Abstract
Background: High-density lipoprotein cholesterol (HDL-C) is known for its cardiovascular and neuroprotective effects, but its association with cognitive function remains unclear, particularly in relation to genetic factors such as apolipoprotein E ε4 (APOE4). We aimed to investigate the association between serum HDL-C levels and cognition and to examine the moderating effect of APOE4 on this relationship. Methods: This cross-sectional study included 196 dementia-free older adults (aged 65–90) recruited from a memory clinic and the community. Cognitive function was assessed across multiple domains using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery. Serum HDL-C levels were measured, and APOE4 genotyping was performed. Multiple linear regression analyses were conducted, adjusting for age, sex, APOE4 status, education, diagnosis, vascular risk, nutritional status, physical activity, and blood biomarkers. Results: Higher HDL-C levels were significantly associated with better episodic memory (B = 0.109, 95% confidence interval [CI]: 0.029–0.189, p = 0.008) and global cognition (B = 0.130, 95% CI: 0.001–0.261, p = 0.049). These associations were significantly moderated by APOE4 status. In APOE4-positive individuals, HDL-C was strongly associated with both episodic memory (B = 0.357, 95% CI: 0.138–0.575, p = 0.003) and global cognition (B = 0.519, 95% CI: 0.220–0.818, p = 0.002), but no such associations were observed in APOE4-negative participants. Conclusions: This study indicates a significant association between serum HDL-C levels and cognitive function, particularly in episodic memory and global cognition, with APOE4 status potentially moderating this relationship. While these findings may suggest a protective role of HDL-C in individuals at increased genetic risk due to APOE4, they should be interpreted with caution given the cross-sectional design. Future longitudinal and mechanistic studies are warranted to clarify causality and potential clinical implications.
1. Introduction
High-density lipoprotein cholesterol (HDL-C) is well recognized for its cardiovascular benefits and has recently garnered attention for its potential neuroprotective effects [1,2]. This lipoprotein contributes to cognitive resilience by reducing neuroinflammation, mitigating oxidative stress, and supporting cholesterol metabolism in the brain [3,4]. However, studies investigating the relationship between HDL-C and cognitive function or dementia have produced inconsistent findings [5,6,7,8,9,10,11,12].
These inconsistencies may arise from heterogeneity in study designs, variations in cognitive test domains, genetic factors such as the apolipoprotein E (APOE) genotype, and demographic differences in age and sex. Some research suggests that HDL-C may have domain-specific effects, with its influence varying across cognitive domains [12,13,14]. While higher HDL-C levels have been associated with better memory performance in some studies [13], another study has reported stronger associations with attention and executive functions [14]. Additionally, a separate study identified sex-specific associations of HDL-C with multiple cognitive domains, including attention, executive functions, and memory, further underscoring the complexity of these relationships [12].
The apolipoprotein E ε4 allele (APOE4), a major genetic risk factor for Alzheimer’s disease (AD) and cognitive decline, has been implicated in impairments in lipid transport, amyloid-beta clearance, and neurovascular integrity [15,16,17]. These mechanisms may attenuate HDL-C’s neuroprotective effects, such as promoting cholesterol efflux and reducing neuroinflammation. Although APOE4 is widely studied as a risk factor, relatively few studies have explicitly tested its moderating effect on the relationship between HDL-C and cognitive function. One study reported interactions between HDL-C and APOE genotype limited to memory [14], but broader moderation effects across multiple cognitive domains remain poorly understood. Understanding the interaction between HDL-C and APOE4 has important clinical implications. If HDL-C modulates APOE4-related cognitive vulnerability, it may support early risk stratification and guide lifestyle or lipid-targeted interventions in genetically at-risk populations.
This study aims to examine the association between serum HDL-C levels and cognitive function across specific domains—including episodic memory, executive function, and global cognition—and determine whether this relationship is moderated by APOE4 status in older adults without dementia.
2. Materials and Methods
2.1. Participants
This study is a cross-sectional analysis of baseline data from the General Lifestyle and Alzheimer’s Disease (GLAD) study, an ongoing prospective cohort that began in 2020. As of July 2022, a total of 196 community-dwelling older adults aged 65–90 years who had not been clinically diagnosed with dementia were enrolled. Of these, 83 participants were classified as cognitively normal (CN), and 113 were identified as having mild cognitive impairment (MCI).
Participants were recruited through two complementary approaches. First, individuals who attended a dementia screening program at the memory clinic of Hallym University Dongtan Sacred Heart Hospital in Hwaseong, South Korea were invited for eligibility assessment. Second, additional volunteers were recruited from the local community through referrals by existing participants, family members, or acquaintances. These community-based participants were selected to ensure diversity in demographic characteristics and cognitive status, with the goal of improving the epidemiological representativeness of the study sample among older Korean adults.
The CN group consisted of participants with a Clinical Dementia Rating [18] score of 0 and no diagnosis of MCI or dementia. All participants with MCI met the current consensus criteria for amnestic MCI, including memory complaints confirmed by an informant, objective memory impairment, preservation of global cognitive function, independence in functional activities, and the absence of dementia. In the objective memory impairment assessment, the age-, education-, and sex-adjusted z-score was <−1.0 on at least one of four episodic memory subtests—word list memory, word list recall, word list recognition, and the constructional recall test)—from the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery [19,20,21]. All MCI individuals had a Clinical Dementia Rating score of 0.5. The exclusion criteria were the presence of a major psychiatric illness, a significant neurological or medical condition, or a comorbidity that could affect mental functioning; illiteracy; the presence of visual/hearing difficulties and/or severe communication or behavioral problems that would make clinical examinations difficult; and the use of an investigational drug.
