Mutual Impact of Dietary Antioxidants and TNF-α rs1800629 on Insulin Levels in Adults with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Dietary Intake and CDAI
2.3. Anthropometric and Body Composition Measurements
2.4. Biochemical Analysis and Clinical Data
2.5. Genotyping of the TNF-α -308 G/A
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ANCOVA | analysis of covariance |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| AU | arbitrary units |
| BMI | body mass index |
| CI | confidence interval |
| CDAI | Composite Dietary Antioxidant Index |
| CRP | C-reactive protein |
| DBP | diastolic blood pressure |
| FABP | fatty acid-binding protein |
| FM/LM | fat mass/lean mass |
| GGT | gamma-glutamyl transferase |
| HC | hip circumference |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| IL-6 | interleukin 6 |
| IRS-1 | insulin receptor substrate-1 |
| KCNJ11 | potassium channel, inwardly rectifying subfamily J member 11 |
| Kcal | kilocalories |
| LDL-C | low-density lipoprotein cholesterol |
| NF-κB | nuclear factor kappa B |
| PCR | polymerase chain reaction |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| ROS | reactive oxygen species |
| RT-PCR | Real-time polymerase chain reaction |
| SBP | systolic blood pressure |
| SNV | single nucleotide variants |
| TC | total cholesterol |
| TG | triglycerides |
| TNF-α | tumor necrosis factor-alpha |
| TyG | TG and glucose index |
| WHR | Waist-to-hip ratio |
References
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 8 November 2024).
- Wild, C.P. The Exposome: From Concept to Utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Ruck, L.; Wiegand, S.; Kühnen, P. Relevance and Consequence of Chronic Inflammation for Obesity Development. Mol. Cell. Pediatr. 2023, 10, 16. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin Resistance: A Metabolic Pathway to Chronic Liver Disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.D.; Hasan, P.M.Z.; Shamsi, A. Role of Polyphenols in Combating Type 2 Diabetes and Insulin Resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R. Review on the Role of Polyphenols in Preventing and Treating Type 2 Diabetes: Evidence from In Vitro and In Vivo Studies. Nutrients 2024, 16, 3159. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity in Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Wright, M.E.; Mayne, S.T.; Stolzenberg-Solomon, R.Z.; Li, Z.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Development of a Comprehensive Dietary Antioxidant Index and Application to Lung Cancer Risk in a Cohort of Male Smokers. Am. J. Epidemiol. 2004, 160, 68–76. [Google Scholar] [CrossRef]
- Luu, H.N.; Wen, W.; Li, H.; Dai, Q.; Yang, G.; Cai, Q.; Xiang, Y.-B.; Gao, Y.-T.; Zheng, W.; Shu, X.-O. Are Dietary Antioxidant Intake Indices Correlated to Oxidative Stress and Inflammatory Marker Levels? Antioxid. Redox Signal. 2015, 22, 951. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Ding, L.; Zhang, Z.; Sun, Y. The Dietary Inflammatory Index and New-Onset Hypertension in Chinese Adults: A Nationwide Cohort Study. Food Funct. 2023, 14, 10759–10769. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Si, J.; Liu, Y.; Kang, L.; Xu, B. Association between Composite Dietary Antioxidant Index and Hypertension: Insights from NHANES. Clin. Exp. Hypertens. 2023, 45, 2233712. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, A.; Askari, M.; Mozaffari, H.; Homayounfrar, R.; Nikparast, A.; Ghazi, M.L.; Nejad, M.M.; Alizadeh, S. Dietary Inflammatory Index in Relation to Type 2 Diabetes: A Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 9953115. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, H.; Chen, Y.; Sang, H.; Tang, Y.; Zhao, Y. Composite Dietary Antioxidant Index Was Negatively Associated with the Prevalence of Diabetes Independent of Cardiovascular Diseases. Diabetol. Metab. Syndr. 2023, 15, 183. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, J.; Lai, R.; Wang, X.; Chen, X.; Tian, W.; Liu, Q.; Li, J.; Ju, J.; Xu, H. Association between Dietary Inflammatory Index and Atherosclerosis Cardiovascular Disease in U.S. adults. Front. Nutr. 2022, 9, 1044329. [Google Scholar] [CrossRef]
- Lin, Z.; Xie, Y.; Lin, Y.; Chen, X. Association between Composite Dietary Antioxidant Index and Atherosclerosis Cardiovascular Disease in Adults: A Cross-Sectional Study. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2165–2172. [Google Scholar] [CrossRef]
- Martín, M.Á.; Ramos, S. Dietary Flavonoids and Insulin Signaling in Diabetes and Obesity. Cells 2021, 10, 1474. [Google Scholar] [CrossRef]
- Harding, A.-H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma Vitamin C Level, Fruit and Vegetable Consumption, and the Risk of New-Onset Type 2 Diabetes Mellitus: The European Prospective Investigation of Cancer-Norfolk Prospective Study. Arch. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Canas, J.A.; Beydoun, H.A.; Chen, X.; Shroff, M.R.; Zonderman, A.B. Serum Antioxidant Concentrations and Metabolic Syndrome Are Associated among U.S. Adolescents in Recent National Surveys. J. Nutr. 2012, 142, 1693–1704. [Google Scholar] [CrossRef]
- de Santos, A.C.; Passos, A.F.F.; Holzbach, L.C.; Cominetti, C. Selenium Intake and Glycemic Control in Young Adults with Normal-Weight Obesity Syndrome. Front. Nutr. 2021, 8, 696325. [Google Scholar] [CrossRef] [PubMed]
- WHO Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 22 November 2024).
