The Impact of Flavonoids and Omega-3 in Mitigating Frailty Syndrome to Improve Treatment Outcomes in Peripheral Artery Disease (PAD) Patients
Abstract
1. Introduction
2. Frailty Syndrome Definition, Prevalence, and Causes
3. The Relationship Between Frailty Syndrome and Chronic Vascular Diseases
3.1. Peripheral Arterial Disease
3.2. Frailty Syndrome and PAD
3.3. Frailty and Treatment Outcomes in PAD
4. Mediterranean Diet and Its Effects on Frailty Syndrome and Chronic Vascular Diseases
4.1. Role of Omega-3 (Fish) in CVD
4.2. Role of Fruits and Vegetables (Contains Flavonoids) in CVD
5. Discussion
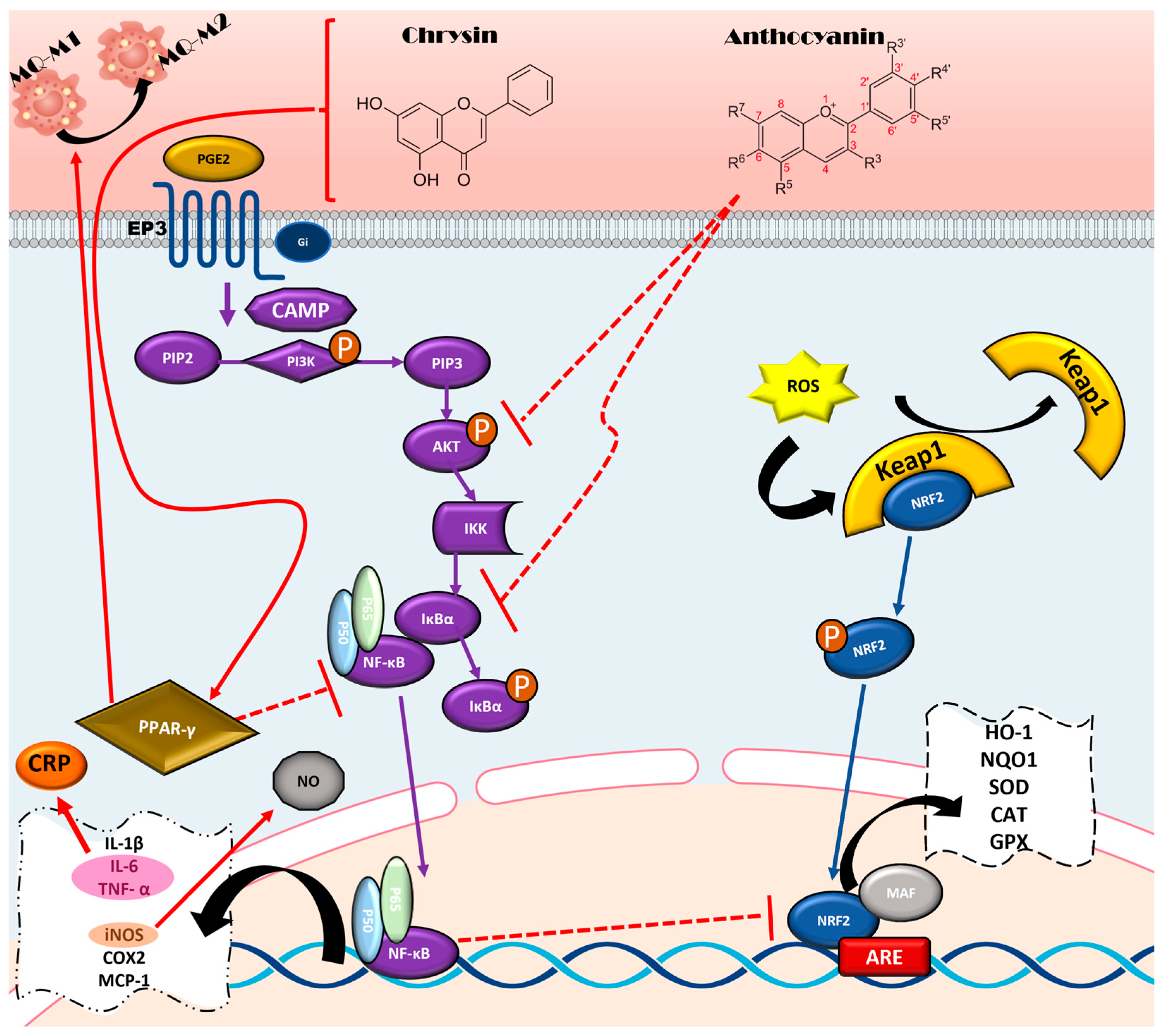
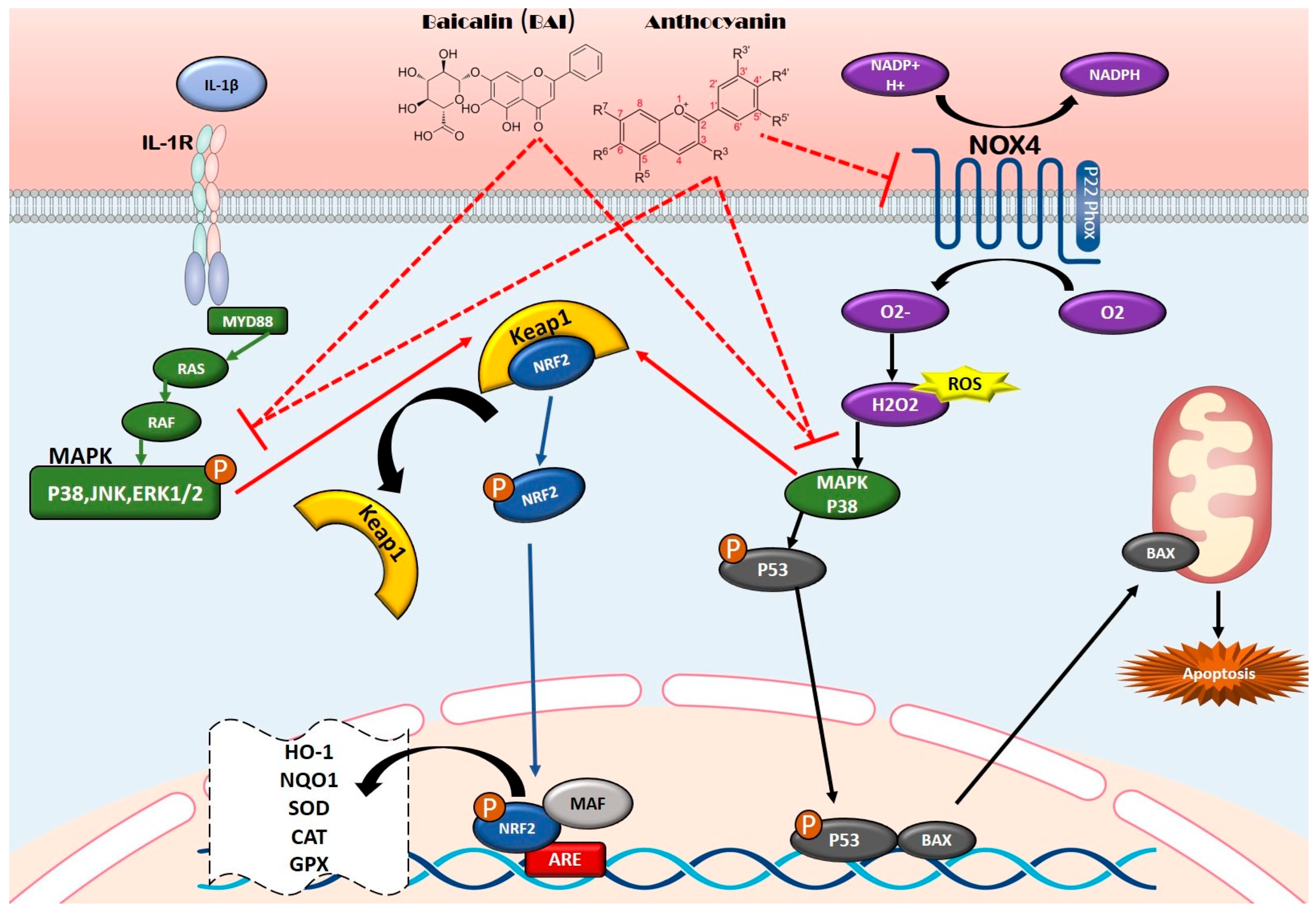
5.1. Anti-Inflammatory Properties and Mechanisms of Flavonoids
5.1.1. NF-κB Pathway
5.1.2. Regulation of Macrophage Polarization by PPARγ Pathway
5.1.3. IL-17
5.2. Antioxidant Properties and Mechanisms of Flavonoids
5.3. Anti-Inflammatory Mechanisms of Omega-3 Fatty Acids
5.4. Antioxidant Properties and Mechanisms of Omega-3 Fatty Acids
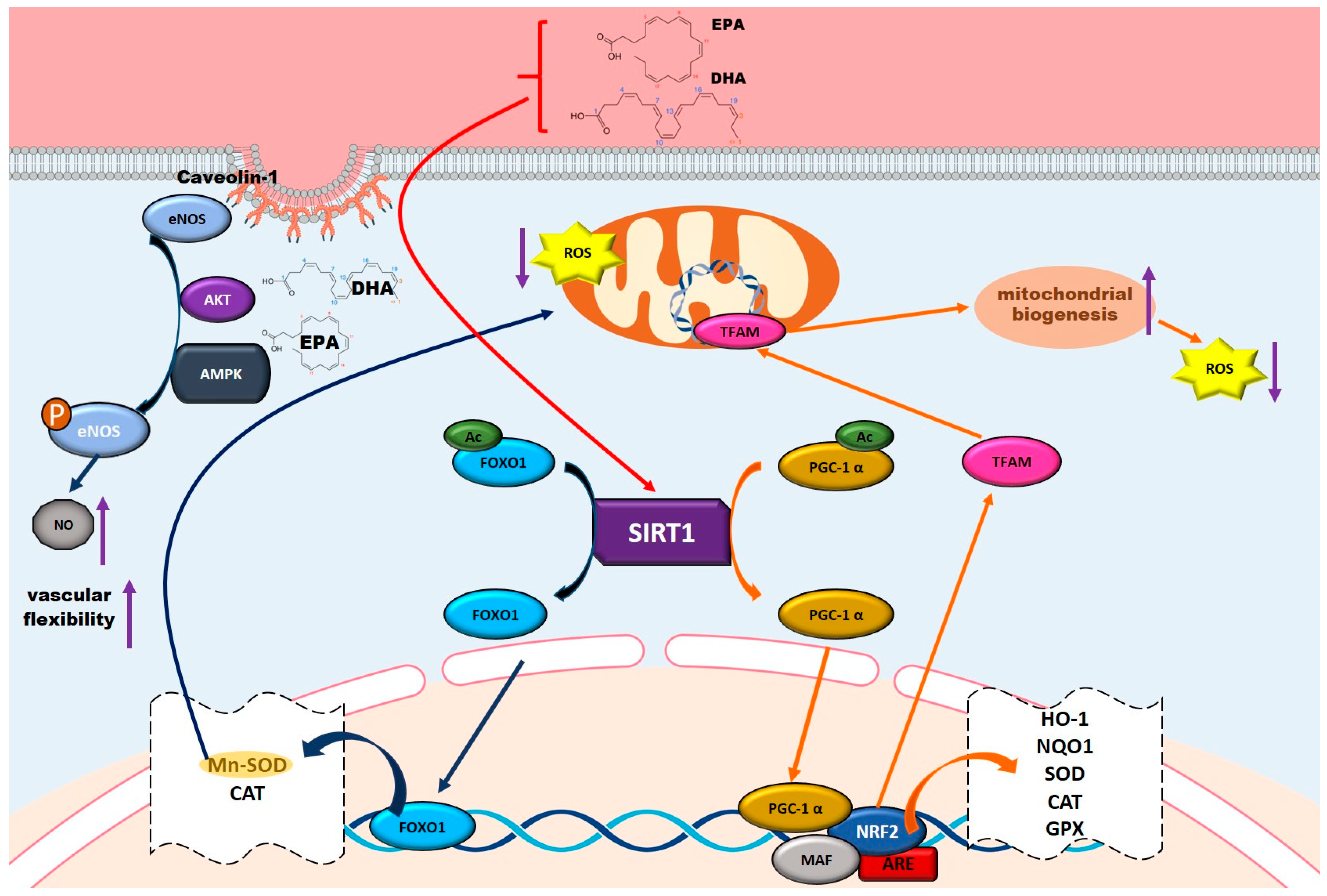
5.5. Vascular Protective Role of Omega-3 Fatty Acids
5.5.1. Activating the PI3K-AKT-mTOR Pathway for Vascular Repair
5.5.2. Enhancing NO Production and Endothelial Function
5.6. The Bioavailability of Anthocyanins and Omega-3
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAD | Peripheral artery disease |
| MedDiet | Mediterranean diet |
| OS | Oxidative stress (OS) |
| RONS | reactive oxygen and nitrogen species |
| hs-CRP | High-sensitivity C reactive protein |
| GSSG | oxidized glutathione |
| MDA | Malondialdehyde |
| 4-HNE | 4-hydroxy-2-nonenal |
| SOD | superoxide dismutase |
| IL-6 | interleukin-6 |
| ROS | reactive oxygen species |
| TNF-α | tumor necrosis factor alpha |
| IL-1β | interleukin-1 beta |
| IL-18 | interleukin-18 |
| IL-8 | interleukin-8 |
| CXCL10 | chemokine (C-X-C) motif ligand 10 |
| CVD | cardiovascular disease |
| T2D | type 2 diabetes |
| MetS | metabolic syndrome |
| NHS | Nurses’ Health Study |
| CHD | coronary heart disease |
| HPFS | Health Professional Follow-up Study |
| MI | myocardial infarction |
| AHA | American Heart Association |
| ESC | European Society of Cardiology |
| IC | Intermittent Claudication |
| GFI | Groningen Frailty Indicator |
| mEFT | modified Essential Frailty Toolset |
| CLTI | Chronic limb-threatening ischemia |
| mFI | Modified Frailty Index |
| ABI | Ankle-Brachial Index |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| sVCAM-1 | Soluble Vascular Cell Adhesion Molecule-1 |
| sICAM-1 | Soluble Intercellular Adhesion Molecule-1 |
| pCAM-1 | Platelet Cell Adhesion Molecule-1 |
| COX-2 | Cyclooxygenase-2 |
| ERK | Extracellular Signal-Regulated Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| SAPK | Stress-Activated Protein Kinase |
| JNK | c-Jun N-terminal Kinase |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| GPx | Glutathione Peroxidase |
| CAT | Catalase |
| ARE | antioxidant response element |
| HO-1 | Heme Oxygenase-1 |
| Nox4 | NADPH oxidase 4 |
| AMPK | AMP-Activated Protein Kinase |
| ALA | alpha-linolenic acid |
| EPA | Eicosapentaenoic Acid |
| DHA | Docosahexaenoic Acid |
| AA | arachidonic acid |
| PI3K-AKT-Mtor | Phosphoinositide 3-Kinase-Protein Kinase B (AKT)-Mammalian Target of Rapamycin |
| VEGF-A | Vascular Endothelial Growth Factor A |
| n-3 PUFAs | Omega-3 polyunsaturated fatty acids |
| SIRT1 | Sirtuin 1 |
| FOXO | Forkhead Box O |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| NO | nitric oxide |
References
