Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment
Abstract
1. Introduction
2. Gut Microbiota and Mental Health: Functional Roles, Gut–Brain Axis Interactions, and Sex-Related Differences Across the Lifespan
2.1. Gut Microbiota and Mental Health
2.2. Gut Microbiota Composition
2.3. Microbial Distribution Along the Gastrointestinal Tract
2.4. The Role of the Microbiota in Regulating the Gut–Brain Axis
2.5. Sex Differences in the Intestinal Microbiota Throughout Life
2.5.1. Sex-Related Differences in Gut Microbiota Composition During Childhood (1–12 Years)
- Sex-dependent effects of vitamin A supplementation
- Temperamental traits and infant microbiota
2.5.2. Sex-Related Differences in Gut Microbiota Composition During Adolescence (12–17 Years)
2.5.3. Sex-Related Differences in Gut Microbiota Composition During Adulthood
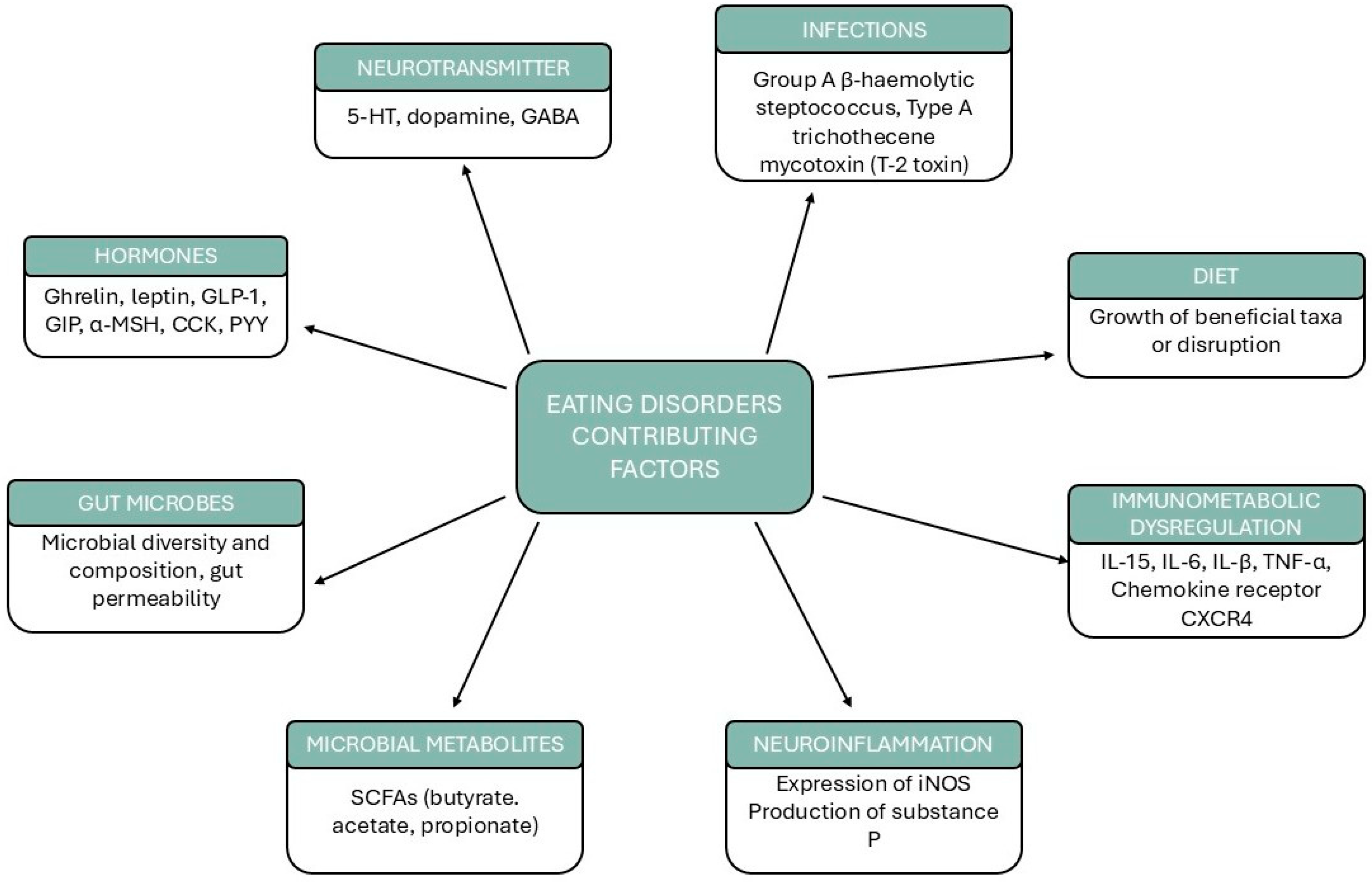
3. Microbiota Alterations in Eating Disorders
3.1. Anorexia Nervosa: Microbiota Alterations in Undernutrition
3.2. Microbiota–Brain Interactions in Psychopathology
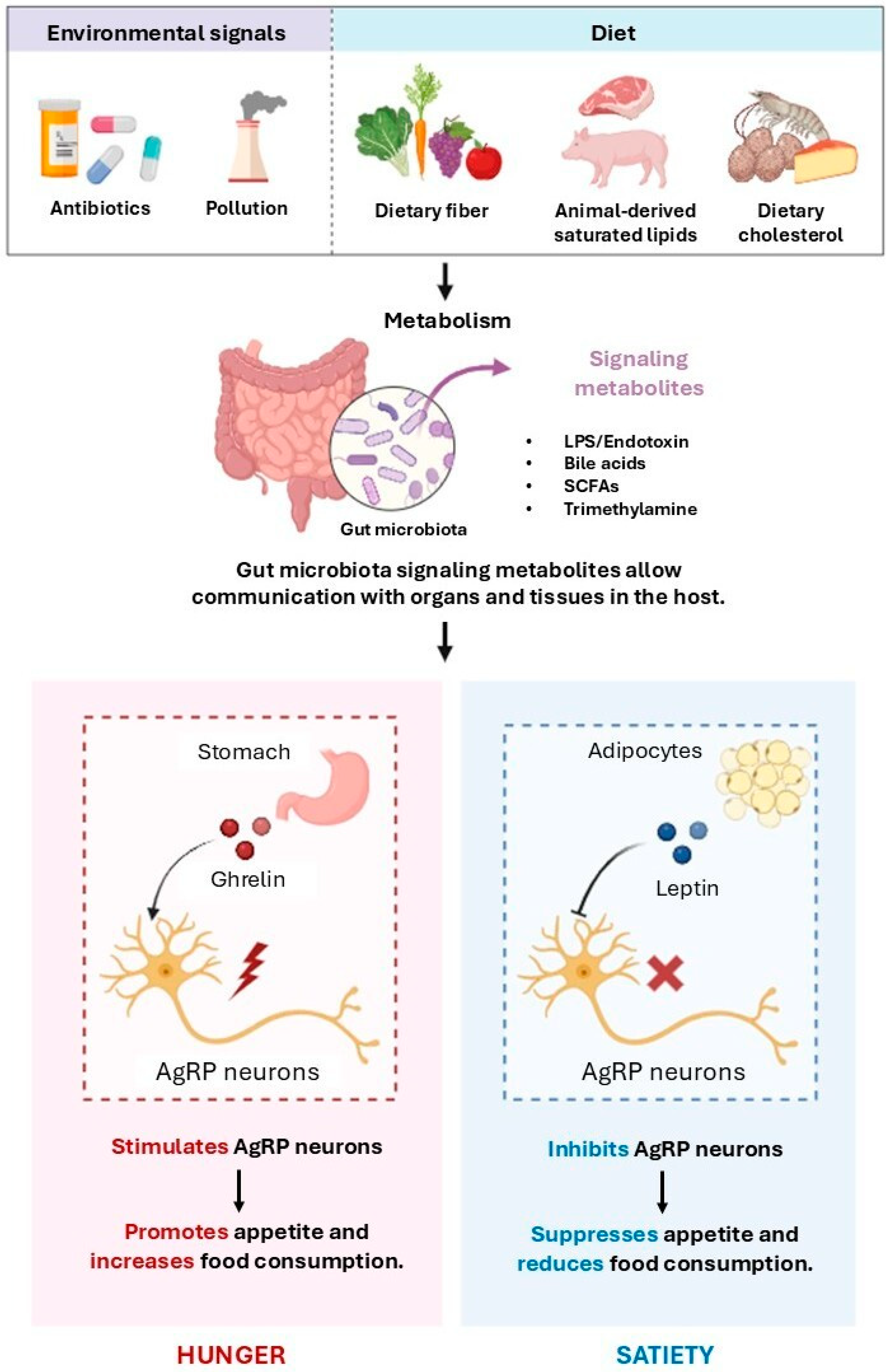
SCFAs, Appetite Regulation, and Metabolic Homeostasis
4. Specific Considerations in Women with EDs
4.1. Estrogen and Progesterone Influences on Microbiota: Menstrual Cycle, Pregnancy, and Menopause and Microbial Shifts in Women
4.2. Diet
5. Potential Microbiota-Targeted Interventions
5.1. Prebiotics, Probiotics, and Dietary Modification Strategies
5.2. Fecal Microbiota Transplantation (FMT): Current Evidence
5.3. Precision Nutrition, Personalized Microbiome-Based Interventions for Women, and Sex-Specific Microbial Biomarkers
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; Muehleman, V. Eating Disorders and Metabolic Diseases. Int. J. Environ. Res. Public Health 2023, 20, 2446. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Mondin, A.M.; Billeci, M.; Fusco, A.; De Prisco, M.; Caiazza, C.; Micanti, F.; Calati, R.; Carvalho, A.F.; de Bartolomeis, A. Psychopharmacology of Eating Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Affect. Disord. 2023, 338, 526–545. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The Role of the Gut Microbiome, Immunity, and Neuroinflammation in the Pathophysiology of Eating Disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Troisi, J.; Serena, G.; Fasano, A.; Dalle Grave, R.; Cascino, G.; Marciello, F.; Calugi, S.; Scala, G.; Corrivetti, G.; et al. The Gut Microbiome and Metabolomics Profiles of Restricting and Binge-Purging Type Anorexia Nervosa. Nutrients 2021, 13, 507. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Sang, L.-X.; Sun, S.-Y. Gut Microbiota and Female Health. World J. Gastroenterol. 2024, 30, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.-Y.; Brunelle, L.; Pilon, G.; Cautela, B.G.; Tompkins, T.A.; Drapeau, V.; Marette, A.; Tremblay, A. Lacticaseibacillus Rhamnosus HA-114 Improves Eating Behaviors and Mood-Related Factors in Adults with Overweight during Weight Loss: A Randomized Controlled Trial. Nutr. Neurosci. 2023, 26, 667–679. [Google Scholar] [CrossRef]
- Grau-Del Valle, C.; Fernández, J.; Solá, E.; Montoya-Castilla, I.; Morillas, C.; Bañuls, C. Association between Gut Microbiota and Psychiatric Disorders: A Systematic Review. Front. Psychol. 2023, 14, 1215674. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Merlo, G.; Bachtel, G.; Sugden, S.G. Gut Microbiota, Nutrition, and Mental Health. Front. Nutr. 2024, 11, 1337889. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.-G.; Li, J.; Cheng, J.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Gan, R.-Y.; Li, H.-B. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 2023, 15, 3258. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the Diversity of the Human Microbiota with Targeted Next-Generation Sequencing. Brief. Bioinform. 2016, 19, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. In Gastrointestinal Pharmacology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–16. [Google Scholar]
- Ohno, H.; Satoh-Takayama, N. Stomach Microbiota, Helicobacter Pylori, and Group 2 Innate Lymphoid Cells. Exp. Mol. Med. 2020, 52, 1377–1382. [Google Scholar] [CrossRef]
- Procházková, N.; Falony, G.; Dragsted, L.O.; Licht, T.R.; Raes, J.; Roager, H.M. Advancing Human Gut Microbiota Research by Considering Gut Transit Time. Gut 2023, 72, 180–191. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Ussery, D.W.; Nielsen, J.; Nookaew, I. A Closer Look at Bacteroides: Phylogenetic Relationship and Genomic Implications of a Life in the Human Gut. Microb. Ecol. 2011, 61, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Severance, E.; Tveiten, D.; Lindström, L.; Yolken, R.; Reichelt, K. The Gut Microbiota and the Emergence of Autoimmunity: Relevance to Major Psychiatric Disorders. Curr. Pharm. Des. 2016, 22, 6076–6086. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Hooper, L.V. Epithelial Cells and Their Neighbors. IV. Bacterial Contributions to Intestinal Epithelial Barrier Integrity. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, G779–G784. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal Microbiota of 6-week-old Infants Across Europe: Geographic Influence Beyond Delivery Mode, Breast-feeding, and Antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Chiesa, G. The Gut Microbiota Affects Host Pathophysiology as an Endocrine Organ: A Focus on Cardiovascular Disease. Nutrients 2019, 12, 79. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-Aminobutyric Acid-Producing Lactobacilli Positively Affect Metabolism and Depressive-like Behaviour in a Mouse Model of Metabolic Syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Wang, G.-J. Food Addiction A Common Neurobiological Mechanism with Drug Abuse. Front. Biosci. 2018, 23, 4618. [Google Scholar] [CrossRef]
- Faraji, N.; Payami, B.; Ebadpour, N.; Gorji, A. Vagus Nerve Stimulation and Gut Microbiota Interactions: A Novel Therapeutic Avenue for Neuropsychiatric Disorders. Neurosci. Biobehav. Rev. 2025, 169, 105990. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Lopetuso, L.R.; Del Zompo, F.; Mangiola, F.; Boškoski, I.; Bruno, G.; Petito, V.; Laterza, L.; Cammarota, G.; et al. Gut Microbial Flora, Prebiotics, and Probiotics in IBD: Their Current Usage and Utility. Biomed. Res. Int. 2013, 2013, 435268. [Google Scholar] [CrossRef]
- Bolon, B. Cellular and Molecular Mechanisms of Autoimmune Disease. Toxicol. Pathol. 2012, 40, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The Impact of Gut Microbiota on Brain and Behaviour. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Mané-Damas, M.; Hoffmann, C.; Zong, S.; Tan, A.; Molenaar, P.C.; Losen, M.; Martinez-Martinez, P. Autoimmunity in Psychotic Disorders. Where We Stand, Challenges and Opportunities. Autoimmun. Rev. 2019, 18, 102348. [Google Scholar] [CrossRef]
- Ramanathan, S.; Brilot, F.; Irani, S.R.; Dale, R.C. Origins and Immunopathogenesis of Autoimmune Central Nervous System Disorders. Nat. Rev. Neurol. 2023, 19, 172–190. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of Microbiota on Central Nervous System and Neurological Diseases: The Gut-Brain Axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine Pathway Metabolism and the Microbiota-Gut-Brain Axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Dale, R.C.; Thomas, T.; Patel, S.; Han, V.X.; Kothur, K.; Troedson, C.; Gupta, S.; Gill, D.; Malone, S.; Waak, M.; et al. CSF Neopterin and Quinolinic Acid Are Biomarkers of Neuroinflammation and Neurotoxicity in FIRES and Other Infection-triggered Encephalopathy Syndromes. Ann. Clin. Transl. Neurol. 2023, 10, 1417–1432. [Google Scholar] [CrossRef]
