Influence of Preoperative Diagnosis of Nutritional Disorders on Short-Term Outcomes After Hip Arthroplasty: A Cohort Study of Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Variables and Methods
2.4. Statistics
3. Results
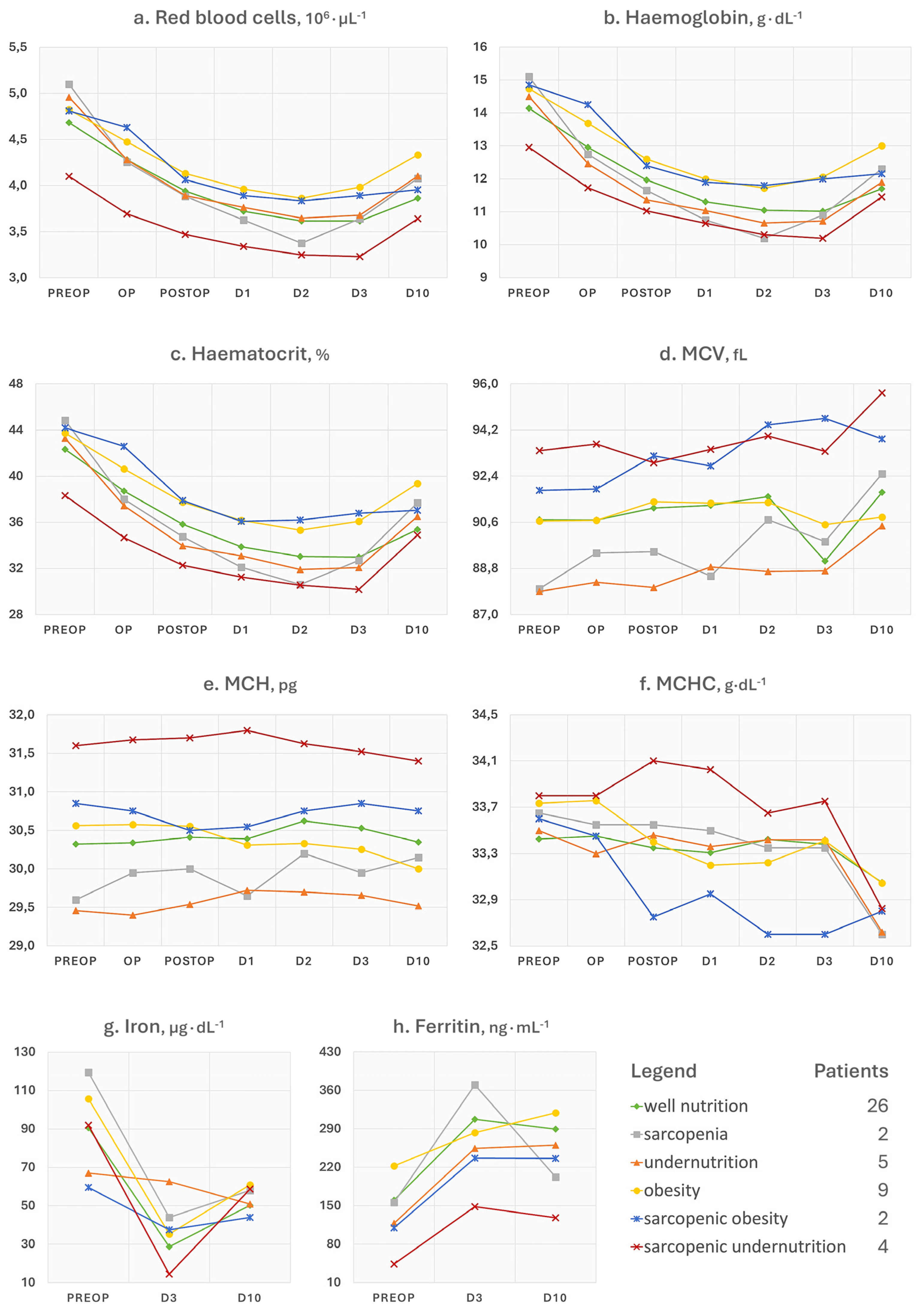
3.1. Red Blood Cell and Iron Status Indices
3.2. Immune and Inflammation Parameters
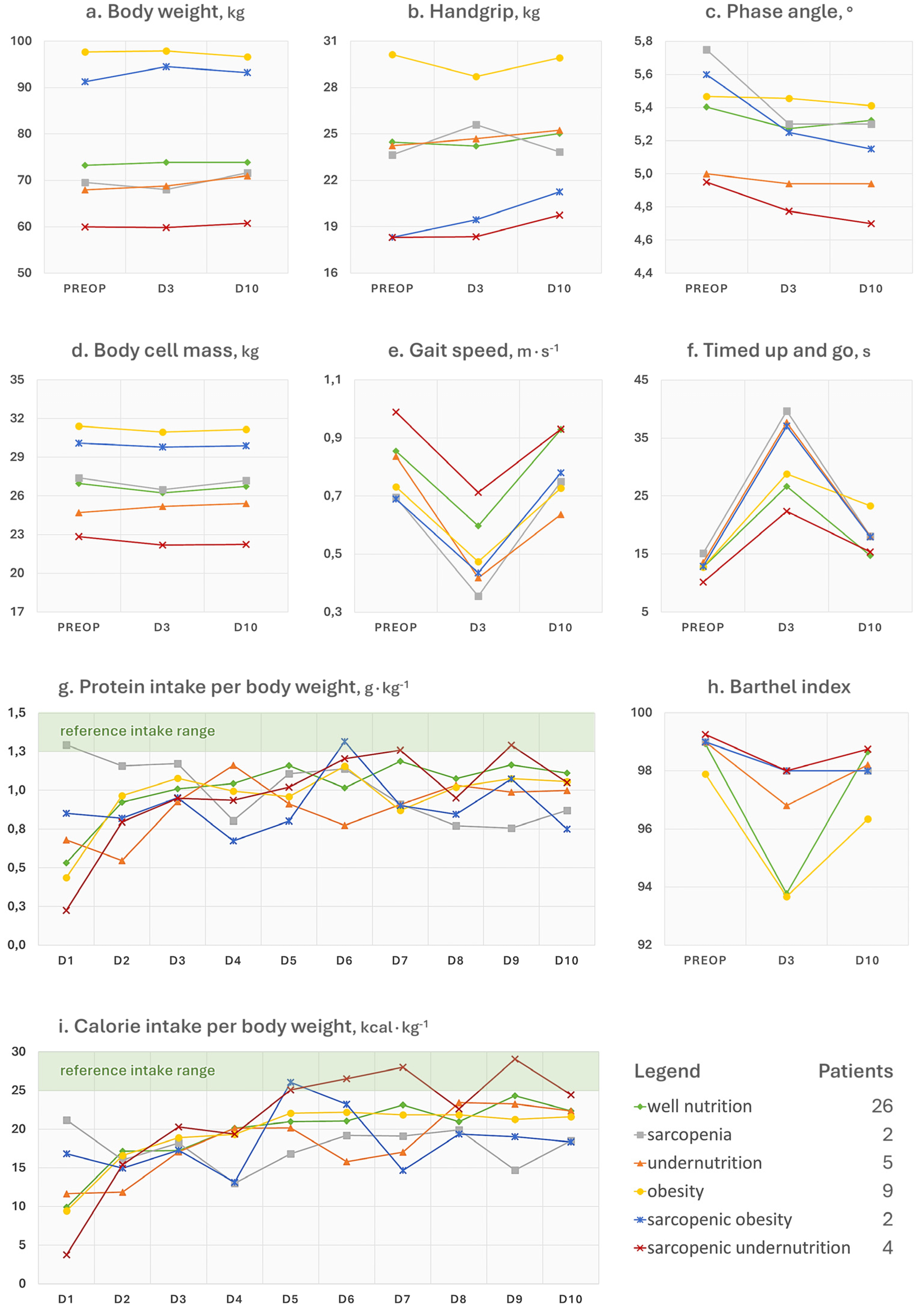
3.3. Physical Function
3.4. Postoperative Dietary Intakes
4. Discussion
- Ferritin is a 24-unit globular protein that takes up to 4300 iron atoms to be deposited in its core. Although its serum concentration represents a small fraction of the body’s ferritin pool, low circulating levels may indicate a depletion of iron stores in the absence of infection or vitamin C deficit. This iron exhaustion may be evident at admission for patients with sarcopenic undernutrition (ferritin < 100 ng∙mL−1), with the highest levels being conversely seen in obese patients (Figure 1h). This additional observation is in line with our main findings of the negative influence of sarcopenia and malnutrition phenotypes on the recovery after surgery. It has been known for some time that if ferritin levels, but also haematocrit [18] and mean corpuscular volume [19], are low at hospital admission, patients will be more likely to require transfusions and encounter adverse events after major orthopaedic surgery [20]. Since ferritin is also an acute-phase protein, a postoperative increase is considered physiological as long as it does not reach excessively high levels, which was the case in patients with sarcopenia.