- Maury, E.; Brichard, S.M. Adipokine Dysregulation, Adipose Tissue Inflammation and Metabolic Syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.C.; González, C.; Pirola, C.J. Meta-Analysis on the G-308A Tumor Necrosis Factor Alpha Gene Variant and Phenotypes Associated with the Metabolic Syndrome. Obes. Res. 2005, 13, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Ayelign, B.; Genetu, M.; Wondmagegn, T.; Adane, G.; Negash, M.; Berhane, N. TNF-α (−308) Gene Polymorphism and Type 2 Diabetes Mellitus in Ethiopian Diabetes Patients. Diabetes Metab. Syndr. Obes. 2019, 12, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Paul, S.; Das, M.; Saha, T.; Faruque, M.O.; Hassan, Z. Tumour Necrosis Factor-α-308G/A Polymorphism Is Associated with Insulin Secretory Defects in Bangladeshi Prediabetic/Diabetic Subjects. J. Taibah Univ. Med. Sci. 2022, 17, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Sentinelli, F.; Capici, F.; Arca, M.; Berni, A.; Vecci, E.; Mario, U.D.; Baroni, M.G. The G-308A Variant of the Tumor Necrosis Factor-α (TNF-α) Gene Is Not Associated with Obesity, Insulin Resistance and Body Fat Distribution. BMC Med. Genet. 2001, 2, 10. [Google Scholar] [CrossRef]
- De Luis, D.A.; Aller, R.; Izaola, O.; Romero, E. Association of the TNF-alpha-308 G/A polymorphisms with metabolic responses secondary to a high protein/low carbohydrate versus a standard hypocaloric diet. Nutr. Hosp. 2016, 33, 267. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112016000300015&lng=es&nrm=iso&tlng=en (accessed on 1 November 2024).
- Wang, H.G.; Yang, J.; Han, H.; Xu, F.; Bian, Y.; Zhang, H.; Wang, J.L. TNF-αG-308A Polymorphism Is Associated with Insulin Resistance: A Meta-Analysis. Genet. Mol. Res. 2015, 14, 563–573. Available online: https://www.geneticsmr.org/articles/tnf-g308a-polymorphism-is-associated-with-insulin-resistance-a-metaanalysis.pdf (accessed on 1 November 2024). [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased Adipose Tissue Expression of Tumor Necrosis Factor-Alpha in Human Obesity and Insulin Resistance. J. Clin. Investig. 1995, 95, 2409. [Google Scholar] [CrossRef]
- Wilson, A.G.; Symons, J.A.; McDowell, T.L.; McDevitt, H.O.; Duff, G.W. Effects of a Polymorphism in the Human Tumor Necrosis Factor Alpha Promoter on Transcriptional Activation. Proc. Natl. Acad. Sci. USA 1997, 94, 3195–3199. [Google Scholar] [CrossRef]
- Kanety, H.; Feinstein, R.; Papa, M.Z.; Hemi, R.; Karasik, A. Tumor Necrosis Factor α-Induced Phosphorylation of Insulin Receptor Substrate-1 (IRS-1): Possible Mechanism for Suppression of Insulin-Stimulated Tyrosine Phosphorylation of IRS-1. J. Biol. Chem. 1995, 270, 23780–23784. [Google Scholar] [CrossRef]
- Rui, L.; Aguirre, V.; Kim, J.K.; Shulman, G.I.; Lee, A.; Corbould, A.; Dunaif, A.; White, M.F. Insulin/IGF-1 and TNF-Alpha Stimulate Phosphorylation of IRS-1 at Inhibitory Ser307 via Distinct Pathways. J. Clin. Investig. 2001, 107, 181–189. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Barak, Y.; Hevener, A.; Olson, P.; Liao, D.; Le, J.; Nelson, M.; Ong, E.; Olefsky, J.M.; Evans, R.M. Adipose-Specific Peroxisome Proliferator-Activated Receptor Gamma Knockout Causes Insulin Resistance in Fat and Liver but Not in Muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 15712–15717. [Google Scholar] [CrossRef] [PubMed]
- Almind, K.; Inoue, G.; Pedersen, O.; Kahn, C.R. A Common Amino Acid Polymorphism in Insulin Receptor Substrate-1 Causes Impaired Insulin Signaling. Evidence from Transfection Studies. J. Clin. Investig. 1996, 97, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin Stimulates Glucose Utilization and Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Weiss, E.P.; Brown, M.D.; Shuldiner, A.R.; Hagberg, J.M. Fatty Acid Binding Protein-2 Gene Variants and Insulin Resistance: Gene and Gene-Environment Interaction Effects. Physiol. Genom. 2002, 10, 145–157. [Google Scholar] [CrossRef]
- Del Bosque-Plata, L.; Martínez-Martínez, E.; Espinoza-Camacho, M.Á.; Gragnoli, C. The Role of TCF7L2 in Type 2 Diabetes. Diabetes 2021, 70, 1220–1228. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Hoebee, B.; Seidell, J.C.; Schaap, M.M.; De Bruin, T.W.A.; Feskens, E.J.M. Common Variants in the ATP-Sensitive K+ Channel Genes KCNJ11 (Kir6.2) and ABCC8 (SUR1) in Relation to Glucose Intolerance: Population-Based Studies and Meta-Analyses. Diabet. Med. 2005, 22, 590–598. [Google Scholar] [CrossRef]
- Rs1800629 RefSNP Report-dbSNP-NCBI. Available online: https://www.ncbi.nlm.nih.gov/snp/rs1800629 (accessed on 22 November 2024).