- Marano, G.; Traversi, G.; Gaetani, E.; Gasbarrini, A.; Mazza, M. Gut Microbiota in Women: The Secret of Psychological and Physical Well-Being. World J. Gastroenterol. 2023, 29, 5945–5952. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M. Function of the Microbiota. Best. Pract. Res. Clin. Gastroenterol. 2013, 27, 5–16. [Google Scholar] [CrossRef]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.L.; Lee, A. The Microbial Ecology of the Large Bowel of Breastfed and Formula-Fed Infants During the First Year of Life. J. Med. Microbiol. 1982, 15, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Solano-Aguilar, G.I.; Lakshman, S.; Shao, J.; Chen, C.; Beshah, E.; Dawson, H.D.; Vinyard, B.; Schroeder, S.G.; Jang, S.; Molokin, A.; et al. Correction: Solano-Aguilar et al. Fruit and Vegetable Supplemented Diet Modulates the Pig Transcriptome and Microbiome after a Two-Week Feeding Intervention. Nutrients 2022, 14, 4513. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R. Vitamin A as an Anti-Inflammatory Agent. Proc. Nutr. Soc. 2002, 61, 397–400. [Google Scholar] [CrossRef]
- Huda, M.N.; Ahmad, S.M.; Kalanetra, K.M.; Taft, D.H.; Alam, M.J.; Khanam, A.; Raqib, R.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Neonatal Vitamin A Supplementation and Vitamin A Status Are Associated with Gut Microbiome Composition in Bangladeshi Infants in Early Infancy and at 2 Years of Age. J. Nutr. 2019, 149, 1075–1088. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Yu, D.; Zhang, T.; Lin, Q.; Li, Q.; Wu, X.; Su, Z.; Zhang, Q.; Xiang, Q.; et al. Vitamin A Promotes Leydig Cell Differentiation via Alcohol Dehydrogenase 1. Front. Endocrinol. 2018, 9, 644. [Google Scholar] [CrossRef]
- Sizonenko, P.C.; Paunier, L. Hormonal Changes in Puberty III: Correlation of Plasma Dehydroepiandrosterone, Testosterone, FSH, and LH with Stages of Puberty and Bone Age in Normal Boys and Girls and in Patients with Addison’s Disease or Hypogonadism or with Premature or Late Adrenarche. J. Clin. Endocrinol. Metab. 1975, 41, 894–904. [Google Scholar] [CrossRef]
- Konieczna, P.; Ferstl, R.; Ziegler, M.; Frei, R.; Nehrbass, D.; Lauener, R.P.; Akdis, C.A.; O’Mahony, L. Immunomodulation by Bifidobacterium Infantis 35624 in the Murine Lamina Propria Requires Retinoic Acid-Dependent and Independent Mechanisms. PLoS ONE 2013, 8, e62617. [Google Scholar] [CrossRef]
- Rothbart, M.K.; Posner, M.I. The Developing Brain in a Multitasking World. Dev. Rev. 2015, 35, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Christian, H.; Zubrick, S.R.; Foster, S.; Giles-Corti, B.; Bull, F.; Wood, L.; Knuiman, M.; Brinkman, S.; Houghton, S.; Boruff, B. The Influence of the Neighborhood Physical Environment on Early Child Health and Development: A Review and Call for Research. Health Place 2015, 33, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and Function of the Healthy Pre-Adolescent Pediatric Gut Microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.E.; Allen, N.B.; Wilbrecht, L.; Suleiman, A.B. Importance of Investing in Adolescence from a Developmental Science Perspective. Nature 2018, 554, 441–450. [Google Scholar] [CrossRef]
- Yuan, Y.; Oishi, S.; Cronin, C.; Müllensiefen, D.; Atkinson, Q.; Fujii, S.; Savage, P.E. Perceptual vs. Automated Judgments of Music Copyright Infringement 2020. In Proceedings of the 21st ISMIR Conference, Montreal, QC, Canada, 11–16 October 2020. [Google Scholar]
- McGee, J.S.; Huttenhower, C. Of Mice and Men and Women: Sexual Dimorphism of the Gut Microbiome. Int. J. Womens Dermatol. 2021, 7, 533–538. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Spor, A.; Koren, O.; Ley, R. Unravelling the Effects of the Environment and Host Genotype on the Gut Microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in Fecal Microbiota in Different European Study Populations in Relation to Age, Gender, and Country: A Cross-Sectional Study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef]
- Oki, E.; Murata, A.; Yoshida, K.; Maeda, K.; Ikejiri, K.; Munemoto, Y.; Sasaki, K.; Matsuda, C.; Kotake, M.; Suenaga, T.; et al. A Randomized Phase III Trial Comparing S-1 versus UFT as Adjuvant Chemotherapy for Stage II/III Rectal Cancer (JFMC35-C1: ACTS-RC). Ann. Oncol. 2016, 27, 1266–1272. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal Microbial Determinants of Fecal and Systemic Estrogens and Estrogen Metabolites: A Cross-Sectional Study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Scavello, I.; Maseroli, E.; Di Stasi, V.; Vignozzi, L. Sexual Health in Menopause. Medicina 2019, 55, 559. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in Gut Microbiota Associated with Age, Sex, and Stool Consistency in Healthy Japanese Subjects. J. Gastroenterol. 2019, 54, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, T.; Huang, G.; Cai, D.; Liang, X.; Su, H.; Zhu, Z.; Li, D.; Yang, Y.; Shen, P.; et al. Gut Microbiota Community and Its Assembly Associated with Age and Diet in Chinese Centenarians. J. Microbiol. Biotechnol. 2015, 25, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Ma, X.; Sankaran, K.; Ru, Y.; Chen, L.; Baiocchi, M.; Zhu, S. Sex-Specific Association between Gut Microbiome and Fat Distribution. Nat. Commun. 2019, 10, 2408. [Google Scholar] [CrossRef]
- Pujo, J.; Petitfils, C.; Le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; Van Hul, M.; Barreau, F.; Blanpied, C.; et al. Bacteria-Derived Long Chain Fatty Acid Exhibits Anti-Inflammatory Properties in Colitis. Gut 2021, 70, 1088–1097. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Chisanga, D.; Blume, J.; Gloury, R.; Britt, K.; Henstridge, D.C.; Zhan, Y.; Torres, S.V.; Liene, S.; Collins, N.; et al. Sex-Specific Adipose Tissue Imprinting of Regulatory T Cells. Nature 2020, 579, 581–585. [Google Scholar] [CrossRef]
- Moore, C.A.; Bokor, B.R. Anorexia Nervosa. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- van Eeden, A.E.; van Hoeken, D.; Hoek, H.W. Incidence, Prevalence and Mortality of Anorexia Nervosa and Bulimia Nervosa. Curr. Opin. Psychiatry 2021, 34, 515–524. [Google Scholar] [CrossRef]
- Ayrolles, A.; Clarke, J.; Godart, N.; André-Carletti, C.; Barbe, C.; Bargiacchi, A.; Blanchet, C.; Bergametti, F.; Bertrand, V.; Caldagues, E.; et al. Early-Onset Anorexia Nervosa: A Scoping Review and Management Guidelines. J. Eat. Disord. 2024, 12, 182. [Google Scholar] [CrossRef]
- Attia, E.; Walsh, B.T. Eating Disorders. JAMA 2025, 333, 1242. [Google Scholar] [CrossRef]
- Zimmermann-Rösner, A.; Prehn-Kristensen, A. The Microbiome in Child and Adolescent Psychiatry. Z. Kinder Jugendpsychiatr Psychother. 2024, 52, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Kodancha, P.; Das, S. Gut Microbiome Changes in Anorexia Nervosa: A Comprehensive Review. Pathophysiology 2024, 31, 68–88. [Google Scholar] [CrossRef]
- Scala, M.; Tabone, M.; Paolini, M.; Salueña, A.; Iturra, R.A.; Ferreiro, V.R.; Alvarez-Mon, M.Á.; Serretti, A.; del Rocío Gonzalez Soltero, M.; Rodriguez-Jimenez, R. Unlocking the Link Between Gut Microbiota and Psychopathological Insights in Anorexia Nervosa: A Systematic Review. Eur. Eat. Disord. Rev. 2025, 33, 700–718. [Google Scholar] [CrossRef]
- Prochazkova, P.; Roubalova, R.; Dvorak, J.; Kreisinger, J.; Hill, M.; Tlaskalova-Hogenova, H.; Tomasova, P.; Pelantova, H.; Cermakova, M.; Kuzma, M.; et al. The Intestinal Microbiota and Metabolites in Patients with Anorexia Nervosa. Gut Microbes 2021, 13, 1902771. [Google Scholar] [CrossRef]