- Kengne, A.P.; Echouffo-Tcheugui, J.B. Differential burden of peripheral artery disease. Lancet Glob. Health 2019, 7, e980–e981. [Google Scholar] [CrossRef]
- Heald, C.; Fowkes, F.; Murray, G.; Price, J.; Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 2006, 189, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Eid, M.A.; Mehta, K.S.; Goodney, P.P. Epidemiology of peripheral artery disease. Semin. Vasc. Surg. 2021, 34, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.E.; Arnaoutakis, D.J.; Smith, A.D.; Semel, M.; Nguyen, L.L. Estimated annual health care expenditures in individuals with peripheral arterial disease. J. Vasc. Surg. 2018, 67, 558–567. [Google Scholar] [CrossRef]
- Kim, C.; Yang, Y.S.; Ryu, G.W.; Choi, M. Risk factors associated with amputation-free survival for patients with peripheral arterial disease: A systematic review. Eur. J. Cardiovasc. Nurs. 2021, 20, 295–304. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin. Geriatr. Med. 2011, 27, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Bandinelli, S.; Corsi, A.M.; Giallauria, F.; Guralnik, J.M.; Zuliani, G.; Cattabiani, C.; Parrino, S.; et al. SHBG, sex hormones, and inflammatory markers in older women. J. Clin. Endocrinol. Metab. 2011, 96, 1053–1059. [Google Scholar] [CrossRef]
- Stenholm, S.; Maggio, M.; Lauretani, F.; Bandinelli, S.; Ceda, G.P.; Di Iorio, A.; Giallauria, F.; Guralnik, J.M.; Ferrucci, L. Anabolic and catabolic biomarkers as predictors of muscle strength decline: The InCHIANTI study. Rejuvenation Res. 2010, 13, 3–11. [Google Scholar] [CrossRef]
- Giallauria, F.; Di Lorenzo, A.; Venturini, E.; Pacileo, M.; D’andrea, A.; Garofalo, U.; De Lucia, F.; Testa, C.; Cuomo, G.; Iannuzzo, G.; et al. Frailty in Acute and Chronic Coronary Syndrome Patients Entering Cardiac Rehabilitation. J. Clin. Med. 2021, 10, 1696. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. S6), 1402S–1406S. [Google Scholar] [CrossRef]
- Jansen-Chaparro, S.; López-Carmona, M.D.; Cobos-Palacios, L.; Sanz-Cánovas, J.; Bernal-López, M.R.; Gómez-Huelgas, R. Statins and Peripheral Arterial Disease: A Narrative Review. Front. Cardiovasc. Med. 2021, 8, 777016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin Hypertens. 2018, 24, 9, Erratum in Clin. Hypertens. 2018, 24, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef]
- Xue, Q.-L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, B.E.; Zunzunegui, M.-V.; Béland, F.; Bamvita, J.-M. Life course social and health conditions linked to frailty in Latin American older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1399–1406. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Gutierrez Avila, G.; Alfaro-Acha, A.; Amor Andres, M.S.; De Los Angeles De La Torre Lanza, M.; Escribano Aparicio, M.V.; Humanes Aparicio, S.; Larrion Zugasti, J.L.; Gomez-Serranillo Reus, M.; Rodriguez-Artalejo, F.; et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J. Nutr. Health Aging 2011, 15, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borras, C.; Gomez-Cabrera, M.C. A free radical theory of frailty. Free. Radic. Biol. Med. 2018, 124, 358–363. [Google Scholar] [CrossRef]
- Wu, I.; Shiesh, S.; Kuo, P.; Lin, X. High oxidative stress is correlated with frailty in elderly Chinese. J. Am. Geriatr. Soc. 2009, 57, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Romano, A.; Greco, A.; Rollo, T.; Bellanti, F.; Altomare, E.; Vendemiale, G. Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. Int. J. Immunopathol. Pharmacol. 2009, 22, 819–827. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Gómez-Díaz, R.; González-Molina, Á.; Vidal-Serrano, S.; Díez-Manglano, J.; Salgado, F.; Soto-Martín, M.; Ollero-Baturone, M.; on behalf of the Proteo Researchers. Oxidative Stress, Telomere Shortening, and Apoptosis Associated to Sarcopenia and Frailty in Patients with Multimorbidity. J. Clin. Med. 2020, 9, 2669. [Google Scholar] [CrossRef]
