lifelines study. Diabetologia 2018, 61, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Bacos, K.; Rönn, T. Epigenetics of type 2 diabetes mellitus and weight change—a tool for precision medicine? Nat. Rev. Endocrinol. 2022, 18, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Formichi, C.; Nigi, L.; Grieco, G.E.; Maccora, C.; Fignani, D.; Brusco, N.; Licata, G.; Sebastiani, G.; Dotta, F. Non-Coding RNAs: Novel Players in Insulin Resistance and Related Diseases. Int. J. Mol. Sci. 2021, 22, 7716. [Google Scholar] [CrossRef] [PubMed]
- Macvanin, M.T.; Gluvic, Z.; Bajic, V.; Isenovic, E.R. Novel insights regarding the role of noncoding RNAs in diabetes. World J. Diabetes 2023, 14, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, L.; He, Y.; Zhou, T.; Cheng, X.; Huang, W.; Xu, Y. Novel histone post-translational modifications in diabetes and complications of diabetes: The underlying mechanisms and implications. Biomed. Pharmacother. 2022, 156, 113984. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luan, Y.; Feng, Q.; Chen, X.; Qin, B.; Ren, K.D.; Luan, Y. Epigenetics and Beyond: Targeting Histone Methylation to Treat Type 2 Diabetes Mellitus. Front. Pharmacol. 2022, 12, 807413. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Krause, B.J.; Cataldo, L.R.; Vega, J.; Salas-Pérez, F.; Mennickent, P.; Gallegos, R.; Milagro, F.I.; Prieto-Hontoria, P.; Riezu-Boj, J.I.; et al. PPARGC1A Gene Promoter Methylation as a Biomarker of Insulin Secretion and Sensitivity in Response to Glucose Challenges. Nutrients 2020, 12, 2790. [Google Scholar] [CrossRef] [PubMed]
- Younesian, S.; Mohammadi, M.H.; Younesian, O.; Momeny, M.; Ghaffari, S.H.; Bashash, D. DNA methylation in human diseases. Heliyon 2024, 10, e32366. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Nano, J.; Voortman, T.; Braun, K.V.E.; Ligthart, S.; Stranges, S.; Bramer, W.M.; Troup, J.; Chowdhury, R.; Dehghan, A.; et al. The role of global and regional DNA methylation and histone modifications in glycemic traits and type 2 diabetes: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, L.; Jacobsen, S.C.; Rönn, T.; Brøns, C.; Vaag, A. PPARGC1A DNA methylation in subcutaneous adipose tissue in low birth weight subjects—impact of 5 days of high-fat overfeeding. Metabolism 2014, 63, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Morais, T.; Sandovici, I.; Seabra, A.L.; Constância, M.; Monteiro, M.P. Adipose Tissue Epigenetic Profile in Obesity-Related Dysglycemia—A Systematic Review. Front. Endocrinol. 2021, 12, 681649. [Google Scholar] [CrossRef] [PubMed]
- Gancheva, S.; Ouni, M.; Jelenik, T.; Koliaki, C.; Szendroedi, J.; Toledo, F.G.S.; Markgraf, D.F.; Pesta, D.H.; Mastrototaro, L.; De Filippo, E.; et al. Dynamic changes of muscle insulin sensitivity after metabolic surgery. Nat. Commun. 2019, 10, 4179. [Google Scholar] [CrossRef] [PubMed]