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Abdalhabib, E.K.; Algarni, A.; Saboor, M.; Alanazi, F.; Ibrahim, I.K.; Alfeel, A.H.; Alanazi, A.M.; Alanazi, A.M.; Alruwaili, A.M.; Alanazi, M.H.; et al. Association of TNF-α Rs1800629 with Adult Acute B-Cell Lymphoblastic Leukemia. Genes 2022, 13, 1237. [Google Scholar] [CrossRef]
- Yu, S.; Xue, M.; Yan, Z.; Song, B.; Hong, H.; Gao, X. Correlation between TNF-α −308 and +489 Gene Polymorphism and Acute Exacerbation of Chronic Obstructive Pulmonary Diseases. BioMed. Res. Int. 2021, 2021, 6661281. [Google Scholar] [CrossRef]
- Mir, H.; Koul, P.A.; Bhat, D.; Shah, Z.A. A Case-Control Study of Tumor Necrosis Factor-Alpha Promoter Polymorphism and Its Serum Levels in Patients with Chronic Obstructive Pulmonary Disease in Kashmir, North India. Lung India 2020, 37, 204–209. [Google Scholar] [CrossRef]
- Aller, R.; de Luis, D.A.; Izaola, O.; Sagrado, M.G.; Conde, R.; Gago, T.A.; Pacheco, D.; González, J.M.; Velasco, M.C. G308A Polymorphism of TNF-Alpha Gene Is Associated with Insulin Resistance and Histological Changes in Non Alcoholic Fatty Liver Disease Patients. Ann. Hepatol. 2010, 9, 439–444. [Google Scholar] [CrossRef]
- Joffe, Y.T.; van der Merwe, L.; Collins, M.; Carstens, M.; Evans, J.; Lambert, E.V.; Goedecke, J.H. The −308 G/A Polymorphism of the Tumour Necrosis Factor-α Gene Modifies the Association between Saturated Fat Intake and Serum Total Cholesterol Levels in White South African Women. Genes Nutr. 2011, 6, 353–359. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Aller de la Fuente, R.; Izaola Jáuregui, O.; González Sagrado, M.; Conde Vicente, R.; de la Fuente Salvador, B.; Ovalle, H.F. Allelic Frequency of G380A Polymorphism of Tumor Necrosis Factor Alpha Gene and Relation with Cardiovascular Risk Factors and Adipocytokines in Obese Patients. Nutr. Hosp. 2011, 26, 711–715. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112011000400007&lng=es&nrm=iso&tlng=en (accessed on 1 November 2024).
- Gupta, V.; Gupta, A.; Jafar, T.; Gupta, V.; Agrawal, S.; Srivastava, N.; Kumar, S.; Singh, A.K.; Natu, S.M.; Agarwal, C.G.; et al. Association of TNF-α Promoter Gene G-308A Polymorphism with Metabolic Syndrome, Insulin Resistance, Serum TNF-α and Leptin Levels in Indian Adult Women. Cytokine 2012, 57, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary Assessment Methods: Dietary Records. Nutr. Hosp. 2015, 31 (Suppl. 3), 38–45. Available online: https://pubmed.ncbi.nlm.nih.gov/25719769/ (accessed on 1 November 2024).
- Mataix-Verdú, J. (Ed.) Tabla de Composición de Alimentos 4a Edición Corregida Y Aumentada; Editorial Universidad de Granada: Granada, Spain, 2003; ISBN 978-84-338-3050-0. [Google Scholar]
- Zhang, H.-Q.; Shi, J.; Yue, T.; Weng, J.-H.; Wang, X.-L.; Wang, H.; Su, X.-Y.; Zheng, X.-Y.; Luo, S.-H.; Ding, Y.; et al. Association between Composite Dietary Antioxidant Index and Stroke among Individuals with Diabetes. World J. Diabetes 2024, 15, 1742–1752. [Google Scholar] [CrossRef]
- Maugeri, A.; Hruskova, J.; Jakubik, J.; Kunzova, S.; Sochor, O.; Barchitta, M.; Agodi, A.; Bauerova, H.; Medina-Inojosa, J.R.; Vinciguerra, M. Dietary Antioxidant Intake Decreases Carotid Intima Media Thickness in Women but Not in Men: A Cross-Sectional Assessment in the Kardiovize Study. Free Radic. Biol. Med. 2019, 131, 274–281. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 Fatty Acids Supplementation and Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, J. International Standards for Anthropometric Assessment; University of New South Wales Press: Sidney, Australia, 2011; Volume 137, ISBN 978-0-620-36207-8. [Google Scholar]
- Body Mass Index—BMI. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 15 April 2022).