- Concerning NLR, it is considered representative of a chronic inflammatory status in the absence of trauma, and its values can be assigned to five levels: normal (<2), low (2–3.99), mild (4–5.99), moderate (6–7.99), and severe (≥8) [6]. In Figure 2f, patients with sarcopenic undernutrition are seen to be the only ones to suffer from a severe chronic inflammation at baseline. This derives from a combination of low lymphocytes (reference interval 21.8–53.1%), which has been a well-known marker of malnutrition for decades [21], and high neutrophils (reference interval 34.0–67.9%). The peak after surgery was more relevant in those who had been diagnosed with sarcopenia and undernutrition. Similarly to ferritin, these findings recall the concepts related to the acute-phase response to surgery (e.g., haematological, hormonal, metabolic, and immunologic changes) and the increased vulnerability to stress of individuals with poor body function and reserves [22]. Specifically, the surgery-derived stress is known to push the metabolism towards a negative whole-body protein balance because of an increase in protein breakdown, a concomitant release of amino acids into circulation with an impaired uptake in skeletal muscles, greater urinary nitrogen losses, a shift of protein synthesis in favour of the acute-phase reactants, and a depression of other proteins’ synthesis [23]. It is, therefore, plausible to think that the patient with sarcopenia will experience not only an altered—possibly exaggerated—immune response after major surgery but also a greater depletion of the lean mass in the postoperative period.
- The postoperative trends of the phase angle (Figure 3c), which is known to be directly associated with muscle mass in different age groups and health conditions [24], can help to appreciate the influence of sarcopenia on the musculoskeletal system post-surgery. It can be noted, in fact, that the non-sarcopenic patients (well nourished, pure obesity, and undernutrition) were the only ones who did not display a visible worsening of the phase angle after surgery. For what concerns the physical performance, it strongly depends on the type of aids used by the patient (e.g., crutches) as well as the possible fear of putting weight on the operated limb or the fear of falling. Other than the gait speed, we calculated the Barthel index (Figure 3h), which is a 10-item questionnaire used to evaluate the patient’s independence for what concerns feeding, bathing, grooming, dressing, reaching and using the bathroom (bowel and bladder control and toilet use), moving from bed to chair and back, ambulating on level surfaces, and climbing the stairs. Since higher scores are indicative of a greater independence, it may be recognisable for patients with pure obesity a tendency to have lower functional independence at the 10-day visit compared to their counterparts without a diagnosis. However, the difference of a few points in the index is not clinically relevant and it would remain to investigate what the recovery trends are in the long term. On the other hand, database research on 13,348 Japanese patients undergoing surgical procedures for femoral fracture found that those with a BMI over ≥27.5 kg∙m−2 appeared to have significantly higher functional scores at discharge than their counterparts with a lower BMI [25]. This would recall the protective effect of having a few—not too many—excess kilos in older age [6], since it can represent a useful energy reserve in the event of a trauma. However, adipose tissue is also linked to a condition of low-grade inflammation orchestrated by adipocyte-derived adipokines, which participate in the whole-body immunological crosstalk [26].