- Ling, C. Epigenetic regulation of insulin action and secretion—role in the pathogenesis of type 2 diabetes. J. Intern. Med. 2020, 288, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Willmer, T.; Johnson, R.; Louw, J.; Pheiffer, C. Blood-Based DNA Methylation Biomarkers for Type 2 Diabetes: Potential for Clinical Applications. Front. Endocrinol. 2018, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, J.; Balbino, K.P.; Hermsdorff, H.H.M.; Juvanhol, L.L.; Martinez, J.A.; Steemburgo, T. Relationship between the FTO Genotype and Early Chronic Kidney Disease in Type 2 Diabetes: The Mediating Role of Central Obesity, Hypertension, and High Albuminuria. Lifestyle Genom. 2021, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.J.; Jagielnicki, M.; Yeager, M. Structure/Function Analysis of human ZnT8 (SLC30A8): A Diabetes Risk Factor and Zinc Transporter. Curr. Res. Struct. Biol. 2020, 2, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Wondafrash, D.Z.; Nire’a, A.T.; Tafere, G.G.; Desta, D.M.; Berhe, D.A.; Zewdie, K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. 2020, 13, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Qie, R.; Chen, Q.; Wang, T.; Chen, X.; Wang, J.; Cheng, R.; Lin, J.; Zhao, Y.; Liu, D.; Qin, P.; et al. Association of ABCG1 gene methylation and its dynamic change status with incident type 2 diabetes mellitus: The Rural Chinese Cohort Study. J. Hum. Genet. 2021, 66, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Eberlé, D.; Clément, K.; Meyre, D.; Sahbatou, M.; Vaxillaire, M.; Le Gall, A.; Ferré, P.; Basdevant, A.; Froguel, P.; Foufelle, F. SREBF-1 gene polymorphisms are associated with obesity and type 2 diabetes in French obese and diabetic cohorts. Diabetes 2004, 53, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Raciti, G.A.; Desiderio, A.; Longo, M.; Leone, A.; Zatterale, F.; Prevenzano, I.; Miele, C.; Napoli, R.; Beguinot, F. DNA Methylation and Type 2 Diabetes: Novel Biomarkers for Risk Assessment? Int. J. Mol. Sci. 2021, 22, 11652. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.; Day, F.R.; Perry, J.R.B.; Loh, M.; Chu, A.Y.; Lehne, B.; Paul, D.S.; Lotta, L.A.; Stewart, I.D.; Kerrison, N.D.; et al. Epigenome-Wide Association Study of Incident Type 2 Diabetes in a British Population: EPIC-Norfolk Study. Diabetes 2019, 68, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Fraszczyk, E.; Spijkerman, A.M.W.; Zhang, Y.; Brandmaier, S.; Day, F.R.; Zhou, L.; Wackers, P.; Dollé, M.E.T.; Bloks, V.W.; Gào, X.; et al. Epigenome-wide association study of incident type 2 diabetes: A meta-analysis of five prospective European cohorts. Diabetologia 2022, 65, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. The association of genetically controlled CpG methylation (cg158269415) of protein tyrosine phosphatase, receptor type N2 (PTPRN2) with childhood obesity. Sci. Rep. 2019, 9, 4855. [Google Scholar] [CrossRef] [PubMed]
