Vitamin D’s Impact on Cancer Incidence and Mortality: A Systematic Review
Abstract
1. Introduction
1.1. Systemic Challenges and Clinical Trial Designs Using Vitamin D
1.2. The Importance of Adjusting for Confounders in Clinical Research
1.3. Vitamin D—Cancer Risk Reduction vs. Mortality
1.4. Systematic Review Process
1.4.1. Literature Search
1.4.2. Rationale for the Study
1.4.3. Objective of the Study
1.4.4. Search Strategy
1.4.5. Protocol and Manuscript Selection
1.4.6. Data Abstraction and Synthesis
1.4.7. Literature Search and Analytical Outcomes
1.4.8. Scope of This Review and Outcomes
2. Vitamin D Requirements—Sun Exposure, Biological Functions, and Cancer
- I.
- Not obese (average wt.: BMI, <29): 70–90 IU/kg BW
- II.
- Moderately obese (BMI, 30–39): 100–130 IU/kg BW
- III.
- Morbid obesity (BMI, over 40): 140–180 IU/kg BW
2.1. Sun Exposure and Generation of Vitamin D
2.2. Causal Role of Vitamin D Deficiency in the Development of Select Cancers
2.3. Vitamin D Plus Calcium—Effect on Cancer
2.4. Vitamin D 1,25(OH)2D Interactions and Cell Proliferation
2.5. Effects of Vitamin D on Cell Proliferation and Metastasis
2.6. Vitamin D Sufficiency–Protective Against Cancer
2.7. Effectiveness of Vitamin D in Different Cancer Types
2.8. Ultraviolet B, Vitamin D, and Prevalence of Cancer
2.9. Sun Exposure, Genetics, and Skin Cancer
2.10. Sun Exposure Reduces Cancer Risks
2.11. Additional Mechanisms of Vitamin D in Cancer Risk Reduction
3. Cancer Mortality Relationships
3.1. Major Challenges Associated with Nutrient Clinical Trials
3.2. The Factors Hindering Large Vitamin D RCTs from Generating Meaningful Data
3.3. Negative RCTs Do Not Mean That the Nutrient Is Not Efficacious
3.4. Rethinking Research Methods: Limitations of RCTs in Micronutrient Evaluation
4. Broader Outcomes from Vitamin D Clinical Studies
4.1. Effects of Vitamin D on Preventing Specific Cancer Types
4.2. Miscellaneous Cancers
4.3. Epidemiological and Meta-Analysis Data
4.4. Correlations of Serum 25(OH)D Levels with Cancer Incidence
4.5. Melanoma and Insulin-like Growth Factor
4.6. Prostate Cancer Risks and a J or U-Shape Curve
4.7. Prevention of Cancer Risk Reduction
4.8. Clinical Trials on Cancer Prevention
5. Improving Clinical Outcomes
5.1. Varying 25(OH)D Levels Required for Preventing Different Diseases
5.2. The Role of Vitamin D-Binding—Protein in Cancer
5.3. Adverse Effects of Vitamin
6. Discussion
7. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxy vitamin D |
| BMI | Body mass index |
| CKD | Chronic kidney disease |
| CVD | Cardiovascular disease |
| IU | International unit |
| kg BW | Kilogram, body weight |
| RCTs | Randomized controlled clinical trials |
| T2D | Type 2 diabetes mellitus |
| UV | Ultraviolet |
| VDR | Vitamin D receptor |
References
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Brennan-Speranza, T.C. Vitamin D and skeletal muscle: Current concepts from preclinical studies. JBMR Plus 2021, 5, e10575. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L.; Drezner, M.K.; Binkley, N.C. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J. Steroid Biochem. Mol. Biol. 2007, 103, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the brain: Genomic and non-genomic actions. Mol. Cell Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 92–102. [Google Scholar] [CrossRef]
- Morris, H.A.; Anderson, P.H. Autocrine and paracrine actions of vitamin D. Clin. Biochem. Rev. 2010, 31, 129–138. [Google Scholar] [PubMed]
- Zmijewski, M.A. Nongenomic activities of vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.C.; Davis, C.T.; Zhu, W.; Bowman-Kirigin, J.A.; Walker, A.E.; Tai, Z.; Thomas, K.R.; Donato, A.J.; Lesniewski, L.A.; Li, D.Y. Dietary Vitamin D and Its Metabolites Non-Genomically Stabilize the Endothelium. PLoS ONE 2015, 10, e0140370. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.J.; Gonzalez-Sancho, J.M.; Bonilla, F.; Munoz, A. Interaction of vitamin D with membrane-based signaling pathways. Front. Physiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the immune system: Genomic and non-genomic actions. Mini Rev. Med. Chem. 2015, 15, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.; Nemere, I. Membrane receptors for vitamin D metabolites. Crit. Rev. Eukaryot. Gene Expr. 2007, 17, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Wimalawansa, S.J.; Carlberg, C. Highlights from the 5th International Conference on Vitamin D Deficiency, Nutrition and Human Health, Abu Dhabi, United Arab Emirates, March 24–25, 2016. J. Steroid Biochem. Mol. Biol. 2018, 175, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Khare, S.; Sizar, O.; Givler, A. Vitamin Deficiency; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wimalawansa, S.J.; Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, Mitigation, Opportunities and Building Resilience “From Adversity to Serendipity,” Perspectives of Global Relevance Based on Research, Experience, and Successes in Combating COVID-19 in Sri Lanka, Colombo, Sri Lanka, 27 January 2021; pp. 171–198. [Google Scholar]
- Wimalawansa, S.J. Effective and practical ways to overcome vitamin deficiency. Fam. Med. Community Health 2020, 8, 1–8. [Google Scholar]
- Al-Daghri, N.M.; Al-Saleh, Y.; Khan, N.; Sabico, S.; Aljohani, N.; Alfawaz, H.; Alsulaimani, M.; Al-Othman, A.M.; Alokail, M.S. Sun exposure, skin color and vitamin D status in Arab children and adults. J. Steroid Biochem. Mol. Biol. 2016, 164, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Bittar, F.B.; Castro, C.H.M.; Szejnfeld, V.L. Screening for vitamin D deficiency in a tropical area: Results of a sun exposure questionnaire. BMC Endocr. Disord. 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr. Osteoporos. Rep. 2012, 10, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Wimalawansa, S.J.; Holick, M.F. Vitamin D supplements and reasonable solar UVB should be recommended to prevent escalating incidence of chronic diseases. Br. Med. J. 2015, 350, h321. [Google Scholar] [CrossRef]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Raymond-Lezman, J.R.; Riskin, S.I. Benefits and risks of sun exposure to maintain adequate Vitamin D levels. Cureus 2023, 15, e38578. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Sun exposure, vitamin D and cancer risk reduction. Eur. J. Cancer 2013, 49, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Grasso, A.A.; Blanco, S.; Fantini, G.; Torelli, F.; Grasso, M. Relationship between sun exposure and kidney cancer: Preliminary experience with the evaluation of recreational UV exposure. Urologia 2014, 81, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Infections and autoimmunity-The immune system and vitamin D: A systematic review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Controlling chronic diseases and acute Infections with vitamin D sufficiency. Nutrients 2023, 15, 3623. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H.B.; Boucher, B.J.; Cannell, J.J.; Foresta, C.; Grubler, M.R.; Kotsa, K.; et al. Vitamin D and chronic diseases: The current state of the art. Arch. Toxicol. 2017, 91, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Haussler, C.A.; Bartik, L.; Whitfield, G.K.; Hsieh, J.C.; Slater, S.; Jurutka, P.W. Vitamin D receptor: Molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 2008, 66, S98–S112. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.R.; Davies, M.; Hayes, M.E.; Hickey, C.D.; Lumb, G.A.; Mawer, E.B.; Adams, P.H. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin. Endocrinol. 1992, 37, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.C.; Cormier, C.; Kindermans, C.; Gao, P.; Cantor, T.; Forette, F.; Baulieu, E.E. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J. Clin. Endocrinol. Metab. 2001, 86, 3086–3090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of vitamin D and calcium supplementation on cancer incidence in older women: A randomized clinical trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-hydroxyvitamin D concentrations >/=40 ng/ml are associated with >65% lower cancer risk: Pooled analysis of randomized trial and prospective cohort study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations >/=60 vs <20 ng/ml (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Enhancing the Design of Nutrient Clinical Trials for Disease Prevention-A Focus on Vitamin D: A Systematic Review. Nutr. Rev. 2025. [CrossRef] [PubMed]
- Wimalawansa, S.J.; Weiss, S.T.; Hollis, B.W. Integrating Endocrine, Genomic, and Extra-Skeletal Benefits of Vitamin D into National and Regional Clinical Guidelines. Nutrients 2024, 16, 3969. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2018, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; Macfadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical appraisal of large vitamin D randomized controlled trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J. How follow-up period in prospective cohort studies affects relationship between baseline serum 25(OH)D concentration and risk of stroke and major cardiovascular events. Nutrients 2024, 16, 3759. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R. Vitamin D: Evidence-based health benefits and recommendations for population guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Ebeling, P.R.; McLeod, D.S.A.; English, D.; Romero, B.D.; Baxter, C.; Armstrong, B.K.; Hartel, G.; Kimlin, M.; O’Connell, R.L.; et al. The effect of monthly vitamin D supplementation on fractures: A tertiary outcome from the population-based, double-blind, randomised, placebo-controlled D-Health trial. Lancet Diabetes Endocrinol. 2023, 11, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Armstrong, B.K.; Baxter, C.; Duarte Romero, B.; Ebeling, P.; English, D.R.; Kimlin, M.G.; McLeod, D.S.; RL, O.C.; van der Pols, J.C.; et al. The D-Health Trial: A randomized trial of vitamin D for prevention of mortality and cancer. Contemp. Clin. Trials 2016, 48, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D deficiency meets Hill’s criteria for causation in SARS-CoV-2 susceptibility, complications, and mortality: A systematic review. Nutrients 2025, 17, 599. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections-Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL. Pathol. Lab. Med. Int. 2022, 14, 37–60. [Google Scholar] [CrossRef]
- Mo, X.; He, C.; Han, F.; Yan, H.; Chen, X.; Wang, Y.; Zhou, M. Association of serum 25-hydroxy-vitamin D concentration and risk of mortality in cancer survivors in the United States. BMC Cancer 2024, 24, 545. [Google Scholar] [CrossRef] [PubMed]
- Mot, C.I.; Horhat, D.I.; Balica, N.C.; Hirtie, B.; Varga, N.I.; Prodan-Barbulescu, C.; Alexandru, A.; Ciurariu, E.; Galis, R. Vitamin D and clinical outcomes in head and neck cancer: A systematic review. Nutrients 2025, 17, 1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dou, B.; Sun, X.; Chen, X. Interaction effects between serum 25(OH)D and CRP status on cancer related mortality in adult cancer survivors. Sci. Rep. 2025, 15, 14798. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Kuznia, S.; Brenner, H. Efficacy of vitamin D(3) supplementation on cancer mortality in the general population and the prognosis of patients with cancer: Protocol of a systematic review and individual patient data meta-analysis of randomised controlled trials. BMJ Open 2021, 11, e041607. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Zhao, Y.; Yang, P.; Zhang, X.; Giovannucci, E.L. Vitamin D and human health: Evidence from Mendelian randomization studies. Eur. J. Epidemiol. 2024, 39, 467–490. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Timofeeva, M.; Farrington, S.M.; Li, X.; Xu, W.; Campbell, H.; Houlston, R.S.; Tomlinson, I.P.; Theodoratou, E.; et al. Bidirectional Mendelian randomisation analysis of the relationship between circulating vitamin D concentration and colorectal cancer risk. Int. J. Cancer 2022, 150, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Emmanouilidou, G.; Kalopitas, G.; Bakaloudi, D.R.; Karanika, E.; Theocharidou, E.; Germanidis, G.; Chourdakis, M. Vitamin D as a chemopreventive agent in colorectal neoplasms. A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Ther. 2022, 237, 108252. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Welch, V.; Petticrew, M.; Tugwell, P.; Moher, D.; O’Neill, J.; Waters, E.; White, H.; the PRISMA-Equity Bellagio Group. PRISMA-Equity 2012 extension: Reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012, 9, e1001333. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Verma, A.; Bivens, C.B.; Schwartz, Z.; Boyan, B.D. Rapid steroid hormone actions via membrane receptors. Biochim. Biophys. Acta 2016, 1863, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Moukayed, M.; Grant, W.B. Molecular link between vitamin D and cancer prevention. Nutrients 2013, 5, 3993–4021. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Felty, Q.; Narayan, S.; Jayakar, P. Signature of mitochondria of steroidal hormones-dependent normal and cancer cells: Potential molecular targets for cancer therapy. Front. Biosci. 2007, 12, 154–173. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Henn, M.; Martin-Gorgojo, V.; Martin-Moreno, J.M. Vitamin D in cancer prevention: Gaps in current nowledge and room for Hope. Nutrients 2022, 14, 4512. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef] [PubMed]
