Polyphenols as Antiviral Agents: Their Potential Against a Range of Virus Types
Abstract
1. Introduction
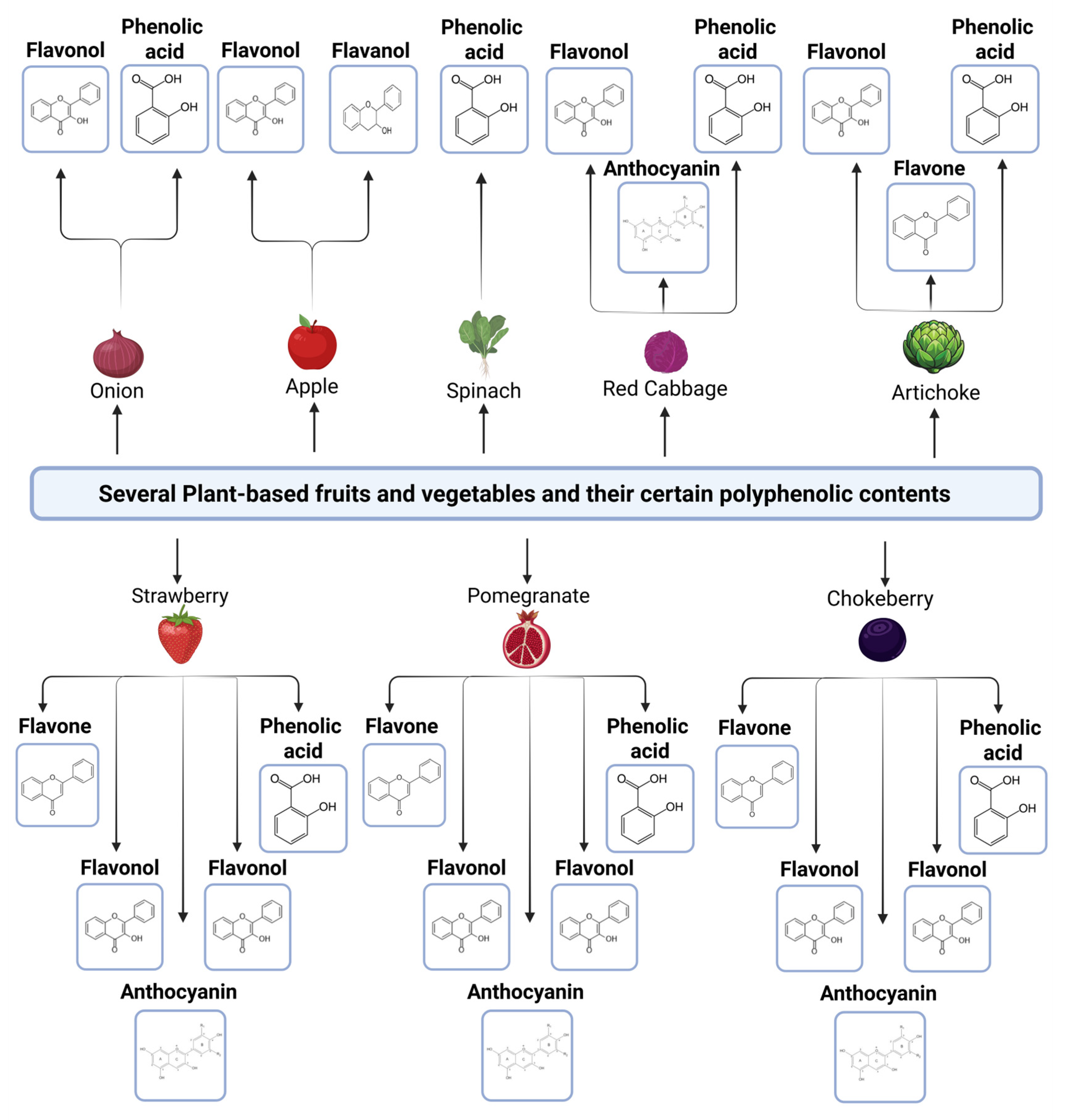
2. Overview of Polyphenols
- (a)
- Classification of Polyphenols and Sources of Polyphenols: Dietary Sources
- (b)
- General Biological Activities of Polyphenols Relevant to Antiviral Activity
3. Common Antiviral Polyphenols
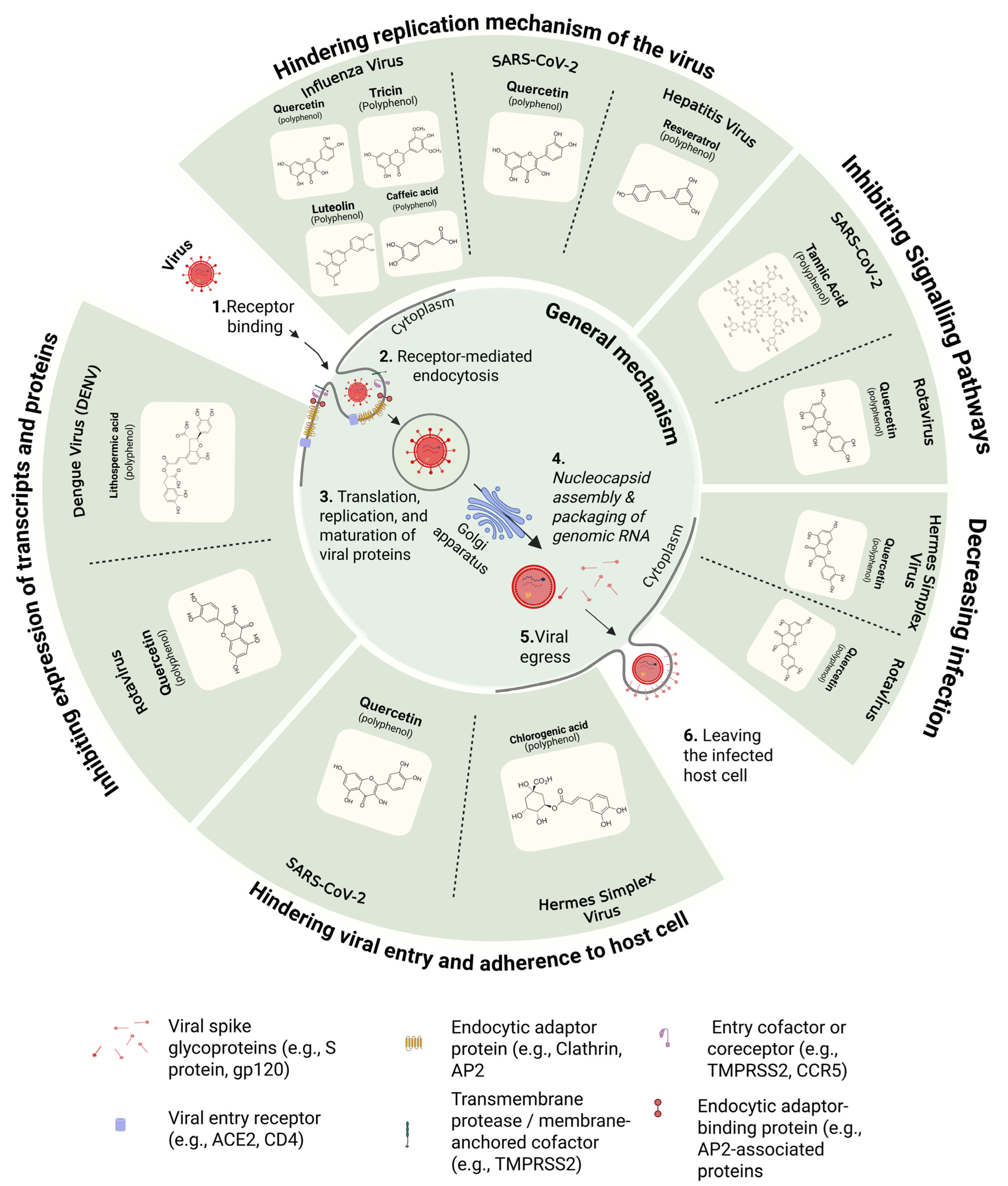
4. Mechanisms of Antiviral Action of Polyphenols Associated with Several Common Viruses
- (i)
- SARS-CoV-2
- (ii)
- Influenza Virus
- (iii)
- Hepatitis Virus
- (iv)
- Herpes Simplex Virus
- (v)
- Dengue Virus (DENV)
- (vi)
- Rotavirus
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chodkowski, M.; Nowak, S.; Janicka, M.; Sobczak, M.; Granica, S.; Bańbura, M.W.; Krzyzowska, M.; Cymerys, J. In Vitro Antiviral Activity of Kalanchoe Daigremontiana Extract against Human Herpesvirus Type 1. Int. J. Mol. Sci. 2024, 25, 7507. [Google Scholar] [CrossRef] [PubMed]
- Prieto, K.; Arévalo, C.; Lasso, P.; Carlosama, C.; Urueña, C.; Fiorentino, S.; Barreto, A. Plant Extracts Modulate Cellular Stress to Inhibit Replication of Mouse Coronavirus MHV-A59. Heliyon 2024, 10, e23403. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, G.; Gerardi, C.; Uberti-Foppa, C.; Lopalco, L. Can Natural Polyphenols Help in Reducing Cytokine Storm in COVID-19 Patients? Molecules 2020, 25, 5888. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, M.; Han, F.; Liao, W.; Duan, X. Natural Polyphenols for Drug Delivery and Tissue Engineering Construction: A Review. Eur. J. Med. Chem. 2024, 266, 116141. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Mori, T.; Kanbara, H.; Habe, T.; Ota, N.; Kurebayashi, Y.; Suzuki, T. Green Tea Catechins Adsorbed on the Murine Pharyngeal Mucosa Reduce Influenza A Virus Infection. J. Funct. Foods 2020, 68, 103894. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S.; Rangan, L. Pongamia pinnata L. Seed-Derived Karanjin as Prominent Antiviral Agent against Newcastle Disease Virus. Virology 2024, 600, 110272. [Google Scholar] [CrossRef]
- Ekowati, J.; Tejo, B.A.; Maulana, S.; Kusuma, W.A.; Fatriani, R.; Ramadhanti, N.S.; Norhayati, N.; Nofianti, K.A.; Sulistyowaty, M.I.; Zubair, M.S.; et al. Potential Utilization of Phenolic Acid Compounds as Anti-Inflammatory Agents through TNF-α Convertase Inhibition Mechanisms: A Network Pharmacology, Docking, and Molecular Dynamics Approach. ACS Omega 2023, 8, 46851–46868. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Afnan; Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, Cardio-Protective and Anti-Inflammatory Potential of Natural-Sources-Derived Phenolic Acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef]
- Sun, X.; Ye, H.; Liu, J.; Wu, L.; Lin, D.; Yu, Y.; Gao, F. Assessment of Anti-Diabetic Activity of Peanut Shell Polyphenol Extracts. J. Zhejiang Univ.-Sci. B 2018, 19, 764–775. [Google Scholar] [CrossRef]
- Galanakis, C.M. (Ed.) Food Bioactives and Health; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-57468-0. [Google Scholar]
- Verma, C. (Ed.) Science and Engineering of Polyphenols: Fundamentals and Industrial Scale Application, 1st ed.; Wiley: Hoboken, NJ, USA, 2024; ISBN 978-1-394-20390-1. [Google Scholar]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317. [Google Scholar] [CrossRef]
- Goldberg, D. Critical Reviews in Clinical Laboratory Sciences. Crit. Rev. Clin. Lab. Sci. 2010, 47, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Tago, R.; Yamauchi, S.; Maruyama, M.; Akiyama, K.; Sugahara, T.; Kishida, T.; Koba, Y. Structure-Antibacterial Activity Relationship for 9-O,9′-O-Demethyl (+)-Virgatusin. Biosci. Biotechnol. Biochem. 2008, 72, 1032–1037. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A Review on Its Nutraceutical Properties and Utilization in Various Food Products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Hao, L.; Li, S.; Chen, G.; Nie, A.; Zeng, L.; Xiao, Z.; Hu, X. Study on the Mechanism of Quercetin in Sini Decoction Plus Ginseng Soup to Inhibit Liver Cancer and HBV Virus Replication through CDK1. Chem. Biol. Drug Des. 2024, 103, e14567. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Ciesek, S.; Von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The Green Tea Polyphenol, Epigallocatechin-3-Gallate, Inhibits Hepatitis C Virus Entry. Hepatology 2010, 54, 1947–1955. [Google Scholar] [CrossRef]
- Yap, J.K.W.; Kehoe, S.T.; Woodman, C.B.J.; Dawson, C.W. The Major Constituent of Green Tea, Epigallocatechin-3-Gallate (EGCG), Inhibits the Growth of HPV18-Infected Keratinocytes by Stimulating Proteasomal Turnover of the E6 and E7 Oncoproteins. Pathogens 2021, 10, 459. [Google Scholar] [CrossRef]
- Bolat, E.; Sarıtaş, S.; Duman, H.; Eker, F.; Akdaşçi, E.; Karav, S.; Witkowska, A.M. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients 2024, 16, 2550. [Google Scholar] [CrossRef]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus Synthetic Drugs; Beliefs and Facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar] [PubMed]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Mulay, A.; Konda, B.; Garcia, G.; Yao, C.; Beil, S.; Villalba, J.M.; Koziol, C.; Sen, C.; Purkayastha, A.; Kolls, J.K.; et al. SARS-CoV-2 Infection of Primary Human Lung Epithelium for COVID-19 Modeling and Drug Discovery. Cell Rep. 2021, 35, 109055. [Google Scholar] [CrossRef] [PubMed]