- The SOST and DKK1 (Figure 2g,h) were dosed in this study because they are both osteoimmunological biomarkers involved in bone remodelling [27]. Specifically, they are mediators linking the immune system to bone tissue, and inhibit the Wnt pathway, reduce osteoblast activity, and in turn promote osteoclastogenesis and bone resorption. By monitoring these two biomarkers, previous investigations in joint replacement surgery aimed at quantifying osteointegration of the endoprosthesis in order to predict early aseptic loosening [28]. Typically, for both of them, no peak should be observed in the immediate postoperative period, since an early elevation has been associated with bone nonunion [27]. Remarkably, preoperative SOST and DKK1 levels in patients with obesity were the highest among study participants. This relationship was already observed in a past cross-sectional investigation [29], further associating high levels of SOST with insulin resistance in skeletal muscles [30]. Although adipose tissue may represent a useful energy reserve, it is also true that the resulting metabolic inflammation, which we did not find in our patients based on leukocyte profiles, should not be underestimated.
4.1. Clinical Implications
4.2. Strengths and Limitations
5. Conclusions
Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Rosenberger, C.; Rechsteiner, M.; Dietsche, R.; Breidert, M. Energy and protein intake in 330 geriatric orthopaedic patients: Are the current nutrition guidelines applicable? Clin. Nutr. ESPEN 2019, 29, 86–91. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Wainwright, T.; Lombardi, G. Definition of malnutrition from routinely-collected data for orthopedic surgery research: The global leadership initiative on malnutrition (GLIM) tool and others. Front. Nutr. 2023, 10, 1200049. [Google Scholar] [CrossRef] [PubMed]
- Heimroth, J.C.; Neufeld, E.V.; Sodhi, N.; Walden, T.; Willinger, M.L.; Boraiah, S. Relationship between Preoperative Nutritional Status and Predicting Short-Term Complications Following Revision Total Hip Arthroplasty. J. Arthroplast. 2023, 38, 1326–1329. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W. Association between malnutrition status and total joint arthroplasty periprosthetic joint infection and surgical site infection: A systematic review meta-analysis. J. Orthop. Surg. Res. 2024, 19, 660. [Google Scholar] [CrossRef]
- Chiavarini, M.; Ricciotti, G.M.; Genga, A.; Faggi, M.I.; Rinaldi, A.; Toscano, O.D.; D’Errico, M.M.; Barbadoro, P. Malnutrition-Related Health Outcomes in Older Adults with Hip Fractures: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1069. [Google Scholar] [CrossRef]
- Briguglio, M.; Latella, M.; Cordani, C.; Petrillo, S.; Langella, F.; Cecchinato, R.; Berjano, P.; Pregliasco, F.E.; Middleton, R.G.; Wainwright, T.W. To lose weight or to weight the loss? Insights into the use of the body mass index in preoperative assessment before major orthopaedic surgery. Int. J. Orthop. Trauma. Nurs. 2025, 58, 101192. [Google Scholar] [CrossRef]
- DeMik, D.E.; Marinier, M.C.; Glass, N.A.; Elkins, J.M. Prevalence of Sarcopenia and Sarcopenic Obesity in an Academic Total Joint Arthroplasty Practice. Arthroplast. Today 2022, 16, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Brzeszczynski, F.; Brzeszczynska, J.; Duckworth, A.D.; Murray, I.R.; Simpson, A.H.R.W.; Hamilton, D.F. The effect of sarcopenia on outcomes following orthopaedic surgery: A systematic review. Bone Jt. J. 2022, 104-B, 321–330. [Google Scholar] [CrossRef]