- Nadiger, N.; Veed, J.K.; Chinya Nataraj, P.; Mukhopadhyay, A. DNA methylation and type 2 diabetes: A systematic review. Clin. Epigenet. 2024, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Metabolic programming of offspring by vitamin B12/folate imbalance during pregnancy. Diabetologia 2008, 51, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Matte, A.; Perfilyev, A.; de Mello, V.D.; Käkelä, P.; Pihlajamäki, J.; Ling, C. Epigenetic Alterations in Human Liver From Subjects With Type 2 Diabetes in Parallel With Reduced Folate Levels. J. Clin. Endocrinol. Metab. 2015, 100, E1491–E1501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, C.; Lu, L.; Yang, K.; Reis, J.; He, K. Intakes of Folate, Vitamin B6, and Vitamin B12 in Relation to Diabetes Incidence Among American Young Adults: A 30-Year Follow-up Study. Diabetes Care 2020, 43, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Lemas, D.J.; Wiener, H.W.; O’Brien, D.M.; Hopkins, S.; Stanhope, K.L.; Havel, P.J.; Allison, D.B.; Fernandez, J.R.; Tiwari, H.K.; Boyer, B.B. Genetic polymorphisms in carnitine palmitoyltransferase 1A gene are associated with variation in body composition and fasting lipid traits in Yup’ik Eskimos. J. Lipid Res. 2012, 53, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Liang, K. Mitochondrial CPT1A: Insights into structure, function, and basis for drug development. Front. Pharmacol. 2023, 14, 1160440. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Q.; Parnell, L.D.; Smith, C.E.; Guo, T.; Sayols-Baixeras, S.; Aslibekyan, S.; Tiwari, H.K.; Irvin, M.R.; Bender, C.; Fei, D.; et al. Carbohydrate and fat intake associated with risk of metabolic diseases through epigenetics of CPT1A. Am. J. Clin. Nutr. 2020, 112, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Goeman, J.J.; Monajemi, R.; Gu, H.; Putter, H.; Zhang, Y.; Slieker, R.C.; Stok, A.P.; Thijssen, P.E.; Müller, F.; et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 2014, 5, 5592. [Google Scholar] [CrossRef] [PubMed]
- Aslibekyan, S.; Demerath, E.W.; Mendelson, M.; Zhi, D.; Guan, W.; Liang, L.; Sha, J.; Pankow, J.S.; Liu, C.; Irvin, M.R.; et al. Epigenome-wide study identifies novel methylation loci associated with body mass index and waist circumference. Obesity 2015, 23, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Frazier-Wood, A.C.; Aslibekyan, S.; Absher, D.M.; Hopkins, P.N.; Sha, J.; Tsai, M.Y.; Tiwari, H.K.; Waite, L.L.; Zhi, D.; Arnett, D.K. Methylation at CPT1A locus is associated with lipoprotein subfraction profiles. J. Lipid Res. 2014, 55, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Irvin, M.R.; Zhi, D.; Joehanes, R.; Mendelson, M.; Aslibekyan, S.; Claas, S.A.; Thibeault, K.S.; Patel, N.; Day, K.; Jones, L.W.; et al. Epigenome-wide association study of fasting blood lipids in the Genetics of Lipid-lowering Drugs and Diet Network