- Terliesner, N.; Unterwalder, N.; Edelmann, A.; Corman, V.; Knaust, A.; Rosenfeld, L.; Gratopp, A.; Ringe, H.; Martin, L.; Von Bernuth, H.; et al. Viral Infections in Hospitalized Children in Germany during the COVID-19 Pandemic: Association with Non-Pharmaceutical Interventions. Front. Pediatr. 2022, 10, 935483. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral Activity of Green Tea and Black Tea Polyphenols in Prophylaxis and Treatment of COVID-19: A Review. Phytomedicine 2021, 85, 153286. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Zeng, Y.-T.; Lin, C.-J.; Harroun, S.G.; Anand, A.; Chang, L.; Wu, C.-J.; Lin, H.-J.; Huang, C.-C. Partial Carbonization of Quercetin Boosts the Antiviral Activity against H1N1 Influenza A Virus. J. Colloid Interface Sci. 2022, 622, 481–493. [Google Scholar] [CrossRef]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 9, 1721. [Google Scholar] [CrossRef]
- Siew, Z.Y.; Asudas, E.; Khoo, C.T.; Cho, G.H.; Voon, K.; Fang, C.-M. Fighting Nature with Nature: Antiviral Compounds That Target Retroviruses. Arch. Microbiol. 2024, 206, 130. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-J.; Kim, D.-H. 5,7-Dihydroxy-6-Methoxy-Flavonoids Eliminate HIV-1 D3-Transfected Cytoprotective Macrophages by Inhibiting the PI3K/Akt Signaling Pathway: Dihydroxymethoxyflavonoids Eliminate HIV1-Transfected Macrophages. Phytother. Res. 2015, 29, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An Analysis of FDA-Approved Drugs: Natural Products and Their Derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Saini, R.; Ali, M.I.; Pant, M.; Warghane, A. Current Status of Potential Antiviral Drugs Derived from Plant, Marine, and Microbial Sources. Anti-Infect. Agents 2024, 22, e090124225414. [Google Scholar] [CrossRef]
- Raposo, R.; Chinnici, F.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Carbú, M.; Guerrero, R.F.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Cantos-Villar, E. Sulfur Free Red Wines through the Use of Grapevine Shoots: Impact on the Wine Quality. Food Chem. 2018, 243, 453–460. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Valls-Fonayet, J.; Richard, T.; Cantos-Villar, E. A Rapid Quantification of Stilbene Content in Wine by Ultra-High Pressure Liquid Chromatography—Mass Spectrometry. Food Control 2020, 108, 106821. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.-W.; Bae, J.-Y.; Heo, J.; Kim, D.; Han, S.-Z.; Park, M.-S. Aronia Melanocarpa and Its Components Demonstrate Antiviral Activity against Influenza Viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, R.; Morimoto, R.; Horio, Y.; Sumitani, H.; Isegawa, Y. Inhibition of Influenza Virus Replication by Apiaceae Plants, with Special Reference to Peucedanum Japonicum (Sacna) Constituents. J. Ethnopharmacol. 2022, 292, 115243. [Google Scholar] [CrossRef]
- Santos Pereira, R.; Vasconcelos Costa, V.; Luiz Menezes Gomes, G.; Rodrigues Valadares Campana, P.; Maia De Pádua, R.; Barbosa, M.; Oki, Y.; Heiden, G.; Fernandes, G.W.; Menezes De Oliveira, D.; et al. Anti-Zika Virus Activity of Plant Extracts Containing Polyphenols and Triterpenes on Vero CCL-81 and Human Neuroblastoma SH-SY5Y Cells. Chem. Biodivers. 2022, 19, e202100842. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Lévèques, A.; Rein, M.; Teml, A.; Schäfer, C.; Hofmann, U.; Li, H.; Schwab, M.; Eichelbaum, M.; Williamson, G. Intestinal Absorption, Metabolism, and Excretion of (–)-Epicatechin in Healthy Humans Assessed by Using an Intestinal Perfusion Technique. Am. J. Clin. Nutr. 2013, 98, 924–933. [Google Scholar] [CrossRef]
- De Freitas Queiroz Barros, H.D.; Maróstica Junior, M.R. Phenolic Compound Bioavailability Using In Vitro and In Vivo Models. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–126. ISBN 978-0-12-814774-0. [Google Scholar]
- Ozkan, G.; Ceyhan, T.; Çatalkaya, G.; Rajan, L.; Ullah, H.; Daglia, M.; Capanoglu, E. Encapsulated Phenolic Compounds: Clinical Efficacy of a Novel Delivery Method. Phytochem. Rev. 2024, 23, 781–819. [Google Scholar] [CrossRef]
- Eker, F.; Akdaşçi, E.; Duman, H.; Bechelany, M.; Karav, S. Gold Nanoparticles in Nanomedicine: Unique Properties and Therapeutic Potential. Nanomaterials 2024, 14, 1854. [Google Scholar] [CrossRef]
- Duman, H.; Akdaşçi, E.; Eker, F.; Bechelany, M.; Karav, S. Gold Nanoparticles: Multifunctional Properties, Synthesis, and Future Prospects. Nanomaterials 2024, 14, 1805. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Jaouhari, Y.; Bordiga, M.; Karav, S. The Impact of Freeze Drying on Bioactivity and Physical Properties of Food Products. Appl. Sci. 2024, 14, 9183. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.-Y.; Zhou, S.-H.; Cheng, G.; Wei, R.-F.; Zhou, Y.-M.; Zhang, Y.; Xie, T.-L.; Zhang, L. The Metabolomic Profiling of the Flavonoid Compounds in Red Wine Grapes and the Impact of Training Systems in the Southern Subtropical Region of China. Int. J. Mol. Sci. 2024, 25, 8624. [Google Scholar] [CrossRef]
- Lu, J.; Tang, Y.; Li, H.; Chen, X.; Qin, P.; Xu, J.; Li, W.; Chen, L. Identifying Exifone as a Dual-Target Agent Targeting Both SARS-CoV-2 3CL Protease and the ACE2/S-RBD Interaction Among Clinical Polyphenolic Compounds. Int. J. Mol. Sci. 2025, 26, 2243. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y. Tannic Acids and Proanthocyanidins in Tea Inhibit SARS-CoV-2 Variants Infection. Am. J. Cancer Res. 2024, 14, 2555–2569. [Google Scholar] [CrossRef] [PubMed]
- Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Milić, A.; Šumić, Z.; Žunić, D.; Adamović, B.; Ilin, Ž. Biostimulants Improve Bulb Yield, Concomitantly Affecting the Total Phenolics, Flavonoids, and Antioxidant Capacity of Onion (Allium Cepa). Horticulturae 2024, 10, 391. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Li, C.; Chu, Q.; Cheng, S.; Su, L.; Shao, D.; Guo, X.; He, Z.; Zhou, X. Effect of Light Intensity on Celery Growth and Flavonoid Synthesis. Front. Plant Sci. 2024, 14, 1326218. [Google Scholar] [CrossRef] [PubMed]