- Briguglio, M.; Sirtori, P.; Mangiavini, L.; Buzzi, S.; Cordani, C.; Zerni, M.F.; Wainwright, T.W.; Ursino, N.; Peretti, G.M.; Banfi, G. How Do Older Patients with End-Stage Osteoarthritis of the Hip Eat Prior to Hip Replacement? A Preliminary Snapshot that Highlights a Poor Diet. Nutrients 2023, 15, 4868. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Sirtori, P.; Mangiavini, L.; Wainwright, T.; Peretti, G.; Banfi, G. Undernutrition, sarcopenia, sarcopenic obesity, and sarcopenic undernutrition: A cross-sectional view on patients before total joint arthroplasty. Orthop. Nurs. 2024, 43, 276–283. [Google Scholar] [CrossRef]
- Fernández Miró, M.; Cabrejo Gavidia, V.; Carrascosa Piquer, O.; Valero Lanau, J.; Toapanta Valencia, M.; Aguado Jodar, A. Malnutrition is associated with postoperative complications in elderly patients undergoing total hip arthroplasty. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2023, 70 (Suppl. S3), 59–66. [Google Scholar] [CrossRef]
- Husted, H.; Jørgensen, C.C.; Gromov, K.; Kehlet, H.; Lundbeck Foundation Center for Fast-track Hip and Knee Replacement Collaborative Group. Does BMI influence hospital stay and morbidity after fast-track hip and knee arthroplasty? Acta Orthop. 2016, 87, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Hasil, L.; Keane, C.; Brassard, D.; Kiernan, F.; Bellafronte, N.T.; Culos-Reed, S.N.; Gramlich, L.; Ljungqvist, O.; Fenton, T.R. A multimodal prehabilitation class for Enhanced Recovery after Surgery: A pragmatic randomised type 1 hybrid effectiveness-implementation trial. Br. J. Anaesth. 2025. [Google Scholar] [CrossRef]
- Wu, W.C.; Schifftner, T.L.; Henderson, W.G.; Eaton, C.B.; Poses, R.M.; Uttley, G.; Sharma, S.C.; Vezeridis, M.; Khuri, S.F.; Friedmann, P.D. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 2007, 297, 2481–2488. [Google Scholar] [CrossRef]
- Shanbhag, S.P.; Solano, M.A.; Botros, M.A.; Khanuja, H.S. Treating Preoperative Anemia to Improve Patient Outcomes after Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2019, 27, e1077–e1085. [Google Scholar] [CrossRef]
- Bisbe, E.; Basora, M.; Colomina, M.J.; Spanish Best Practice in Peri-operative Anaemia Optimisation Panel. Peri-operative treatment of anaemia in major orthopaedic surgery: A practical approach from Spain. Blood Transfus. 2017, 15, 296–306. [Google Scholar] [CrossRef][Green Version]
- Seltzer, M.H.; Bastidas, J.A.; Cooper, D.M.; Engler, P.; Slocum, B.; Fletcher, H.S. Instant nutritional assessment. JPEN J. Parenter. Enter. Nutr. 1979, 3, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Amrock, L.G.; Deiner, S. Perioperative frailty. Int. Anesthesiol. Clin. 2014, 52, 26–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillis, C.; Carli, F. Promoting Perioperative Metabolic and Nutritional Care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.C.; Alves Junior, C.A.S.; Silva, A.M.; Silva, D.A.S. Phase angle and body composition: A scoping review. Clin. Nutr. ESPEN 2023, 56, 237–250. [Google Scholar] [CrossRef]