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of Fat-Free Mass Using Bioelectrical Impedance Measurements of the Human Body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. Available online: https://pubmed.ncbi.nlm.nih.gov/4337382/ (accessed on 1 November 2024). [CrossRef] [PubMed]
- Guerrero-Romero, F.; Villalobos-Molina, R.; Jiménez-Flores, J.R.; Simental-Mendia, L.E.; Méndez-Cruz, R.; Murguía-Romero, M.; Rodríguez-Morán, M. Fasting Triglycerides and Glucose Index as a Diagnostic Test for Insulin Resistance in Young Adults. Arch. Med. Res. 2016, 47, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Rocella, E.J. Recommendations for Blood Pressure Measurement in Humans and Experimental Animals. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef]
- de Luis, D.A.; Sagrado, M.G.; Vallejo, L.A.; Carcedo, L.M.G.; Izaola, O.; Cuellar, L.; Terroba, M.C.; Aller, R. Influence of G308A Polymorphism of Tumor Necrosis Factor-Alpha Gene on Inflammatory Markers in Postsurgical Head and Neck Cancer Patients with Early Enteral Nutrition. Nutrition 2007, 23, 529–532. [Google Scholar] [CrossRef]
- Devyatkin, V.A.; Shklyar, A.A.; Fursova, A.Z.; Rumyantseva, Y.V.; Kozhevnikova, O.S. Allele-Specific PCR with Fluorescently Labeled Probes: Criteria for Selecting Primers for Genotyping. Vavilovskii Zhurnal Genet. Sel. 2024, 28, 351–359. [Google Scholar] [CrossRef]
- Jiménez-Ortega, R.F.; Meneses-León, J.; Hernández, S.; Thebar-Moreno, P.; Aparicio-Bautista, D.I.; Becerra-Cervera, A.; Aguilar-Salinas, C.; Salmerón, J.; Rivera-Paredez, B.; Velázquez-Cruz, R. High Dietary Antioxidant Index Associated with Reduced Insulin Resistance in Female Mexican Children and Adolescents. Nutr. Res. 2024, 132, 53–66. [Google Scholar] [CrossRef]
- Xu, Y.; Zhuang, Y.; Zhang, H. Single and Mixed Associations of Composite Antioxidant Diet on Triglyceride-Glucose Index. Lipids Health Dis. 2024, 23, 254. [Google Scholar] [CrossRef]
- Zeng, Z.; Zdzieblik, D.; Centner, C.; Brauchle, C.; Gollhofer, A.; König, D. Changing Dietary Habits Increases the Intake of Antioxidant Vitamins and Reduces the Concentration of Reactive Oxygen Species in Blood: A Pilot Study. Int. J. Food Prop. 2020, 23, 1337–1346. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.C.; Callas, P.W.; Hanley, A.J.G.; Festa, A.; Haffner, S.M.; Wagenknecht, L.E.; Tracy, R.P. Circulating Levels of TNF-α Are Associated with Impaired Glucose Tolerance, Increased Insulin Resistance, and Ethnicity: The Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 2012, 97, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, T.D.R.; Díaz, E.I.; Velarde, M.S.; Prado, M.M.; Bazán, M.C.; Abregú, A.V. [Tumor necrosis factor-alpha in a children population with overweight]. Medicina (B Aires) 2013, 73, 310–314. Available online: https://pubmed.ncbi.nlm.nih.gov/23924528/ (accessed on 1 November 2024). [PubMed]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-Alpha and Obesity. Curr. Dir. Autoimmun. 2010, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Aller, R.; Izaola, O.; Gonzalez Sagrado, M.; Conde, R. Role of G308 Promoter Variant of Tumor Necrosis Factor Alpha Gene on Weight Loss and Metabolic Parameters after a High Monounsaturated versus a High Polyunsaturated Fat Hypocaloric Diets. Med. Clin. 2013, 141, 189–193. [Google Scholar] [CrossRef]
- Lee, S.C.; Pu, Y.B.; Thomas, G.N.; Lee, Z.S.K.; Tomlinson, B.; Cockram, C.S.; Critchley, J.A.J.H.; Chan, J.C.N. Tumor Necrosis Factor Alpha Gene G-308A Polymorphism in the Metabolic Syndrome. Metabolism 2000, 49, 1021–1024. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative Stress and Inflammation Interactions in Human Obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Wang, J. Metabolism and Chronic Inflammation: The Links Between Chronic Heart Failure and Comorbidities. Front. Cardiovasc. Med. 2021, 8, 650278. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Razis, A.F.A.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative Stress, Insulin Resistance, Dyslipidemia and Type 2 Diabetes Mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Navas-Carretero, S.; San-Cristobal, R.; Alvarez-Alvarez, I.; Celis-Morales, C.; Livingstone, K.M.; O’Donovan, C.B.; Mavrogianni, C.; Lambrinou, C.P.; Manios, Y.; Traczyck, I.; et al. Interactions of Carbohydrate Intake and Physical Activity with Regulatory Genes Affecting Glycaemia: A Food4Me Study Analysis. Lifestyle Genom. 2021, 14, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Jacobs, D.R.; Nichaman, M.Z. An Assessment of Caloric Intake as an Indicator of Physical Activity. Prev. Med. 1989, 18, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Physical Activity, Food Intake, and Body Weight Regulation: Insights from Doubly Labeled Water Studies. Nutr. Rev. 2010, 68, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, D.; Nóbrega, C.; Manco, L.; Padez, C. The Contribution of Genetics and Environment to Obesity. Br. Med. Bull. 2017, 123, 159–173. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The Genetics of Obesity: From Discovery to Biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Comuzzie, A.G.; Williams, J.T.; Martin, L.J.; Blangero, J. Searching for Genes Underlying Normal Variation in Human Adiposity. J. Mol. Med. 2001, 79, 57–70. [Google Scholar] [CrossRef]
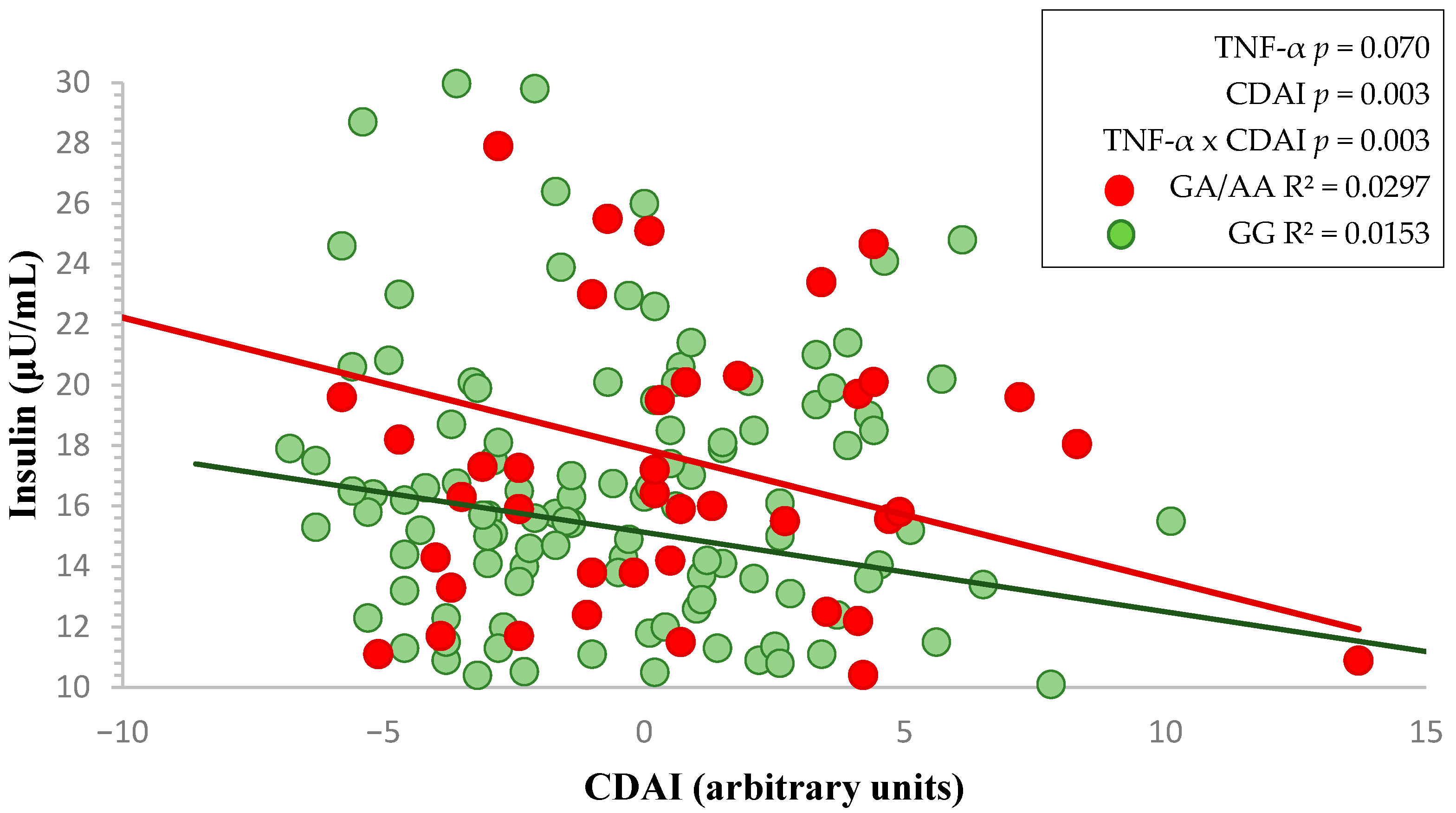
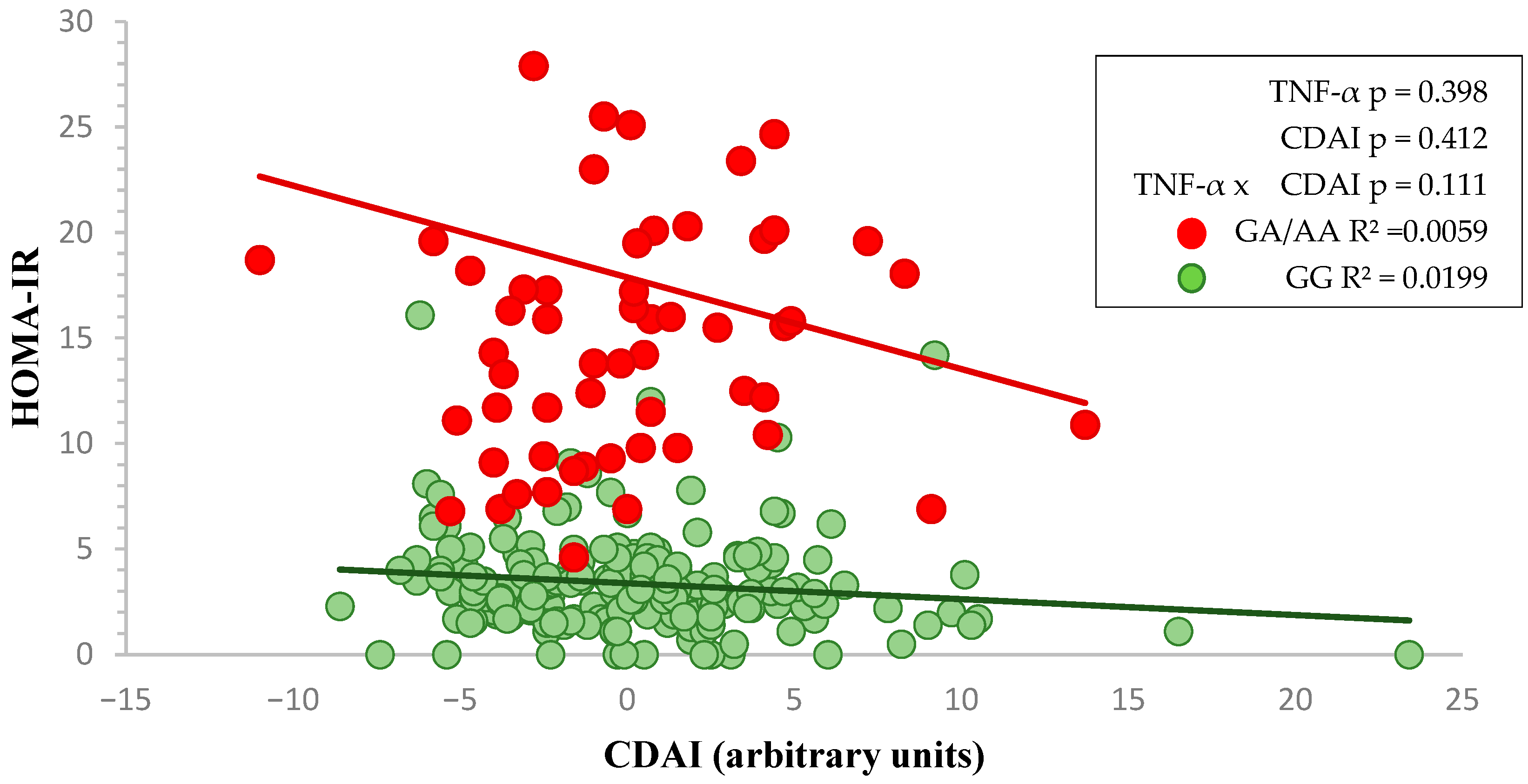
| BMI < 35 kg/m2 (n = 115) | BMI ≥ 35 kg/m2 (n = 173) | p | CDAI < −0.3 AU (n = 130) | CDAI ≥ −0.3 AU (n = 127) | p | GG Genotype (n = 214) | GA/AA Genotype (n = 76) | p | |
|---|---|---|---|---|---|---|---|---|---|
| Women (%) | 56.6 | 69.9 | 0.024 | 62.3 | 73.2 | 0.064 | 66.0 | 60.5 | 0.404 |
| Weight (kg) | 89.5 ± 11.5 | 107.5 ± 17.6 | <0.001 | 99.0 ± 17.0 | 100.7 ± 17.8 | 0.426 | 99.8 ± 17.0 | 102.2 ± 19.9 | 0.315 |
| BMI (kg/m2) | 32.7 ± 1.5 | 40.8 ± 4.5 | <0.001 | 37.5 ± 5.2 | 37.7 ± 5.3 | 0.751 | 37.5 ± 5.3 | 37.9 ± 5.6 | 0.527 |
| Fat Mass (kg) | 33.1 ± 8.7 | 47.5 ± 10.9 | <0.001 | 43.2 ± 11.8 | 40.8 ± 12.5 | 0.145 | 41.0 ± 11.8 | 44.0 ± 13.5 | 0.111 |
| Lean Mass (%) | 61.9 ± 9.7 | 54.6 ± 7.2 | <0.001 | 55.4 ± 7.9 | 58.9 ± 9.0 | 0.003 | 57.8 ± 9.0 | 56.6 ± 8.9 | 0.384 |
| FM/LM Ratio | 0.89 ± 0.12 | 1.05 ± 0.22 | <0.001 | 0.98 ± 0.16 | 0.99 ± 0.23 | 0.951 | 0.97 ± 0.20 | 1.04 ± 0.20 | 0.025 |
| Waist Circumference (cm) | 103.8 ± 10.1 | 119.2 ± 11.9 | <0.001 | 112.8 ± 13.8 | 113.8 ± 13.0 | 0.587 | 112.3 ± 13.4 | 116.0 ± 13.7 | 0.048 |
| WHR | 0.92 ± 0.08 | 0.95 ± 0.09 | 0.020 | 0.92 ± 0.09 | 0.95 ± 0.09 | 0.067 | 0.93 ± 0.09 | 0.95 ± 0.09 | 0.323 |
| Glucose (mg/dL) | 95.9 ± 19.3 | 97.4 ± 14.3 | 0.445 | 97.2 ± 15.9 | 96.3 ± 18.4 | 0.688 | 96.9 ± 17.4 | 96.4 ± 13.4 | 0.838 |
| Insulin (μU/mL) | 14.4 ± 10.7 | 18.3 ± 13.1 | 0.007 | 16.7 ± 9.9 | 15 ± 8.2 | 0.151 | 16.1 ± 11.8 | 19.0 ± 13.4 | 0.087 |
| HOMA-IR | 3.1 ± 2.9 | 4.3 ± 3.6 | 0.002 | 3.4 ± 3.2 | 3.3 ± 2.2 | 0.052 | 3.6 ± 3.3 | 4.3 ± 3.6 | 0.116 |
| TC (mg/dL) | 199.4 ± 37.1 | 196.5 ± 38.3 | 0.541 | 197.2 ± 41.1 | 196 ± 35.3 | 0.808 | 197.4 ± 37.8 | 197.5 ± 38.3 | 0.989 |