- Foschi, M.; Marsili, L.; Luciani, I.; Gornati, G.; Scappaticci, C.; Ruggieri, F.; D’Archivio, A.A.; Biancolillo, A. Optimization of the Cold Water Extraction Method for High-Value Bioactive Compounds from Chamomile (Matricaria chamomilla L.) Flower Heads Through Chemometrics. Molecules 2024, 29, 4925. [Google Scholar] [CrossRef]
- Curtasu, M.V.; Nørskov, N.P. Quantitative Distribution of Flavan-3-Ols, Procyanidins, Flavonols, Flavanone and Salicylic Acid in Five Varieties of Organic Winter Dormant Salix Spp. by LC-MS/MS. Heliyon 2024, 10, e25129. [Google Scholar] [CrossRef]
- Arora, B.; Lather, V.; Pathalingappa, M.B.; Walia, R. Enhancement of Aqueous Solubility of Hesperidin and Naringenin Utilizing Hydrotropic Solubilization Technique: Characterization and In Vitro Evaluation. J. Asian Nat. Prod. Res. 2024, 26, 1207–1218. [Google Scholar] [CrossRef]
- Mohammed, H.; Abdullah, A.S.; AL-Mozie’l, M.S.G. Protection Effect of Soy Isoflavones (Genistein and Daidzein) on Hematologic Parameters in Acute Kidney Injury. Acad. Open 2024, 9. [Google Scholar] [CrossRef]
- Jiang, N.; Gomez, L.; Grotewold, E. Extraction and Quantification of Total Anthocyanins, Determination of Anthocyanidin Core Structures, and Characterization of Specific Anthocyanins from Maize. Cold Spring Harb. Protoc. 2025, 2025. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.L.; Mohammed, R.K. The Study of the Antibacterial Effect of Anthocyanin Pigment Extracted From Red Cabbage (Brassica Oleracea Var. Capitata f. Rubra) and Red Radish Peels (Raphanus Sativus. Var. Sativus). IOP Conf. Ser. Earth Environ. Sci. 2024, 1371, 052089. [Google Scholar] [CrossRef]
- Hariri, M.; Amirkalali, B.; Gholami, A. Effects of Purified Anthocyanins Supplementation on Serum Concentration of Inflammatory Mediators: A Systematic Review and Dose–Response Meta-analysis on Randomized Clinical Trials. Phytother. Res. 2024, 38, 1494–1508. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Chang, S.K.; Chen, Y.; Sheng, Z.; Jiang, Y.; Yang, B. The Structure Characteristics, Biosynthesis and Health Benefits of Naturally Occurring Rare Flavonoids. Crit. Rev. Food Sci. Nutr. 2024, 64, 2490–2512. [Google Scholar] [CrossRef]
- Arzuk, E.; Armağan, G. Genistein and Daidzein Induce Ferroptosis in MDA-MB-231 Cells. J. Pharm. Pharmacol. 2024, 76, 1599–1608. [Google Scholar] [CrossRef]
- Mehrabi, M.; Esmaeili, S.; Ezati, M.; Abassi, M.; Rasouli, H.; Nazari, D.; Adibi, H.; Khodarahmi, R. Antioxidant and Glycohydrolase Inhibitory Behavior of Curcumin-Based Compounds: Synthesis and Evaluation of Anti-Diabetic Properties in Vitro. Bioorg. Chem. 2021, 110, 104720. [Google Scholar] [CrossRef]
- Lassouane, N.; Aïd, F.; Quinet, M.; Lutts, S. Phenolic Acids and Flavonoids Classes in Acacia Arabica (Lam) Willd. Seedling during Water Stress and Subsequent Re-Hydration. Plant Soil 2024, 496, 449–471. [Google Scholar] [CrossRef]
- Kika, J.; Jakubczyk, K.; Ligenza, A.; Maciejewska-Markiewicz, D.; Szymczykowska, K.; Janda-Milczarek, K. Matcha Green Tea: Chemical Composition, Phenolic Acids, Caffeine and Fatty Acid Profile. Foods 2024, 13, 1167. [Google Scholar] [CrossRef]
- Özel, H.B.; Baş Topcu, K.S.; Dere, S.; Genç, N.; Kisa, D. In Vitro and in Silico Based Assessment of Biological Activity of Endemic Allium Species: LC-MS/MS Analysis of Onions. Food Biosci. 2024, 59, 104209. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vidović, B.B.; Gašić, U.M.; Milenković, M.; Kostić, A.Ž.; Stanojević, S.P.; Ilić, T.; Pešić, M.B. A Systematic UHPLC Q-ToF MS Approach for the Characterization of Bioactive Compounds from Freeze-Dried Red Goji Berries (L. barbarum L.) Grown in Serbia: Phenolic Compounds and Phenylamides. Food Chem. 2024, 456, 140044. [Google Scholar] [CrossRef]
- AboelAinin, M.A.; El-Ashmony, R.M.S.; Tantawy, I.A.A.; Mohamed, H.S.; Galal, A.A. Acetic Acid and Hydrogen Peroxide Improved Defense-Related Biochemical Responses of Onion Bulbs to Black Mold Rot Caused by Aspergillus niger L. Int. J. Veg. Sci. 2024, 30, 411–428. [Google Scholar] [CrossRef]
- Wang, D.; Wang, G.; Lu, X.; Liu, Z.; Sun, S.; Guo, H.; Tian, W.; Li, Z.; Wang, L.; Li, L.; et al. Dynamic Changes in Polyphenols in Fruit Development of Red Flesh Apple ‘Hongxun 2’. Horticulturae 2024, 10, 1125. [Google Scholar] [CrossRef]
- Ceylan, F.D.; Günal-Köroğlu, D.; Saricaoglu, B.; Ozkan, G.; Capanoglu, E.; Calina, D.; Sharifi-Rad, J. Anticancer Potential of Hydroxycinnamic Acids: Mechanisms, Bioavailability, and Therapeutic Applications. Naunyn. Schmiedebergs Arch. Pharmacol. 2025, 398, 469–495. [Google Scholar] [CrossRef]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical Investigation and Antioxidant Properties of Unripe Tomato Cultivars (Solanum lycopersicum L.). Food Chem. 2024, 438, 137863. [Google Scholar] [CrossRef] [PubMed]
- Beilankouhi, S.; Pourfarzad, A.; Ghanbarzadeh, B.; Rasouli, M.; Hamishekar, H. Identification of Polyphenol Composition in Grape (Vitis Vinifera Cv. Bidaneh Sefid) Stem Using Green Extraction Methods and LC–MS/MS Analysis. Food Sci. Nutr. 2024, 12, 6789–6798. [Google Scholar] [CrossRef]
- Hanzouli, F.; Daldoul, S.; Zemni, H.; Boubakri, H.; Vincenzi, S.; Mliki, A.; Gargouri, M. Stilbene Production as Part of Drought Adaptation Mechanisms in Cultivated Grapevine (Vitis vinifera L.) Roots Modulates Antioxidant Status. Plant Biol. 2025, 27, 102–115. [Google Scholar] [CrossRef]
- Gołąbek-Grenda, A.; Juzwa, W.; Kaczmarek, M.; Olejnik, A. Resveratrol and Its Natural Analogs Mitigate Immune Dysregulation and Oxidative Imbalance in the Endometriosis Niche Simulated in a Co-Culture System of Endometriotic Cells and Macrophages. Nutrients 2024, 16, 3483. [Google Scholar] [CrossRef]