- Nishioka, S.; Wakabayashi, H.; Maeda, K.; Shamoto, H.; Taketani, Y.; Kayashita, J.; Momosaki, R. Body mass index and recovery of activities of daily living in older patients with femoral fracture: An analysis of a national inpatient database in Japan. Arch. Gerontol. Geriatr. 2020, 87, 104009. [Google Scholar] [CrossRef]
- Tilg, H.; Ianiro, G.; Gasbarrini, A.; Adolph, T.E. Adipokines: Masterminds of metabolic inflammation. Nat. Rev. Immunol. 2025, 25, 250–265. [Google Scholar] [CrossRef]
- Starlinger, J.; Santol, J.; Kaiser, G.; Sarahrudi, K. Close negative correlation of local and circulating Dickkopf-1 and Sclerostin levels during human fracture healing. Sci. Rep. 2024, 14, 6524. [Google Scholar] [CrossRef]
- Cucchi, D.; Menon, A.; Galliera, E.; Messina, C.; Zanini, B.; Marazzi, M.G.; Massaccesi, L.; Compagnoni, R.; Corsi Romanelli, M.M.; Randelli, P. A Prospective Assessment of Periprosthetic Bone Mineral Density and Osteoimmunological Biomarkers Variations after Total Knee Replacement Surgery. J. Clin. Densitom. 2019, 22, 86–95. [Google Scholar] [CrossRef]
- Kalem, M.N.; Kalem, Z.; Akgun, N.; Bakırarar, B. The relationship between postmenopausal women’s sclerostin levels and their bone density, age, body mass index, hormonal status, and smoking and consumption of coffee and dairy products. Arch. Gynecol. Obstet. 2017, 295, 785–793. [Google Scholar] [CrossRef]
- Daniele, G.; Winnier, D.; Mari, A.; Bruder, J.; Fourcaudot, M.; Pengou, Z.; Tripathy, D.; Jenkinson, C.; Folli, F. Sclerostin and Insulin Resistance in Prediabetes: Evidence of a Cross Talk between Bone and Glucose Metabolism. Diabetes Care 2015, 38, 1509–1517. [Google Scholar] [CrossRef]
- Briguglio, M.; Wainwright, T.W. Towards Personalised Nutrition in Major Orthopaedic Surgery: Elements of Care Process. Nutrients 2025, 17, 700. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Cohort (n = 48) | Females (n = 22) | Males (n = 26) |
|---|---|---|---|
| Age, years | 71.54 (6.41) [61; 84] | 71.73 (6.71) [63; 84] | 71.38 (6.27) [61; 83] |
| BMI, kg∙m−2 | 27.09 (4.89) [20; 40] | 25.71 (4.49) [20; 37] | 28.26 (4.99) [22; 40] |
| CCI | 3.42 (1.19) [2; 7] | 3.14 (0.99) [2; 5] | 3.65 (1.32) [2; 7] |
| LOS, days * | 3.90 (0.72) [3; 5] | 4.00 (0.62) [3; 5] | 3.81 (0.80) [3; 5] |
| Parameter | Well Nutrition (n = 26) | Undernutrition (n = 5) | Sarcopenia (n = 2) | Obesity (n = 9) | Sarcopenic Undernutrition (n = 4) | Sarcopenic Obesity (n = 2) |
|---|---|---|---|---|---|---|
| RBC | 4.68 (0.52) | 4.96 (0.50) | 5.10 (0.18) | 4.82 (0.41) | 4.10 (0.45) | 4.81 (0.21) |
| Hb | 14.14 (1.34) | 14.50 (0.58) | 15.10 (0.42) | 14.73 (1.36) | 12.95 (1.57) | 14.85 (1.20) |
| Ht | 42.33 (4.02) | 43.28 (1.28) | 44.85 (0.49) | 43.74 (4.54) | 38.33 (4.90) | 44.20 (3.39) |
| MCV | 90.70 (4.55) | 87.90 (7.64) | 88.00 (4.10) | 90.66 (4.49) | 93.40 (4.15) | 91.85 (3.04) |
| MCH | 30.32 (1.80) | 29.46 (3.06) | 29.60 (0.28) | 30.57 (1.61) | 31.60 (1.94) | 30.85 (1.20) |
| MCHC | 33.43 (1.12) | 33.50 (1.07) | 33.65 (1.34) | 33.73 (1.19) | 33.80 (1.18) | 33.60 (0.14) |
| Iron | 90.64 (27.36) | 67.00 (14.54) | 119.50 (51.62) | 105.67 (23.32) | 92.00 (19.78) | 59.50 (23.33) |
| Ferritin | 160.35 (123.08) | 118.00 (83.39) | 156.00 (94.75) | 222.22 (208.49) | 43.75 (14.93) | 110.00 (76.37) |
| Parameter | Well Nutrition (n = 26) | Undernutrition (n = 5) | Sarcopenia (n = 2) | Obesity (n = 9) | Sarcopenic Undernutrition (n = 4) | Sarcopenic Obesity (n = 2) |