- Ucar, N.; Holick, M.F. Illuminating the connection: Cutaneous vitamin D(3) synthesis and Its role in skin cancer prevention. Nutrients 2025, 17, 386. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gorgojo, A.; Martin-Moreno, J.M. Insights into the role of vitamin D in the prevention and control of cancer and other chronic noncommunicable diseases: Shedding further light on a captivating subject. Nutrients 2024, 16, 2166. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Nurnberg, B. Solar UV-radiation, vitamin D and skin cancer surveillance in organ transplant recipients (OTRs). Adv. Exp. Med. Biol. 2008, 624, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, W.; Ma, J.; Liu, J.; Sha, H.; Zhou, S.; Wang, F.; Ma, Q. Vitamin D—Pivotal nutraceutical in the regulation of cancer metastasis and angiogenesis. Curr. Med. Chem. 2013, 20, 4109–4120. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.L.; Zhou, H.; Kalak, R.; Zheng, Y.; Conigrave, A.D.; Seibel, M.J.; Dunstan, C.R. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010, 70, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Vitamin D may reduce prostate cancer metastasis by several mechanisms including blocking Stat3. Am. J. Pathol. 2008, 173, 1589–1590. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Fakhoury, H.M.A.; Moukayed, M.; Pilz, S.; Al-Daghri, N.M. Evidence That Increasing Serum 25(OH)D Concentrations to 30 ng/mL in the Kingdom of Saudi Arabia and the United Arab Emirates Could Greatly Improve Health Outcomes. Biomedicines 2023, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.M.; Looker, A.C.; Chang, S.C.; Graubard, B.I. Prospective study of serum vitamin D and cancer mortality in the United States. J. Natl. Cancer Inst. 2007, 99, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; English, D.R.; Armstrong, B.K.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; O’Connell, R.; et al. The D-Health Trial: A randomised controlled trial of the effect of vitamin D on mortality. lancet. Diabetes Endocrinol. 2022, 10, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; LaCroix, A.Z.; Wu, L.; Horwitz, M.; Danielson, M.E.; Bauer, D.C.; Lee, J.S.; Jackson, R.D.; Robbins, J.A.; Wu, C.; et al. Serum 25 hydroxyvitamin D concentrations and the risk of hip Fractures: The women’s health initiative. Ann. Intern. Med. 2008, 149, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Grant, W.B. Vitamin D and cancer: An historical overview of the epidemiology and mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: Case-control versus nested case-control studies. Anticancer. Res. 2015, 35, 1153–1160. [Google Scholar] [PubMed]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, M.J.; Alonso, E.N.; Gandini, N.A.; Fermento, M.E.; Villegas, M.E.; Quevedo, M.A.; Arevalo, J.; Lopez Romero, A.; Rivadulla, M.L.; Gomez, G.; et al. The UVB1 Vitamin D analogue inhibits colorectal carcinoma progression. J. Steroid Biochem. Mol. Biol. 2016, 163, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guo, X.; Yu, X.; Liu, S.; Cui, X.; Zhang, B.; Liang, H. 25-hydroxyvitamin D and Total cancer incidence and mortality: A meta-Analysis of prospective cohort studies. Nutrients 2019, 11, 2295. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, J.D.; Manson, J.E.; Scragg, R. Vitamin D and clinical cancer outcomes: A review of meta-analyses. JBMR Plus 2021, 5, e10420. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ordonez-Mena, J.M.; Chen, T.; Schottker, B.; Arndt, V.; Brenner, H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: A systematic review and meta-analysis. Prev. Med. 2013, 57, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Chen, Y.H.; Liang, F.W.; Wu, Y.C.; Wang, J.J.; Lim, S.W.; Ho, C.H. Determinants of cancer incidence and mortality among people with vitamin D deficiency: An epidemiology study using a real-world population database. Front. Nutr. 2023, 10, 1294066. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Magno, S.; Romano, F.; Barrea, L.; Colao, A.M.; Muscogiuri, G.; Savastano, S. Vitamin D deficiency: A potential risk factor for cancer in obesity? Int. J. Obes. 2022, 46, 707–717. [Google Scholar] [CrossRef] [PubMed]
- McGrowder, D.; Tulloch-Reid, M.K.; Coard, K.C.M.; McCaw-Binns, A.M.; Ferguson, T.S.; Aiken, W.; Harrison, L.; Anderson, S.G.; Jackson, M.D. Vitamin D deficiency at diagnosis increases all-cause and prostate cancer-specific mortality in Jamaican men. Cancer Control 2022, 29, 10732748221131225. [Google Scholar] [CrossRef] [PubMed]
- Kittivisuit, S.; Sripornsawan, P.; Songthawee, N.; Chavananon, S.; Yam-Ubon, U.; McNeil, E.B.; Jaruratanasirikul, S.; Chotsampancharoen, T. Vitamin D deficiency in childhood cancer survivors: Results from Southern Thailand. Nutrients 2023, 15, 1328. [Google Scholar] [CrossRef] [PubMed]
- van der Rhee, H.; Coebergh, J.W.; de Vries, E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur. J. Cancer 2013, 49, 1422–1436. [Google Scholar] [CrossRef] [PubMed]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Cancela, L.; Nemere, I.; Norman, A.W. 1 alpha,25(OH)2 vitamin D3: A steroid hormone capable of producing pleiotropic receptor-mediated biological responses by both genomic and nongenomic mechanisms. J. Steroid Biochem. 1988, 30, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: Everything You Need to Know; Karunaratne & Sons: Homagama, Sri Lanka, 2012; ISBN 978-955-9098-94-2. [Google Scholar]
- Oliveria, S.A.; Saraiya, M.; Geller, A.C.; Heneghan, M.K.; Jorgensen, C. Sun exposure and risk of melanoma. Arch. Dis. Child. 2006, 91, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Shuk, E.; Burkhalter, J.E.; Baguer, C.F.; Holland, S.M.; Pinkhasik, A.; Brady, M.S.; Coit, D.G.; Ariyan, C.E.; Hay, J.L. Factors associated with inconsistent sun protection in first-degree relatives of melanoma survivors. Qual. Health Res. 2012, 22, 934–945. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F.; et al. Maternal 25(OH)D concentrations >/=40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of vitamin D beyond the skeletal function: A review of the molecular and clinical studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Klein, P.; Grossbard, M.L. Vitamin D and breast cancer. Oncologist 2012, 17, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating vitamin D and colorectal cancer risk: An international pooling Project of 17 cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.D. Vitamin D and calcium in the prevention of prostate and colon cancer: New approaches for the identification of needs. J. Nutr. 2005, 135, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Ma, C.; Yuan, C.; Shi, Q.; Wolpin, B.M.; Zhang, Y.; Fuchs, C.S.; Meyer, J.; Zemla, T.; Cheng, E.; et al. Plasma 25-hydroxyvitamin D levels and survival in stage III colon cancer: Findings from CALGB/SWOG 80702 (Alliance). Clin. Cancer Res. 2023, 29, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Vaghari-Tabari, M.; Mohammadzadeh, I.; Qujeq, D.; Majidinia, M.; Alemi, F.; Younesi, S.; Mahmoodpoor, A.; Maleki, M.; Yousefi, B.; Asemi, Z. Vitamin D in respiratory viral infections: A key immune modulator? Crit. Rev. Food Sci. Nutr. 2023, 63, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Infante, M.; Ricordi, C. Editorial—Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.I.; Xiong, Y. Influence of vitamin D on cancer risk and treatment: Why the variability? Trends Cancer Res. 2018, 13, 43–53. [Google Scholar] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Holick, M.F. Why the IOM recommendations for vitamin D are deficient. J. Bone Miner. Res. 2011, 26, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, P.J.; Ekwaru, J.P. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients 2014, 6, 4472–4475. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? An examination using Hill’s criteria for causality. Dermatoendocrinol 2009, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Stoitzfus, J.; Swan, B.A. Optimizing vitamin D status to reduce colorectal cancer risk: An evidentiary review. Clin. J. Oncol. Nurs. 2009, 13, E3–E17. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 2006, 35, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Garland, C.; Gorham, E. Vitamin D Supplementation and Cancer Risk. JAMA 2017, 318, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.V.; Lyles, B.; Lin, M.F.; Weigel, N.L. Vitamin D receptor agonists induce prostatic acid phosphatase to reduce cell growth and HER-2 signaling in LNCaP-derived human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2005, 97, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Kimball, S.M. The association between serum 25(OH)D status and blood pressure in participants of a community-based program taking vitamin D supplements. Nutrients 2017, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Dominguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.; Amend, J. Results of daily oral dosing with up to 60,000 international units (iu) of vitamin D3 for 2 to 6 years in 3 adult males. J. Steroid Biochem. Mol. Biol. 2017, 173, 308–312. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, T.; Gredner, T.; Kuznia, S.; Schottker, B.; Mons, U.; Brenner, H. Vitamin D supplementation to the older adult population in Germany has the cost-saving potential of preventing almost 30000 cancer deaths per year. Mol. Oncol. 2021, 15, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014, 149, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.L.; Friedberg, J.W.; Calvi, L.M.; van Wijngaarden, E.; Fisher, S.G. A case-control study of ultraviolet radiation exposure, vitamin D, and lymphoma risk in adults. Cancer Causes Control 2010, 21, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Re: Prospective study of ultraviolet radiation exposure and risk of breast cancer in the United States. Environ. Res. 2017, 152, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Zamoiski, R.D.; Freedman, D.M.; Linet, M.S.; Kitahara, C.M.; Liu, W.; Cahoon, E.K. Prospective study of ultraviolet radiation exposure and risk of breast cancer in the United States. Environ. Res. 2016, 151, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J. Are Hill’s criteria for causality satisfied for vitamin D and periodontal disease? Dermatoendocrinol 2010, 2, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, H.E.; Banwell, B. Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim. Biophys. Acta 2011, 1812, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Bergmann, P.; Boonen, S.; Devogelaer, J.P.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Rozenberg, S.; Reginster, J.Y. Extraskeletal benefits and risks of calcium, vitamin D and anti-osteoporosis medications. Osteoporos. Int. 2012, 23 (Suppl. S1), S1–S23. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Bertoldo, F.; Bischoff-Ferrari, H.A.; Bruyere, O.; Cooper, C.; Cutolo, M.; Kanis, J.A.; Kaufman, J.M.; Reginster, J.Y.; Rizzoli, R.; et al. Vitamin D supplementation in the prevention and management of major chronic diseases not related to mineral homeostasis in adults: Research for evidence and a scientific statement from the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Endocrine 2017, 56, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Wimalawansa, S.J.; Holick, M.F.; Cannell, J.J.; Pludowski, P.; Lappe, J.M.; Pittaway, M.; May, P. Emphasizing the health benefits of vitamin D for those with neurodevelopmental disorders and intellectual disabilities. Nutrients 2015, 7, 1538–1564. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Cross, H.S.; Garland, C.F.; Gorham, E.D.; Moan, J.; Peterlik, M.; Porojnicu, A.C.; Reichrath, J.; Zittermann, A. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog. Biophys. Mol. Biol. 2009, 99, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: Everything You Need to Know… and More. Available online: https://www.grassrootshealth.net/blog/vitamin-d-everything-need-know/?_ga=2.249464128.698298248.1700688605-1993470031.1700688605 (accessed on 15 November 2024).