The study protocol was approved by the Institutional Review Board of the Hallym University Dongtan Sacred Heart Hospital and was conducted it in accordance with the recommendations of the current version of the Declaration of Helsinki. The participants or their legal representatives provided informed consent.
2.2. Clinical Assessments
All participants underwent standardized clinical assessments by trained psychiatrists based on the GLAD study’s clinical assessment protocol, which incorporates the CERAD clinical and neuropsychological battery [19,20]. The CERAD neuropsychological battery [21] was administered by licensed psychologists who had experience working with older adults. All clinical decisions and assessments were based on the consensus of a panel of psychiatrists and psychologists with expertise in dementia.
Cognitive outcomes were assessed across three domains: episodic memory, non-memory, and global cognition. Episodic memory was evaluated using four tests from the CERAD battery—word list memory, word list recall, word list recognition, and constructional recall—and their summed score was termed the episodic memory score (EMS). Episodic memory decline represents an early cognitive marker of AD [22,23,24,25,26,27]. Non-memory cognition was assessed using three tests—verbal fluency (executive function/attention/language), the modified Boston Naming Test (language), and constructional praxis (visuospatial function)—with the combined score referred to as the non-memory score (NMS) [28]. Global cognition was measured using the CERAD total score (TS), calculated by summing the scores of all seven tests in the battery [29]. To standardize interpretation across the manuscript, these cognitive scores—EMS, NMS, and TS—are consistently used to represent episodic memory, non-memory, and global cognitive function, respectively.
Vascular risks were assessed based on data collected by trained researchers during systematic interviews of the participants and their family members. The vascular risk score (VRS) was calculated based on the number of vascular risks, including hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, transient ischemic attack, and stroke, present and was reported as a percentage [30]. The Body Mass Index (BMI) was determined using the individual’s weight in kilograms divided by their height in meters squared. Physical activities were evaluated using the Korean version of the Physical Activity Scale for the Elderly (PASE) [31,32], which has been tested for its reliability and validity. Trained researchers evaluated the participants’ frequency, duration, and intensity of activities performed during leisure, household, and occupational activities during the previous week. A weighted total physical activity score based on PASE subscores reflecting these activities was calculated.
Nutritional status was assessed using the Mini Nutritional Assessment (MNA) tool [33], a validated instrument designed for use in older populations. The MNA comprises two components: a screening section (maximum score of 14) and an assessment section (maximum score of 16). The sum of these two sections yields the MNA total score, which ranges from 0 to 30. The MNA total score was subsequently included to assess the potential confounding effect of overall nutritional status on the relationship between HDL-C and cognitive outcomes. The MNA includes evaluation of any change in food intake over the past 3 months due to loss of appetite, digestive problems, or chewing/swallowing difficulties—and dietary patterns, including intake of protein and fruits or vegetables. Protein intake levels were categorized as ‘low’, ‘medium’, or ‘high’ based on adherence to three key consumption markers: daily dairy, weekly legumes or eggs (two or more servings), and daily meat, fish, or poultry; intake was classified as low (0–1 marker met), medium (2 markers met), and high (all 3 markers met). Fruit and vegetable intake was classified as ‘high’ if participants reported consuming two or more servings per day, and ‘low’ otherwise. To acquire accurate information for all assessments, reliable informants were also interviewed.
2.3. Measuring Serum Levels of HDL-C and Other Blood Biomarkers
Morning (8–9 A.M.) blood samples were collected through venipuncture. HDL-C and low-density lipoprotein-cholesterol (LDL-C), albumin, and glucose were also measured using a COBAS c702 analyzer and dedicated reagents (Roche Diagnostics, Mannheim, Germany).
2.4. APOE4 Genotyping
Blood samples were collected in EDTA anticoagulated vacutainer tube. Genomic DNA was extracted using QIAamp DSP DNA Blood mini kit (QIAGEN, Hilden, Germany) and QIAcube HT System (QIAGEN, Hilden, Germany). The APOE genotyping was performed using a Seeplex ApoE ACE Genotyping Kit (Seegene, Seoul, Republic of Korea) and ProFlex PCR system (ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction. The PCR product was analyzed using a capillary electrophoresis device (QIAxcel Advanced System, QIAGEN, Hilden, Germany), and interpreted as ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4, or ε4/ε4 according to the electrophoresis pattern and manufacturer’s instruction. Participants were defined as APOE4-positive if they had at least one ε4 allele.
2.5. Statistical Analysis
To examine the relationships between HDL-C levels and cognitive function, multiple linear regression analyses were performed using HDL-C as the independent variable—treated either as a continuous (in mg/dL) or a categorical variable depending on the specific analysis—and cognitive scores (EMS, NMS, and TS) as dependent variables. In the primary analyses, HDL-C was modeled as a continuous variable to assess linear associations. For additional analyses, HDL-C was categorized into three groups: ‘low’ (<50 mg/dL), based on the Mayo Clinic criteria (https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/in-depth/hdl-cholesterol/art-20046388 (accessed on 5 May 2025)), which is particularly relevant given the predominantly female study population (over 70%). Among those with HDL-C ≥ 50 mg/dL, two additional strata were defined using the median: ‘medium’ (50–60 mg/dL) and ‘high’ (>60 mg/dL), to ensure a balanced distribution of participants across the categories.