- D’Amico, E.; Cinquini, C.; Petrini, M.; Barone, A.; Iezzi, G.; D’Ercole, S.; De Filippis, B.; Pierfelice, T.V. The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids. Metabolites 2024, 14, 350. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, Z.; Song, Z.; Li, Y.; Shim, Y.Y.; Reaney, M.J.T.; Lee, Y.Y.; Wang, Y.; Zhang, N. Bioconversion of Lignans in Flaxseed Cake by Fermented Tofu Microbiota and Isolation of Enterococcus Faecium Strain ZB26 Responsible for Converting Secoisolariciresinol Diglucoside to Enterodiol. Food Chem. 2024, 457, 140077. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Liu, B.; Li, Y.; Wang, M.; Sun, Q. Lignan Intake and Type 2 Diabetes Incidence Among US Men and Women. JAMA Netw. Open 2024, 7, e2426367. [Google Scholar] [CrossRef] [PubMed]
- Sintim, H.O. Seed Quality and Relative Lignan Profiles of Sesame Prospected from Northern Ghana. Heliyon 2024, 10, e39108. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.-W.; Sung, J.; Kim, Y. Optimal Extraction Conditions and Quantification of Lignan Phytoestrogens in Cereal Grains Using Targeted LC-MS/MS. Front. Nutr. 2024, 11, 1409309. [Google Scholar] [CrossRef]
- Oguz, M.C. Stimulating Endogenous Hormone Content by Plant Extracts: Increased In Vitro Regeneration of Flax (Linum usitatissimum) Cultivars. J. Crop Sci. Biotechnol. 2025, 28, 93–105. [Google Scholar] [CrossRef]
- Nittayananta, W.; Lerdsamran, H.; Chutiwitoonchai, N.; Promsong, A.; Srichana, T.; Netsomboon, K.; Prasertsopon, J.; Kerdto, J. A Novel Film Spray Containing Curcumin Inhibits SARS-CoV-2 and Influenza Virus Infection and Enhances Mucosal Immunity. Virol. J. 2024, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Vardhini, N.M.; Punia, J.; Jat, S.; Pawar, S.D.; Devi, N.; Radhakrishnanand, P.; Murty, U.S.; Saini, A.; Sethi, K.K.; Kumar, P. Purification and Characterization of Pure Curcumin, Desmethoxycurcumin, and Bisdemethoxycurcumin from North-East India Lakadong Turmeric (Curcuma Longa). J. Chromatogr. A 2023, 1708, 464358. [Google Scholar] [CrossRef]
- Idowu-Adebayo, F.; Fogliano, V.; Linnemann, A. Turmeric-Fortified Cow and Soya Milk: Golden Milk as a Street Food to Support Consumer Health. Foods 2022, 11, 558. [Google Scholar] [CrossRef]
- Watrelot, A.A. Tannin Content in Vitis Species Red Wines Quantified Using Three Analytical Methods. Molecules 2021, 26, 4923. [Google Scholar] [CrossRef]
- Rouxinol, M.I.; Martins, M.R.; Murta, G.C.; Mota Barroso, J.; Rato, A.E. Quality Assessment of Red Wine Grapes through NIR Spectroscopy. Agronomy 2022, 12, 637. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.-L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef]
- Radulescu, C.; Olteanu, R.L.; Buruleanu, C.L.; Tudorache, M.N.; Dulama, I.D.; Stirbescu, R.M.; Bucurica, I.A.; Stanescu, S.G.; Banica, A.L. Polyphenolic Screening and the Antioxidant Activity of Grape Pomace Extracts of Romanian White and Red Grape Varieties. Antioxidants 2024, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Stannard, H.; Koszalka, P.; Deshpande, N.; Desjardins, Y.; Baz, M. Pre-Clinical Evaluation of the Antiviral Activity of Epigalocatechin-3-Gallate, a Component of Green Tea, against Influenza A(H1N1)Pdm Viruses. Viruses 2023, 15, 2447. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoo, B.-G.; Chen, F.; Byun, E.-B.; Song, H.-Y. Fermented Codonopsis Lanceolata Root Extract Exhibits Anti-Viral Effects against Influenza A Infection by Inhibiting Neuraminidase Activity and Inflammatory Responses. Food Biosci. 2024, 61, 104617. [Google Scholar] [CrossRef]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; Da Cunha Lima, B.F.; Rodrigues, B.M.; et al. Antiviral, Anti-Inflammatory and Antioxidant Effects of Curcumin and Curcuminoids in SH-SY5Y Cells Infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef]
- Rajendrasozhan, S. Antioxidant, Antibacterial and Antiviral Effect of the Combination of Ginger and Garlic Extracts. Bioinformation 2024, 20, 11–17. [Google Scholar] [CrossRef]
- Thomasi, R.M.D.O.; Teixeira, T.R.; Lopes, G.F.M.; Mendonça, S.C.; Gomes, B.A.; Leitão, S.G.; Oliveira, T.A.D.; Fonseca, S.T.D.D.; Taranto, A.G.; Ferreira, J.M.S.; et al. Antiviral Activity of Flavonoids from Bauhinia Holophylla Leaves against Zika Virus. Microbiol. Res. 2024, 15, 582–597. [Google Scholar] [CrossRef]
- Da Conceição, P.J.P.; Ayusso, G.M.; Carvalho, T.; Duarte Lima, M.L.; Marinho, M.D.S.; Moraes, F.R.; Galán-Jurado, P.E.; González-Santamaría, J.; Bittar, C.; Zhang, B.; et al. In Vitro Evaluation of the Antiviral Activity of Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) Against Mayaro Virus. Viruses 2025, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, M.M.; Fuentes, P.; Chiappetta, D.; Flichman, D.M. Contrasting Effect of Curcumin on Hepatitis B Virus Replication According to the Hepatoma Cell Line. Pathogens 2025, 14, 203. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.-J.; Kim, J.H.; Kwon, H.-J.; Kim, J.H.; Park, K.H.; Rho, M.-C.; Lee, W.S. Neuraminidase Inhibitory Activities of Flavonols Isolated from Rhodiola Rosea Roots and Their in Vitro Anti-Influenza Viral Activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Kim, Y.; Narayanan, S.; Chang, K.-O. Inhibition of Influenza Virus Replication by Plant-Derived Isoquercetin. Antivir. Res. 2010, 88, 227–235. [Google Scholar] [CrossRef]