| LDL-c (mg/dL) | 125.2 ± 33.1 | 121.6 ± 33.9 | 0.386 | 122.9 ± 36.5 | 121.2 ± 31.4 | 0.700 | 123.4 ± 33.7 | 121.3 ± 33.8 | 0.643 |
| HDL-c (mg/dL) | 50.7 ± 13.8 | 48.6 ± 12.5 | 0.195 | 50.5 ± 13.4 | 49.3 ± 13.3 | 0.512 | 49.4 ± 13 | 49.3 ± 13.1 | 0.932 |
| TG (mg/dL) | 111.3 ± 53.5 | 134.2 ± 75.3 | 0.003 | 119.8 ± 57.3 | 127.9 ± 79.0 | 0.357 | 122.9 ± 61.3 | 130.9 ± 85.2 | 0.387 |
| TyG index | 10.5 ± 1.2 | 10.9 ± 1.2 | 0.002 | 10.7 ± 1.11 | 10.8 ± 1.3 | 0.660 | 10.7 ± 1.2 | 10.8 ± 1.2 | 0.666 |
| AST (U/L) | 22.3 ± 8.8 | 22.8 ± 10.4 | 0.702 | 21.8 ± 7.7 | 23.2 ± 10.7 | 0.249 | 22.5 ± 9.5 | 22.8 ± 10.5 | 0.842 |
| ALT (U/L) | 30.4 ± 13.5 | 34.4 ± 22.2 | 0.074 | 31.6 ± 18.2 | 34.5 ± 20.2 | 0.236 | 32.7 ± 18.6 | 33.6 ± 21.6 | 0.723 |
| GGT (U/L) | 29.1 ± 16.6 | 30.4 ± 23.3 | 0.630 | 27.1 ± 16.8 | 31.2 ± 21.7 | 0.209 | 29.2 ± 21.8 | 31.6 ± 17.6 | 0.416 |
| CRP (mg/dL) | 3.6 ± 5.5 | 5.4 ± 12.0 | 0.266 | 5.9 ± 14.1 | 3.6 ± 5.0 | 0.177 | 3.8 ± 6.0 | 7.5 ± 16.8 | 0.151 |
| SBP (mmHg) | 122.1 ± 14.5 | 132.6 ± 16.9 | <0.001 | 130.3 ± 16.4 | 127.2 ± 17.4 | 0.188 | 129.1 ± 17.5 | 127.1 ± 14.7 | 0.397 |
| DBP (mmHg) | 76.9 ± 10.9 | 83.9 ± 12 | <0.001 | 82.5 ± 11.4 | 79.8 ± 12.9 | 0.101 | 81.7 ± 12.0 | 79.5 ± 12.3 | 0.196 |
| BMI < 35 kg/m2 (n = 115) | BMI ≥ 35 kg/m2 (n = 173) | p | CDAI < −0.3 AU (n = 130) | CDAI ≥ −0.3 AU (n = 127) | p | GG Genotype (n = 214) | GA/AA Genotype (n = 76) | p | |
|---|---|---|---|---|---|---|---|---|---|
| Energy (Kcal) | 1956 ± 733 | 2033± 787 | 0.438 | 1643 ± 484 | 2353 ± 828 | <0.001 | 2012 ± 814 | 2001 ± 612 | 0.916 |
| Protein (% energy) | 19.5 ± 0.5 | 19.6 ± 0.4 | 0.905 | 19.3 ± 0.5 | 19.7 ± 0.5 | 0.579 | 19.6 ± 0.4 | 19.2 ± 0.7 | 0.558 |
| Lipids (% energy) | 41.3 ± 0.9 | 40.7 ± 0.7 | 0.649 | 44.1 ± 0.9 | 38.4 ± 0.8 | <0.001 | 40.9 ± 0.7 | 40.9 ± 1.1 | 0.960 |
| Carbohydrate (% energy) | 39.4 ± 1.0 | 39.7 ± 0.8 | 0.832 | 36.9 ± 1.0 | 41.8 ± 0.9 | <0.001 | 39.5 ± 0.7 | 39.9 ± 1.3 | 0.779 |
| Fiber (g) | 16.4 ± 0.6 | 15.2 ± 0.5 | 0.143 | 11.9 ± 0.5 | 19.3 ± 0.5 | <0.001 | 15.6 ± 0.5 | 16 ± 0.8 | 0.664 |
| Vitamin A (μg) | 1617.2 ± 109.9 | 1398.2 ± 86.5 | 0.119 | 1027.0 ± 96.7 | 1923.1 ± 95.0 | <0.001 | 1531.0 ± 79.5 | 1340.1 ± 135.5 | 0.226 |
| Vitamin C (mg) | 138.0 ± 11.0 | 145.4 ± 8.7 | 0.595 | 97.7 ± 9.7 | 186.1 ± 9.5 | <0.001 | 146.6 ± 8.0 | 132.3 ± 13.6 | 0.366 |
| Vitamin E (mg) | 8.9 ± 0.4 | 8.0 ± 0.3 | 0.076 | 7.1 ± 0.4 | 9.5 ± 0.4 | <0.001 | 8.3 ± 0.3 | 8.4 ± 0.5 | 0.946 |