- Grant, W.B.; Boucher, B.J.; Pludowski, P.; Wimalawansa, S.J. The emerging evidence for non-skeletal health benefits of vitamin D supplementation in adults. Nat. Rev. Endocrinol. 2022, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Chlebowski, R.T.; Wactawski-Wende, J.; Robbins, J.A.; Rodabough, R.J.; Chen, Z.; Johnson, K.C.; O’Sullivan, M.J.; Jackson, R.D.; Manson, J.E. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: The Women’s Health Initiative. J. Womens Health (Larchmt) 2013, 22, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Brunner, R.L.; Wactawski-Wende, J.; Caan, B.J.; Cochrane, B.B.; Chlebowski, R.T.; Gass, M.L.; Jacobs, E.T.; LaCroix, A.Z.; Lane, D.; Larson, J.; et al. The effect of calcium plus vitamin D on risk for invasive cancer: Results of the Women’s Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr. Cancer 2011, 63, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Na, S.Y.; Kim, K.B.; Lim, Y.J.; Song, H.J. Vitamin D and colorectal cancer: Current perspectives and future sirections. J. Cancer Prev. 2022, 27, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Huncharek, M.; Muscat, J.; Kupelnick, B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: A meta-analysis of 26,335 cases from 60 observational studies. Nutr. Cancer 2009, 61, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. Calcium and vitamin D supplements and health outcomes: A reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am. J. Clin. Nutr. 2011, 94, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Goulao, B.; Stewart, F.; Ford, J.A.; MacLennan, G.; Avenell, A. Cancer and vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Balk, E.M.; Brendel, M.; Ip, S.; Lau, J.; Lee, J.; Lichtenstein, A.; Patel, K.; Raman, G.; Tatsioni, A.; et al. Vitamin D and calcium: A systematic review of health outcomes. Evid. Rep. Technol. Assess. (Full Rep.) 2009, 183, 1–420. [Google Scholar]
- Cruz-Pierard, S.M.; Nestares, T.; Amaro-Gahete, F.J. Vitamin D and calcium as key potential factors related to colorectal cancer prevention and treatment: A systematic peview. Nutrients 2022, 14, 4934. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Ricordi, C.; Baidal, D.A.; Alejandro, R.; Lanzoni, G.; Sears, B.; Caprio, M.; Fabbri, A. VITAL study: An incomplete picture? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- Al Nozha, O.M. Vitamin D and extra-skeletal health: Causality or consequence. Int. J. Health Sci. 2016, 10, 443–452. [Google Scholar] [CrossRef]
- Paulsen, E.M.; Rylander, C.; Brustad, M.; Jensen, T.E. Pre-diagnostic intake of vitamin D and incidence of colorectal cancer by anatomical subsites: The Norwegian Women and Cancer Cohort Study (NOWAC). Br. J. Nutr. 2023, 130, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Caleya, J.F.; Ortega-Valin, L.; Fernandez-Villa, T.; Delgado-Rodriguez, M.; Martin-Sanchez, V.; Molina, A.J. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: Systematic review and meta-analysis of case-control studies. Cancer Causes Control 2022, 33, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, X.; Zanello, L.P. 1alpha,25-Dihydroxyvitamin D(3) antiproliferative actions involve vitamin D receptor-mediated activation of MAPK pathways and AP-1/p21(waf1) upregulation in human osteosarcoma. Cancer Lett. 2007, 254, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bikle, D.D. Inhibition of 1,25-dihydroxyvitamin-D-induced keratinocyte differentiation by blocking the expression of phospholipase C-gamma1. J. Investig. Dermatol. 2001, 117, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, J.; Uskokovic, M.; Lemp, N.; Vadgama, J.; Koeffler, H.P. Novel Gemini-vitamin D3 analog inhibits tumor cell growth and modulates the Akt/mTOR signaling pathway. J. Steroid Biochem. Mol. Biol. 2006, 100, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Tuckey, R.C.; Nguyen, M.N.; Thorpe, E.M., Jr.; Slominski, A.T. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J. Cell Physiol. 2010, 223, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Roles of Solar UVB and Vitamin D in Reducing Cancer Risk and Increasing Survival. Anticancer. Res. 2016, 36, 1357–1370. [Google Scholar] [PubMed]
- Steele, C.B.; Thomas, C.C.; Henley, S.J.; Massetti, G.M.; Galuska, D.A.; Agurs-Collins, T.; Puckett, M.; Richardson, L.C. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity—United States, 2005-2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njolstad, I.; Lochen, M.L.; Marz, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Serrano, D.; Pilz, S.; Gandini, S. Vitamin D supplementation and cancer: Review of randomized controlled trials. Anti-Cancer Agents Med. Chem. 2013, 13, 118–125. [Google Scholar] [CrossRef]
- Pilz, S. Editorial: Vitamin d and cancer: Current evidence and future perspective. Anticancer. Agents Med. Chem. 2013, 13, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomas, N.; Babio, N.; Salas-Salvado, J.; Macias-Gonzalez, M. Vitamin D intake and the risk of colorectal cancer: An updated meta-analysis and systematic review of case-control and prospective cohort studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Shahril, M.R.; Shahar, S.; Fenech, M.; Sharif, R. Association between diet-related behaviour and risk of colorectal cancer: A scoping review. J. Cancer Prev. 2022, 27, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Trump, D.L.; Johnson, C.S.; Feldman, D. The role of vitamin D in cancer prevention and treatment. Rheum. Dis. Clin. North. Am. 2012, 38, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Vanoirbeek, E.; Krishnan, A.; Eelen, G.; Verlinden, L.; Bouillon, R.; Feldman, D.; Verstuyf, A. The anti-cancer and anti-inflammatory actions of 1,25(OH)2D3. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.W.; Ping, J.D.; Wang, Y.F.; Liu, X.N.; Li, N.; Hu, Z.L.; Ming, L. Vitamin D suppress the production of vascular endothelial growth factor in mast cell by inhibiting PI3K/Akt/p38 MAPK/HIF-1alpha pathway in chronic spontaneous urticaria. Clin. Immunol. 2020, 215, 108444. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Seifer, D.B.; Grazi, R.V.; Irani, S.; Rosenwaks, Z.; Tal, R. Vitamin D secreases serum VEGF correlating with clinical Improvement in vitamin D-seficient women with PCOS: A randomized placebo-controlled trial. Nutrients 2017, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin D in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Spath, L.; Ulivieri, A.; Lavra, L.; Fidanza, L.; Carlesimo, M.; Giubettini, M.; Narcisi, A.; Luciani, E.; Bucci, B.; Pisani, D.; et al. Antiproliferative effects of 1alpha-OH-vitD(3) in malignant melanoma: Potential therapeutic implications. Sci. Rep. 2017, 7, 40370. [Google Scholar] [CrossRef] [PubMed]
- Anshida, V.P.; Kumari, R.A.; Murthy, C.S.; Samuel, A. Extracellular matrix degradation by host matrix metalloproteinases in restorative dentistry and endodontics: An overview. J. Oral. Maxillofac. Pathol. 2020, 24, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Manousakis, E.; Miralles, C.M.; Esquerda, M.G.; Wright, R.H.G. CDKN1A/p21 in breast cancer: Part of the problem, or part of the solution? Int. J. Mol. Sci. 2023, 24, 7488. [Google Scholar] [CrossRef] [PubMed]
- Cheema, H.A.; Fatima, M.; Shahid, A.; Bouaddi, O.; Elgenidy, A.; Rehman, A.U.; Oussama Kacimi, S.E.; Hasan, M.M.; Lee, K.Y. Vitamin D supplementation for the prevention of total cancer incidence and mortality: An updated systematic review and meta-analysis. Heliyon 2022, 8, e11290. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.A.; Evans, C.V.; Ivlev, I.; Rushkin, M.C.; Thomas, R.G.; Martin, A.; Lin, J.S. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA 2022, 327, 2334–2347. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Keil, A.P.; Harmon, Q.E.; Jackson, C.L.; White, A.J.; Diaz-Santana, M.V.; Taylor, J.A.; Sandler, D.P. Vitamin D supplement use and risk of breast cancer by race-ethnicity. Epidemiology 2022, 33, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Albanes, D.; Mondul, A.M.; Yu, K.; Parisi, D.; Horst, R.L.; Virtamo, J.; Weinstein, S.J. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am. J. Public Health 2014, 104, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Porojnicu, A.C.; Dahlback, A.; Moan, J. Sun exposure and cancer survival in Norway: Changes in the risk of death with season of diagnosis and latitude. Adv. Exp. Med. Biol. 2008, 624, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Porojnicu, A.C.; Lagunova, Z.; Robsahm, T.E.; Berg, J.P.; Dahlback, A.; Moan, J. Changes in risk of death from breast cancer with season and latitude: Sun exposure and breast cancer survival in Norway. Breast Cancer Res. Treat. 2007, 102, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gaksch, M.; Hartaigh, B.O.; Tomaschitz, A.; Marz, W. Vitamin D in preventive medicine. Anticancer. Res. 2015, 35, 1161–1170. [Google Scholar] [PubMed]
- Garland, C.F.; Garland, F.C.; Gorham, E.D. Calcium and vitamin D. Their potential roles in colon and breast cancer prevention. Ann. N. Y. Acad. Sci. 1999, 889, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Health Quality, O. Clinical utility of vitamin d testing: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2010, 10, 1–93. [Google Scholar]
- Lagunova, Z.; Porojnicu, A.C.; Dahlback, A.; Berg, J.P.; Beer, T.M.; Moan, J. Prostate cancer survival is dependent on season of diagnosis. Prostate 2007, 67, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Garland, C.F. The association of solar ultraviolet B (UVB) with reducing risk of cancer: Multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer. Res. 2006, 26, 2687–2699. [Google Scholar] [PubMed]