- Montes, A.C.; Boga, J.A.; Millo, C.B.; González, A.R.; Ochoa, Y.P.; Naredo, I.V.; Reig, M.M.; Rizos, L.R.; Jurado, P.M.S.; Solano, J.J.; et al. Potential early biomarkers of sarcopenia among independent older adults. Maturitas 2017, 104, 117–122. [Google Scholar] [CrossRef]
- Liu, C.K.; Lyass, A.; Larson, M.G.; Massaro, J.M.; Wang, N.; D’Agostino, R.B.; Benjamin, E.J.; Murabito, J.M. Biomarkers of oxidative stress are associated with frailty: The Framingham Offspring Study. AGE 2016, 38, 1. [Google Scholar] [CrossRef]
- Dzięgielewska-Gęsiak, S.; Muc-Wierzgoń, M. Inflammation and Oxidative Stress in Frailty and Metabolic Syndromes—Two Sides of the Same Coin. Metabolites 2023, 13, 475. [Google Scholar] [CrossRef]
- Martínez-Ezquerro, J.D.; Rodríguez-Castañeda, A.; Ortiz-Ramírez, M.; Sánchez-García, S.; Rosas-Vargas, H.; Sánchez-Arenas, R.; la Torre, P.G.-D. Oxidative Stress, Telomere Length, and Frailty in an Old Age Population. Rev. Investig. Clin. Organo Hosp. Enfermedades Nutr. 2019, 71, 393–401. [Google Scholar] [CrossRef]
- Tembo, M.C.; Holloway-Kew, K.L.; Bortolasci, C.C.; Sui, S.X.; Brennan-Olsen, S.L.; Williams, L.J.; Kotowicz, M.A.; Pasco, J.A. Total Antioxidant Capacity and Frailty in Older Men. Am. J. Men’s Health 2020, 14, 1557988320946592. [Google Scholar] [CrossRef]
- Tembo, M.C.; Holloway-Kew, K.L.; Bortolasci, C.C.; Brennan-Olsen, S.L.; Williams, L.J.; Kotowicz, M.A.; Pasco, J.A. Association between serum interleukin-6 and frailty in older men: Cross-sectional data. Eur. Geriatr. Med. 2021, 12, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Jiang, L.; Chen, J.; Xiao, M.; Wang, W.; Liu, P.; Wu, J. Serum Inflammatory Factors and Oxidative Stress Factors Are Associated with Increased Risk of Frailty and Cognitive Frailty in Patients with Cerebral Small Vessel Disease. Front. Neurol. 2022, 12, 786277. [Google Scholar] [CrossRef]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Pérez, D.; Sánchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.P.; Fernández-Tajes, J.; Pásaro, E.; Laffon, B.; Valdiglesias, V. Association of inflammatory mediators with frailty status in older adults: Results from a systematic review and meta-analysis. GeroScience 2020, 42, 1451–1473. [Google Scholar] [CrossRef]
- Ribeiro, É.C.T.; Sangali, T.D.; Clausell, N.O.; Perry, I.S.; Souza, G.C. C-Reactive Protein and Frailty in Heart Failure. Am. J. Cardiol. 2022, 166, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Mailliez, A.; Guilbaud, A.; Puisieux, F.; Dauchet, L.; Boulanger, É. Circulating biomarkers characterizing physical frailty: CRP, hemoglobin, albumin, 25OHD and free testosterone as best biomarkers. Results of a meta-analysis. Exp. Gerontol. 2020, 139, 111014. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Chen, D.; Jiang, X.; Xiong, Z. Inflammatory biomarkers in older adults with frailty: A systematic review and meta-analysis of cross-sectional studies. Aging Clin. Exp. Res. 2022, 34, 971–987. [Google Scholar] [CrossRef]