To examine the independent association between HDL-C levels and cognitive unction, multiple linear regression models were adjusted for a range of covariates that are known to affect both HDL-C and cognitive function. These included age, sex, APOE4 status, education, clinical diagnosis (CN or MCI), vascular risk factors, BMI, physical activity, dietary patterns (e.g., protein and fruit/vegetable intake), and blood nutritional markers such as albumin, glucose, and LDL-C levels [13,34,35,36,37,38,39,40]. Two models were used, each controlling for the covariates in a stepwise manner. The first model included age, sex, APOE4 status, education, clinical diagnosis, as covariates; and the second model included those covariates plus VRS, BMI, PASE total score, protein intake, fruit or vegetables intake, albumin, fasting glucose, and LDL-C levels. To confirm the robustness of our regression analyses, we assessed the assumptions of normality and homoscedasticity of residuals and verified the absence of collinearity by utilizing normal probability plots, scatter plots, and variance inflation factor (VIF) values.
The moderating effect of APOE4-positivity on the association between HDL-C levels and cognitive function was examined using multiple linear regression models that included HDL-C, APOE4 status, and their interaction term (HDL-C × APOE4). These models also adjusted for all other previously described covariates, excluding APOE4 as a covariate. When a significant interaction was observed, stratified analyses were conducted separately within APOE4-positive and APOE4-negative subgroups to further explore group-specific associations.
The same sensitivity analyses were also performed in individuals with no decrease in food intake over the past 3 months for any reason to eliminate any influence of a physical or mental condition potentially related to the HDL-C levels and cognitive function status. All statistical analyses were performed using SPSS Statistics software ver. 28 (IBM, Armonk, NY, USA), with p < 0.05 were considered statistically significant. Given the assessment of multiple cognitive outcomes, a Bonferroni correction was applied (adjusted significance threshold: p < 0.0125 based on four comparisons), and relevant findings are noted in the Section 4.
3. Results
3.1. Participants
Table 1 outlines the demographic and clinical characteristics of the study cohort, comprising 156 APOE4-negative and 40 APOE4-positive participants. There were no statistically significant differences in the demographic variables or other clinical characteristics between these two groups.

Table 1.
Demographic and clinical characteristics of the participants according to APOE4 status.
3.2. Association of the Serum HDL-C Levels with Cognitive Function
Serum HDL-C levels were positively associated with TS (B = 0.130, 95% confidence interval [CI]: 0.001–0.261, p = 0.049) and EMS (B = 0.109, 95% CI: 0.029–0.189, p = 0.008) after adjusting all covariates (Model 2). In contrast, no significant associations were found for verbal fluency, the modified Boston Naming Test, or constructional praxis (Table 2; Figure 1A,B).

Table 2.
Results of the multiple linear regression analyses of the association between the HDL-cholesterol level and cognitive decline.
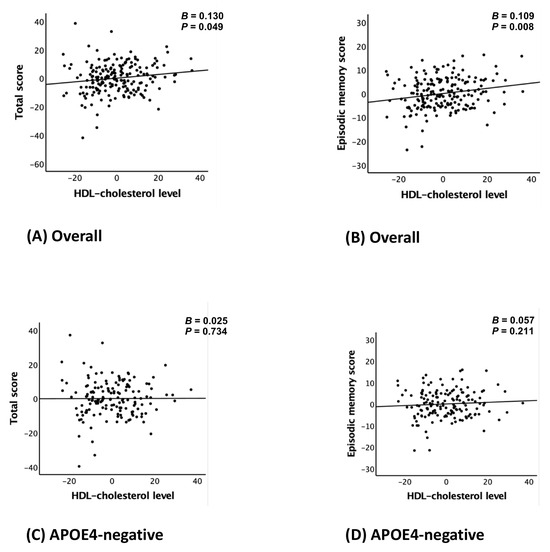
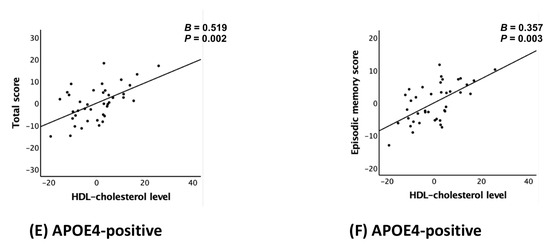
Figure 1.
Partial regression plot for the association between the HDL-cholesterol level and CERAD total score (A,C,E) or episodic memory score (B,D,F) in non-demented older adults: (A,B) overall, (C,D) APOE4-negative, and (E,F) APOE4-positive group. Abbreviations: high-density lipoprotein (HDL); CERAD, the Consortium to Establish a Registry for Alzheimer’s Disease; APOE4, apolipoprotein ε4 allele. Footnotes: error bars indicate standard errors. Multiple linear regression analyses were performed after adjusting for all confounders.
3.3. APOE4 Moderation of the Association Between the Serum HDL-C Levels and Cognitive Function
The interaction between serum HDL-C levels and APOE4 positivity significantly influenced TS (B = 0.379, 95% CI: 0.082–0.675, p = 0.013) and EMS (B = 0.236, 95% CI: 0.057–0.416, p = 0.010), but had no significant effect on verbal fluency, the modified Boston Naming Test, or constructional praxis. These findings indicate that APOE4 status modulates the relationship between serum HDL-C levels and both episodic memory and global cognition, while showing no moderating effects on non-memory cognitive tests (Table 3). In addition to the interaction effects, APOE4-positivity was independently associated with significantly lower TS (B = −22.733, 95% CI: −39.451–−6.015, p = 0.008) and EMS (B = −15.813, 95% CI: −25.820–−5.806, p = 0.002). These findings reaffirm the detrimental impact of APOE4 on episodic and global cognitive function, independent of serum HDL-C levels.