- Waltz, P.; Chodick, G. Assessment of ecological regression in the study of colon, breast, ovary, non-Hodgkin’s lymphoma, or prostate cancer and residential UV. Eur. J. Cancer Prev. 2008, 17, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Moukayed, M.; Grant, W.B. The roles of UVB and vitamin D in reducing risk of cancer incidence and mortality: A review of the epidemiology, clinical trials, and mechanisms. Rev. Endocr. Metab. Disord. 2017, 18, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wu, S. The Mechanisms of Carnosol in Chemoprevention of Ultraviolet B-Light-Induced Non-Melanoma Skin Cancer Formation. Sci. Rep. 2018, 8, 3574. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrer-Mayorga, G.; Larriba, M.J.; Crespo, P.; Munoz, A. Mechanisms of action of vitamin D in colon cancer. J. Steroid Biochem. Mol. Biol. 2018, 185, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Larriba, M.J.; Munoz, A. Vitamin D and colon cancer. Endocr. Relat. Cancer 2012, 19, R51–R71. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, L.; Zhang, L.; Hu, W.; Shen, J.; Xiao, Z.; Wu, X.; Chan, F.L.; Cho, C.H. 1,25-Dihydroxyvitamin D3 suppresses gastric cancer cell growth through VDR- and mutant p53-mediated induction of p21. Life Sci. 2017, 179, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, R.W.; Moseley, V.R.; Sun, S.; Wargovich, M.J. Vitamin D receptor and retinoid X receptor alpha status and vitamin D insufficiency in models of murine colitis. Cancer Prev. Res. 2013, 6, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Haug, U.; Schomburg, L.; Köhrle, J.; Perna, L.; Müller, H.; Holleczek, B.; Brenner, H. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am. J. Clin. Nutr. 2013, 97, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N. Is vitamin D the fountain of youth? Endocr. Pract. 2009, 15, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Garland, C.F. Evidence supporting the role of vitamin D in reducing the risk of cancer. J. Intern. Med. 2002, 252, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Beach, M.; Mandel, J.S.; van Stolk, R.U.; Haile, R.W.; Sandler, R.S.; Rothstein, R.; Summers, R.W.; Snover, D.C.; Beck, G.J.; et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N. Engl. J. Med. 1999, 340, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Epidemiology of vitamin D and colorectal cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 11–19. [Google Scholar] [CrossRef]
- Sperati, F.; Vici, P.; Maugeri-Sacca, M.; Stranges, S.; Santesso, N.; Mariani, L.; Giordano, A.; Sergi, D.; Pizzuti, L.; Di Lauro, L.; et al. Vitamin D supplementation and breast cancer prevention: A systematic review and meta-analysis of randomized clinical trials. PLoS ONE 2013, 8, e69269. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A.M.; Gross, G.; Twiss, J.; Waltman, N.; Ott, C.; Moore, T.E. Postmenopausal survivors of breast cancer at risk for osteoporosis: Nutritional intake and body size. Cancer Nurs. 2002, 25, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Porojnicu, A.C.; Robsahm, T.E.; Dahlback, A.; Berg, J.P.; Christiani, D.; Bruland, O.S.; Moan, J. Seasonal and geographical variations in lung cancer prognosis in Norway. Does Vitamin D from the sun play a role? Lung Cancer 2007, 55, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, M.K.; Gharehgozlou, R.; Fadaei, S.; Hajian, P.; Mirzaei, H.R. Association between serum vitamin D levels and prognostic factors in nonmetastatic breast cancer patients. J. Res. Med. Sci. 2022, 27, 56. [Google Scholar] [CrossRef] [PubMed]
- Peila, R.; Rohan, T.E. Association of prediagnostic serum levels of vitamin D with risk of ductal carcinoma In situ of the breast in the UK Biobank cohort study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Zhong, H.; Li, X. Association of 25-hydroxyvitamin D level with survival outcomes in female breast cancer patients: A meta-analysis. J. Steroid Biochem. Mol. Biol. 2021, 212, 105947. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Sukik, L.; Hoque, B.; Boutefnouchet, L.; Shi, Z. Association of serum 25-hydroxyvitamin D concentration with breast cancer risk in postmenopausal women in the US. J. Pers. Med. 2022, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cai, W.; Xing, J.; Zhao, C. Lower vitamin D levels and VDR variants are risk factors for breast cancer: An updated meta-analysis. Nucleosides Nucleotides Nucleic Acids 2023, 42, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Fera, N.; Murugan, N.J.; Voutsadakis, I.A. Vitamin D levels in newly diagnosed breast cancer patients according to tumor sub-types. J. Diet. Suppl. 2023, 20, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Marik, P. Cancer Care: The role of repurposed drugs and metabolic interventions in treating cancer (Second Edition). Available online: https://imahealth.org/wp-content/uploads/2023/06/Cancer-Care-FLCCC-Dr-Paul-Marik-v2.pdf (accessed on 15 November 2024).
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control 2012, 23, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.R.; Hankinson, S.E.; Bertone-Johnson, E.R.; Ding, E.L. Plasma vitamin D levels, menopause, and risk of breast cancer: Dose-response meta-analysis of prospective studies. Medicine 2013, 92, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, K.; Veierod, M.B.; Brustad, M.; Braaten, T.; Engelsen, O.; Lund, E. Vitamin D-effective solar UV radiation, dietary vitamin D and breast cancer risk. Int. J. Cancer 2011, 128, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Fagherazzi, G.; Mesrine, S.; Boutron-Ruault, M.C.; Clavel-Chapelon, F. Joint effects of dietary vitamin D and sun exposure on breast cancer risk: Results from the French E3N cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Juzeniene, A.; Lagunova, Z.; Porojnicu, A.C.; Moan, J.E. Vitamin D levels in Norway may be inadequate to reduce risk of breast cancer. Int. J. Cancer 2011, 128, 2249–2250. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Garland, F.C. Relationship between low ultraviolet B irradiance and higher breast cancer risk in 107 countries. Breast J. 2008, 14, 255–260. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Harmon, Q.E.; Jackson, C.L.; Diaz-Santana, M.V.; Taylor, J.A.; Weinberg, C.R.; Sandler, D.P. Vitamin D concentrations and breast cancer incidence among Black/African American and non-Black Hispanic/Latina women. Cancer 2022, 128, 2463–2473. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Squires, H.; Carroll, C.; Papaioannou, D.; Booth, A.; Logan, R.F.; Maguire, C.; Hind, D.; Tappenden, P. Chemoprevention of colorectal cancer: Systematic review and economic evaluation. Health Technol. Assess. 2010, 14, 1–206. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Relation between prediagnostic serum 25-hydroxyvitamin D level and incidence of breast, colorectal, and other cancers. J. Photochem. Photobiol. B 2010, 101, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Neuhausen, S.L.; Hoffman, M.; Caan, B.; Curtin, K.; Ma, K.N.; Samowitz, W. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int. J. Cancer 2004, 111, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yuan, C.; Nguyen, L.H.; Ng, K.; Giovannucci, E.L. Prediagnostic vitamin D status and colorectal cancer survival by vitamin D binding protein isoforms in US cohorts. J. Clin. Endocrinol. Metab. 2023, 108, e223–e229. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomas, N.; Fernandez de la Puente, M.; Babio, N.; Macias-Gonzalez, M.; Salas-Salvado, J. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.E.; Bertrand, K.A.; Petrick, J.L.; Gerlovin, H.; White, L.F.; Adams-Campbell, L.L.; Rosenberg, L.; Roy, H.K.; Palmer, J.R. Predicted vitamin D status and colorectal cancer Incidence in the black women’s health study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, L.; Liang, Y.; Huang, T.; Zhang, H.; Fan, S.; Sun, W.; Wang, Y. Relationship of vitamin D intake, serum 25(OH) D, and solar ultraviolet-B radiation with the risk of gastric cancer: A meta-analysis. J. Cancer Res. Ther. 2022, 18, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Kevin, A.; Anandhi, A.; Lakshminarayanan, S.; Sureshkumar, S.; Kamalanathan, S. Association between serum 25-hydroxy vitamin D level and gastric adenocarcinoma—A cross sectional study. Prz. Gastroenterol. 2021, 16, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Y.; Zou, X. Correlation between serum 25-hydroxyvitamin D Levels and gastric cancer: A systematic review and meta-analysis. Curr. Oncol. 2022, 29, 8390–8400. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Huynh, N.N.Y.; Nguyen, D.D.; Ta, N.H.; Van Nguyen, T.; Dang, H.T.; Le, N.T. Vitamin D intake and gastric cancer in Vietnam: A case-control study. BMC Cancer 2022, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.M.; Ngan, R.K.; Ng, W.T.; Lin, J.H.; Kwong, D.L.; Yuen, K.T.; Lee, C.K.; Leung, J.N.; Ip, D.K.; Chan, Y.H.; et al. Low vitamin D exposure and risk of nasopharyngeal carcinoma: Observational and genetic evidence from a multicenter case-control study. Clin. Nutr. 2021, 40, 5180–5188. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayona, R.; Bes-Rastrollo, M.; Fernandez-Lazaro, C.I.; Bastyr, M.; Madariaga, A.; Pons, J.J.; Martinez-Gonzalez, M.A.; Toledo, E. Vitamin D and risk of obesity-related cancers: Results from the SUN (‘Seguimiento Universidad de Navarra’) project. Nutrients 2022, 14, 2561. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.C.; Fernbach, M.; Breitbart, W.S.; Nelson, C. Prognosis in metastatic lung cancer: Vitamin D deficiency and depression-a cross-sectional analysis. BMJ Support. Palliat. Care 2022, 12, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Lin, J.; Fu, R.; Qi, S.; Fu, X.; Yuan, L.; Qian, L. The role of vitamin D intake on the prognosis and incidence of lung cancer: A systematic review and meta-analysis. J. Nutr. Sci. Vitaminol. 2021, 67, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Jacobs, E.J.; Arslan, A.A.; Qi, D.; Patel, A.V.; Helzlsouer, K.J.; Weinstein, S.J.; McCullough, M.L.; Purdue, M.P.; Shu, X.O.; et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 81–93. [Google Scholar] [CrossRef] [PubMed]