- Reiner, A.P.; Aragaki, A.K.; Gray, S.L.; Wactawski-Wende, J.; Cauley, J.A.; Cochrane, B.B.; Kooperberg, C.L.; Woods, N.F.; LaCroix, A.Z. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am. J. Med. 2009, 122, 947–954. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Frailty biomarkers in humans and rodents: Current approaches and future advances. Mech. Ageing Dev. 2019, 180, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.E.; Clarke, S.L.; Wu, K.-H.H.; Kanoni, S.; Zajac, G.J.M.; Ramdas, S.; Surakka, I.; Ntalla, I.; Vedantam, S.; Winkler, T.W.; et al. The power of genetic diversity in genome-wide association studies of lipids. Nature 2021, 600, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Hellwege, J.N.; Keaton, J.M.; Park, J.; Qiu, C.; Warren, H.R.; Torstenson, E.S.; Kovesdy, C.P.; Sun, Y.V.; Wilson, O.D.; et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat. Genet. 2019, 51, 51–62. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The diabetes mellitus–atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Schloss, M.J.; Swirski, F.K.; Nahrendorf, M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ. Res. 2020, 126, 1242–1259. [Google Scholar] [CrossRef]
- Domínguez, F.; Fuster, V.; Fernández-Alvira, J.M.; Fernández-Friera, L.; López-Melgar, B.; Blanco-Rojo, R.; Fernández-Ortiz, A.; García-Pavía, P.; Sanz, J.; Mendiguren, J.M.; et al. Association of sleep duration and quality with subclinical atherosclerosis. J. Am. Coll. Cardiol. 2019, 73, 134–144. [Google Scholar] [CrossRef]
- Bhatnagar, A. Environmental determinants of cardiovascular disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Biddinger, K.J.; Emdin, C.A.; Haas, M.E.; Wang, M.; Hindy, G.; Ellinor, P.T.; Kathiresan, S.; Khera, A.V.; Aragam, K.G. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw. Open 2022, 5, e223849. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Khotina, V.A.; VakiliGhartavol, R.; Glanz, V.Y.; Sukhorukov, V.N.; Orekhov, A.N. Interplay and Causative Rela-tionship Between Frailty and Atherosclerosis. J. Angiother. 2024, 8, 1–11. [Google Scholar]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. (Eds.) Overview of classification systems in peripheral artery disease. In Seminars in Interventional Radiology; Thieme Medical Publishers: New York, NY, USA, 2014. [Google Scholar]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Sigvant, B.; Wiberg-Hedman, K.; Bergqvist, D.; Rolandsson, O.; Andersson, B.; Persson, E.; Wahlberg, E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J. Vasc. Surg. 2007, 45, 1185–1191. [Google Scholar] [CrossRef]
- Aboyans, V.; Fowkes, F.J.I.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Nordanstig, J.; Behrendt, C.; Bradbury, A.; De Borst, G.; Fowkes, F.; Golledge, J.; Gottsater, A.; Hinchliffe, R.J.; Nikol, S.; Norgren, L. Peripheral arterial disease (PAD)—A challenging manifestation of atherosclerosis. Prev. Med. 2023, 171, 107489. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, J.; Guo, J.; Su, Z.; Wang, J.; Guo, J.; Shi, X.; Gu, Y. Impact of geriatric nutritional risk index on prognosis in peripheral artery disease patients undergoing endovascular therapy. J. Clin. Hypertens. 2023, 25, 497–503. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Ji, H.; Du, W.; You, C.; Chen, J.; Jiang, J.; Shan, Y.; Pan, Q.; Cao, R.; et al. Frailty and risk of systemic atherosclerosis: A bidirectional Mendelian randomization study. PLoS ONE 2024, 19, e0304300. [Google Scholar] [CrossRef] [PubMed]
- Thiede, R.; Toosizadeh, N.; Mills, J.L.; Zaky, M.; Mohler, J.; Najafi, B. Gait and balance assessments as early indicators of frailty in patients with known peripheral artery disease. Clin. Biomech. 2016, 32, 1–7. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Williams, D.T.; Harding, K.G.; Price, P. An Evaluation of the efficacy of methods used in screening for lower-limb arterial disease in diabetes. Diabetes Care 2005, 28, 2206–2210. [Google Scholar] [CrossRef]