- Mondot, S.; Lachkar, L.; Doré, J.; Blottière, H.M.; Hanachi, M. Roseburia, a Decreased Bacterial Taxon in the Gut Microbiota of Patients Suffering from Anorexia Nervosa. Eur. J. Clin. Nutr. 2022, 76, 1486–1489. [Google Scholar] [CrossRef]
- Baenas, I.; Camacho-Barcia, L.; Miranda-Olivos, R.; Solé-Morata, N.; Misiolek, A.; Jiménez-Murcia, S.; Fernández-Aranda, F. Probiotic and Prebiotic Interventions in Eating Disorders: A Narrative Review. Eur. Eat. Disord. Rev. 2024, 32, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, Y.; Miyata, N.; Nakashima, M.; Hata, T.; Takakura, S.; Yoshihara, K.; Suematsu, T.; Nomoto, K.; Miyazaki, K.; Tsuji, H.; et al. Persistence of Gut Dysbiosis in Individuals with Anorexia Nervosa. PLoS ONE 2023, 18, e0296037. [Google Scholar] [CrossRef] [PubMed]
- Huwart, S.J.P.; Morales-Puerto, N.; Everard, A. Gut Microbiota-Related Neuroinflammation at the Crossroad of Food Reward Alterations: Implications for Eating Disorders. Gut 2025, 1–13. [Google Scholar] [CrossRef]
- Li, Z.; Bi, T. Causal Effects of Gut Microbiota, Metabolites, Immune Cells, Liposomes, and Inflammatory Proteins on Anorexia Nervosa: A Mediation Joint Multi-Omics Mendelian Randomization Analysis. J. Affect. Disord. 2025, 368, 343–358. [Google Scholar] [CrossRef]
- Käver, L.; Voelz, C.; Specht, H.E.; Thelen, A.C.; Keller, L.; Dahmen, B.; Andreani, N.A.; Tenbrock, K.; Biemann, R.; Borucki, K.; et al. Cytokine and Microbiome Changes in Adolescents with Anorexia Nervosa at Admission, Discharge, and One-Year Follow-Up. Nutrients 2024, 16, 1596. [Google Scholar] [CrossRef] [PubMed]
- Shahid, F.; Afridi, Z.; Ali, M.; Saddique, M.N.; Iqbal, J.; Jha, A. Beneath the Surface: Unveiling the Gut–Brain Axis in Anorexia and Depression. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2025, 30, 14. [Google Scholar] [CrossRef]
- Bozzola, E.; Barni, S.; Marchili, M.R.; Hellmann, R.; Giudice, E.D.; De Luca, G.; Cupertino, V. Anorexia Nervosa in Children and Adolescents: An Early Detection of Risk Factors. Ital. J. Pediatr. 2024, 50, 221. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, H.; Zhao, M.; Zhang, J.; Qu, N. Gut Microbiotas, Inflammatory Factors, and Mental-Behavioral Disorders: A Mendelian Randomization Study. J. Affect. Disord. 2025, 371, 113–123. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, M.; Yu, B.; Wang, Y.; Yan, Z.; Gao, R. Anorexia Nervosa and Bulimia Nervosa: A Mendelian Randomization Study of Gut Microbiota. Front. Microbiol. 2024, 15, 1396932. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Støving, R.K.; Berreira Ibraim, S.; Hyötyläinen, T.; Thirion, F.; Arora, T.; Lyu, L.; Stankevic, E.; Hansen, T.H.; Déchelotte, P.; et al. The Gut Microbiota Contributes to the Pathogenesis of Anorexia Nervosa in Humans and Mice. Nat. Microbiol. 2023, 8, 787–802. [Google Scholar] [CrossRef]
- Quagebeur, R.; Dalile, B.; Raes, J.; Van Oudenhove, L.; Verbeke, K.; Vrieze, E. The Role of Short-Chain Fatty Acids (SCFAs) in Regulating Stress Responses, Eating Behavior, and Nutritional State in Anorexia Nervosa: Protocol for a Randomized Controlled Trial. J. Eat. Disord. 2023, 11, 191. [Google Scholar] [CrossRef]
- Helal, P.; Xia, W.; Sardar, P.; Conway-Morris, A.; Conway-Morris, A.; Pedicord, V.A.; Serfontein, J. Changes in the Firmicutes to Bacteriodetes Ratio in the Gut Microbiome in Individuals with Anorexia Nervosa Following Inpatient Treatment: A Systematic Review and a Case Series. Brain Behav. 2024, 14, e70014. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Han, Y.; Liu, C.; Cao, S.; Cui, Y.; Zhu, X.; Wang, Z.; Liu, B.; Shi, Y. Gut Microbiota-Derived Gamma-Aminobutyric Acid Improves Host Appetite by Inhibiting Satiety Hormone Secretion. Msystems 2024, 9, e01015-24. [Google Scholar] [CrossRef]
- Himmerich, H.; Treasure, J. Anorexia Nervosa: Diagnostic, Therapeutic, and Risk Biomarkers in Clinical Practice. Trends Mol. Med. 2024, 30, 350–360. [Google Scholar] [CrossRef]
- Vitetta, L.; Bambling, M.; Strodl, E. Persister Intestinal Bacteria, Epigenetics and Major Depression. Front. Biosci. Landmark 2025, 30, 26837. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.F.; de Almeida Nogueira, Y.J.; Romano-Silva, M.A.; Marques de Miranda, D. Effects of Antipsychotics on the Gastrointestinal Microbiota: A Systematic Review. Psychiatry Res. 2024, 336, 115914. [Google Scholar] [CrossRef]
- Andreani, N.A.; Sharma, A.; Dahmen, B.; Specht, H.E.; Mannig, N.; Ruan, V.; Keller, L.; Baines, J.F.; Herpertz-Dahlmann, B.; Dempfle, A.; et al. Longitudinal Analysis of the Gut Microbiome in Adolescent Patients with Anorexia Nervosa: Microbiome-Related Factors Associated with Clinical Outcome. Gut Microbes 2024, 16, 2304158. [Google Scholar] [CrossRef] [PubMed]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in Anorexia Nervosa: The Triangle between Bacterial Species, Metabolites and Psychological Tests. PLoS ONE 2017, 12, e0179739. [Google Scholar] [CrossRef]
- Schulz, N.; Belheouane, M.; Dahmen, B.; Ruan, V.A.; Specht, H.E.; Dempfle, A.; Herpertz-Dahlmann, B.; Baines, J.F.; Seitz, J. Gut Microbiota Alteration in Adolescent Anorexia Nervosa Does Not Normalize with Short-term Weight Restoration. Int. J. Eat. Disord. 2021, 54, 969–980. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef]
- van de Pol, J.A.A.; van Best, N.; Mbakwa, C.A.; Thijs, C.; Savelkoul, P.H.; Arts, I.C.W.; Hornef, M.W.; Mommers, M.; Penders, J. Gut Colonization by Methanogenic Archaea Is Associated with Organic Dairy Consumption in Children. Front. Microbiol. 2017, 8, 355. [Google Scholar] [CrossRef]
- Hanachi, M.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Levenez, F.; Cournède, N.; Doré, J.; Melchior, J.-C. Altered Host-Gut Microbes Symbiosis in Severely Malnourished Anorexia Nervosa (AN) Patients Undergoing Enteral Nutrition: An Explicative Factor of Functional Intestinal Disorders? Clin. Nutr. 2019, 38, 2304–2310. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed Moods: A Review of the Interactions between Inflammation and Mood Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Tarchi, L.; Cassioli, E.; Rossi, E.; Merola, G.P.; Ficola, A.; Cordasco, V.Z.; Ricca, V.; Castellini, G. A Transdiagnostic and Diagnostic-Specific Approach on Inflammatory Biomarkers in Eating Disorders: A Meta-Analysis and Systematic Review. Psychiatry Res. 2024, 340, 116115. [Google Scholar] [CrossRef]
- Kalkan, A.E.; BinMowyna, M.N.; Raposo, A.; Ahmad, M.F.; Ahmed, F.; Otayf, A.Y.; Carrascosa, C.; Saraiva, A.; Karav, S. Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review. Nutrients 2025, 17, 1305. [Google Scholar] [CrossRef]
- van Norren, K.; Dwarkasing, J.T.; Witkamp, R.F. The Role of Hypothalamic Inflammation, the Hypothalamic–Pituitary–Adrenal Axis and Serotonin in the Cancer Anorexia–Cachexia Syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Slezacek, J.; Fusani, L.; Kaiya, H.; Quillfeldt, P. A First Glimpse into Circulating Ghrelin Patterns of Thin-Billed Prion Chicks (Pachyptila Belcheri). J. Comp. Physiol. B 2025, 195, 209–213. [Google Scholar] [CrossRef]
- Fakhoury, M.; Salman, I.; Najjar, W.; Merhej, G.; Lawand, N. The Lateral Hypothalamus: An Uncharted Territory for Processing Peripheral Neurogenic Inflammation. Front. Neurosci. 2020, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Jing, L.; Xu, Y.; Zhang, K.; Li, Y.; Sun, N.; Liu, P.; Zhang, H. Gut Microbiota and Inflammatory Factor Characteristics in Major Depressive Disorder Patients with Anorexia. BMC Psychiatry 2024, 24, 334. [Google Scholar] [CrossRef]