- van Duijnhoven, F.J.B.; Jenab, M.; Hveem, K.; Siersema, P.D.; Fedirko, V.; Duell, E.J.; Kampman, E.; Halfweeg, A.; van Kranen, H.J.; van den Ouweland, J.M.W.; et al. Circulating concentrations of vitamin D in relation to pancreatic cancer risk in European populations. Int. J. Cancer 2018, 142, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Risch, H.A.; Bosetti, C.; Anderson, K.E.; Petersen, G.M.; Bamlet, W.R.; Cotterchio, M.; Cleary, S.P.; Ibiebele, T.I.; La Vecchia, C.; et al. Vitamin D and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2015, 26, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, K.A.; Chang, E.T.; Abel, G.A.; Zhang, S.M.; Spiegelman, D.; Qureshi, A.A.; Laden, F. Sunlight exposure, vitamin D, and risk of non-Hodgkin lymphoma in the Nurses’ Health Study. Cancer Causes Control 2011, 22, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Helzlsouer, K.J.; Chow, W.H.; Freedman, D.M.; Hankinson, S.E.; Hartge, P.; Hartmuller, V.; Harvey, C.; Hayes, R.B.; Horst, R.L.; et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: Design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Alkhenizan, A.; Hafez, K. The role of vitamin E in the prevention of cancer: A meta-analysis of randomized controlled trials. Ann. Saudi Med. 2007, 27, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. An ecologic study of cancer mortality rates in Spain with respect to indices of solar UVB irradiance and smoking. Int. J. Cancer 2007, 120, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, M.T.; Turner, J.J.; Falster, M.O.; Meagher, N.S.; Joske, D.J.; Grulich, A.E.; Giles, G.G.; Vajdic, C.M. Latitude gradients for lymphoid neoplasm subtypes in Australia support an association with ultraviolet radiation exposure. Int. J. Cancer 2013, 133, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Honda, A.; Kurokawa, M. Impact of vitamin D level at diagnosis and transplantation on the prognosis of hematological malignancy: A meta-analysis. Blood Adv. 2022, 6, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, F.; Newton-Bishop, J.; O’Connell, S.; Hawkins, J.E.; Guideline Development, G. Melanoma: Summary of NICE guidance. BMJ 2015, 351, h3708. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Darder, I.; Carrera, C.; Alamon-Reig, F.; Puig, S.; Malvehy, J.; Podlipnik, S. Vitamin D deficiency in melanoma patients is associated with worse overall survival: A retrospective cohort study. Melanoma Res. 2022, 32, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Moro, R.; Sanchez-Silva, A.; Aguerralde-Martin, M.; Gonzalez-Cuevas, R.; Peruilh-Bagolini, L.; Traves, V.; Manrique-Silva, E.; Requena, C.; Nagore, E. Prognostic value of vitamin D serum levels in cutaneous melanoma. Actas Dermosifiliogr. 2022, 113, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Newton-Bishop, J.A.; Beswick, S.; Randerson-Moor, J.; Chang, Y.-M.; Affleck, P.; Elliott, F.; Chan, M.; Leake, S.; Karpavicius, B.; Haynes, S.; et al. Serum 25-Hydroxyvitamin D(3) Levels Are Associated with Breslow Thickness at Presentation and Survival From Melanoma. J. Clin. Oncol. 2009, 27, 5439–5444. [Google Scholar] [CrossRef] [PubMed]
- Malloy, P.J.; Feldman, D. Genetic disorders and defects in vitamin D action. Rheum. Dis. Clin. N. Am. 2012, 38, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Rheum. Dis. Clin. N. Am. 2012, 38, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Muindi, J.R.; Adjei, A.A.; Wu, Z.R.; Olson, I.; Huang, H.; Groman, A.; Tian, L.; Singh, P.K.; Sucheston, L.E.; Johnson, C.S.; et al. Serum Vitamin D Metabolites in Colorectal Cancer Patients Receiving Cholecalciferol Supplementation: Correlation with Polymorphisms in the Vitamin D Genes. Horm. Cancer 2013, 4, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.J.; Freedman, D.M.; Bhatti, P.; Doody, M.M.; Fu, Y.P.; Chang, S.C.; Linet, M.S.; Sigurdson, A.J. Sunlight, polymorphisms of vitamin D-related genes and risk of breast cancer. Anticancer. Res. 2013, 33, 543–551. [Google Scholar] [PubMed]
- Pande, M.; Thompson, P.A.; Do, K.A.; Sahin, A.A.; Amos, C.I.; Frazier, M.L.; Bondy, M.L.; Brewster, A.M. Genetic variants in the vitamin D pathway and breast cancer disease-free survival. Carcinogenesis 2013, 34, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.A.; Damsgaard, C.T.; Dalskov, S.M.; Sorensen, L.B.; Hjorth, M.F.; Ritz, C.; Kjolbaek, L.; Andersen, R.; Tetens, I.; Krarup, H.; et al. Vitamin D status and its determinants during autumn in children at northern latitudes: A cross-sectional analysis from the optimal well-being, development and health for Danish children through a healthy New Nordic Diet (OPUS) School Meal Study. Br. J. Nutr. 2016, 115, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Huotari, A.; Herzig, K.H. Vitamin D and living in northern latitudes--an endemic risk area for vitamin D deficiency. Int. J. Circumpolar Health 2008, 67, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G.; Downs, N.J.; Parisi, A.V. Comparison of human facial UV exposure at high and low latitudes and the potential impact on dermal vitamin D production. Photochem. Photobiol. Sci. 2003, 2, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Giovannucci, E. Calcium, vitamin D and colorectal cancer chemoprevention. Best. Pract. Res. Clin. Gastroenterol. 2011, 25, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Gammon, M.D.; Steck, S.E.; Hershman, D.L.; Cremers, S.; Dworakowski, E.; Shane, E.; Terry, M.B.; Desai, M.; Teitelbaum, S.L.; et al. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev. Res. 2009, 2, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Helzlsouer, K.J. Overview of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Cellular and molecular effects of vitamin D on carcinogenesis. Arch. Biochem. Biophys. 2012, 523, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Trukova, K.; Lammersfeld, C.A.; Braun, D.P.; Gupta, D. Impact of oral vitamin D supplementation on serum 25-hydroxyvitamin D levels in oncology. Nutr. J. 2010, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Manzano, N.; Salas, Y.; Angel, M.; Diaz-Couselo, F.A.; Zylberman, M. Vitamin D deficiency in patients admitted to the general ward with breast, lung, and colorectal cancer in Buenos Aires, Argentina. Arch. Osteoporos. 2016, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Lammersfeld, C.A.; Braun, D.P.; Gupta, D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutr. J. 2011, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D and sunlight: Strategies for cancer prevention and other health benefits. Clin. J. Am. Soc. Nephrol. 2008, 3, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; DeSmet, M.; Johnson, R.; Li, Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012, 441, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Willett, W.C. Cancer incidence and mortality and vitamin D in black and white male health professionals. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 575–579. [Google Scholar] [CrossRef] [PubMed]
- George, P.S.; Pearson, E.R.; Witham, M.D. Effect of vitamin D supplementation on glycaemic control and insulin resistance: A systematic review and meta-analysis. Diabet. Med. 2012, 29, e142–e150. [Google Scholar] [CrossRef] [PubMed]
- Manousopoulou, A.; Al-Daghri, N.M.; Garbis, S.D.; Chrousos, G.P. Vitamin D and cardiovascular risk among adults with obesity: A systematic review and meta-analysis. Eur. J. Clin. Invest. 2015, 45, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Kunutsor, S.; Franco, O.H.; Chowdhury, R. Vitamin D, type 2 diabetes and other metabolic outcomes: A systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 2013, 72, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Kienreich, K.; Rutters, F.; de Jongh, R.; van Ballegooijen, A.J.; Grubler, M.; Tomaschitz, A.; Dekker, J.M. Role of vitamin D in the development of insulin resistance and type 2 diabetes. Curr. Diab Rep. 2013, 13, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.C.; Liao, M.T.; Lu, K.C.; Wu, C.C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012, 2012, 634195. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Gysemans, C.; Bouillon, R.; Mathieu, C. Vitamin D and diabetes. Rheum. Dis. Clin. N. Am. 2012, 38, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Cunha, N.; Campos, S.; Serrao, V. Vitamin D levels in a cohort of Portuguese melanoma patients relate to time of follow-up from diagnosis, sun-exposure behaviour, and use of photoprotection. Eur. J. Dermatol. 2018, 28, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Orlow, I.; Shi, Y.; Kanetsky, P.A.; Thomas, N.E.; Luo, L.; Corrales-Guerrero, S.; Cust, A.E.; Sacchetto, L.; Zanetti, R.; Rosso, S.; et al. The interaction between vitamin D receptor polymorphisms and sun exposure around time of diagnosis influences melanoma survival. Pigment. Cell Melanoma Res. 2018, 31, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Mandelcorn-Monson, R.; Marrett, L.; Kricker, A.; Armstrong, B.K.; Orlow, I.; Goumas, C.; Paine, S.; Rosso, S.; Thomas, N.; Millikan, R.C.; et al. Sun exposure, vitamin D receptor polymorphisms FokI and BsmI and risk of multiple primary melanoma. Cancer Epidemiol. 2011, 35, e105–e110. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R.; de Vries, E.; Erdmann, F.; Espina, C.; Auvinen, A.; Kesminiene, A.; Schuz, J. European Code against Cancer 4th Edition: Ultraviolet radiation and cancer. Cancer Epidemiol. 2015, 39 (Suppl. S1), S75–S83. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Weighing the evidence linking UVB irradiance, vitamin D, and cancer risk. Mayo Clin. Proc. 2011, 86, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, S.; Wolbers, F.; Brouckaert, O.; Vermes, I.; Franke, H.R. Cancer prevalence in osteoporotic women with low serum vitamin D levels. Menopause 2011, 18, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. The Institute of Medicine did not find the vitamin D-cancer link because it ignored UV-B dose studies. Public Health Nutr. 2011, 14, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Milner, J.A. Nutrigenomics, vitamin D and cancer prevention. J. Nutr. Nutr. 2011, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Y.; Lu, C.; Wang, Y.; Peng, L.; Jiang, M.; Tang, Y.; Zhao, Q. Meta-analysis of the correlation between vitamin D and lung cancer risk and outcomes. Oncotarget 2017, 8, 81040–81051. [Google Scholar] [CrossRef] [PubMed]