Table 3.
Results of multiple linear regression analyses that included interaction terms for the association between HDL-cholesterol and APOE4-positivity in predicting cognitive decline.
3.4. Subgroup Analyses Based on APOE4 Status
The analysis stratified by APOE4 status revealed significant associations between serum HDL-C levels and both TS (B = 0.519, 95% CI: 0.220–0.818, p = 0.002) and EMS (B = 0.357, 95% CI: 0.138–0.575, p = 0.003) in the APOE4-positive group after adjusting all covariates (models 2), whereas no such associations were identified in the APOE4-negative group. These results are shown in Table 4 and Figure 1C–F, which provide complementary statistical and visual representations.

Table 4.
Results of the multiple linear regression analyses of the association between the HDL-cholesterol level and cognitive decline according to APOE4 subgroup.
3.5. Association of the Serum HDL-C Level Groups with Cognitive Function
In the categorical analysis of HDL-C levels, participants in the high HDL-C group had significantly higher EMS compared to those in the low HDL-C group (B = 3.239, 95% CI: 0.766–5.713, p = 0.011) after adjusting all covariates (models 2). Although a positive trend was observed for total cognitive scores (TS) in the high HDL-C group, the association did not reach statistical significance (B = 3.982, 95% CI: −0.214–8.178, p = 0.063) (Table 5). Stratified analyses by APOE4 status revealed that among APOE4-positive individuals, those with high HDL-C levels showed significantly higher TS (B = 13.945, 95% CI: 3.754–24.137, p = 0.010) and EMS (B = 10.677, 95% CI: 3.632–17.722, p = 0.005) after adjusting all covariates (models 2), whereas no significant associations were observed in the APOE4-negative group (Table 5).

Table 5.
Results of the multiple linear regression analyses of the association between the stratified HDL-cholesterol level and cognitive decline according to APOE4 subgroup.
3.6. Sensitivity Analyses
The sensitivity analysis conducted on participants who had not experienced a decrease in food intake over the past 3 months yielded similar results for both TS and EMS (Table 6).

Table 6.
Results of the multiple linear regression analyses of the association between the HDL-cholesterol level and cognitive decline according to APOE4 subgroup in older adults without a 3-month decline in food intake (n = 182).
4. Discussion
This study explored the relationship between serum HDL-C levels and cognitive function across various domains, with a specific focus on EMS, NMS, and TS. Additionally, it examined the moderating effects of APOE4 on these associations. Key findings revealed a positive association between higher HDL-C levels and EMS, as well as TS, but not NMS. APOE4 status significantly moderated these relationships, with stronger associations observed in APOE4-positive participants. Sensitivity analyses excluding participants with decreased food intake over the past three months supported the robustness of these findings.
Our findings align with prior research on HDL-C’s domain-specific effects on cognitive function. For instance, several studies have reported positive associations between HDL-C levels and memory performance [13,37], consistent with the current study’s EMS results. Conversely, one study has demonstrated stronger associations with attention and executive functions [14]. Additionally, a separate study highlighted sex-specific associations with multiple cognitive domains, including attention, executive functions, and memory [12]. These variations suggest that HDL-C’s cognitive benefits may differ across populations, sexes, and cognitive domains. Furthermore, earlier meta-analyses have noted inconsistent findings, often attributed to methodological heterogeneity and confounding factors [5]. To address these limitations, the present study controlled for variables such as LDL-C, vascular risks, and nutritional factors, aiming to reduce potential biases and provide clearer insights into HDL-C’s cognitive implications.
The study’s finding that APOE4 moderates the relationship between HDL-C levels and cognitive function underscores the complexity of this relationship. Among APOE4-positive individuals, those with higher HDL-C levels demonstrated better performance in EMS and TS, suggesting that HDL-C may be associated with a reduced impact of APOE4’s detrimental effects on lipid transport, amyloid-beta clearance, and neurovascular health [16,17]. Importantly, the strongest effects were observed in episodic memory, which aligns with the earliest cognitive decline associated with AD [22,23,24,25,26,27]. This study builds on previous research, which noted domain-specific HDL-C effects in APOE genotypes [14], and highlights the clinical importance of episodic memory as a potential early marker for AD. In contrast to these significant associations observed for episodic memory and global cognition, no associations were found between HDL-C levels and other cognitive domains such as verbal fluency, naming, and constructional praxis. These null findings highlight the domain-specific nature of HDL-C’s effects and suggest that its potential cognitive benefits may be limited to memory-related processes. Further research is needed to clarify whether this selectivity reflects underlying neurobiological mechanisms or sample-specific characteristics.
However, conflicting findings have also been reported. For instance, a study focusing on middle-aged individuals did not observe a significant moderating effect of APOE4 [13]. One possible explanation is that the shorter follow-up period in this study may not have allowed sufficient time for APOE4’s moderating effects to manifest. Additionally, the absence of APOE4’s moderating effects in middle-aged populations might be due to differences in neuropathological processes between middle age and older adulthood. During middle age, compensatory mechanisms such as greater neuroplasticity or the availability of alternative pathways for lipid metabolism and amyloid-beta clearance may mitigate APOE4’s negative impact [41,42]. Conversely, these mechanisms might weaken in later life, allowing APOE4-related vulnerabilities to emerge more clearly. Differences in vascular health, which is often better in middle age, could also contribute to the lack of observable effects in younger populations [43].