study. Circulation 2014, 130, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Q.; Wojczynski, M.K.; Parnell, L.D.; Hidalgo, B.A.; Irvin, M.R.; Aslibekyan, S.; Province, M.A.; Absher, D.M.; Arnett, D.K.; Ordovás, J.M. Epigenome-wide association study of triglyceride postprandial responses to a high-fat dietary challenge. J. Lipid Res. 2016, 57, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sha, J.; Hidalgo, B.; Aslibekyan, S.; Do, A.N.; Zhi, D.; Sun, D.; Zhang, T.; Li, S.; Chen, W.; et al. Association of DNA Methylation at CPT1A Locus with Metabolic Syndrome in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) Study. PLoS ONE 2016, 11, e0145789. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Xu, G.B.; Chen, H.; Pan, Y.X. Epigenetic regulation of carnitine palmitoyltransferase 1 (Cpt1a) by high fat diet. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Munetsuna, E.; Yamada, H.; Ando, Y.; Yamazaki, M.; Taromaru, N.; Nagura, A.; Ishikawa, H.; Suzuki, K.; Teradaira, R.; et al. High fructose consumption induces DNA methylation at PPARα and CPT1A promoter regions in the rat liver. Biochem. Biophys. Res. Commun. 2015, 468, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Nishio, Y.; Nakamura, T.; Maegawa, H.; Kikkawa, R.; Kashiwagi, A. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1180–E1190. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Bellumori, M.; Pucci, L.; Gabriele, M.; Longo, V.; Paoli, P.; Melani, F.; Mulinacci, N.; Innocenti, M. Does fermentation really increase the phenolic content in cereals? A study on millet. Foods 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.; Pérez-Álvarez, J.Á.; Pateiro, M.; Viuda-Matos, M.; Fernández-López, J.; Lorenzo, J.M. Satiety from healthier and functional foods. Trends Food Sci. Technol. 2021, 113, 397–410. [Google Scholar] [CrossRef]
- Modesti, M.; Tonacci, A.; Sansone, F.; Billeci, L.; Bellincontro, A.; Cacopardo, G.; Sanmartin, C.; Taglieri, I.; Venturi, F. E-senses, panel tests and wearable sensors: A teamwork for food quality assessment and prediction of consumer’s choices. Chemosensors 2022, 10, 244. [Google Scholar] [CrossRef]
- Tonacci, A.; Scalzini, G.; Díaz-Guerrero, P.; Sanmartin, C.; Taglieri, I.; Ferroni, G.; Flamini, G.; Odello, L.; Billeci, L.; Venturi, F. Chemosensory analysis of emotional wines: Merging of explicit and implicit methods to measure emotions aroused by red wines. Food Res. Int. 2024, 190, 114611. [Google Scholar] [CrossRef] [PubMed]
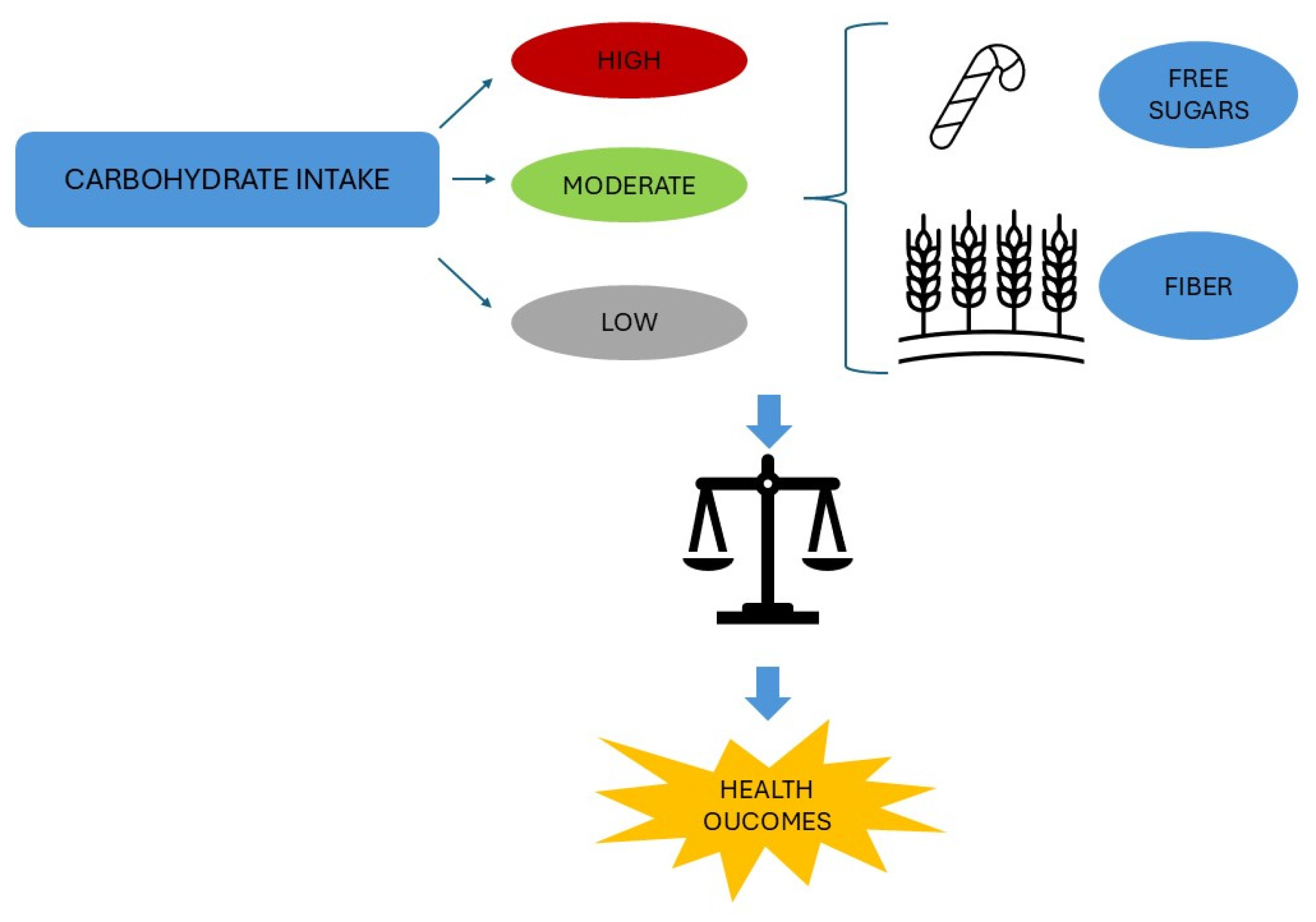

| Clues | Reference | Pitfalls | Reference |
|---|---|---|---|
| High intake of carbohydrates significantly associated with an increased risk of T2D | [27] | Carbohydrate intake not associated with an increased risk of T2D | [162,163,168] |
| GI and GL significantly and positively associated with risk of T2D | [161,163,164,165] | No significant association of GI and GL with T2D incidence | [163] |