- Ochnik, M.; Franz, D.; Sobczyński, M.; Naporowski, P.; Banach, M.; Orzechowska, B.; Sochocka, M. Inhibition of Human Respiratory Influenza A Virus and Human Betacoronavirus-1 by the Blend of Double-Standardized Extracts of Aronia Melanocarpa (Michx.) Elliot and Sambucus nigra L. Pharmaceuticals 2022, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.-Y.; Kim, Y.M.; Park, S.-J.; Rho, M.-C.; Kim, S.-J.; Lee, W.S. Influenza Virus Neuraminidase Inhibitory Activity of Phlorotannins from the Edible Brown Alga Ecklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The Green Tea Molecule EGCG Inhibits Zika Virus Entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Salavatiha, Z.; Gogoi, U.; Mohebbi, A. An Overview of Anti-Hepatitis B Virus Flavonoids and Their Mechanisms of Action. Front. Cell. Infect. Microbiol. 2024, 14, 1356003. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yu, Z.-Y.; Chen, Y.-C.; Hung, S.-L. Effects of Epigallocatechin-3-Gallate and Acyclovir on Herpes Simplex Virus Type 1 Infection in Oral Epithelial Cells. J. Formos. Med. Assoc. 2021, 120, 2136–2143. [Google Scholar] [CrossRef]
- He, Y.; Hao, M.; Yang, M.; Guo, H.; Rayman, M.P.; Zhang, X.; Zhang, J. Influence of EGCG Oxidation on Inhibitory Activity against the SARS-CoV-2 Main Protease. Int. J. Biol. Macromol. 2024, 274, 133451. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Li, Q.-S.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules 2021, 26, 3962. [Google Scholar] [CrossRef]
- Setyawati, I.; Setiawan, A.G.; Nemchinova, M.; Vidilaseris, K. The Potential Inhibitory Mechanism of EGCG against the Chikungunya Virus Targeting Non-Structural Protein 2 through Molecular Dynamics Simulation. Sci. Rep. 2024, 14, 29797. [Google Scholar] [CrossRef]
- Chowdhury, P.; Sahuc, M.-E.; Rouillé, Y.; Rivière, C.; Bonneau, N.; Vandeputte, A.; Brodin, P.; Goswami, M.; Bandyopadhyay, T.; Dubuisson, J.; et al. Theaflavins, Polyphenols of Black Tea, Inhibit Entry of Hepatitis C Virus in Cell Culture. PLoS ONE 2018, 13, e0198226. [Google Scholar] [CrossRef]
- Liu, S.; Chen, R.; Hagedorn, C.H. Tannic Acid Inhibits Hepatitis C Virus Entry into Huh7.5 Cells. PLoS ONE 2015, 10, e0131358. [Google Scholar] [CrossRef]
- Hesari, A.; Ghasemi, F.; Salarinia, R.; Biglari, H.; Tabar Molla Hassan, A.; Abdoli, V.; Mirzaei, H. Effects of Curcumin on NF-κB, AP-1, and Wnt/Β-catenin Signaling Pathway in Hepatitis B Virus Infection. J. Cell. Biochem. 2018, 119, 7898–7904. [Google Scholar] [CrossRef] [PubMed]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Extract Diminishes Atherogenic Risk in Type 2 Diabetes Mellitus Patients With Obesity. medRxiv 2024. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive Compounds from Pomegranate Peels—Biological Properties, Structure–Function Relationships, Health Benefits and Food Applications—A Comprehensive Review. J. Funct. Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Sundararajan, A.; Ganapathy, R.; Huan, L.; Dunlap, J.R.; Webby, R.J.; Kotwal, G.J.; Sangster, M.Y. Influenza Virus Variation in Susceptibility to Inactivation by Pomegranate Polyphenols Is Determined by Envelope Glycoproteins. Antivir. Res. 2010, 88, 1–9. [Google Scholar] [CrossRef]
- Kwon, E.-B.; Kim, Y.S.; Han, S.M.; Kim, S.-G.; Choi, J.-G. The Protective Effect of Tilia Amurensis Honey on Influenza A Virus Infection through Stimulation of Interferon-Mediated IFITM3 Signaling. Biomed. Pharmacother. 2022, 153, 113259. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic Potential of Resveratrol against Emerging Respiratory Viral Infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Arena, G.; Lacanna, E.; Oliviero, G.; Colavita, F.; Mattia, E. Resveratrol Inhibits Epstein Barr Virus Lytic Cycle in Burkitt’s Lymphoma Cells by Affecting Multiple Molecular Targets. Antivir. Res. 2012, 96, 196–202. [Google Scholar] [CrossRef]
- Yiu, C.-Y.; Chen, S.-Y.; Chang, L.-K.; Chiu, Y.-F.; Lin, T.-P. Inhibitory Effects of Resveratrol on the Epstein-Barr Virus Lytic Cycle. Molecules 2010, 15, 7115–7124. [Google Scholar] [CrossRef]
- Wu, C.-C.; Fang, C.-Y.; Hsu, H.-Y.; Chen, Y.-J.; Chou, S.-P.; Huang, S.-Y.; Cheng, Y.-J.; Lin, S.-F.; Chang, Y.; Tsai, C.-H.; et al. Luteolin Inhibits Epstein-Barr Virus Lytic Reactivation by Repressing the Promoter Activities of Immediate-Early Genes. Antivir. Res. 2016, 132, 99–110. [Google Scholar] [CrossRef]
- Nomura, E.; Hosoda, A.; Morishita, H.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Taniguchi, H. Synthesis of Novel Polyphenols Consisted of Ferulic and Gallic Acids, and Their Inhibitory Effects on Phorbol Ester-Induced Epstein–Barr Virus Activation and Superoxide Generation. Bioorg. Med. Chem. 2002, 10, 1069–1075. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Liu, Z.; Le, Z.; Nie, T.; Qiao, D.; Su, Y.; Mai, H.; Chen, Y.; Liu, L. Therapeutic Nanovaccines Sensitize EBV-Associated Tumors to Checkpoint Blockade Therapy. Biomaterials 2020, 255, 120158. [Google Scholar] [CrossRef]
- Beik, A.; Joukar, S.; Najafipour, H. A Review on Plants and Herbal Components with Antiarrhythmic Activities and Their Interaction with Current Cardiac Drugs. J. Tradit. Complement. Med. 2020, 10, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Zhou, G.; Zhu, Z.; Cui, Y.; Pu, R. Antiviral Activities of Resveratrol against Rotavirus In Vitro and In Vivo. Phytomedicine 2020, 77, 153230. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Galvéz-Ruíz, J.C.; Ikner, L.A.; Umsza-Guez, M.A.; De Paula Castro, T.L.; Gerba, C.P. In Vitro Antiviral Effect of Mexican and Brazilian Propolis and Phenolic Compounds against Human Coronavirus 229E. Int. J. Environ. Health Res. 2023, 33, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Fatimawali; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T.B. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential Use of Polyphenols in the Battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Carrera Montoya, J.; Fritzlar, S.; Flavel, M.; Londrigan, S.L.; Mackenzie, J.M. Polyphenol Rich Sugarcane Extract (PRSE) Has Potential Antiviral Activity against Influenza A Virus in Vitro. Virology 2024, 590, 109969. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, R.; Ballini, A.; Jirillo, E.; Santacroce, L. Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics 2024, 13, 603. [Google Scholar] [CrossRef]