Beyond these interaction effects, APOE4-positivity itself was strongly associated with lower global and episodic memory scores, reinforcing its established role in cognitive vulnerability. The significant main effects observed in both TS and EMS suggest that APOE4 may contribute to broad cognitive decline regardless of HDL-C levels, and further highlight the relevance of stratifying by APOE genotype in cognitive aging research.
Evidence suggests that HDL-C is involved in various neuroprotective processes that may contribute to cognitive resilience, particularly in individuals at increased risk for AD. Among the proposed mechanisms, the most compelling human-based evidence supports HDL-C’s role in promoting amyloid-beta clearance and preserving vascular integrity [16,17]. In this context, HDL-C enhances lipid transport and facilitates amyloid-beta efflux through pathways involving apolipoprotein E and the ABCA1/ABCG1 transporters [16,44,45], which is especially relevant in APOE4 carriers with impaired lipid metabolism and reduced amyloid clearance efficiency [16]. In addition, HDL-C appears to exert vasoprotective effects—such as maintaining endothelial function and reducing neurovascular inflammation—that may help preserve blood–brain barrier integrity and cerebral perfusion [17,46]. These vascular benefits may be particularly important in APOE4 carriers, who are more vulnerable to neurovascular dysfunction. Although additional mechanisms—such as antioxidant activity via paraoxonase 1 (PON1) [47,48], signaling modulation through sphingosine-1-phosphate (S1P) [49], and activation of the PI3K/Akt pathway to promote neuronal survival and synaptic plasticity [50]—have been proposed, current human evidence remains limited. In addition, HDL-C may regulate neuroinflammation through astrocytic and microglial pathways [6,51], and influence brain-derived neurotrophic factor (BDNF) expression, which supports hippocampal integrity and memory [52]. Given the reduced BDNF levels observed in APOE4 carriers, this neurotrophic mechanism may represent an additional route through which HDL-C mitigates synaptic vulnerability. Future studies should explicitly test these pathways using longitudinal and biomarker-based approaches. Overall, our findings support the hypothesis that HDL-C may reduce APOE4-related AD risk via mechanisms involving lipid metabolism, amyloid clearance, vascular protection, and neurotrophic and anti-inflammatory support.
Although our findings suggest a beneficial association between HDL-C and cognitive function, evidence from longitudinal and interventional studies remains inconclusive. Prospective cohort studies have reported that higher HDL-C levels are associated with slower cognitive decline and a reduced risk of dementia [10]. In contrast, Mendelian randomization studies have shown inconsistent results, with some reporting no causal effect of genetically elevated HDL-C on AD risk [53,54]. Similarly, randomized controlled trials aiming to raise HDL-C levels—particularly through cholesteryl ester transfer protein inhibitors—have not demonstrated clear cognitive benefits [55]. These discrepancies underscore the importance of HDL functionality, including cholesterol efflux capacity, anti-inflammatory effects, and particle composition, rather than HDL-C concentration alone. Therefore, while our findings support the hypothesis that HDL-C may be associated with APOE4-related cognitive vulnerability, they should be interpreted with caution and validated through future mechanistic and longitudinal studies.
Dietary patterns such as the Mediterranean diet may enhance HDL functionality and support cognitive health. Rich in unsaturated fats and polyphenols, this diet has been shown to improve HDL particle composition, cholesterol efflux, and antioxidant capacity [56,57,58,59,60]. These effects may help explain its association with slower cognitive decline and reduced AD risk [61,62]. Benefits may be especially relevant for APOE4 carriers, who are prone to lipid dysregulation and neuroinflammation. Nutrition-based strategies targeting HDL function may thus offer a promising approach to cognitive prevention in at-risk individuals. Future research should integrate HDL function, diet, and genetic risk to identify modifiable pathways in cognitive aging.
This study has several strengths, including its robust methodology, comprehensive adjustments for confounders, and sensitivity analyses to account for potential biases from dietary decline. The use of standardized cognitive assessments and stratification by APOE4 status allowed for nuanced insights. To the best of our knowledge, this study is the first to show a moderating effect of APOE4 on the association between the serum HDL-C levels and episodic memory or global cognition in humans.
This study has several limitations that should be considered. First, as a cross-sectional study, it cannot establish causal relationships between HDL-C levels and cognitive function. Longitudinal studies are needed to clarify the temporal dynamics of this association. Second, the study did not measure AD-related pathological biomarkers, such as amyloid-beta and tau proteins, which could provide deeper insights into the relationship between HDL-C levels and AD pathogenesis. Third, it remains unclear whether APOE4 acts primarily as a moderator or as a principal driver in the observed association between HDL-C levels and cognitive function. However, the positive association between HDL-C and episodic memory in APOE4-positive participants is a notable finding. Fourth, previous studies have suggested that extremely high HDL-C levels (>80 mg/dL) may negatively affect cognitive function, but this was not explored in detail here due to the small sample size of participants with very high HDL-C (n = 3). Fifth, HDL-C was categorized based on the Mayo Clinic criteria for women, which may not be directly generalizable to Korean men or to the broader Korean population. These thresholds were chosen to ensure a balanced distribution of participants across groups, particularly considering the predominantly female sample. Nevertheless, our primary analyses treated HDL-C as a continuous variable and yielded consistent associations with cognitive outcomes, supporting the robustness of our findings regardless of the categorization scheme. Sixth, participants were recruited from a memory clinic and through community outreach, which may have introduced selection bias and limited the generalizability of the findings. The sample may include individuals with greater cognitive concerns or higher health awareness compared to the broader older adult population. For instance, a gender imbalance was observed, with a higher proportion of female participants compared to male participants. Therefore, future studies using population-based samples are warranted to confirm the robustness and applicability of these findings. Seventh, although multiple cognitive outcomes were assessed, no formal correction for multiple comparisons was applied due to the exploratory nature of the study. Notably, the association between HDL-C and EMS remained statistically significant even after Bonferroni adjustment (p < 0.0125), based on four cognitive outcomes. Nevertheless, the findings should be interpreted with caution given the potential risk of type I error. Eighth, although significant associations were observed in the APOE4-positive subgroup, the relatively small sample size (n = 40) may limit the stability and generalizability of the estimates. The wide confidence intervals around some of the beta coefficients reflect statistical uncertainty, and the results should therefore be interpreted with caution. Nevertheless, the consistency of findings across both Model 1 and Model 2—before and after adjusting for vascular, metabolic, and nutritional covariates—provides some support for the robustness of the observed associations. Finally, while dietary and nutritional factors could influence HDL-C levels, the sensitivity analysis excluding participants with dietary decline and the inclusion of dietary biomarkers and patterns as covariates produced similar results, reducing the likelihood of these factors confounding the findings.