|---|---|---|---|---|---|---|
| Neut | 59.70 (6.41) | 68.08 (4.94) | 56.15 (11.53) | 61.18 (5.48) | 77.92 (6.19) | 60.90 (2.40) |
| Lymp | 28.02 (5.52) | 21.90 (4.25) | 32.80 (11.31) | 26.92 (4.09) | 12.40 (6.25) | 26.90 (0.71) |
| NLR | 2.29 (0.91) | 3.24 (0.89) | 1.88 (1.00) | 2.35 (0.59) | 8.40 (5.96) | 2.27 (0.15) |
| Mono | 8.91 (2.00) | 8.08 (1.97) | 8.25 (0.49) | 9.82 (3.67) | 8.50 (1.39) | 8.75 (1.91) |
| Eosi | 2.66 (2.90) | 1.40 (1.14) | 2.15 (0.07) | 1.51 (0.72) | 0.72 (0.61) | 2.70 (0.28) |
| Baso | 0.71 (0.28) | 0.54 (0.27) | 0.65 (0.21) | 0.61 (0.28) | 0.45 (0.31) | 0.75 (0.07) |
| SOST | 21.42 (7.53) | 20.45 (7.36) | 19.62 (5.76) | 27.83 (13.34) | 15.74 (8.83) | 32.43 (NA) |
| DKK1 | 34.15 (15.34) | 22.53 (3.30) | 31.16 (9.6) | 48.91 (35.78) | 20.53 (10.24) | 30.33 (NA) |
| Parameter | Well Nutrition (n = 26) | Undernutrition (n = 5) | Sarcopenia (n = 2) | Obesity (n = 9) | Sarcopenic Undernutrition (n = 4) | Sarcopenic Obesity (n = 2) |
|---|---|---|---|---|---|---|
| W | 73.23 (11.62) | 67.94 (14.43) | 69.55 (7.71) | 97.71 (13.47) | 59.95 (8.49) | 91.25 (19.45) |
| HGS | 24.47 (10.12) | 24.24 (13.29) | 23.65 (0.35) | 30.14 (7.36) | 18.30 (5.61) | 18.30 (7.21) |
| PhA | 5.40 (0.80) | 5.00 (0.58) | 5.75 (0.21) | 5.47 (0.77) | 4.95 (0.31) | 5.60 (0.85) |
| BCM | 26.97 (6.82) | 24.70 (4.91) | 27.40 (0.71) | 31.43 (6.71) | 22.85 (5.37) | 30.10 (12.02) |
| 10MWT | 0.85 (0.21) | 0.84 (0.30) | 0.70 (0.23) | 0.73 (0.16) | 0.99 (0.24) | 0.69 (0.28) |
| TUG | 12.70 (4.64) | 13.55 (3.75) | 15.12 (1.59) | 12.79 (4.29) | 10.16 (2.38) | 12.85 (0.91) |
| BI | 98.92 (1.98) | 99.00 (1.00) | 99.00 (1.41) | 97.89 (3.48) | 99.25 (1.50) | 99.00 (1.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briguglio, M.; Latella, M.; Sirtori, P.; Mangiavini, L.; De Luca, P.; Geroldi, M.; De Vecchi, E.; Lombardi, G.; Petrillo, S.; Wainwright, T.W.; et al. Influence of Preoperative Diagnosis of Nutritional Disorders on Short-Term Outcomes After Hip Arthroplasty: A Cohort Study of Older Adults. Nutrients 2025, 17, 2319. https://doi.org/10.3390/nu17142319
Briguglio M, Latella M, Sirtori P, Mangiavini L, De Luca P, Geroldi M, De Vecchi E, Lombardi G, Petrillo S, Wainwright TW, et al. Influence of Preoperative Diagnosis of Nutritional Disorders on Short-Term Outcomes After Hip Arthroplasty: A Cohort Study of Older Adults. Nutrients. 2025; 17(14):2319. https://doi.org/10.3390/nu17142319
Chicago/Turabian StyleBriguglio, Matteo, Marialetizia Latella, Paolo Sirtori, Laura Mangiavini, Paola De Luca, Manuela Geroldi, Elena De Vecchi, Giovanni Lombardi, Stefano Petrillo, Thomas W. Wainwright, and et al. 2025. "Influence of Preoperative Diagnosis of Nutritional Disorders on Short-Term Outcomes After Hip Arthroplasty: A Cohort Study of Older Adults" Nutrients 17, no. 14: 2319. https://doi.org/10.3390/nu17142319
APA StyleBriguglio, M., Latella, M., Sirtori, P., Mangiavini, L., De Luca, P., Geroldi, M., De Vecchi, E., Lombardi, G., Petrillo, S., Wainwright, T. W., Peretti, G. M., & Banfi, G. (2025). Influence of Preoperative Diagnosis of Nutritional Disorders on Short-Term Outcomes After Hip Arthroplasty: A Cohort Study of Older Adults. Nutrients, 17(14), 2319. https://doi.org/10.3390/nu17142319