- Xue, Q.; Qin, M.Z.; Jia, J.; Liu, J.P.; Wang, Y. Association between frailty and the cardio-ankle vascular index. Clin. Interv. Aging 2019, 14, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.B.; Hu, F.Y.; Arya, S.; Gillespie, T.W.; Rajani, R.R. Preoperative frailty is predictive of complications after major lower extremity amputation. J. Vasc. Surg. 2017, 65, 804–811. [Google Scholar] [CrossRef]
- Campbell, W.B.; Marriott, S.; Eve, R.; Mapson, E.; Sexton, S.; Thompson, J.F. Factors influencing the early outcome of major lower limb amputation for vascular disease. Ann. R. Coll. Surg. Engl. 2001, 83, 309. [Google Scholar]
- Helm, P.; Engel, T.; Holm, A.; Kristiansen, V.B.; Rosendahl, S. Function after lower limb amputation. Acta Orthop. Scand. 1986, 57, 154–157. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic lower extremity ischemia and its association with the frailty syndrome in patients with diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jie, Y.; Wang, P.; Sun, Y.; Wang, X.; Fan, Y. Impact of frailty on all-cause mortality or major amputation in patients with lower extremity peripheral artery disease: A meta-analysis. Ageing Res. Rev. 2022, 79, 101656. [Google Scholar] [CrossRef]
- Drudi, L.M.; Ades, M.; Mancini, R.; Boudrias, C.; Obrand, D.I.; Steinmetz, O.K.; Afilalo, J. Frailty assessment in older adults undergoing interventions for peripheral arterial disease. J. Vasc. Surg. 2019, 70, 1594–1602.e1. [Google Scholar] [CrossRef]
- Höbaus, C.; Roller-Wirnsberger, R.; Schernthaner, G.-H. Peripheral arterial disease and loss of physical function: Just two old friends? Atherosclerosis 2017, 257, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Abdelhafiz, A.H.; Rodríguez-Mañas, L. Frailty and sarcopenia-newly emerging and high impact complications of diabetes. J. Diabetes Its Complicat. 2017, 31, 1465–1473. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, Y.; Wang, D.; Zheng, Y.; Liu, H.; Wu, H. Research progress of frailty assessment and intervention in the aged. Chin. J. Mod. Nurs. 2021, 27, 561–565. [Google Scholar]
- Zhu, Z.B.; Yu, H.B.; Jiang, M.B.; Wu, H.B.; Wang, J.B.; Xu, F.M. Status and influencing factors of frailty in patients with restenosis after percutaneous transluminal angioplasty for peripheral arterial disease: A cross-sectional study. Medicine 2023, 102, e34465. [Google Scholar] [CrossRef]
- van Aalst, F.M.; Verwijmeren, L.; van Dongen, E.P.; de Vries, J.-P.P.; de Groot, E.; Noordzij, P.G. Frailty and functional outcomes after open and endovascular procedures for patients with peripheral arterial disease: A systematic review. J. Vasc. Surg. 2020, 71, 297–306.e1. [Google Scholar] [CrossRef]
- Patel, M.R.; Conte, M.S.; Cutlip, D.E.; Dib, N.; Geraghty, P.; Gray, W.; Hiatt, W.R.; Ho, M.; Ikeda, K.; Ikeno, F.; et al. Evaluation and Treatment of Patients with Lower Extremity Peripheral Artery Disease. J. Am. Coll. Cardiol. 2015, 65, 931–941. [Google Scholar] [CrossRef]
- Adam, D.J.; Beard, J.D.; Cleveland, T.; Bell, J.; Bradbury, A.W.; Forbes, J.F.; Fowkes, F.G.R.; Gillepsie, I.; Ruckley, C.V.; Raab, G.; et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet 2005, 366, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Schneider, P.A.; Conte, M.S. Advances in Revascularization for Peripheral Artery Disease: Revascularization in PAD. Circ. Res. 2021, 128, 1885–1912. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 75–83. [Google Scholar] [CrossRef]