To further validate this, we conducted additional regression analyses incorporating the total MNA score as a comprehensive indicator of nutritional status. The associations between HDL-C levels and both episodic memory and global cognition remained robust—particularly among APOE4-positive individuals—even after adjusting for the MNA score (Tables S1 and S2). These findings support the independent relevance of HDL-C to cognitive outcomes beyond general nutritional condition.
Future research should focus on longitudinal studies to establish causality and explore temporal relationships between HDL-C levels and cognitive trajectories. Investigating the impact of interventions aimed at modulating HDL-C, such as lifestyle or pharmacological approaches, in diverse populations and across APOE genotypes could yield valuable insights. Furthermore, examining potential thresholds for HDL-C levels, including the effects of very high HDL-C, is crucial for refining clinical recommendations.
5. Conclusions
This study indicates a significant association between serum HDL-C levels and cognitive function, particularly in episodic memory and global cognition, with APOE4 status potentially moderating this relationship. While these findings may suggest a protective role of HDL-C in individuals at increased genetic risk due to APOE4, they should be interpreted with caution given the cross-sectional design. Future longitudinal and mechanistic studies are warranted to clarify causality and potential clinical implications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17142321/s1, Table S1: Results of the multiple linear regression analyses of the association between the HDL-cholesterol level and cognitive decline according to APOE4 subgroup with additional adjustment for MNA total score. Table S2: Results of the multiple linear regression analyses of the association between the stratified HDL-cholesterol level and cognitive decline according to APOE4 subgroup with additional adjustment for MNA total score.
Author Contributions
J.W.K. conceived and designed the study. Y.M.C., H.J.C., M.K., B.C.L., G.-H.S., S.G.K., H.S.K., J.H., D.Y. and J.W.K. were involved in acquisition, or analysis and interpretation of the data and helped to draft the manuscript. Y.M.C., H.J.C., M.K., B.C.L., G.-H.S., S.G.K., H.S.K., J.H., D.Y. and J.W.K. were major contributors in writing the manuscript and critically revising the manuscript for intellectual content. J.W.K. served as principal investigator and supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Hallym University Research Fund (grant no. HURF-2024-43). The funding sources had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit it for publication.
Institutional Review Board Statement
This study protocol was approved by the institutional review board of the Hallym University Dongtan Sacred Heart Hospital (2020-12-004, 1 December 2020) and was conducted it in accordance with the recommendations of the current version of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all participants or their legal representatives.
Data Availability Statement
The study data are not freely accessible because the IRB of the Hallym University Dongtan Sacred Heart Hospital prevents public sharing of such data for privacy reasons. However, the data are available on reasonable request after IRB approval. Requests for data access can be submitted to an independent administrative coordinator by e-mail (yoon4645@gmail.com).