- Freff, J.; Bröker, L.; Leite Dantas, R.; Schwarte, K.; Bühlmeier, J.; Kraft, I.; Hinney, A.; Buhlmann, U.; Arolt, V.; Dannlowski, U.; et al. Expression of CXCR4 on CD4+ T Cells Predicts Body Composition Parameters in Female Adolescents with Anorexia Nervosa. Front. Psychiatry 2022, 13, 960905. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Song, W.-X.; Wang, Y.-S.; Liu, Y.; Chen, F.-J.; Chen, Y.-H.; Jiang, Y.-B.; Zhang, C.; Yang, X. A Review of Anorexia Induced by T-2 Toxin. Food Chem. Toxicol. 2023, 179, 113982. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Su, Y.; Li, T. An Update on T2-Toxins: Metabolism, Immunotoxicity Mechanism and Human Assessment Exposure of Intestinal Microbiota. Heliyon 2022, 8, e10012. [Google Scholar] [CrossRef]
- Conde, K.; Fang, S.; Xu, Y. Unraveling the Serotonin Saga: From Discovery to Weight Regulation and beyond—A Comprehensive Scientific Review. Cell Biosci. 2023, 13, 143. [Google Scholar] [CrossRef]
- Pierzgalski, A.; Bryła, M.; Kanabus, J.; Modrzewska, M.; Podolska, G. Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms. Toxins 2021, 13, 768. [Google Scholar] [CrossRef]
- Huang, T.; Li, A.; Zhang, S.; Fan, J.; Hua, Z.; Wang, X.; Zhang, C.; Yang, X. The Role of Gut Microbiota in Anorexia Induced by T-2 Toxin. Ecotoxicol. Environ. Saf. 2024, 281, 116612. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Huang, Z.; Chen, J.-K.; Tucker, L.; Zhang, Q. Comprehensive Insights into Emerging Advances in the Neurobiology of Anorexia. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-Chain Fatty Acids Stimulate Leptin Production in Adipocytes through the G Protein-Coupled Receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.; Tazoe, H.; Hayashi, H.; Kashiwabara, H.; Tooyama, K.; Suzuki, Y.; Kuwahara, A. Expression of the Short-Chain Fatty Acid Receptor, GPR43, in the Human Colon. J. Mol. Histol. 2008, 39, 135–142. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut Hormone PYY3-36 Physiologically Inhibits Food Intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Verdich, C.; Flint, A.; Gutzwiller, J.-P.; Naslund, E.; Beglinger, C.; Hellstrom, P.M.; Long, S.J.; Morgan, L.M.; Holst, J.J.; Astrup, A. A Meta-Analysis of the Effect of Glucagon-Like Peptide-1 (7–36) Amide on Ad Libitum Energy Intake in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 4382–4389. [Google Scholar] [CrossRef]
- Verhoef, S.P.M.; Meyer, D.; Westerterp, K.R. Effects of Oligofructose on Appetite Profile, Glucagon-like Peptide 1 and Peptide YY3-36 Concentrations and Energy Intake. Br. J. Nutr. 2011, 106, 1757–1762. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Huang, G.; Wang, H.; Chen, H.; Su, Y.; Yu, K.; Zhu, W. Propionate Stimulates the Secretion of Satiety Hormones and Reduces Acute Appetite in a Cecal Fistula Pig Model. Anim. Nutr. 2022, 10, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, M.; Tang, M.; Gao, C.; Wang, H.; Man, S.; Lu, F. Oligosaccharide and Short-Chain Fatty Acid: A Double-Edged Sword in Obese Mice by Regulating Food Intake and Fat Synthesis. Food Res. Int. 2022, 159, 111619. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.; Hanssen, R.; Qubad, M.; Bouzouina, A.; Schack, V.; Sochor, H.; Schiweck, C.; Aichholzer, M.; Matura, S.; Slattery, D.A.; et al. Impact of Insulin and Insulin Resistance on Brain Dopamine Signalling and Reward Processing—An Underexplored Mechanism in the Pathophysiology of Depression? Neurosci. Biobehav. Rev. 2023, 149, 105179. [Google Scholar] [CrossRef]
- Tadayonnejad, R.; Majid, D.-A.; Tsolaki, E.; Rane, R.; Wang, H.; Moody, T.D.; Pauli, W.M.; Pouratian, N.; Bari, A.A.; Murray, S.B.; et al. Mesolimbic Neurobehavioral Mechanisms of Reward Motivation in Anorexia Nervosa: A Multimodal Imaging Study. Front. Psychiatry 2022, 13, 806327. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy Metabolism in Health and Diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Rahman, N.; Mian, M.F.; Nazli, A.; Kaushic, C. Human Vaginal Microbiota Colonization Is Regulated by Female Sex Hormones in a Mouse Model. Front. Cell Infect. Microbiol. 2023, 13, 1307451. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, W.; Yuan, Y.; Zhu, W.; Shang, A. Vaginal Microecological Characteristics of Women in Different Physiological and Pathological Period. Front. Cell Infect. Microbiol. 2022, 12, 959793. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.S.; de Lima, A.B.F.; de Brito, J.C.R.; Sarmento, A.C.A.; Gonçalves, A.K.S.; Eleutério, J. Postmenopausal Vaginal Microbiome and Microbiota. Front. Reprod. Health 2022, 3, 780931. [Google Scholar] [CrossRef]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The Role of Gut and Genital Microbiota and the Estrobolome in Endometriosis, Infertility and Chronic Pelvic Pain. Hum. Reprod. Update 2021, 28, 92–131. [Google Scholar] [CrossRef]
- Hammouda, Z.K.; Wasfi, R.; Abdeltawab, N.F. Hormonal Drugs: Influence on Growth, Biofilm Formation, and Adherence of Selected Gut Microbiota. Front. Cell Infect. Microbiol. 2023, 13, 1147585. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The Impact of the Gut Microbiota on the Reproductive and Metabolic Endocrine System. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Kornman, K.S.; Loesche, W.J. Effects of Estradiol and Progesterone on Bacteroides Melaninogenicus and Bacteroides Gingivalis. Infect. Immun. 1982, 35, 256–263. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.M.P.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium Scindens: A Human Gut Microbe with a High Potential to Convert Glucocorticoids into Androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.S.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. Msphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Plummer, E.L.; Vodstrcil, L.A.; Bradshaw, C.S. Unravelling the Vaginal Microbiome, Impact on Health and Disease. Curr. Opin. Obstet. Gynecol. 2024, 36, 338–344. [Google Scholar] [CrossRef]
- Morsli, M.; Gimenez, E.; Magnan, C.; Salipante, F.; Huberlant, S.; Letouzey, V.; Lavigne, J.-P. The Association between Lifestyle Factors and the Composition of the Vaginal Microbiota: A Review. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1869–1881. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Saraf, V.S.; Sheikh, S.A.; Ahmad, A.; Gillevet, P.M.; Bokhari, H.; Javed, S. Vaginal Microbiome: Normalcy vs. Dysbiosis. Arch. Microbiol. 2021, 203, 3793–3802. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Metwally, E.; Chughtai, M.I.; Mazhar, M.U.; Khan, S.A. Relationship between Gut Microbiota and Host-Metabolism: Emphasis on Hormones Related to Reproductive Function. Anim. Nutr. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Schieren, A.; Koch, S.; Pecht, T.; Simon, M.-C. Impact of Physiological Fluctuations of Sex Hormones During the Menstrual Cycle on Glucose Metabolism and the Gut Microbiota. Exp. Clin. Endocrinol. Diabetes 2024, 132, 267–278. [Google Scholar] [CrossRef]
- Terry, S.M.; Barnett, J.A.; Gibson, D.L. A Critical Analysis of Eating Disorders and the Gut Microbiome. J. Eat. Disord. 2022, 10, 154. [Google Scholar] [CrossRef]
- Sebastiani, G.; Andreu-Fernández, V.; Herranz Barbero, A.; Aldecoa-Bilbao, V.; Miracle, X.; Meler Barrabes, E.; Balada Ibañez, A.; Astals-Vizcaino, M.; Ferrero-Martínez, S.; Gómez-Roig, M.D.; et al. Eating Disorders During Gestation: Implications for Mother’s Health, Fetal Outcomes, and Epigenetic Changes. Front. Pediatr. 2020, 8, 587. [Google Scholar] [CrossRef]