- Zgaga, L.; O’Sullivan, F.; Cantwell, M.M.; Murray, L.J.; Thota, P.N.; Coleman, H.G. Markers of Vitamin D Exposure and Esophageal Cancer Risk: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. The likely role of vitamin D from solar ultraviolet-B irradiance in increasing cancer survival. Anticancer. Res. 2006, 26, 2605–2614. [Google Scholar] [PubMed]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Garland, F.C. Ultraviolet B irradiance and vitamin D status are inversely associated with incidence rates of pancreatic cancer worldwide. Pancreas 2010, 39, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, S.; Valle, N.; Ferrer-Mayorga, G.; Lombardia, L.; Herrera, M.; Dominguez, O.; Segura, M.F.; Bonilla, F.; Hernando, E.; Munoz, A. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Human. Mol. Genet. 2012, 21, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Waterhouse, M.; Rahman, S.; Baxter, C.; Duarte Romero, B.; McLeod, D.S.A.; Ebeling, P.R.; English, D.R.; Hartel, G.; O’Connell, R.L.; et al. The effect of vitamin D supplementation on the gut microbiome in older Australians—Results from analyses of the D-Health Trial. Gut Microbes 2023, 15, 2221429. [Google Scholar] [CrossRef] [PubMed]
- Brusnic, O.; Onisor, D.; Boicean, A.; Hasegan, A.; Ichim, C.; Guzun, A.; Chicea, R.; Todor, S.B.; Vintila, B.I.; Anderco, P.; et al. Fecal microbiota transplantation: Insights into colon carcinogenesis and immune regulation. J. Clin. Med. 2024, 13, 6578. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Role of solar UVB irradiance and smoking in cancer as inferred from cancer incidence rates by occupation in Nordic countries. Dermatoendocrinol 2012, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Epstein, E.; Nielsen, K.; Landin-Olsson, M.; Ingvar, C.; Olsson, H. Avoidance of sun exposure as a risk factor for major causes of death: A competing risk analysis of the Melanoma in Southern Sweden cohort. J. Intern. Med. 2016, 280, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Yin, X.; Zou, C.; Li, H. Effects of behavioral change techniques on diet and physical activity in colorectal cancer patients: A systematic review and meta-analysis. Support. Care Cancer 2022, 31, 29. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Guijarro, A.; Nencioni, A. Advances in diet and physical activity in breast cancer prevention and treatment. Nutrients 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Ambagaspitiya, S.S.; Appuhamillage, G.A.; Wimalawansa, S.J. Impact of vitamin D on skin aging, and age-related dermatological conditions. Front. Biosci. (Landmark Ed.) 2025, 30, 25463. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Kim, J.H. Sunlight exposure in association with risk of lymphoid malignancy: A meta-analysis of observational studies. Cancer Causes Control 2021, 32, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Mai, J.Z.; Fang, M.; Chernyavskiy, P.; Kennerley, V.; Cahoon, E.K.; Cockburn, M.G.; Kendall, G.M.; Kimlin, M.G. Solar ultraviolet radiation exposure, and incidence of childhood acute lymphocytic leukaemia and non-Hodgkin lymphoma in a US population-based dataset. Br. J. Cancer 2024, 130, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Jordan, S.J.; Lucas, R.; Webb, P.M.; Neale, R.; Australian Ovarian Cancer Study, G. Association between ambient ultraviolet radiation and risk of epithelial ovarian cancer. Cancer Prev. Res. 2012, 5, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Lucas, R.; Kimlin, M.; Whiteman, D.; Neale, R.; Australian Cancer, S. Association between ambient ultraviolet radiation and risk of esophageal cancer. Am. J. Gastroenterol. 2012, 107, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Balk, S.J. Ultraviolet Radiation: A Hazard to Children and Adolescents: A hazard to children and adolescents. Pediatrics 2011, 127, e791–e817. [Google Scholar] [CrossRef] [PubMed]
- Godar, D.E.; Landry, R.J.; Lucas, A.D. Increased UVA exposures and decreased cutaneous vitamin D(3) levels may be responsible for the increasing incidence of melanoma. Med. Hypotheses 2009, 72, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Dinas, P.C.; on behalf of the Students of Module 5104 (Introduction to Systematic Reviews); Karaventza, M.; Liakou, C.; Georgakouli, K.; Bogdanos, D.; Metsios, G.S. Combined effects of physical activity and diet on cancer patients: A systematic review and Meta-dnalysis. Nutrients 2024, 16, 1749. [Google Scholar] [CrossRef] [PubMed]
- Markozannes, G.; Cividini, S.; Aune, D.; Becerra-Tomas, N.; Kiss, S.; Balducci, K.; Vieira, R.; Cariolou, M.; Jayedi, A.; Greenwood, D.C.; et al. The role of physical activity, sedentary behaviour, diet, adiposity and body composition on health-related quality of life and cancer-related fatigue after diagnosis of colorectal cancer: A Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. ESMO Open 2025, 10, 104301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Trivedi, T.; Lin, R.C.; Fong-Yee, C.; Nolte, R.; Manibo, J.; Chen, Y.; Hossain, M.; Horas, K.; Dunstan, C.; et al. Loss of the vitamin D receptor in human breast and prostate cancers strongly induces cell apoptosis through downregulation of Wnt/beta-catenin signaling. Bone Res. 2017, 5, 17023. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Garland, C.F.; Holick, M.F. Comparisons of estimated economic burdens due to insufficient solar ultraviolet irradiance and vitamin D and excess solar UV irradiance for the United States. Photochem. Photobiol. 2005, 81, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Gorham, E.D.; Garland, C.F. Vitamin D and the limits of randomized controlled trials. Public Health Nutr. 2011, 14, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Gorham, E.D.; Garland, C.F.; Garland, F.C.; Grant, W.B.; Mohr, S.B.; Lipkin, M.; Newmark, H.L.; Giovannucci, E.; Wei, M.; Holick, M.F. Optimal vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am. J. Prev. Med. 2007, 32, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Szeto, F.L.; Zhang, W.; Ye, H.; Kong, J.; Zhang, Z.; Sun, X.J.; Li, Y.C. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E820–E828. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.S.; Silveiro, S.P. Maternal-fetal impact of vitamin D deficiency: A critical review. Matern. Child Health J. 2015, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low vitamin D status: Definition, prevalence, consequences, and correction. Rheum. Dis. Clin. N. Am. 2012, 38, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Manger, P.T.; Khare, R.K. Fok I and Bsm I gene polymorphism of vitamin D receptor and essential hypertension: A mechanistic link. Clin. Hypertens. 2023, 29, 5. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nishimura, K.I.; Mori, J.I. Update on recent progress in vitamin D research. Molecular basis of epigenetic regulation by vitamin D via its nuclear receptor. Clin. Calcium 2017, 27, 1543–1550. [Google Scholar] [PubMed]
- Grant, W.B.; Boucher, B.J. Randomized controlled trials of vitamin D and cancer incidence: A modeling study. PLoS ONE 2017, 12, e0176448. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.L.; Peacock, J.L.; Rees, J.R.; Bostick, R.M.; Robertson, D.J.; Bresalier, R.S.; Baron, J.A. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: A randomized clinical trial. JAMA Oncol. 2017, 3, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Mondul, A.M.; Layne, T.M.; Yu, K.; Huang, J.; Stolzenberg-Solomon, R.Z.; Ziegler, R.G.; Purdue, M.P.; Huang, W.Y.; Abnet, C.C.; et al. Prediagnostic serum vitamin D, vitamin D binding protein isoforms, and cancer survival. JNCI Cancer Spectr. 2022, 6, pkac019. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- National Heart, L.; Blood Institute, P.C.T.N.; Ginde, A.A.; Brower, R.G.; Caterino, J.M.; Finck, L.; Banner-Goodspeed, V.M.; Grissom, C.K.; Hayden, D.; Hough, C.L.; et al. Early high-dose vitamin D(3) for critically Ill, vitamin D-deficient patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [CrossRef]
- Scragg, R.K.R. Overview of results from the Vitamin D Assessment (ViDA) study. J. Endocrinol. Investig. 2019, 42, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Desouza, C.; Chatterjee, R.; Vickery, E.M.; Nelson, J.; Johnson, K.C.; Kashyap, S.R.; Lewis, M.R.; Margolis, K.; Pratley, R.; Rasouli, N.; et al. The effect of vitamin D supplementation on cardiovascular risk in patients with prediabetes: A secondary analysis of the D2d study. J. Diabetes Its Complicat. 2022, 36, 108230. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Birschmann, I.; Schulz, U.; Berthold, H.K.; et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): A 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur. Heart J. 2017, 38, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Barry, E.L.; Mott, L.A.; Rees, J.R.; Sandler, R.S.; Snover, D.C.; Bostick, R.M.; Ivanova, A.; Cole, B.F.; Ahnen, D.J.; et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N. Engl. J. Med. 2015, 373, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Verheyen, N.; Grubler, M.R.; Tomaschitz, A.; Marz, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding nutritional epidemiology and its role in policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R.; Frongillo, E.A.; Adams, S.A.; Turner-McGrievy, G.M.; Hurley, T.G.; Miller, D.R.; Ockene, I.S. Perspective: Randomized controlled trials are not a panacea for diet-related research. Adv. Nutr. 2016, 7, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grubler, M.R.; Marz, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Bhattoa, H.P.; Boucher, B.J. Seasonal variations of U.S. mortality rates: Roles of solar ultraviolet-B doses, vitamin D, gene expression, and infections. J. Steroid Biochem. Mol. Biol. 2017, 173, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Chartres, N.; Scrinis, G.; Bero, L.A. Study sponsorship and the nutrition research agenda: Analysis of randomized controlled trials included in systematic reviews of nutrition interventions to address obesity. Public Health Nutr. 2017, 20, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lipsyc-Sharf, M.; Zong, X.; Wang, X.; Hur, J.; Song, M.; Wang, M.; Smith-Warner, S.A.; Fuchs, C.; Ogino, S.; et al. Total vitamin D Intake and risks of early-onset colorectal cancer and precursors. Gastroenterology 2021, 161, 1208–1217.e1209. [Google Scholar] [CrossRef] [PubMed]