- Valdiviezo-Campos, J.E.; Rodriguez-Aredo, C.D.; Ruiz-Reyes, S.G.; Venegas-Casanova, E.A.; Bussmann, R.W.; Ganoza-Yupanqui, M.L. Identification of Polyphenols by UPLC-MS/MS and Their Potential in Silico Antiviral Activity from Medicinal Plants in Trujillo, Peru. J. Pharm. Pharmacogn. Res. 2024, 12, 323–347. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Berdyshev, D.V.; Krylova, N.V.; Iunikhina, O.V.; Kalinovskiy, A.I.; Shchelkanov, M.Y.; Mishchenko, N.P.; Aminin, D.L.; et al. Polyphenols from Maackia Amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection. Int. J. Mol. Sci. 2024, 25, 4142. [Google Scholar] [CrossRef]
- Okumuş, N.; Erdoğmuş, S.F.; Doğan, H.H.; Altintaş, Ö.E.; Çelik, S.; Duman, R.; Ünlü, Ü. Anti HSV-1 Activity of Cistus Laurifolius and Development of Antiviral Herbal Lip Balm. Rev. Bras. Farmacogn. 2024, 34, 625–636. [Google Scholar] [CrossRef]
- Zima, K.; Khaidakov, B.; Sochocka, M.; Ochnik, M.; Lemke, K.; Kowalczyk, P. Exploring the Potency of Polyphenol-Rich Blend from Lonicera Caerulea Var. Kamtschatica Sevast., Aronia Melanocarpa, and Echinacea Purpurea: Promising Anti-Inflammatory, Antioxidant, and Antiviral Properties. Heliyon 2024, 10, e35630. [Google Scholar] [CrossRef]
- Aljohani, A.K.; Maghrabi, N.A.; Alrehili, O.M.; Alharbi, A.S.; Alsihli, R.S.; Alharthe, A.M.; Albladi, R.S.; Alosaimi, K.A.; Albadrani, B.M.; Miski, S.F.; et al. Ajwa Date Extract (Phoenix dactylifera L.): Phytochemical Analysis, Antiviral Activity against Herpes Simplex Virus-I and Coxsackie B4 Virus, and in Silico Study. Saudi Med. J. 2025, 46, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Augustus, A.R.; Radhakrishnan, Y.; Bhaskar, J.P.; Ramamurthi, S.; Shunmugiah, K.P. Tannic Acid Modulates SARS-CoV-2 Pathogenesis by Curbing Key Host Receptors and Oxidative Stress. Toxicol. In Vitro 2025, 103, 105971. [Google Scholar] [CrossRef]
- Keshavarz, M.; Ghorbani, M.; Shamsizadeh, F.; Namdari, H.; Salimi, V.; Rezaei, F. Effects and Mechanisms of Silibinin on Influenza A/H1N1 Pathogenesis in a Mouse Model. J. Trop. Med. 2025, 2025, 6618423. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Esaulkova, Y.L.; Ilyina, P.A.; Zarubaev, V.V.; Sheikin, V.V.; Petruk, A.A.; Rubtsova, E.D.; Veklich, T.N. Antiviral Potential of Spiraea Extracts (Prepared by Repercolation) Against Influenza A (H1N1) Virus. Foods 2024, 13, 4008. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Ochiai, H.; Sakai, S.; Nakajima, K.; Terasawa, K. Inhibitory Effect of Ferulic Acid and Isoferulic Acid on Murine Interleukin-8 Production in Response to Influenza Virus Infections In Vitro and In Vivo. Planta Med. 1995, 61, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, Y.-X.; Liu, A.-L.; Wang, H.-D.; Wang, Y.-L.; Du, G.-H. Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles Speciosa. Molecules 2010, 15, 8507–8517. [Google Scholar] [CrossRef]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Phenolic Compounds Disrupt Spike-Mediated Receptor-Binding and Entry of SARS-CoV-2 Pseudo-Virions. PLoS ONE 2021, 16, e0253489. [Google Scholar] [CrossRef]
- Maaroufi, I.; Jamsransuren, D.; Hashida, K.; Matsuda, S.; Ogawa, H.; Takeda, Y. An Abies Extract Containing Nonvolatile Polyphenols Shows Virucidal Activity against SARS-CoV-2 That Is Enhanced in Increased pH Conditions. Pathogens 2023, 12, 1093. [Google Scholar] [CrossRef]
- Chen, C.; Yu, X.; Kuo, C.; Min, J.; Chen, S.; Ma, L.; Liu, K.; Guo, R. Overview of Antiviral Drug Candidates Targeting Coronaviral 3C-like Main Proteases. FEBS J. 2021, 288, 5089–5121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Pawara, R.; Surana, S.; Patel, H. The Repurposed ACE2 Inhibitors: SARS-CoV-2 Entry Blockers of COVID-19. Top. Curr. Chem. 2021, 379, 40. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.V.; Chan, M.; Banadyga, L.; He, S.; Zhu, W.; Chrétien, M.; Mbikay, M. Quercetin Inhibits SARS-CoV-2 Infection and Prevents Syncytium Formation by Cells Co-Expressing the Viral Spike Protein and Human ACE2. Virol. J. 2024, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Park, Y.-I.; Cha, Y.-E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea Polyphenols EGCG and Theaflavin Inhibit the Activity of SARS-CoV-2 3CL-Protease In Vitro. Evid. Based Complement. Alternat. Med. 2020, 2020, 5630838. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological Effects and Mechanisms of Tannic Acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, X.; Meng, Y.; Liao, F.; Li, Z.; Zheng, W.; Wang, W.; Dai, W.; Zhang, S.; Li, G. Lithospermic Acid Inhibits Dengue Virus Infection through Binding with Envelope Proteins. Microb. Pathog. 2024, 197, 107055. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Kim, C.; Yoon, S.-W.; Jeon, S.; Kweon, M.-N.; Seong, B.-L.; Seo, S.-U.; Jang, Y.H. Antiviral Activity of the Water Extract and Ethanol Extract of Sorbus Commixta against Influenza A Virus in Vitro. Heliyon 2024, 10, e39049. [Google Scholar] [CrossRef]
- Hayden, F.G.; Palese, P. Influenza Virus. In Clinical Virology; Richman, D.D., Whitley, R.J., Hayden, F.G., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 943–976. ISBN 978-1-68367-407-8. [Google Scholar]