- Ehlert, B.A.; Najafian, A.; Orion, K.C.; Malas, M.B.; Black, J.H.; Abularrage, C.J. Validation of a modified Frailty Index to predict mortality in vascular surgery patients. J. Vasc. Surg. 2016, 63, 1595–1601.e2. [Google Scholar] [CrossRef] [PubMed]
- Drudi, L.M.; Ades, M.; Landry, T.; Gill, H.L.; Grenon, S.M.; Steinmetz, O.K.; Afilalo, J. Scoping review of frailty in vascular surgery. J. Vasc. Surg. 2019, 69, 1989–1998. [Google Scholar] [CrossRef]
- Brahmbhatt, R.; Brewster, L.P.; Shafii, S.; Rajani, R.R.; Veeraswamy, R.; Salam, A.; Dodson, T.F.; Arya, S. Gender and frailty predict poor outcomes in infrainguinal vascular surgery. J. Surg. Res. 2016, 201, 156–165. [Google Scholar] [CrossRef]
- Gonzalez, L.; Kassem, M.; Owora, A.H.; Seligson, M.T.; Richards, C.Y.; Monita, M.M.; Cardounell, S.Z.; Brangman, S.A.; Gahtan, V. Frailty and biomarkers of frailty predict outcome in veterans after open and endovascular revascularization. J. Surg. Res. 2019, 243, 539–552. [Google Scholar] [CrossRef]
- Rothenberg, K.A.; George, E.L.; Trickey, A.W.; Barreto, N.B.; Johnson, T.M.; Hall, D.E.; Johanning, J.M.; Arya, S. Assessment of the Risk Analysis Index for prediction of mortality, major complications, and length of stay in patients who underwent vascular surgery. Ann. Vasc. Surg. 2020, 66, 442–453. [Google Scholar] [CrossRef]
- Koh, B.J.; Lee, Q.; Wee, I.J.; Syn, N.; Lee, K.S.; Ng, J.J.; LA Wong, A.; Soong, J.T.; Choong, A.M. Frailty scoring in vascular and endovascular surgery: A systematic review. Vasc. Med. 2022, 27, 302–307. [Google Scholar] [CrossRef]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Changes in Diet Quality Scores and Risk of Cardiovascular Disease Among US Men and Women. Circulation 2015, 132, 2212–2219. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Tormo, M.; Sánchez, M.; Rodríguez, L.; Quirós, J.; Navarro, C.; Molina, E.; Martínez, C.; Marín, P.; et al. Major dietary patterns and risk of coronary heart disease in middle-aged persons from a Mediterranean country: The EPIC-Spain cohort study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 192–199. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Bamia, C.; Norat, T.; Overvad, K.; Schmidt, E.B.; Tjønneland, A.; Halkjær, J.; Clavel-Chapelon, F.; Vercambre, M.-N.; Boutron-Ruault, M.-C.; et al. Modified Mediterranean diet and survival after myocardial infarction: The EPIC-Elderly study. Eur. J. Epidemiol. 2007, 22, 871–881. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757, Erratum in Circulation 2003, 107, 512. [Google Scholar] [CrossRef]
- Bucher, H.C.; Hengstler, P.; Schindler, C.; Meier, G. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am. J. Med. 2002, 112, 298–304. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, L.-Q.; Xiao, T.-H.; Zhang, Y.-X.; Zhu, M.; Zhang, R.; Li, K.; Wang, Y.; Li, Y. Effects of omega-3 fatty acid for sudden cardiac death prevention in patients with cardiovascular disease: A contemporary meta-analysis of randomized, controlled trials. Cardiovasc. Drugs Ther. 2011, 25, 259–265. [Google Scholar] [CrossRef]
- Filion, K.B.; El Khoury, F.; Bielinski, M.; Schiller, I.; Dendukuri, N.; Brophy, J.M. Omega-3 fatty acids in high-risk cardiovascular patients: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2010, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Thompson, R.L.; Harrison, R.A.; Summerbell, C.D.; Ness, A.R.; Moore, H.J.; Worthington, H.V.; Durrington, P.N.; Higgins, J.P.T.; Capps, N.E.; et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ 2006, 332, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Oomen, C.M.; Feskens, E.J.M.; Nen, L.R.S.; Fidanza, F.; Nissinen, A.M.; Menotti, A.; Kok, F.J.; Kromhout, D.; Räsänen, L. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am. J. Epidemiol. 2000, 151, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Vonschacky, C.; Harris, W. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc. Res. 2007, 73, 310–315. [Google Scholar] [CrossRef]