- Abuduwaili, M.; Xing, Z.; Xia, B.; Fei, Y.; Zhu, J.; Su, A. Correlation between Pre-Operative 25-Hydroxyvitamin D Levels and Poor Prognostic Factors for Papillary Thyroid Cancer. J. Investig. Surg. 2022, 35, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Fullmer, M.; Su, A.; Bachrach, S.; Hossain, J.; Kecskemethy, H.H. Newly diagnosed children with cancer have lower 25-vitamin D levels than their cancer-Free peers: A comparison across age, race, and sex. Cancers 2022, 14, 2378. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, A.; Virtanen, J.K.; Hantunen, S.; Nurmi, T.; Kokko, P.; Tuomainen, T.P. How competing risks affect the epidemiological relationship between vitamin D and prostate cancer incidence? A population-based study. Andrologia 2022, 54, e14410. [Google Scholar] [CrossRef] [PubMed]
- Maturana-Ramirez, A.; Aitken-Saavedra, J.; Guevara-Benitez, A.L.; Espinoza-Santander, I. Hypovitaminosis D, oral potentially malignant disorders, and oral squamous cell carcinoma: A systematic review. Med. Oral. Patol. Oral. Cir. Bucal 2022, 27, e135–e141. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.L.; Friedberg, J.W.; Calvi, L.M.; van Wijngaarden, E.; Fisher, S.G. Vitamin D and non-Hodgkin lymphoma risk in adults: A review. Cancer Investig. 2009, 27, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Maurer, M.J.; Link, B.K.; Habermann, T.M.; Ansell, S.M.; Micallef, I.N.; Kelly, J.L.; Macon, W.R.; Nowakowski, G.S.; Inwards, D.J.; et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J. Clin. Oncol. 2010, 28, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Shekarriz-Foumani, R.; Khodaie, F. The Correlation of Plasma 25-Hydroxyvitamin D Deficiency with Risk of Breast Neoplasms: A Systematic Review. Iran. J. Cancer Prev. 2016, 9, e4469. [Google Scholar] [CrossRef] [PubMed]
- Momivand, M.; Razaghi, M.; Mohammadi, F.; Hoseinzadeh, E.; Najafi-Vosough, R. The status of serum 25(OH)D levels is related to breast cancer. Cancer Treat. Res. Commun. 2024, 42, 100870. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Wen, X.; Zhang, Y.; Wei, X.; Liu, T. Vitamin D deficiency and increased risk of bladder carcinoma: A meta-analysis. Cell Physiol. Biochem. 2015, 37, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.C.; Hodge, A.M.; Fanidi, A.; Albanes, D.; Mai, X.M.; Shu, X.O.; Weinstein, S.J.; Larose, T.L.; Zhang, X.; Han, J.; et al. No association between circulating concentrations of vitamin D and risk of lung cancer: An analysis in 20 prospective studies in the Lung Cancer Cohort Consortium (LC3). Ann. Oncol. 2018, 29, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Travis, R.C.; Perez-Cornago, A.; Appleby, P.N.; Albanes, D.; Joshu, C.E.; Lutsey, P.L.; Mondul, A.M.; Platz, E.A.; Weinstein, S.J.; Layne, T.M.; et al. A collaborative analysis of Individual participant data from 19 prospective studies assesses circulating vitamin D and prostate cancer Risk. Cancer Res. 2019, 79, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Gorham, E.D.; Garland, C.F.; Garland, F.C.; Grant, W.B.; Mohr, S.B.; Lipkin, M.; Newmark, H.L.; Giovannucci, E.; Wei, M.; Holick, M.F. Vitamin D and prevention of colorectal cancer. J. Steroid Biochem. Mol. Biol. 2005, 97, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, J.M.; Martin-Gorgojo, A. The role of vitamin D in cancer prevention: Some new clues on a fascinating subject. Nutrients 2023, 15, 2560. [Google Scholar] [CrossRef] [PubMed]
- Hammad, L.N.; Abdelraouf, S.M.; Hassanein, F.S.; Mohamed, W.A.; Schaalan, M.F. Circulating IL-6, IL-17 and vitamin D in hepatocellular carcinoma: Potential biomarkers for a more favorable prognosis? J. Immunotoxicol. 2013, 10, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, F.P.; Schymura, M.J. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer 2006, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Linseisen, J.; Slanger, T.; Kropp, S.; Mutschelknauss, E.J.; Flesch-Janys, D.; Chang-Claude, J. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer--results of a large case-control study. Carcinogenesis 2008, 29, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Grant, W.B.; Giovannucci, E.L.; Lipkin, M.; Newmark, H.; Holick, M.F.; Garland, F.C. Vitamin D and prevention of breast cancer: Pooled analysis. J. Steroid Biochem. Mol. Biol. 2007, 103, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Kricker, A.; Armstrong, B.K.; Hughes, A.M.; Goumas, C.; Smedby, K.E.; Zheng, T.; Spinelli, J.J.; De Sanjose, S.; Hartge, P.; Melbye, M.; et al. Personal sun exposure and risk of non Hodgkin lymphoma: A pooled analysis from the Interlymph Consortium. Int. J. Cancer 2008, 122, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Treskova, I.; Vrzalova, J.; Svobodova, S.; Topolcan, O.; Fuchsova, R.; Rousarova, M.; Treska, V.; Kydlicek, T. Evaluation of IGF1 serum levels in malignant melanoma and healthy subjects. Anticancer. Res. 2014, 34, 5217–5220. [Google Scholar] [PubMed]
- Beasley, J.M.; Gunter, M.J.; LaCroix, A.Z.; Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Vitolins, M.Z.; Strickler, H.D. Associations of serum insulin-like growth factor-I and insulin-like growth factor-binding protein 3 levels with biomarker-calibrated protein, dairy product and milk intake in the Women’s Health Initiative. Br. J. Nutr. 2014, 111, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; De Vita, F.; Lauretani, F.; Butto, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Aglio, E.; Ceda, G.P. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef] [PubMed]
- Peric, M.; Koglin, S.; Dombrowski, Y.; Gross, K.; Bradac, E.; Buchau, A.; Steinmeyer, A.; Zugel, U.; Ruzicka, T.; Schauber, J. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis. PLoS ONE 2009, 4, e6340. [Google Scholar] [CrossRef] [PubMed]
- Tuohimaa, P.; Tenkanen, L.; Ahonen, M.; Lumme, S.; Jellum, E.; Hallmans, G.; Stattin, P.; Harvei, S.; Hakulinen, T.; Luostarinen, T.; et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: A longitudinal, nested case-control study in the Nordic countries. Int. J. Cancer 2004, 108, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Nair-Shalliker, V.; Smith, D.P.; Egger, S.; Hughes, A.M.; Kaldor, J.M.; Clements, M.; Kricker, A.; Armstrong, B.K. Sun exposure may increase risk of prostate cancer in the high UV environment of New South Wales, Australia: A case-control study. Int. J. Cancer 2012, 131, E726–E732. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wei, W.; Wang, G.; Zhou, H.; Fu, Y.; Liu, N. Circulating vitamin D concentration and risk of prostate cancer: A dose-response meta-analysis of prospective studies. Ther. Clin. Risk Manag. 2018, 14, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Schenk, J.M.; Till, C.A.; Tangen, C.M.; Goodman, P.J.; Song, X.; Torkko, K.C.; Kristal, A.R.; Peters, U.; Neuhouser, M.L. Serum 25-hydroxyvitamin D Concentrations and Risk of Prostate Cancer: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.T.; Hibler, E.A.; Lance, P.; Sardo, C.L.; Jurutka, P.W. Association between circulating concentrations of 25(OH)D and colorectal adenoma: A pooled analysis. Int. J. Cancer 2013, 133, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.D.; Tulloch-Reid, M.K.; Lindsay, C.M.; Smith, G.; Bennett, F.I.; McFarlane-Anderson, N.; Aiken, W.; Coard, K.C.M. Both serum 25-hydroxyvitamin D and calcium levels may increase the risk of incident prostate cancer in Caribbean men of African ancestry. Cancer Med. 2015, 4, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Capiod, T.; Barry Delongchamps, N.; Pigat, N.; Souberbielle, J.C.; Goffin, V. Do dietary calcium and vitamin D matter in men with prostate cancer? Nat. Rev. Urol. 2018, 15, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Steck, S.E.; Omofuma, O.O.; Su, L.J.; Maise, A.A.; Woloszynska-Read, A.; Johnson, C.S.; Zhang, H.; Bensen, J.T.; Fontham, E.T.H.; Mohler, J.L.; et al. Calcium, magnesium, and whole-milk intakes and high-aggressive prostate cancer in the North Carolina-Louisiana Prostate Cancer Project (PCaP). Am. J. Clin. Nutr. 2018, 107, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Stampfer, M.J.; Ma, J.; Gann, P.H.; Gaziano, J.M.; Giovannucci, E.L. Dairy products, calcium, and prostate cancer risk in the Physicians’ Health Study. Am. J. Clin. Nutr. 2001, 74, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Grant, W.B.; Willett, W.C. Does the high prevalence of vitamin D deficiency in African Americans contribute to health disparities? Nutrients 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Miller, K.D.; Tossas, K.Y.; Winn, R.A.; Jemal, A.; Siegel, R.L. Cancer statistics for African American/Black people 2022. CA Cancer J. Clin. 2022, 72, 202–229. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in dietary supplement use among US adults from 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Domoslawska-Zylinska, K.; Wlodarczyk, D. Smoking avoidance, physical activity and diet as preventative behaviours for lung, prostate and colorectal cancer—A comparison of the extended parallel process model groups. Int. J. Public Health 2025, 70, 1607278. [Google Scholar] [CrossRef] [PubMed]
- Droufakou, S. How does lifestyle impact incidence of cancer: Preventive measures to consider. Public Health Toxicol. 2022, 2, A58. [Google Scholar] [CrossRef]
- Loprz, P. Do I Need an HPV Vaccine? Available online: https://publichealth.jhu.edu/2024/do-i-need-an-hpv-vaccine#:~:text=Almost%20everyone%20will%20get%20human,such%20infections%20and%20subsequent%20cancers (accessed on 15 March 2025).
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and challenges in integrating nutritional care into oncology practice: Results from a national survey on behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Phothikul, J.; Chung, J.; Faro, J.; Seven, M. Diet and physical activity behaviors of breast cancer survivors: A scoping review. Semin. Oncol. Nurs. 2025, 41, 151763. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of vitamin D3 supplements on development of advanced cancer: A secondary analysis of the VITAL randomized clinical trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Highfill, R.M.; Garland, F.C. Mapping vitamin D deficiency, breast cancer, and colorectal cancer. In Proceedings of the ESRI International User Conference, Redlands, CA, USA, 25–29 July 2005. [Google Scholar]