- Blümel, J.; Burger, R.; Drosten, C.; Gröner, A.; Gürtler, L.; Heiden, M.; Hildebrandt, M.; Jansen, B.; Klamm, H.; Montag-Lessing, T.; et al. Influenza Virus. Transfus. Med. Hemotherapy 2008, 35, 42–49. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral Activity of Chlorogenic Acid against Influenza A (H1N1/H3N2) Virus and Its Inhibition of Neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef]
- Parvez, M.; Al-Dosari, M.; Abdelwahid, M.; Alqahtani, A.; Alanzi, A. Novel Anti-hepatitis B Virus-active Catechin and Epicatechin from Rhus tripartita. Exp. Ther. Med. 2022, 23, 398. [Google Scholar] [CrossRef]
- Pan, P.; Li, J.; Lin, W.; Long, G. Effects of Resveratrol on Hepatitis B Virus Replication: In Vitro and in Vivo Experiments. Intervirology 2022, 65, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, T.; Zhang, X.; Wang, L.; Li, T.; Zhang, X. Resveratrol Mitigates Oxidative Stress and Suppresses HBV Replication via Modulation of the SIRT1-Nrf2 Pathway in Liver Cells. Future Virol. 2025, 20, 83–92. [Google Scholar] [CrossRef]
- Zangooie, S.; Ghanbari, R.; Jalilian, F.A.; Mahmoudvand, S.; Teimoori, A. Antiviral Potential of Phenolic Compounds against HSV-1: In-Vitro Study. Antivir. Ther. 2024, 29, 13596535241271589. [Google Scholar] [CrossRef]
- Malavige, G.N.; Fernando, S.; Fernando, D.J.; Seneviratne, S.L. Dengue Viral Infections. Postgrad. Med. J. 2004, 80, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Menis Candela, F.; Soria, E.A.; Moliva, M.V.; Suárez Perrone, A.; Reinoso, E.B.; Giordano, W.; Sabini, M.C. Anti-DENV-2 Activity of Ethanolic Extracts from Arachis hypogaea L.: Peanut Skin as a Relevant Resource of Bioactive Compounds against Dengue Virus. Plants 2024, 13, 2881. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus Infection. Nat. Rev. Dis. Primer 2017, 3, 17083. [Google Scholar] [CrossRef]
- Parvez, M.K. Antiviral Flavonoids and Polyphenols Driven Novel Anti-HBV Efficacy of Ilex Paraguariensis. Arch. Med. Sci. 2025, 21, 919–929. [Google Scholar] [CrossRef]
- Yi, B.; Chew, B.X.Z.; Chen, H.; Lee, R.C.H.; Fong, Y.D.; Chin, W.X.; Mok, C.K.; Chu, J.J.H. Antiviral Activity of Catechin against Dengue Virus Infection. Viruses 2023, 15, 1377. [Google Scholar] [CrossRef]
- Tamkutė, L.; Haddad, J.G.; Diotel, N.; Desprès, P.; Venskutonis, P.R.; El Kalamouni, C. Cranberry Pomace Extract Exerts Antiviral Activity against Zika and Dengue Virus at Safe Doses for Adult Zebrafish. Viruses 2022, 14, 1101. [Google Scholar] [CrossRef]
- Ali, S.K.; Mahmoud, S.M.; El-Masry, S.S.; Alkhalifah, D.H.M.; Hozzein, W.N.; Aboel-Ainin, M.A. Phytochemical Screening and Characterization of the Antioxidant, Anti-Proliferative and Antibacterial Effects of Different Extracts of Opuntia Ficus-Indica Peel. J. King Saud Univ.-Sci. 2022, 34, 102216. [Google Scholar] [CrossRef]
- Han, J.-H.; Lee, N.; Choi, S.-W.; Yoo, M.; Yun, S.-I.; Chang, H.-J. Antiviral Activity of Polygonum Aviculare Extract against Murine Norovirus as Norovirus Surrogate and Its Application in Model Food. LWT 2024, 211, 116887. [Google Scholar] [CrossRef]
| Virus | Polyphenols | Model | Mechanisms of Antiviral Action | Treatment Concentration | Ref. |
|---|---|---|---|---|---|
| Hepatitis B virus | Curcumin | In vitro (HepG22.15 and Huh-7) | Triggers a cell-type-specific response in hepatoma cell lines and prevents an adaptive cellular optimization that enhances replication of the hepatitis B virus. | 20 µM for 72 h | [91] |
| Catechin/Epicatechin | In vitro (HepG22.15) | Particularly inhibit the viral antigen surface and show antiviral effect. | 50 µM for 5 days | [145] | |
| Polyphenol-rich Ilex paraguariensis extract (quercetin, kaempferol, rutin, caffeic acid, chlorogenic acid) | In vitro (HepG22.15) | Its antiviral phenolic compounds exhibit potential therapeutic efficacy. | 10 µg/mL | [152] | |
| SARS-CoV-2 | Exifone and benserazide hydrochloride | In vitro (protein-based assays: 3CLpro inhibition, ACE2-S-RBD interaction) | Impede the 3CLpro protease activity vital for SARS-CoV-2 replication. | IC50: (exifone: 3.18 µM; benserazide hydrochloride: 0.37 µM) | [47] |
| Tannic acid | In vitro, in silico, in vivo (Danio rerio) | Prevents the virus uptake to cells by regulating the proteins and exhibits an antioxidant role in ROS that is caused by viral infection. | 50 μg/mL | [127] | |
| Curcumin-containing film spray | In vitro (Vero and MDCK cells) | Inhibit inflammation and apoptosis in alveolar epithelial cells, adjust macrophage polarization, and protect alveolar epithelial cell integrity. | EC50: 3.15 µg/mL | [78] | |
| Abies sachalinensis (kaempferol, quercetin derivatives, ferulic acid, p-coumaric acid, lignans) | In vitro (African green monkey kidney cells: Vero) | Exhibits an inhibitory effect on the viral infection. | Original extract (undiluted)/1 min | [133] | |
| Brazilin and theaflavin-3,3′-digallate | In vitro (human alveolar epithelial cell line A549) | Exhibits multiple anti-SARS-CoV-2 activities. | 25 μg/mL | [132] | |
| Influenza | Polydatin | In vitro (Vero E6 African green monkey kidney cells, LGC, and MDCK Madin-Darby canine kidney cells) | Its treatment reduces IL-6 cytokine production by correcting its anti-inflammatory properties during the influenza A virus infection. | 40 µg/mL | [29] |
| Peucedanum japonicum (Sacna extract: quercetin, luteolin, caffeic acid) | In vitro (Madin–Darby canine kidney cell line: MDCK) | Inhibits the viral replication of both types of influenza A and B infection. | 2 mg/mL | [38] | |