- Whelton, S.P.; He, J.; Whelton, P.K.; Muntner, P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am. J. Cardiol. 2004, 93, 1119–1123. [Google Scholar] [CrossRef]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Appel, L.J.; Miller, E.R., III.; Seidler, A.J.; Whelton, P.K. Does supplementation of diet with ‘fish oil’ reduce blood pressure? A meta-analysis of controlled clinical trials. Arch. Intern. Med. 1993, 153, 1429–1438. [Google Scholar] [CrossRef]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.; Atar, D.; Borch-Johnsen, K.; Boysen, G.; Burell, G.; Cifkova, R.; Dallongeville, J.; De Backer, G.; Ebrahim, S.; Gjelsvik, B.; et al. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary: Fourth Joint Task Force of the European Society of Cardiology. and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2007, 28, 2375–2414. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96, Erratum in Circulation 2006, 114, e629. Erratum in Circulation 2006, 114, e27. [Google Scholar] [CrossRef]
- Howard, B.V.; Kritchevsky, D. Phytochemicals and cardiovascular disease. A statement for healthcare professionals from the American Heart Association. Circulation 1997, 95, 2591–2593. [Google Scholar] [CrossRef]
- Beitz, R.; Mensink, G.B.; Fischer, B. Blood pressure and vitamin C and fruit and vegetable intake. Ann. Nutr. Metab. 2003, 47, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; de la Fuente, C.; Martín-Arnau, A.M.; de Irala, J.; Martínez, J.A.; Martínez-González, M.Á. Fruit and vegetable consumption is inversely associated with blood pressure in a Mediterranean population with a high vegetable-fat intake: The Seguimiento Universidad de Navarra (SUN) Study. Br. J. Nutr. 2004, 92, 311–319. [Google Scholar] [CrossRef]
- Miura, K.; Greenland, P.; Stamler, J.; Liu, K.; Daviglus, M.L.; Nakagawa, H. Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men: The Chicago Western Electric Study. Am. J. Epidemiol. 2004, 159, 572–580. [Google Scholar] [CrossRef]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [CrossRef]
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens. 2007, 21, 717–728. [Google Scholar] [CrossRef]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruits, vegetables and coronary heart disease. Nat. Rev. Cardiol. 2009, 6, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Roddam, A.W.; Key, T.J.; Appleby, P.N.; Overvad, K.; Jakobsen, M.U.; Tjønneland, A.; Hansen, L.; Boeing, H.; Weikert, C.; et al. Fruit and vegetable intake and mortality from ischaemic heart disease: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. Eur. Heart J. 2011, 32, 1235–1243. [Google Scholar] [CrossRef]
- John, J.H.; Ziebland, S.; Yudkin, P.; Roe, L.S.; Neil, H.A.W.; Oxford Fruit and Vegetable Study Group. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet 2002, 359, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Neil, H.A.W. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef]
- Han, J.; Luo, L.; Marcelina, O.; Kasim, V.; Wu, S. Therapeutic angiogenesis-based strategy for peripheral artery disease. Theranostics 2022, 12, 5015–5033. [Google Scholar] [CrossRef]