- Vaughan-Shaw, P.G.; O’Sullivan, F.; Farrington, S.M.; Theodoratou, E.; Campbell, H.; Dunlop, M.G.; Zgaga, L. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: Systematic review and meta-analysis. Br. J. Cancer 2017, 116, 1092–1110. [Google Scholar] [CrossRef] [PubMed]
- Vaughan-Shaw, P.G.; Timofeeva, M.; Ooi, L.Y.; Svinti, V.; Grimes, G.; Smillie, C.; Blackmur, J.P.; Donnelly, K.; Theodoratou, E.; Campbell, H.; et al. Differential genetic influences over colorectal cancer risk and gene expression in large bowel mucosa. Int. J. Cancer 2021, 149, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Razzaque, M.S.; Al-Daghri, N.M. Calcium and vitamin D in human health: Hype or real? J. Steroid Biochem. Mol. Biol. 2018, 180, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Jackson, R.; Holdaway, I.M.; Lim, T.; Beaglehole, R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: A community-based study. Int. J. Epidemiol. 1990, 19, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Lawler, T.; Warren Andersen, S. Serum 25-hydroxyvitamin D and cancer risk: A systematic review of mendelian randomization studies. Nutrients 2023, 15, 422. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Baggerly, C.A.; Garland, F.C. Re: Prospective study of vitamin D and cancer mortality in the United States. J. Natl. Cancer Inst. 2008, 100, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control 2005, 16, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Behradmanesh, S.; Ahmadi, A.; Rafieian-Kopaei, M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J. Nephropathol. 2014, 3, 29–33. [Google Scholar] [CrossRef] [PubMed]
- von Hurst, P.R.; Stonehouse, W.; Matthys, C.; Conlon, C.; Kruger, M.C.; Coad, J. Study protocol--metabolic syndrome, vitamin D and bone status in South Asian women living in Auckland, New Zealand: A randomised, placebo-controlled, double-blind vitamin D intervention. BMC Public Health 2008, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cockcroft, J.R.; Elwood, P.C.; Pickering, J.E.; Lovegrove, J.A.; Givens, D.I. Vitamin D intake and risk of CVD and all-cause mortality: Evidence from the Caerphilly Prospective Cohort Study. Public Health Nutr. 2017, 20, 2744–2753. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalidi, B.; Kimball, S.M.; Kuk, J.L.; Ardern, C.I. Metabolically healthy obesity, vitamin D, and all-cause and cardiometabolic mortality risk in NHANES III. Clin. Nutr. 2018, 38, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, X.K.; Liu, Y.; Yue, Y.B.; Yan, M. Correlation between 25-hydroxyvitamin D and nephroblastoma in children and its value in assessing disease prognosis. Zhongguo Dang Dai Er Ke Za Zhi 2023, 25, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A narrative review of the evidence for variations in serum 25-hdroxyvitamin D concentration thresholds for optimal health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Whiting, S.J.; Schwalfenberg, G.K.; Genuis, S.J.; Kimball, S.M. Estimated economic benefit of increasing 25-hydroxyvitamin D concentrations of Canadians to or above 100 nmol/L. Dermatoendocrinol 2016, 8, e1248324. [Google Scholar] [CrossRef] [PubMed]
- Cangoz, S.; Chang, Y.Y.; Chempakaseril, S.J.; Guduru, R.C.; Huynh, L.M.; John, J.S.; John, S.T.; Joseph, M.E.; Judge, R.; Kimmey, R.; et al. Vitamin D and type 2 diabetes mellitus. J. Clin. Pharm. Ther. 2013, 38, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Whiting, S.J.; Barton, C.N. Vitamin D intake: A global perspective of current status. J. Nutr. 2005, 135, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.J.; Zhao, B.; Bielenberg, D.R.; Dridi, S.; Wu, J.; Jiang, W.; Huang, B.; Pirie-Shepherd, S.; Fannon, M. Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells. PLoS ONE 2010, 5, e13428. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Purdue, M.P.; Smith-Warner, S.A.; Mondul, A.M.; Black, A.; Ahn, J.; Huang, W.Y.; Horst, R.L.; Kopp, W.; Rager, H.; et al. Serum 25-hydroxyvitamin D, vitamin D binding protein and risk of colorectal cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer 2015, 136, E654–E664. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.P.; Robinson-Cohen, C.; de Boer, I.H.; Houston, D.K.; Lohman, K.; Liu, Y.; Kritchevsky, S.B.; Cauley, J.A.; Tanaka, T.; Ferrucci, L.; et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA 2012, 308, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.; Wimalawansa, S.J. Minerals and human health: From deficiency to toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Farooq, N. Vitamin D Toxicity. In StatPearls (Internet); The National Center for Biotechnology Information: Treasure Island, FL, USA, 2024. [Google Scholar]
- Shamim, N.; Majid, H.; Khemani, S.; Salim, M.; Muneer, S.; Khan, A.H. Inappropriate supplementation of Vitamin D can result in toxicity: A crosssectional study of paediatrics population. JPMA J. Pak. Med. Assoc. 2023, 73, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; von Hurst, P.R. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients 2015, 7, 5111–5142. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, S582–S586. [Google Scholar] [CrossRef] [PubMed]
- Fuss, M.; Pepersack, T.; Gillet, C.; Karmali, R.; Corvilain, J. Calcium and vitamin D metabolism in granulomatous diseases. Clin. Rheumatol. 1992, 11, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Bansal, A.S.; Looke, D.F.; Whitby, M.; Hogan, P.G. Hypercalcaemia and elevated 1,25(OH)(2)D(3) levels associated with disseminated Mycobacterium avium infection in AIDS. J. Infect. 2001, 42, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.L. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011; p. 20. [Google Scholar]
- Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005, 135, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.A.; Prindiville, S.; Weiss, D.G.; Willett, W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003, 290, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Robertson, A.S.; Rodriguez, C.; Jacobs, E.J.; Chao, A.; Carolyn, J.; Calle, E.E.; Willett, W.C.; Thun, M.J. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the cancer prevention study II nutrition cohort (United States). Cancer Causes Control 2003, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. IOM recommendations vs. vitamin D guidelines applicable to the rest of the world. In Proceedings of the 5th International Conference on Vitamin D, Abu Dhabi, United Arab Emirates, 16–17 March 2017; p. 9. [Google Scholar]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
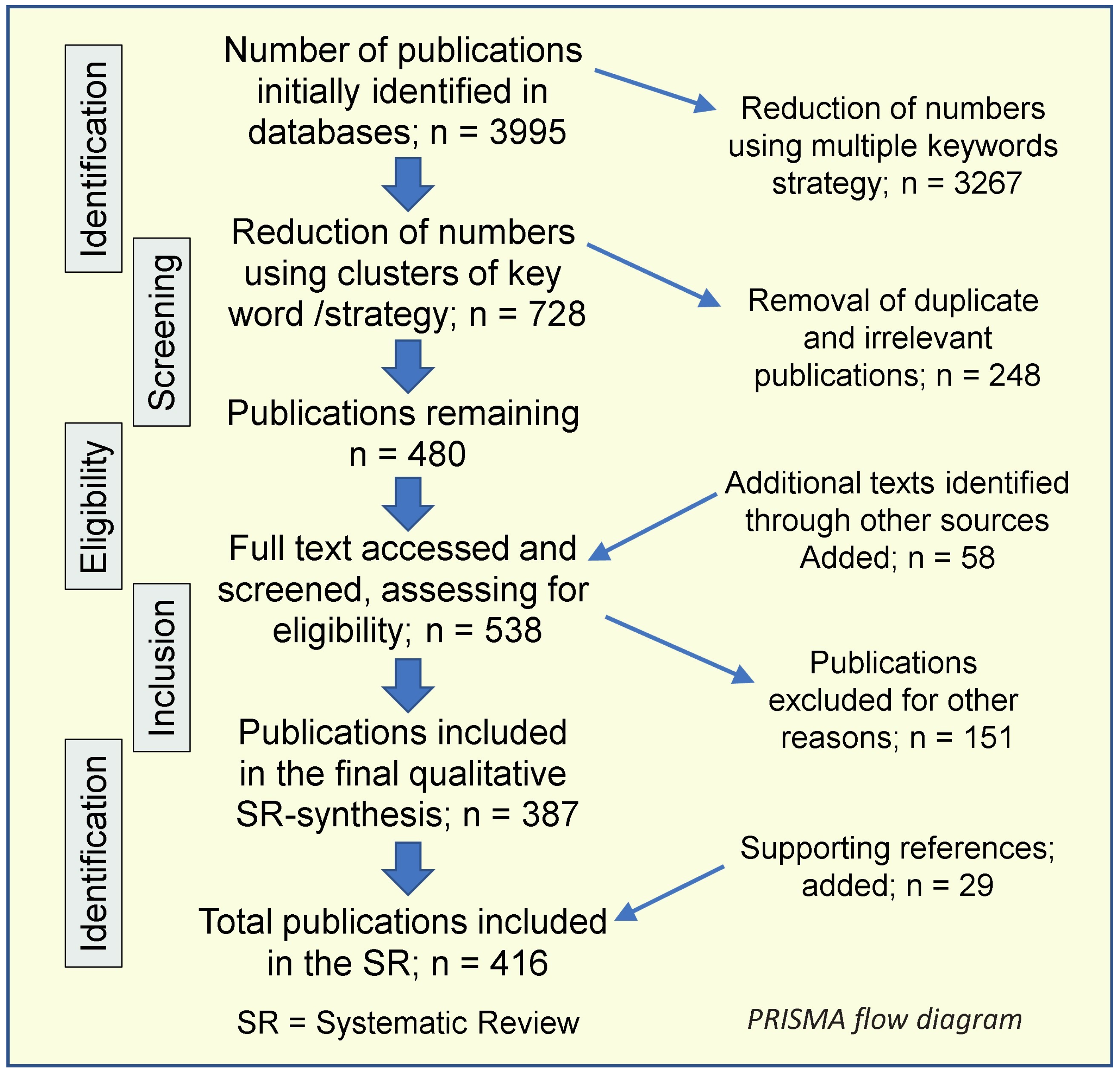
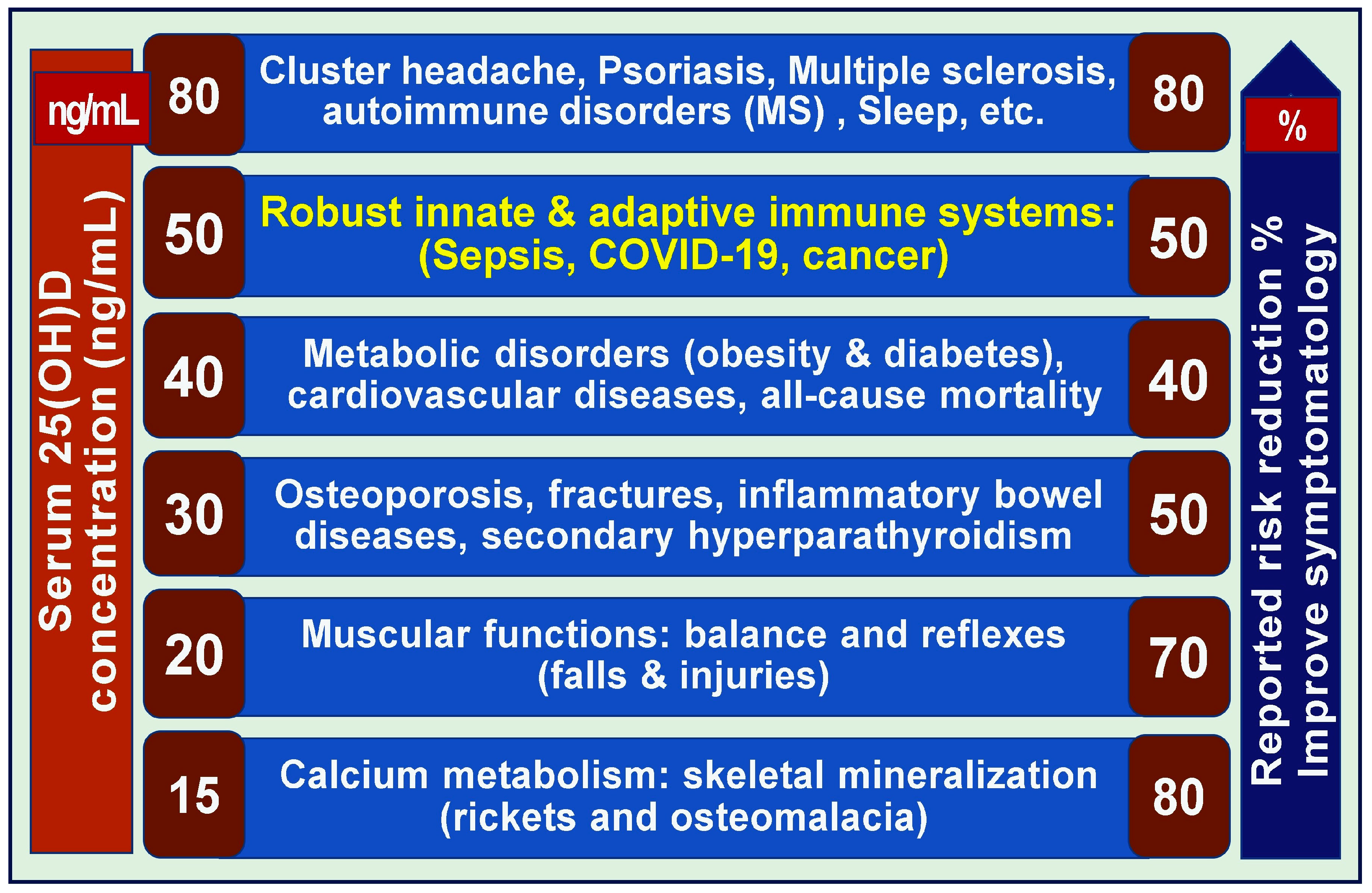
| PICOS Criteria | Conditions | |
|---|---|---|
| 1 | Participants | Adults aged 18 to 80; de novo or diagnosis of a cancer |
| 2 | Intervention | Vitamin D, calcium and vitamin D, calcifediol, solar UVB exposure, Omega-3 fatty acids |
| 3 | Comparison/control | Retrospective, case report, observational, epidemiological, community-based/ecological, and randomized control studies, and longer-term follow-up studies related to cancer |
| 4 | Outcome elements | Morbidity, complications, and death; all-cause mortality. Relationship of serum 25(OH)D to the incidences and changes in cancer prevalence |
| 5 | Study design philosophies | Randomized controlled clinical trials, non-randomized controlled clinical trials, non-randomized non-controlled trials, and prospective and observational studies related to cancer are included. |
| Cancer Type | References |
|---|---|
| All cancers | [94,147,187,189,190,192,193,194,195,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221] |
| Breast cancer and survival | [150,151,163,189,190,192,193,194,215,216,217,218,219,220,222,223,224,225,226,227,228,229] |
| Colorectal cancers | [89,90,109,150,151,163,189,190,192,193,194,229,230,231,232,233,234] |
| Gastric cancers | [235,236,237,238] |
| Oral and nasopharyngeal carcinomas | [239,240] |
| Lung cancer | [241,242] |
| Pancreas and esophagus | [243,244,245] |
| Non-Hodgkins lymphoma | [119,246,247,248,249,250,251] |
| Melanoma | [1,103,252,253,254,255] |
| VDR polymorphisms | [211,256,257,258,259,260] |
| Cancer mortality | [39,41] |
| Relationship to living in higher latitudes | [89,107,189,190,261,262,263,264,265,266,267] |
| Relationship to serum 25(OH)D levels | [89,163,168,187,195,206,207,208,209,224,225,226,268,269,270,271] |
| [70,167,168,272] | |
| UVB/sun exposure and cancer reduction | [70,88,89,110,168,187,189,190,192,193,194,261,262,263,273,274,275,276,277,278,279,280,281,282,283,284] |
| Cancer metastasis | [285,286,287,288] |
| Recommendation | Reference | Recommendation | Reference |
|---|---|---|---|
| Maintaining a healthy weight at any age | [299] | Avoiding all forms of smoking and exposure to second-hand smoke | [299] |
| Engaging in regular physical activity | [308,309] | Breastfeeding infants | |
| Adopting a healthy diet, like the Mediterranean diet | [300] | Protecting the skin from excessive sun exposure | [299] |
| Avoidance or limiting alcohol intake to one drink per day for women and two for men | [299] | Being vaccinated against hepatitis B and HPV | [375] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimalawansa, S.J. Vitamin D’s Impact on Cancer Incidence and Mortality: A Systematic Review. Nutrients 2025, 17, 2333. https://doi.org/10.3390/nu17142333
Wimalawansa SJ. Vitamin D’s Impact on Cancer Incidence and Mortality: A Systematic Review. Nutrients. 2025; 17(14):2333. https://doi.org/10.3390/nu17142333
Chicago/Turabian StyleWimalawansa, Sunil J. 2025. "Vitamin D’s Impact on Cancer Incidence and Mortality: A Systematic Review" Nutrients 17, no. 14: 2333. https://doi.org/10.3390/nu17142333
APA StyleWimalawansa, S. J. (2025). Vitamin D’s Impact on Cancer Incidence and Mortality: A Systematic Review. Nutrients, 17(14), 2333. https://doi.org/10.3390/nu17142333