- Krog, M.C.; Hugerth, L.W.; Fransson, E.; Bashir, Z.; Nyboe Andersen, A.; Edfeldt, G.; Engstrand, L.; Schuppe-Koistinen, I.; Nielsen, H.S. The Healthy Female Microbiome across Body Sites: Effect of Hormonal Contraceptives and the Menstrual Cycle. Human. Reprod. 2022, 37, 1525–1543. [Google Scholar] [CrossRef]
- Hua, X.; Cao, Y.; Morgan, D.M.; Miller, K.; Chin, S.M.; Bellavance, D.; Khalili, H. Longitudinal Analysis of the Impact of Oral Contraceptive Use on the Gut Microbiome. J. Med. Microbiol. 2022, 71, 001512. [Google Scholar] [CrossRef] [PubMed]
- Ratten, L.K.; Plummer, E.L.; Bradshaw, C.S.; Fairley, C.K.; Murray, G.L.; Garland, S.M.; Bateson, D.; Tachedjian, G.; Masson, L.; Vodstrcil, L.A. The Effect of Exogenous Sex Steroids on the Vaginal Microbiota: A Systematic Review. Front. Cell Infect. Microbiol. 2021, 11, 732423. [Google Scholar] [CrossRef]
- Garmendia, J.V.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection. Microorganisms 2024, 12, 1641. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut Microbial Beta-Glucuronidase: A Vital Regulator in Female Estrogen Metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Guan, Z.; Xuanqi, Z.; Zhu, J.; Yuan, W.; Jia, J.; Zhang, C.; Sun, T.; Leng, H.; Jiang, C.; Xu, Y.; et al. Estrogen Deficiency Induces Bone Loss through the Gut Microbiota. Pharmacol. Res. 2023, 196, 106930. [Google Scholar] [CrossRef]
- Jin, C.; Qin, L.; Liu, Z.; Li, X.; Gao, X.; Cao, Y.; Zhao, S.; Wang, J.; Han, T.; Yan, L.; et al. Comparative Analysis of the Vaginal Microbiome of Healthy and Polycystic Ovary Syndrome Women: A Large Cross-Sectional Study. Reprod. Biomed. Online 2023, 46, 1005–1016. [Google Scholar] [CrossRef]
- Peters, B.A.; Lin, J.; Qi, Q.; Usyk, M.; Isasi, C.R.; Mossavar-Rahmani, Y.; Derby, C.A.; Santoro, N.; Perreira, K.M.; Daviglus, M.L.; et al. Menopause Is Associated with an Altered Gut Microbiome and Estrobolome, with Implications for Adverse Cardiometabolic Risk in the Hispanic Community Health Study/Study of Latinos. Msystems 2022, 7, e00273-22. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, G.; Cong, Y.; Yu, Y.; Li, Y. Sex-Related Differences in Inflammatory Bowel Diseases: The Potential Role of Sex Hormones. Inflamm. Bowel Dis. 2022, 28, 1766–1775. [Google Scholar] [CrossRef]
- Wu, A.H.; Tseng, C.; Vigen, C.; Yu, Y.; Cozen, W.; Garcia, A.A.; Spicer, D. Gut Microbiome Associations with Breast Cancer Risk Factors and Tumor Characteristics: A Pilot Study. Breast Cancer Res. Treat. 2020, 182, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Liu, Z.; Shang, Q.; Gao, Y.; Li, X.; Zheng, C.; Deng, X.; Chen, T. The Disordered Vaginal Microbiota Is a Potential Indicator for a Higher Failure of in Vitro Fertilization. Front. Med. 2020, 7, 217. [Google Scholar] [CrossRef]
- Gatenby, C.; Simpson, P. Menopause: Physiology, Definitions, and Symptoms. Best. Pract. Res. Clin. Endocrinol. Metab. 2024, 38, 101855. [Google Scholar] [CrossRef] [PubMed]
- Tramice, A.; Paris, D.; Manca, A.; Guevara Agudelo, F.A.; Petrosino, S.; Siracusa, L.; Carbone, M.; Melck, D.; Raymond, F.; Piscitelli, F. Analysis of the Oral Microbiome during Hormonal Cycle and Its Alterations in Menopausal Women: The “AMICA” Project. Sci. Rep. 2022, 12, 22086. [Google Scholar] [CrossRef]
- Leite, G.; Barlow, G.M.; Parodi, G.; Pimentel, M.L.; Chang, C.; Hosseini, A.; Wang, J.; Pimentel, M.; Mathur, R. Duodenal Microbiome Changes in Postmenopausal Women: Effects of Hormone Therapy and Implications for Cardiovascular Risk. Menopause 2022, 29, 264–275. [Google Scholar] [CrossRef]
- Huhmann, K. Menses Requires Energy: A Review of How Disordered Eating, Excessive Exercise, and High Stress Lead to Menstrual Irregularities. Clin. Ther. 2020, 42, 401–407. [Google Scholar] [CrossRef]
- Lawson, E.A.; Eddy, K.T.; Donoho, D.; Misra, M.; Miller, K.K.; Meenaghan, E.; Lydecker, J.; Herzog, D.; Klibanski, A. Appetite-Regulating Hormones Cortisol and Peptide YY Are Associated with Disordered Eating Psychopathology, Independent of Body Mass Index. Eur. J. Endocrinol. 2011, 164, 253–261. [Google Scholar] [CrossRef]
- Misra, M.; Katzman, D.K.; Estella, N.M.; Eddy, K.T.; Weigel, T.; Goldstein, M.A.; Miller, K.K.; Klibanski, A. Impact of Physiologic Estrogen Replacement on Anxiety Symptoms, Body Shape Perception, and Eating Attitudes in Adolescent Girls With Anorexia Nervosa. J. Clin. Psychiatry 2013, 74, e765–e771. [Google Scholar] [CrossRef]
- Baskaran, C.; Cunningham, B.; Plessow, F.; Singhal, V.; Woolley, R.; Ackerman, K.E.; Slattery, M.; Lee, H.; Lawson, E.A.; Eddy, K.; et al. Estrogen Replacement Improves Verbal Memory and Executive Control in Oligomenorrheic/Amenorrheic Athletes in a Randomized Controlled Trial. J. Clin. Psychiatry 2017, 78, e490–e497. [Google Scholar] [CrossRef]
- Chui, H.T.; Christensen, B.K.; Zipursky, R.B.; Richards, B.A.; Hanratty, M.K.; Kabani, N.J.; Mikulis, D.J.; Katzman, D.K. Cognitive Function and Brain Structure in Females With a History of Adolescent-Onset Anorexia Nervosa. Pediatrics 2008, 122, e426–e437. [Google Scholar] [CrossRef]
- Cano Sokoloff, N.; Eguiguren, M.L.; Wargo, K.; Ackerman, K.E.; Baskaran, C.; Singhal, V.; Clarke, H.; Slattery, M.; Lee, H.; Eddy, K.T.; et al. Bone Parameters in Relation to Attitudes and Feelings Associated with Disordered Eating in Oligo-amenorrheic Athletes, Eumenorrheic Athletes, and Nonathletes. Int. J. Eat. Disord. 2015, 48, 522–526. [Google Scholar] [CrossRef]
- Plessow, F.; Singhal, V.; Toth, A.T.; Micali, N.; Eddy, K.T.; Misra, M. Estrogen Administration Improves the Trajectory of Eating Disorder Pathology in Oligo-Amenorrheic Athletes: A Randomized Controlled Trial. Psychoneuroendocrinology 2019, 102, 273–280. [Google Scholar] [CrossRef]
- Frazier, L.D.; Bazo Perez, M. Unpacking Eating Disorder Risk and Resilience during Menopause: A Biopsychosocial Perspective. Menopause 2025, 32, 443–452. [Google Scholar] [CrossRef]
- Mangweth-Matzek, B.; Kummer, K.K.; Hoek, H.W. Update on the Epidemiology and Treatment of Eating Disorders among Older People. Curr. Opin. Psychiatry 2023, 36, 405–411. [Google Scholar] [CrossRef]
- Carbone, E.A.; D’Amato, P.; Vicchio, G.; De Fazio, P.; Segura-Garcia, C. A Systematic Review on the Role of Microbiota in the Pathogenesis and Treatment of Eating Disorders. Eur. Psychiatry 2021, 64, e2. [Google Scholar] [CrossRef]
- Herman, A.; Bajaka, A. The Role of the Intestinal Microbiota in Eating Disorders—Bulimia Nervosa and Binge Eating Disorder. Psychiatry Res. 2021, 300, 113923. [Google Scholar] [CrossRef]
- Novelle, M.G. Decoding the Role of Gut-Microbiome in the Food Addiction Paradigm. Int. J. Environ. Res. Public Health 2021, 18, 6825. [Google Scholar] [CrossRef]
- Martínez-Olcina, M.; Rubio-Arias, J.A.; Reche-García, C.; Leyva-Vela, B.; Hernández-García, M.; Hernández-Morante, J.J.; Martínez-Rodríguez, A. Eating Disorders in Pregnant and Breastfeeding Women: A Systematic Review. Medicina 2020, 56, 352. [Google Scholar] [CrossRef]
- Watson, H.J.; Thornton, L.M.; Yilmaz, Z.; Baker, J.H.; Coleman, J.R.I.; Adan, R.A.H.; Alfredsson, L.; Andreassen, O.A.; Ask, H.; Berrettini, W.H.; et al. Common Genetic Variation and Age of Onset of Anorexia Nervosa. Biol. Psychiatry Glob. Open Sci. 2022, 2, 368–378. [Google Scholar] [CrossRef]
- Santollo, J.; Daniels, D. Multiple Estrogen Receptor Subtypes Influence Ingestive Behavior in Female Rodents. Physiol. Behav. 2015, 152, 431–437. [Google Scholar] [CrossRef]