| No evidence of publication bias | [27,161,162] | Potential residual confounding | [27,161,162,169] |
| Possibility of misclassification error and bias in the diagnosis and assessment of T2D (mostly based on self-reports) | [27,161,163,165,166] | ||
| Most studies measure dietary intakes at baseline only | [27,161,163,165] | ||
| Nutrition assessment used only FFQ and is therefore susceptible to large random and systemic errors | [27,161,162,163,164] | ||
| Possibility of measurement errors in dietary assessment despite the improvement of methods | [169] | ||
| Heterogeneity between studies due to differences in participant characteristics, geographical areas, and confounding factors | [27,161,164,165,166] | ||
| No possibility to establish to what extent the effect of GL is attributable to carbohydrate intake | [161] | ||
| Most studies conducted in female participants | [27] | ||
| No causal relationship defined due to observational study design | [27,161,162,163,164,165] | ||
| Publication bias between studies | [164,165,168] | ||
| Possibility of misclassification in the assignment of GI and GL to food items | [166] | ||
| Predominance of participants of European American descent | [164,169] |
| Carbohydrate Type | Gene | Variant | Effect of Interaction on T2D Risk | Level of Evidence |
|---|---|---|---|---|
| High intake of total fiber/cereal fiber/whole grains | TCF7L2 | rs7903146 TT rs7903146 CC rs4506565 AA rs12255372 | Harmful Protective Protective Harmful/Protective | Moderate Low Low Low |
| High intake of dietary fiber | NOTCH2 ZEBD2 | rs10923931 rs445705 | Protective Protective | Low Low |
| High whole grain intake | GCKR | rs780094 C/CC | Harmful | Moderate |
| Low carbohydrate diet High carbohydrate intake Low short fatty acid -to-carbohydrate ratio | IRS1 | rs2943641 T rs2943641 CC rs2943641 T rs7578326 G | Protective Protective Protective Protective | Low High Low Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorini, F.; Tonacci, A. The Complex Gene–Carbohydrate Interaction in Type 2 Diabetes: Between Current Knowledge and Future Perspectives. Nutrients 2025, 17, 2350. https://doi.org/10.3390/nu17142350
Gorini F, Tonacci A. The Complex Gene–Carbohydrate Interaction in Type 2 Diabetes: Between Current Knowledge and Future Perspectives. Nutrients. 2025; 17(14):2350. https://doi.org/10.3390/nu17142350
Chicago/Turabian StyleGorini, Francesca, and Alessandro Tonacci. 2025. "The Complex Gene–Carbohydrate Interaction in Type 2 Diabetes: Between Current Knowledge and Future Perspectives" Nutrients 17, no. 14: 2350. https://doi.org/10.3390/nu17142350
APA StyleGorini, F., & Tonacci, A. (2025). The Complex Gene–Carbohydrate Interaction in Type 2 Diabetes: Between Current Knowledge and Future Perspectives. Nutrients, 17(14), 2350. https://doi.org/10.3390/nu17142350