Acknowledgments
We thank the GLAD study participants and their caregivers.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| APOE4 | apolipoprotein E ε4 allele |
| BMI | body mass index |
| CERAD | Consortium to Establish a Registry for Alzheimer’s Disease |
| CN | cognitively normal |
| EMS | episodic memory score |
| GLAD | General Lifestyle and AD |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| MCI | mild cognitive impairment |
| MNA | Mini-Nutritional Assessment |
| NMS | non-memory score |
| PASE | Physical Activity Scale for the Elderly |
| TS | total score of CERAD |
| VRS | vascular risk score |
References
- Hong, B.V.; Agus, J.K.; Tang, X.; Zheng, J.J.; Romo, E.Z.; Lei, S.; Zivkovic, A.M. Precision nutrition and cardiovascular disease risk reduction: The promise of high-density lipoproteins. Curr. Atheroscler. Rep. 2023, 25, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Heinrich, K.; Riwanto, M.; Luscher, T.F.; Landmesser, U. High-density lipoprotein-mediated anti-atherosclerotic and endothelial-protective effects: A potential novel therapeutic target in cardiovascular disease. Curr. Pharm. Des. 2010, 16, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Turri, M.; Marchi, C.; Adorni, M.P.; Calabresi, L.; Zimetti, F. Emerging role of HDL in brain cholesterol metabolism and neurodegenerative disorders. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159123. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and cholesterol handling in the brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Vazquez, O.; Puente-Martinez, A.; Ubillos-Landa, S.; Pacheco-Bonrostro, J.; Santabarbara, J. Cholesterol and Alzheimer’s disease risk: A meta-meta-analysis. Brain Sci. 2020, 10, 386. [Google Scholar] [CrossRef]
- Chernick, D.; Zhong, R.; Li, L. The role of HDL and HDL mimetic peptides as potential therapeutics for Alzheimer’s disease. Biomolecules 2020, 10, 1276. [Google Scholar] [CrossRef]
- Button, E.B.; Robert, J.; Caffrey, T.M.; Fan, J.; Zhao, W.; Wellington, C.L. HDL from an Alzheimer’s disease perspective. Curr. Opin. Lipidol. 2019, 30, 224–234. [Google Scholar] [CrossRef]
- Hottman, D.A.; Chernick, D.; Cheng, S.; Wang, Z.; Li, L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 2014, 72 Pt A, 22–36. [Google Scholar] [CrossRef]
- Hussain, S.M.; Robb, C.; Tonkin, A.M.; Lacaze, P.; Chong, T.T.; Beilin, L.J.; Yu, C.; Watts, G.F.; Ryan, J.; Ernst, M.E.; et al. Association of plasma high-density lipoprotein cholesterol level with risk of incident dementia: A cohort study of healthy older adults. Lancet Reg. Health West. Pac. 2024, 43, 100963. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010, 67, 1491–1497. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.X.; Luchsinger, J.; Mayeux, R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 2004, 61, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; An, Y.; Yu, H.; Che, F.; Zhang, X.; Rong, H.; Xi, Y.; Xiao, R. Sex-specific nonlinear associations between serum lipids and different domains of cognitive function in middle to older age individuals. Metab. Brain Dis. 2017, 32, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Gimeno, D.; Kivimaki, M.; Brunner, E.; Marmot, M.G. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: The Whitehall II study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1556–1562. [Google Scholar] [CrossRef]
- Glans, I.; Nagga, K.; Gustavsson, A.M.; Stomrud, E.; Nilsson, P.M.; Melander, O.; Hansson, O.; Palmqvist, S. Associations of modifiable and non-modifiable risk factors with cognitive functions—A prospective, population-based, 17 years follow-up study of 3,229 individuals. Alzheimers Res. Ther. 2024, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Weisgraber, K.H.; Mahley, R.W. Human apolipoprotein E: The Alzheimer’s disease connection. FASEB J. 1996, 10, 1485–1494. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Mahley, R.W.; Huang, Y. Apolipoprotein E sets the stage: Response to injury triggers neuropathology. Neuron 2012, 76, 871–885. [Google Scholar] [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.U.; Lee, D.Y.; Kim, K.W.; Jhoo, J.H.; Kim, J.H.; Lee, K.H.; Kim, S.Y.; Han, S.H.; Woo, J.I. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): Clinical and neuropsychological assessment batteries. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, P47–P53. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, K.U.; Lee, J.H.; Kim, K.W.; Jhoo, J.H.; Kim, S.Y.; Yoon, J.C.; Woo, S.I.; Ha, J.; Woo, J.I. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J. Int. Neuropsychol. Soc. 2004, 10, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Howieson, D.B.; Dame, A.; Camicioli, R.; Sexton, G.; Payami, H.; Kaye, J.A. Cognitive markers preceding Alzheimer’s dementia in the healthy oldest old. J. Am. Geriatr. Soc. 1997, 45, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Grober, E.; Lipton, R.B.; Hall, C.; Crystal, H. Memory impairment on free and cued selective reminding predicts dementia. Neurology 2000, 54, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Tromp, D.; Dufour, A.; Lithfous, S.; Pebayle, T.; Despres, O. Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2015, 24, 232–262. [Google Scholar] [CrossRef]
- Backman, L.; Small, B.J.; Fratiglioni, L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 2001, 124, 96–102. [Google Scholar] [CrossRef]
- Laakso, M.P.; Hallikainen, M.; Hanninen, T.; Partanen, K.; Soininen, H. Diagnosis of Alzheimer’s disease: MRI of the hippocampus vs delayed recall. Neuropsychologia 2000, 38, 579–584. [Google Scholar] [CrossRef]
- Backman, L.; Jones, S.; Berger, A.K.; Laukka, E.J.; Small, B.J. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology 2005, 19, 520–531. [Google Scholar] [CrossRef]