- Lalonde-Bester, S.; Malik, M.; Masoumi, R.; Ng, K.; Sidhu, S.; Ghosh, M.; Vine, D. Prevalence and Etiology of Eating Disorders in Polycystic Ovary Syndrome: A Scoping Review. Adv. Nutr. 2024, 15, 100193. [Google Scholar] [CrossRef]
- Rolan, E.P.; Mikhail, M.E.; Culbert, K.M.; Burt, S.A.; Klump, K.L. Estrogen Moderation of Genetic Influences on Eating Disorder Symptoms during Gonadarche in Girls: Specific Effects on Binge Eating. Psychoneuroendocrinology 2023, 158, 106384. [Google Scholar] [CrossRef]
- Gómez-Oro, C.; Latorre, M.C.; Arribas-Poza, P.; Ibáñez-Escribano, A.; Baca-Cornejo, K.R.; Gallego-Valle, J.; López-Escobar, N.; Mondéjar-Palencia, M.; Pion, M.; López-Fernández, L.A.; et al. Progesterone Promotes CXCl2-Dependent Vaginal Neutrophil Killing by Activating Cervical Resident Macrophage–Neutrophil Crosstalk. JCI Insight 2024, 9, e177899. [Google Scholar] [CrossRef]
- Diviccaro, S.; FitzGerald, J.A.; Cioffi, L.; Falvo, E.; Crispie, F.; Cotter, P.D.; O’Mahony, S.M.; Giatti, S.; Caruso, D.; Melcangi, R.C. Gut Steroids and Microbiota: Effect of Gonadectomy and Sex. Biomolecules 2022, 12, 767. [Google Scholar] [CrossRef]
- Chen, M.-J.; Chou, C.-H.; Hsiao, T.-H.; Wu, T.-Y.; Li, C.-Y.; Chen, Y.-L.; Chao, K.-H.; Lee, T.-H.; Gicana, R.G.; Shih, C.-J.; et al. Clostridium innocuum, an Opportunistic Gut Pathogen, Inactivates Host Gut Progesterone and Arrests Ovarian Follicular Development. Gut Microbes 2024, 16, 2424911. [Google Scholar] [CrossRef]
- Monnin, N.; Fattet, A.J.; Koscinski, I. Endometriosis: Update of Pathophysiology, (Epi) Genetic and Environmental Involvement. Biomedicines 2023, 11, 978. [Google Scholar] [CrossRef]
- Coombes, Z.; Yadav, V.; McCoubrey, L.; Freire, C.; Basit, A.; Conlan, R.; Gonzalez, D. Progestogens Are Metabolized by the Gut Microbiota: Implications for Colonic Drug Delivery. Pharmaceutics 2020, 12, 760. [Google Scholar] [CrossRef]
- Wan, S.; Sun, Y.; Zong, J.; Meng, W.; Yan, J.; Chen, K.; Wang, S.; Guo, D.; Xiao, Z.; Zhou, Q.; et al. METTL3-Dependent M6A Methylation Facilitates Uterine Receptivity and Female Fertility via Balancing Estrogen and Progesterone Signaling. Cell Death Dis. 2023, 14, 349. [Google Scholar] [CrossRef]
- Kato, S.; Nagasawa, T.; Uehara, O.; Shimizu, S.; Sugiyama, N.; Hasegawa-Nakamura, K.; Noguchi, K.; Hatae, M.; Kakinoki, H.; Furuichi, Y. Increase in Bifidobacterium Is a Characteristic of the Difference in the Salivary Microbiota of Pregnant and Non-Pregnant Women. BMC Oral Health 2022, 22, 260. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, D.; Zhang, A.; Huang, H.; Li, Y.; Xu, D. Candida Albicans-Induced Activation of the TGF-β/Smad Pathway and Upregulation of IL-6 May Contribute to Intrauterine Adhesion. Sci. Rep. 2023, 13, 579. [Google Scholar] [CrossRef]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human Implantation: The Complex Interplay between Endometrial Receptivity, Inflammation, and the Microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Moron, A.F.; Linhares, I.M.; Forney, L.J. Influence of Lactobacillus Crispatus, Lactobacillus Iners and Gardnerella Vaginalis on Bacterial Vaginal Composition in Pregnant Women. Arch. Gynecol. Obstet. 2021, 304, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.; Black, N.; Bennett, P.R.; Brosens, J.; Quenby, S.; MacIntyre, D.A. The Endometrial Microbiota and Early Pregnancy Loss. Human. Reprod. 2024, 39, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H.J.; Bos, A.M.; Himschoot, L.; van Eekelen, R.; Matamoros, S.P.F.; de Boer, M.A.; Oudijk, M.A.; Ris-Stalpers, C.; Cools, P.; Savelkoul, P.H.M.; et al. Vaginal Microbiota and Spontaneous Preterm Birth in Pregnant Women at High Risk of Recurrence. Heliyon 2024, 10, e30685. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, Y.; Fu, Y.; Qian, L. Systematic Review and Meta-Analysis of the Correlation between Intestinal Flora and Gestational Diabetes Mellitus. Ann. Palliat. Med. 2021, 10, 9752–9764. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730–736.e3. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Atkinson, C.; Wähälä, K.; Lampe, J.W. Obesity Prevalence in Relation to Gut Microbial Environments Capable of Producing Equol or O-Desmethylangolensin from the Isoflavone Daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef]
- Nakatsu, C.H.; Armstrong, A.; Clavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal Bacterial Community Changes Associated with Isoflavone Metabolites in Postmenopausal Women after Soy Bar Consumption. PLoS ONE 2014, 9, e108924. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, J.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q.; Wang, S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3072. [Google Scholar] [CrossRef] [PubMed]
- Mate, A.; Reyes-Goya, C.; Santana-Garrido, Á.; Vázquez, C.M. Lifestyle, Maternal Nutrition and Healthy Pregnancy. Curr. Vasc. Pharmacol. 2020, 19, 132–140. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A Novel Theory for the Development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Herpertz-Dahlmann, B.; Mörkl, S. Editorial: Biological Therapies and Eating Disorders. Eur. Eat. Disord. Rev. 2024, 33, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Garzone, S.; Charitos, I.A.; Mandorino, M.; Maggiore, M.E.; Capozzi, L.; Cakani, B.; Dias Lopes, G.C.; Bocchio-Chiavetto, L.; Colella, M. Can We Modulate Our Second Brain and Its Metabolites to Change Our Mood? A Systematic Review on Efficacy, Mechanisms, and Future Directions of “Psychobiotics”. Int. J. Mol. Sci. 2025, 26, 1972. [Google Scholar] [CrossRef]
- Moshfeghinia, R.; Nemati, H.; Ebrahimi, A.; Shekouh, D.; Karami, S.; Eraghi, M.M.; Mohagheghzadeh, H.; Hunter, J.; Pasalar, M. The Impact of Probiotics, Prebiotics, and Synbiotics on Depression and Anxiety Symptoms of Patients with Depression: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2025, 188, 104–116. [Google Scholar] [CrossRef]
- Marano, G.; Rossi, S.; Sfratta, G.; Traversi, G.; Lisci, F.M.; Anesini, M.B.; Pola, R.; Gasbarrini, A.; Gaetani, E.; Mazza, M. Gut Microbiota: A New Challenge in Mood Disorder Research. Life 2025, 15, 593. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Bahari, H.; Akhgarjand, C.; Mirmohammadali, S.N.; Malekahmadi, M. Probiotics and Eating Disorders: A Systematic Review of Humans and Animal Model Studies. J. Eat. Disord. 2024, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Poveda, C.; Jenkins, P.E.; Walton, G.E. An In Vitro Approach to Studying the Microbial Community and Impact of Pre and Probiotics under Anorexia Nervosa Related Dietary Restrictions. Nutrients 2021, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.; Lahaye, E.; El Mehdi, M.; do Rego, J.; do Rego, J.; Fetissov, S.O. Lactobacillus Salivarius and Lactobacillus Gasseri Supplementation Reduces Stress-induced Sugar Craving in Mice. Eur. Eat. Disord. Rev. 2024, 32, 1041–1054. [Google Scholar] [CrossRef]
- Trinh, S.; Käver, L.; Schlösser, A.; Simon, A.; Kogel, V.; Voelz, C.; Beyer, C.; Seitz, J. Gut-Associated Lymphatic Tissue in Food-Restricted Rats: Influence of Refeeding and Probiotic Supplementation. Microorganisms 2023, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Verspohl, V.; van Egmond, M.; Kneisel, L.; Reese, F.; Thelen, A.C.; Korten, N.; Neumann, M.; Schaack, L.; Voelz, C.; Käver, L.; et al. Chronic Starvation Induces Microglial Cell Depletion in an Activity-Based Anorexia Model. Sci. Rep. 2025, 15, 14132. [Google Scholar] [CrossRef]