| Curcumin-containing film spray | In vitro (Vero and MDCK cells) | Inhibit inflammation and apoptosis in alveolar epithelial cells, adjust macrophage polarization, and protect alveolar epithelial cell integrity. | EC50: 6.32 µg/mL (influenza B); 7.24 µg/mL (influenza A/H1N1); 12.5 µg/mL (influenza A/H3N2) | [78] | |
| Polyphenol-rich Spiraea extracts (chlorogenic, gentisic, caffeic, ferulic and cinnamic acids, quercetin, quercitrin, luteolin-7-glucoside) | In vitro (Madin–Darby canine kidney cell line: MDCK) | Shows a highly antiviral effect on the influenza A virus (H1N1) by blocking replication. | 5.9 µg/mL | [129] | |
| Polyphenol-rich sugarcane extract (caffeic acid, chlorogenic acid, ferulic acid, p-coumaric acid, sinapic acid, apigenin, luteolin, tricin, quercetin, rutin, catechin, epicatechin) | In vitro (Madin–Darby canine kidney cell line: MDCK) | Blocks the H3N2 and H1N1 replication. | IC50: 0.45 mg/mL | [120] | |
| Dengue virus | Lithospermic acid | In vitro (Vero: African green monkey kidney cells) | Inhibits viral replication by binding envelope protein and Non-Structural Protein 3 which are important for viral uptake, at the onset of infection. | EC50: 6.50 μg/mL | [140] |
| Catechin | In vitro (human hepatoma cells: (Huh 7); (human lymphoblast cells: K562); (baby hamster kidney: BHK-21); (Aedes albopictus larvae cells: C6/36) | Inhibits dengue virus replication. | IC50: 6.422 µM | [153] | |
| Arachis hypogaea L. extract (resveratrol, caffeic acid, ferulic acid, quercetin, catechin) | In vitro (African green monkey kidney cells: Vero) | Acts in the viral adsorption–penetration stage and inhibits the first steps of infection in the post-penetration stage. | IC50: 3.47 μg/mL | [150] | |
| Cranberry pomace extract (cyanidin, quercetin, myricetin, kaempfer) | In vitro (human lung carcinoma A549 cells); (human hepatoma (Huh 7.5 cells) and in vivo (Danio rerio) | Blocks viral entry by preventing viral attachment to host cells. | 25–2000 µg/mL for A549 and Huh 7.5 cells; up to 2000 µg/mL for zebrafish | [154] | |
| Herpes Simplex Virus Type 1 | Quercetin | In vitro (African green monkey kidney cells: Vero) | Reduce viral infectivity and show significant potential for virus suppression. | 62–125 µM | [148] |
| Ajwa date extract (gallic acid, ferulic acid, caffeic acid, quercetin, kaempferol, catechin, epicatechin) | In vitro (African green monkey kidney cells: Vero) | Protects cells by preventing virus uptake into host cells. | IC50: 113.99 μg/mL | [126] | |
| Kalanchoe daigremontiana extract (gallic, chlorogenic, ferulic, caffeic, and p-coumaric acids) | In vitro (African green monkey kidney cells: Vero); (human HaCaT keratinocytes) | Blocks virus attachment, penetration, and infection. | 0.16 g/mL | [1] | |
| Zika virus | Cranberry pomace extract (gallic acid, caffeic acid, quercetin, cyanidin) | In vitro (human lung epithelial A549 cells); (human-derived Huh-7.5 hepatoma cells) | Acts on viral particles and thus prevents their adhesion to the cell surface, being a potential inhibitor of virus entry into the host cell. | 26 µg/mL | [154] |
| Rotavirus | Opuntia ficus-indica peel (gallic acid, caffeic acid, chlorogenic acid, ferulic acid, p-coumaric acid, quercetin) | In vitro human breast cancer cells (MCF-17) | Anti-proliferative activity and significant reduction in cell viability | 400 µg/mL | [155] |
| Newcastle disease virus | Pongamia pinnata L. seed-derived karanjin | In vitro (chicken embryo fibroblast cells: DF-1) | Enhances antiviral responses and influences glucose metabolism. Reduces virus replication. | 3.125–25 μM | [6] |
| Human Papillomavirus | Epigallocatechin-3-Gallate | In vitro (human foreskin keratinocytes: HFK- HPV18) | Shows anti-viral activity by targeting the E6 and E7 proteins. | 100–150 µM | [21] |
| Mayaro virus | Epigallocatechin-3-Gallate | In vitro (baby hamster kidney: BHK-21 | Shows antiviral activity against Mayaro virus by targeting its replicative cycle. | 8.3–25 µg/mL | [90] |
| Murine norovirus | Polygonum aviculare extract (quercetin, kaempferol, rutin, gallic acid, caffeic acid, ferulic acid) | In vitro (RAW 264.7 cells) and In situ (cabbage surface inoculated with MNV-1) | Efficiently inactivates norovirus and prevents the infection. | IC50 = 78.4 µg/mL | [156] |
| Mouse coronavirus MHV-A59 | P2Et and anamu SC extracts from Caesalpinia spinosa and Petiveria alliacea (tannins, gallic acid derivatives, ellagic acid) | In vitro (B16–F10 murine melanoma cell line) | Exposure of calreticulin on the surface, which is induced during infection. | IC50: 119.6 μg/mL (P2Et extract); 226 μg/mL (anamu SC extract) | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coşkun, N.; Demir, R.; Canbolat, A.A.; Sarıtaş, S.; Pekdemir, B.; Bechelany, M.; Karav, S. Polyphenols as Antiviral Agents: Their Potential Against a Range of Virus Types. Nutrients 2025, 17, 2325. https://doi.org/10.3390/nu17142325
Coşkun N, Demir R, Canbolat AA, Sarıtaş S, Pekdemir B, Bechelany M, Karav S. Polyphenols as Antiviral Agents: Their Potential Against a Range of Virus Types. Nutrients. 2025; 17(14):2325. https://doi.org/10.3390/nu17142325
Chicago/Turabian StyleCoşkun, Nurten, Ranya Demir, Ahmet Alperen Canbolat, Sümeyye Sarıtaş, Burcu Pekdemir, Mikhael Bechelany, and Sercan Karav. 2025. "Polyphenols as Antiviral Agents: Their Potential Against a Range of Virus Types" Nutrients 17, no. 14: 2325. https://doi.org/10.3390/nu17142325
APA StyleCoşkun, N., Demir, R., Canbolat, A. A., Sarıtaş, S., Pekdemir, B., Bechelany, M., & Karav, S. (2025). Polyphenols as Antiviral Agents: Their Potential Against a Range of Virus Types. Nutrients, 17(14), 2325. https://doi.org/10.3390/nu17142325