- Ferman, T.J.; Smith, G.E.; Kantarci, K.; Boeve, B.F.; Pankratz, V.S.; Dickson, D.W.; Graff-Radford, N.R.; Wszolek, Z.; Van Gerpen, J.; Uitti, R.; et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology 2013, 81, 2032–2038. [Google Scholar] [CrossRef]
- Seo, E.H.; Lee, D.Y.; Lee, J.H.; Choo, I.H.; Kim, J.W.; Kim, S.G.; Park, S.Y.; Shin, J.H.; Do, Y.J.; Yoon, J.C.; et al. Total scores of the CERAD neuropsychological assessment battery: Validation for mild cognitive impairment and dementia patients with diverse etiologies. Am. J. Geriatr. Psychiatry 2010, 18, 801–809. [Google Scholar] [CrossRef]
- DeCarli, C.; Mungas, D.; Harvey, D.; Reed, B.; Weiner, M.; Chui, H.; Jagust, W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004, 63, 220–227. [Google Scholar] [CrossRef]
- Choe, M.A.; Kim, J.; Jeon, M.; Chae, Y.R. Evaluation of the Korean Version of Physical Activity Scale for the Elderly (K-PASE). Korean J. Women Health Nurs. 2010, 16, 47. [Google Scholar] [CrossRef]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, Y.; Schwarz, D.; Reinecke, H. LDL-C augments whereas HDL-C prevents inflammatory innate immune memory. Trends Mol. Med. 2022, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Chen, M.T.; He, Y.Y.; Chen, M.; Liang, J.R.; Jia, F.J.; Huang, Q.; Zhou, R.; Hou, C.L. Cognitive impairment and depression precede increased HDL-C levels in middle-aged and older Chinese adults: Cross-lagged panel analyses. Lipids Health Dis. 2024, 23, 288. [Google Scholar] [CrossRef]
- Bruce, D.G.; Davis, W.A.; Davis, T.M.E. Low serum HDL-cholesterol concentrations in mid-life predict late-life cognitive impairment in type 2 diabetes: The Fremantle diabetes study. J. Diabetes Complicat. 2017, 31, 945–947. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Davey, A.; Sullivan, K.J.; Robbins, M.A. Higher HDL cholesterol is associated with better cognitive function: The Maine-Syracuse study. J. Int. Neuropsychol. Soc. 2014, 20, 961–970. [Google Scholar] [CrossRef]
- Wen, J.; Hao, X.; Jia, Y.; Wang, B.; Pang, J.; Liang, F. Sex differences in the association between LDL/HDL with cognitive decline in older adults: National Health and Nutrition Examination Survey. J. Alzheimer’s Dis. 2024, 98, 1493–1502. [Google Scholar] [CrossRef]
- Lara, V.P.; Caramelli, P.; Teixeira, A.L.; Barbosa, M.T.; Carmona, K.C.; Guimaraes, H.C.; Carvalho, M.G.; Fernandes, A.P.; Gomes, K.B. Cortisol, HDL-c, VLDL-c, and APOE polymorphisms as laboratorial parameters associated to cognitive impairment no dementia (CIND) and dementia. J. Clin. Lab. Anal. 2016, 30, 374–380. [Google Scholar] [CrossRef]
- Braga, P.G.S.; Freitas, F.R.; Bachi, A.L.L.; Amirato, G.R.; Baroni, R.V.; Alves, M.; Vieira, R.P.; Vaisberg, M.W.; Aldin, M.N.; Kalil Filho, R.; et al. Regular practice of physical activity improves cholesterol transfers to high-density lipoprotein (HDL) and other HDL metabolic parameters in older adults. Nutrients 2023, 15, 4871. [Google Scholar] [CrossRef]
- Seidler, R.D. Neuroplasticity in middle age: An ecologically valid approach. Front. Hum. Neurosci. 2012, 6, 324. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.O.; Park, D.C. Neuroplasticity and cognitive aging: The scaffolding theory of aging and cognition. Restor. Neurol. Neurosci. 2009, 27, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Marchi, C.; Adorni, M.P.; Caffarra, P.; Ronda, N.; Spallazzi, M.; Barocco, F.; Galimberti, D.; Bernini, F.; Zimetti, F. ABCA1- and ABCG1-mediated cholesterol efflux capacity of cerebrospinal fluid is impaired in Alzheimer’s disease. J. Lipid Res. 2019, 60, 1449–1456. [Google Scholar] [CrossRef]
- Fagan, A.M.; Holtzman, D.M. Astrocyte lipoproteins, effects of apoE on neuronal function, and role of apoE in amyloid-beta deposition in vivo. Microsc. Res. Tech. 2000, 50, 297–304. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Rosenblat, M.; Vaya, J.; Shih, D.; Aviram, M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: A possible role for lysophosphatidylcholine. Atherosclerosis 2005, 179, 69–77. [Google Scholar] [CrossRef]
- Chua, X.Y.; Chai, Y.L.; Chew, W.S.; Chong, J.R.; Ang, H.L.; Xiang, P.; Camara, K.; Howell, A.R.; Torta, F.; Wenk, M.R.; et al. Immunomodulatory sphingosine-1-phosphates as plasma biomarkers of Alzheimer’s disease and vascular cognitive impairment. Alzheimers Res. Ther. 2020, 12, 122. [Google Scholar] [CrossRef]
- Yassine, H.N.; Finch, C.E. APOE alleles and diet in brain aging and Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 150. [Google Scholar] [CrossRef]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum brain-derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Proitsi, P.; Lupton, M.K.; Velayudhan, L.; Newhouse, S.; Fogh, I.; Tsolaki, M.; Daniilidou, M.; Pritchard, M.; Kloszewska, I.; Soininen, H.; et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: A Mendelian randomization analysis. PLoS Med. 2014, 11, e1001713. [Google Scholar] [CrossRef]
- Benn, M.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjaerg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017, 357, j1648. [Google Scholar] [CrossRef]
- Group, H.T.R.C.; Bowman, L.; Hopewell, J.C.; Chen, F.; Wallendszus, K.; Stevens, W.; Collins, R.; Wiviott, S.D.; Cannon, C.P.; Braunwald, E.; et al. Effects of Anacetrapib in patients with atherosclerotic vascular disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Barbaresko, J.; Rienks, J.; Nöthlings, U. Lifestyle indices and cardiovascular disease risk: A meta-analysis. Am. J. Prev. Med. 2018, 55, 555–564. [Google Scholar] [CrossRef]
- Farukhi, Z.M.; Mora, S.; Manson, J.E. Marine omega-3 fatty acids and cardiovascular disease prevention: Seeking clearer water. Mayo Clin. Proc. 2021, 96, 277–279. [Google Scholar] [CrossRef]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Martí, J.; Alcántara, M.; Pujadas-Bastardes, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.A.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).