- Gröbner, E.; Zeiler, M.; Fischmeister, F.P.S.; Kollndorfer, K.; Schmelz, S.; Schneider, A.; Haid-Stecher, N.; Sevecke, K.; Wagner, G.; Keller, L.; et al. The Effects of Probiotics Administration on the Gut Microbiome in Adolescents with Anorexia Nervosa—A Study Protocol for a Longitudinal, Double-blind, Randomized, Placebo-controlled Trial. Eur. Eat. Disord. Rev. 2022, 30, 61–74. [Google Scholar] [CrossRef]
- Ghafouri-Taleghani, F.; Tafreshi, A.S.; Doost, A.H.; Tabesh, M.; Abolhasani, M.; Amini, A.; Saidpour, A. Effects of Probiotic Supplementation Added to a Weight Loss Program on Anthropometric Measures, Body Composition, Eating Behavior, and Related Hormone Levels in Patients with Food Addiction and Weight Regain After Bariatric Surgery: A Randomized Clinical Trial. Obes. Surg. 2024, 34, 3181–3194. [Google Scholar] [CrossRef]
- Komorniak, N.; Kaczmarczyk, M.; Łoniewski, I.; Martynova-Van Kley, A.; Nalian, A.; Wroński, M.; Kaseja, K.; Kowalewski, B.; Folwarski, M.; Stachowska, E. Analysis of the Efficacy of Diet and Short-Term Probiotic Intervention on Depressive Symptoms in Patients after Bariatric Surgery: A Randomized Double-Blind Placebo Controlled Pilot Study. Nutrients 2023, 15, 4905. [Google Scholar] [CrossRef]
- Dang, J.T.; Mocanu, V.; Park, H.; Laffin, M.; Hotte, N.; Karmali, S.; Birch, D.W.; Madsen, K.L. Roux-En-Y Gastric Bypass and Sleeve Gastrectomy Induce Substantial and Persistent Changes in Microbial Communities and Metabolic Pathways. Gut Microbes 2022, 14, 2050636. [Google Scholar] [CrossRef]
- Zhu, R.; Tian, P.; Zhang, H.; Wang, G.; Chen, W. Gut Microbiome-Brain Interactions in Anorexia Nervosa: Potential Mechanisms and Regulatory Strategies. Neuropharmacology 2023, 224, 109315. [Google Scholar] [CrossRef]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of Fecal Microbiota Transplant on Symptoms of Psychiatric Disorders: A Systematic Review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- de Clercq, N.C.; Frissen, M.N.; Davids, M.; Groen, A.K.; Nieuwdorp, M. Weight Gain after Fecal Microbiota Transplantation in a Patient with Recurrent Underweight Following Clinical Recovery from Anorexia Nervosa. Psychother. Psychosom. 2019, 88, 58–60. [Google Scholar] [CrossRef]
- Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Leong, K.S.W.; Tweedie-Cullen, R.Y.; Creagh, C.; Depczynski, M.; Edwards, T.; Vatanen, T.; Thabrew, H.; et al. An Open-Label Pilot Trial of Faecal Microbiome Transfer to Restore the Gut Microbiome in Anorexia Nervosa: Protocol. BMJ Open 2023, 13, e070616. [Google Scholar] [CrossRef]
- Maschek, S.; Østergaard, T.H.; Krych, L.; Zachariassen, L.F.; Sørensen, D.B.; Junker Mentzel, C.M.; Hansen, A.K.; Sjögren, J.M.; Barfod, K.K. Investigating Fecal Microbiota Transplants from Individuals with Anorexia Nervosa in Antibiotic-Treated Mice Using a Cross-over Study Design. J. Eat. Disord. 2025, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Kooij, K.L.; Andreani, N.A.; van der Gun, L.L.; Keller, L.; Trinh, S.; van der Vijgh, B.; Luijendijk, M.; Dempfle, A.; Herpertz-Dahlmann, B.; Seitz, J.; et al. Fecal Microbiota Transplantation of Patients with Anorexia Nervosa Did Not Alter Flexible Behavior in Rats. Int. J. Eat. Disord. 2024, 57, 1868–1881. [Google Scholar] [CrossRef]
- Kan, C.; Treasure, J. Recent Research and Personalized Treatment of Anorexia Nervosa. Psychiatr. Clin. N. Am. 2019, 42, 11–19. [Google Scholar] [CrossRef]
- Feng, B.; Harms, J.; Chen, E.; Gao, P.; Xu, P.; He, Y. Current Discoveries and Future Implications of Eating Disorders. Int. J. Environ. Res. Public Health 2023, 20, 6325. [Google Scholar] [CrossRef]
- Rodriguez, I.; Huckins, L.M.; Bulik, C.M.; Xu, J.; Igudesman, D. Harnessing Precision Nutrition to Individualize Weight Restoration in Anorexia Nervosa. J. Eat. Disord. 2025, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Golinelli, D.; Bucci, A.; Boetto, E.; Maietti, E.; Toscano, F.; Fantini, M.P. Gender Differences and Multiple Determinants of Perceived Physical and Mental Health in Italy. Ann. Di Ig. Med. Prev. E Di Comunita 2021, 33, 456–473. [Google Scholar]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-Specific Association between the Gut Microbiome and High-Fat Diet-Induced Metabolic Disorders in Mice. Biol. Sex. Differ. 2020, 11, 5. [Google Scholar] [CrossRef]
- Choukas-Bradley, S.; Maheux, A.J.; Aubrey, J.S.; Charmaraman, L.; Maas, M.K.; Nesi, J.; Ward, L.M.; Yang, C. Social Media Use, Body Image Concerns, and Disordered Eating Among Adolescents. In Handbook of Children and Screens; Springer Nature: Cham, Switzerland, 2025; pp. 149–156. [Google Scholar]
- Shobeiri, P.; Kalantari, A.; Teixeira, A.L.; Rezaei, N. Shedding Light on Biological Sex Differences and Microbiota–Gut–Brain Axis: A Comprehensive Review of Its Roles in Neuropsychiatric Disorders. Biol. Sex. Differ. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Tennoune, N.; Legrand, R.; Ouelaa, W.; Breton, J.; Lucas, N.; Bole-Feysot, C.; Rego, J.-C.D.; Déchelotte, P.; Fetissov, S.O. Sex-Related Effects of Nutritional Supplementation of Escherichia Coli: Relevance to Eating Disorders. Nutrition 2015, 31, 498–507. [Google Scholar] [CrossRef] [PubMed]
| Studies | Microbial Taxa | Feature | Mechanistic Insights |
|---|---|---|---|
| Zimmermann-Rösner & Prehn-Kristensen, 2024 [75]; Zhao et al., 2024 [76]; Scala et al., 2025 [77]. | Methanobrevibacter smithii | Strongly ↑ in AN; microbial adaptation to caloric restriction: converts CO2 into methane using H2, enhancing energy extraction. Methane slows intestinal motility, contributing to constipation. Negatively correlated with BMI. | CO2 conversion into methane via hydrogen; ↑ energy efficiency; methane slows gut motility → constipation; negatively correlated with BMI. |
| Prochazkova et al., 2021 [78]; Mondot et al., 2022 [79]. | Roseburia | Butyrate-producing genus with anti-inflammatory properties; enhances epithelial barrier integrity and modulates local immunity. ↓ in AN even post-refeeding. | Fermentation of dietary fibers → butyrate production; activation of Tregs; enhancement of epithelial barrier and modulation of anti-inflammatory cytokines. |
| Zimmermann-Rösner & Prehn-Kristensen, 2024 [75]. | Ruminococcus | Involved in fiber degradation and SCFA production. Reduced in AN; associated with dysbiosis and mucosal alteration. | Degradation of complex polysaccharides → SCFAs; supports mucosal integrity and local immune regulation. |
| Baenas et al., 2024 [80]. | Clostridium coccoides | ↑ in restrictive-type AN; implicated in uremic toxin production and potential intestinal damage. | Uremic toxin production; contributes to pro-inflammatory gut dysbiosis. |
| Monteleone et al., 2021 [4]; Morisaki et al., 2023 [81]. | Bifidobacterium | ↑ in binge–purging subtype; higher levels linked to weight recovery. Supports immunity and regulates inflammation. | Carbohydrate metabolism and lactic acid production; promotes growth of beneficial microbes; stimulates tolerogenic dendritic cells. |
| Huwart et al., 2025 [82]. | Faecalibacterium prausnitzii | Butyrate producer; associated with anti-inflammatory effects. ↓ in AN and BN; alleviates binge-like behavior in mice. | Butyrate production → activation of GPR43 and GPR109A receptors; NF-κB inhibition; IL-6 and TNF-α suppression; protection against dysbiosis. |
| Li Z et al., 2025 [83]. | Bacteroides vulgatus | Modulates behavior; ↓ anxiety and disordered eating in animal models. | GABAergic signaling modulation; reduction in anxiety-related neuronal activity (amygdala); influences HPA axis function. |