| Copper (μg) | 1109.0 ± 55.7 | 979.9 ± 43.9 | 0.070 | 797.9 ± 49.1 | 1253.6 ± 48.2 | <0.001 | 1055.4 ± 40.4 | 964.0 ± 68.9 | 0.254 |
| Selenium (μg) | 74.7 ± 3.3 | 73.7 ± 2.6 | 0.819 | 63.9 ± 3 | 84.0 ± 3.0 | <0.001 | 74.0 ± 2.4 | 75.3 ± 4.1 | 0.789 |
| Zinc (mg) | 13.2 ± 7.6 | 23.1 ± 6.0 | 0.310 | 9.1 ± 7.1 | 29.2 ± 7.1 | 0.059 | 16.6 ± 5.5 | 27.5 ± 9.4 | 0.317 |
| Omega-3 (g) | 0.38 ± 0.07 | 0.45 ± 0.06 | 0.481 | 0.21 ± 0.07 | 0.63 ± 0.07 | <0.001 | 0.45 ± 0.05 | 0.34 ± 0.09 | 0.313 |
| CDAI (AU) | 0.08 ± 4.0 | −0.07 ± 4.4 | 0.778 | −3.26 ± 1.83 | 3.16 ± 3.49 | <0.001 | 0.15 ± 4.34 | −0.39 ± 4.05 | 0.370 |
| Insulin | β (95% CI) | p | R2 |
|---|---|---|---|
| Sex | −3.235 (−5.678; −0.793) | 0.010 | 0.014 |
| BMI (kg/m2) | 0.428 (0.223; 0.633) | <0.001 | 0.101 |
| CDAI (AU) | −0.628 (−0.976; −0.281) | <0.001 | 0.020 |
| TNF-α -308 G/A genotypes | 2.347 (0.154; 4.539) | 0.036 | 0.012 |
| Total R2 | 0.152 | ||
| HOMA-IR | β (95% CI) | p | R2 |
| Sex | −0.955 (−1.683; −0.226) | 0.010 | 0.024 |
| BMI (kg/m2) | 0.108 (0.046; 0.170) | 0.001 | 0.105 |
| CDAI (AU) | −0.169 (−0.262; −0.075) | <0.001 | 0.015 |
| TNF-α -308 G/A genotypes | 0.806 (0.144; 1.468) | 0.017 | 0.009 |
| Total R2 | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Ruelas, E.; Vizmanos, B.; López Gómez, J.J.; Rico, D.; Martínez, J.A.; Luis, D.A.D. Mutual Impact of Dietary Antioxidants and TNF-α rs1800629 on Insulin Levels in Adults with Obesity. Nutrients 2025, 17, 2345. https://doi.org/10.3390/nu17142345
Sierra-Ruelas E, Vizmanos B, López Gómez JJ, Rico D, Martínez JA, Luis DAD. Mutual Impact of Dietary Antioxidants and TNF-α rs1800629 on Insulin Levels in Adults with Obesity. Nutrients. 2025; 17(14):2345. https://doi.org/10.3390/nu17142345
Chicago/Turabian StyleSierra-Ruelas, Erika, Barbara Vizmanos, Juan José López Gómez, Daniel Rico, J. Alfredo Martínez, and Daniel A. De Luis. 2025. "Mutual Impact of Dietary Antioxidants and TNF-α rs1800629 on Insulin Levels in Adults with Obesity" Nutrients 17, no. 14: 2345. https://doi.org/10.3390/nu17142345
APA StyleSierra-Ruelas, E., Vizmanos, B., López Gómez, J. J., Rico, D., Martínez, J. A., & Luis, D. A. D. (2025). Mutual Impact of Dietary Antioxidants and TNF-α rs1800629 on Insulin Levels in Adults with Obesity. Nutrients, 17(14), 2345. https://doi.org/10.3390/nu17142345