| Käver et al., 2024 [84]. | Anaerostipes | ↑ in AN; negatively associated with IL-15. Influences immune signaling and inflammation. | Fermentation of complex sugars → SCFAs; IL-15 inhibition; potential interaction with TLR pathways. |
| Shahid et al., 2025 [85]. | Coprococcus | Involved in mood regulation via cytokine and neurotransmitter signaling. ↓ in AN with comorbid depression. | Inflammatory regulation via SCFAs and neurotransmitter synthesis; interaction with gut–brain axis pathways. |
| Bozzola et al., 2024 [86]; Käver et al., 2024 [84]. | Lachnospiraceae | ↑ in AN; inversely related to TNF-α; predicts clinical outcomes and gut function. | SCFA production; interaction with TLR and PRR; inverse correlation with TNF-α and inflammatory biomarkers. |
| Bozzola et al., 2024 [86]; Huwart et al., 2025 [82]. | Enterobacteriaceae | ↓ in acute AN, ↑ in chronic forms. Gram-negative; LPS-linked inflammation. | Gram-negative component; lipopolysaccharide (LPS) stimulates immune response; linked to chronic inflammation. |
| Bozzola et al., 2024 [86]; Käver et al., 2024 [84]. | Romboutsia | ↓ in AN; positively correlates with IL-15; relevant to gut–brain axis regulation. | Modulates IL-15 levels; possibly activates JAK/STAT pathways in mucosal immune responses. |
| Ma et al., 2025 [87]; Huwart et al., 2025 [82] | Clostridium cluster | Associated with AN risk; involved in appetite and neuroactive metabolite regulation and neuroinflammation. | Production of neuroactive metabolites (e.g., tryptophan derivatives); modulation of serotonergic receptors. Fermentation of dietary fibers; SCFA production. |
| Ma et al., 2025 [87]. | Eubacterium hallii | SCFA producer; ↓ in EDs, especially bulimia. | Fermentation of polysaccharides; propionate production; PPAR-γ stimulation and intestinal inflammation reduction. |
| Yu et al., 2024 [88]. | Peptostreptococcaceae | Potential risk factor for AN from MR studies; affects innate immune signaling. | Expression of microbial antigens activating innate immunity; possible effects on mucosal barrier integrity. |
| Fan et al., 2023 [89]; Quagebeur et al., 2023 [90]. | Parabacteroides | SCFA producer; altered in AN; modulates immunity and metabolism. | Fermentation of complex polysaccharides into SCFAs; activation of anti-inflammatory signaling pathways (e.g., GPR41/43). |
| Fan et al., 2023 [89]. | Alistipes | Variable presence in AN; involved in mood and lipid metabolism; potential biomarker. | SCFA production; influence on serotonergic signaling and low-grade inflammation modulation. |
| Scala et al., 2025 [77]. | Turicibacter | Altered in AN; involved in immune regulation and serotonin metabolism. | Involvement in immune and metabolic regulation; possible modulation of serotonin synthesis and gut–brain signaling. |
| Li et al., 2025 [83]. | Klebsiella pneumoniae | Negatively associated with AN; may exert protective microbial competition. | Production of immunomodulatory metabolites; competes with pathogenic microbes; potential anti-inflammatory role. |
| Studies | Study Design | Population | Trial Duration | Type of Intervention | Outcomes |
|---|---|---|---|---|---|
| Nicol et al. [206] | Animal study | Male C57Bl6 mice | 7 days | 1 × 1010 CFU mix of L. salivarius LS7892 and L. gasseri LG6410 | Sugar craving in chronic mild stress condition |
| Trinh et al. [207] | Animal study | Translational activity-based anorexia female Wistar rats | 48 days | 1 × 109 CFU/mL VSL#3® (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus delbrueckii subsp. bulgaricus) | Gut-associated lymphatic tissue in chronic starvation |
| Verspohl et al. [208] | Animal study | Translational activity-based anorexia female Wistar rats | 35 days | Omega-3 FAs or OMNi-BiOTiC® SR-9 (Lactobacillus casei W56, Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Bifidobacterium lactis W51, Lactobacillus salivarius W24, Lactococcus lactis W19, Bifidobacterium lactis W52, Lactobacillus plantarum W62, Bifidobacterium bifidum W23) | Chronic starvation on glial and neuronal cell populations |
| Liu et al. [205] | In vitro study | Colonic model | 65 days | FOS (1.67 g/daily) or Saccharomyces boulardii (5 × 108 CFU) | Dietary restrictions on the intestinal ecosystem |
| Studies | Study Design | Population | Trial Duration | Type of Intervention | Outcomes |
|---|---|---|---|---|---|
| Gröbner et al. [209] | Two-center, longitudinal, double-blind, randomized, controlled trial | 30 F with AN (13–19 years) compared to 30 in age- and sex-matched placebo group | 12 months | Daily OMNi-BiOTiC® SR-9 (Lactobacillus casei W56, Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Bifidobacterium lactis W51, Lactobacillus salivarius W24, Lactococcus lactis W19, Bifidobacterium lactis W52, Lactobacillus plantarum W62, Bifidobacterium bifidum W23) | Gut microbiota composition, weight gain, gastrointestinal complaints, and psychopathology |
| Ghafouri- Taleghani et al. [210] | Triple-blind, randomized, placebo-controlled clinical trial | 25 M/F with food addiction and weight regain after bariatric surgery (18–50 years) compared to 25 F/M in placebo group | 12 weeks | 1.8 × 109 CFU/capsule multi-strain probiotic (Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium longum, Lactobacillus reuteri, Lactobacillus rhamnosus) | Anthropometric measures, biochemical markers, eating behavior, and food addiction |
| Komorniak et al. [211] | Double-blind, randomized, placebo controlled pilot study | 21 M/F adults (≥6 months post-bariatric surgery) with depressive symptoms in the probiotic group and 17 F/M in placebo group | 5 weeks | Sanprobi Barrier (Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Levilactobacillus brevis W63, Lacticaseibacillus casei W56, Ligilactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58) | Psychometric tests, microbiota composition, and gut barrier markers |
| Choi et al. [6] | Parallel, double-blind, randomized, placebo-controlled trial | 37 M/F with overweight undergoing controlled weight loss (18–55 years) in probiotic group and 30 in placebo group | 12 weeks | Lacticaseibacillus rhamnosus HA-114 (10 × 109 CFU/capsule) | Eating behaviors, mood-related aspects, and metabolic biomarkers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marano, G.; Rossi, S.; Sfratta, G.; Acanfora, M.; Anesini, M.B.; Traversi, G.; Lisci, F.M.; Rinaldi, L.; Pola, R.; Gasbarrini, A.; et al. Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment. Nutrients 2025, 17, 2316. https://doi.org/10.3390/nu17142316
Marano G, Rossi S, Sfratta G, Acanfora M, Anesini MB, Traversi G, Lisci FM, Rinaldi L, Pola R, Gasbarrini A, et al. Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment. Nutrients. 2025; 17(14):2316. https://doi.org/10.3390/nu17142316
Chicago/Turabian StyleMarano, Giuseppe, Sara Rossi, Greta Sfratta, Mariateresa Acanfora, Maria Benedetta Anesini, Gianandrea Traversi, Francesco Maria Lisci, Lucio Rinaldi, Roberto Pola, Antonio Gasbarrini, and et al. 2025. "Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment" Nutrients 17, no. 14: 2316. https://doi.org/10.3390/nu17142316
APA StyleMarano, G., Rossi, S., Sfratta, G., Acanfora, M., Anesini, M. B., Traversi, G., Lisci, F. M., Rinaldi, L., Pola, R., Gasbarrini, A., Sani, G., Gaetani, E., & Mazza, M. (2025). Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment. Nutrients, 17(14), 2316. https://doi.org/10.3390/nu17142316