- McGinigle, K.L.; Spangler, E.L.; Ayyash, K.; Vavra, A.K.; Arya, S.; Settembrini, A.M.; Thomas, M.M.; Dell, K.E.; Swiderski, I.J.; Davies, M.G.; et al. A framework for perioperative care for lower extremity vascular bypasses: A Consensus Statement by the Enhanced Recovery after Surgery (ERAS) Society and Society for Vascular Surgery. J. Vasc. Surg. 2023, 77, 1295–1315. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Zhang, L.; Li, S. Advances in nutritional supplementation for sarcopenia management. Front. Nutr. 2023, 10, 1189522. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NF-κB system. Wiley Interdiscip Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 2016, 21, 642–649. [Google Scholar] [CrossRef]
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020, 76, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Chen, X.L.; Yang, H.W.; Ma, Y.R. Effects of nuclear factor κB expression on retinal neovascularization and apoptosis in a diabetic retinopathy rat model. Int. J. Ophthalmol. 2015, 8, 448–452. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Skinhøj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-α) and atherosclerosis. Clin. Exp. Immunol. 2000, 121, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Biernacka, A.; Frangogiannis, N. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar]
- Wang, Z.; Sun, W.; Sun, X.; Wang, Y.; Zhou, M. Kaempferol ameliorates cisplatin-induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-κB pathways. AMB Express 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Delgado-López, F.; González, I.; Pérez-Castro, R.; Romero, J.; Rojas, I. The receptor for advanced glycation end-products: A complex signaling scenario for a promiscuous receptor. Cell. Signal. 2013, 25, 609–614. [Google Scholar] [CrossRef]
- Liang, H.; Yang, X.; Liu, C.; Sun, Z.; Wang, X. Effect of NF-kB signaling pathway on the expression of MIF, TNF-α, IL-6 in the regulation of intervertebral disc degeneration. J. Musculoskelet. Neuronal Interact. 2018, 18, 551–556. [Google Scholar]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and anti-inflammatory properties of anthocyanins extracted from Oryza sativa L. in primary dermal fibroblasts. Oxidative Med. Cell. Longev. 2019, 2019, 2089817. [Google Scholar] [CrossRef]
- Duarte, L.J.; Chaves, V.C.; Nascimento, M.V.P.d.S.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simões, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018, 247, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Lee, S.-G.; Shin, J.-S.; Lee, H.-Y.; Yoon, K.; Ji, Y.W.; Jang, D.S.; Lee, K.-T. p-Coumaroyl anthocyanin mixture isolated from tuber epidermis of Solanum tuberosum attenuates reactive oxygen species and pro-inflammatory mediators by suppressing NF-κB and STAT1/3 signaling in LPS-induced RAW264.7 macrophages. Biol. Pharm. Bull. 2017, 40, 1894–1902. [Google Scholar] [CrossRef]
- Sahu, B.D.; Kalvala, A.K.; Koneru, M.; Kumar, J.M.; Kuncha, M.; Rachamalla, S.S.; Sistla, R.; Mukhopadhyay, P. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS ONE 2014, 9, e105070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chinetti-Gbaguidi, G.; Staels, B. Macrophage polarization in metabolic disorders: Functions and regulation. Curr. Opin. Lipidol. 2011, 22, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Feng, X.; Qin, H.; Shi, Q.; Zhang, Y.; Zhou, F.; Wu, H.; Ding, S.; Niu, Z.; Lu, Y.; Shen, P. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem. Pharmacol. 2014, 89, 503–514. [Google Scholar] [CrossRef]
- Pascual, G.; Fong, A.L.; Ogawa, S.; Gamliel, A.; Li, A.C.; Perissi, V.; Rose, D.W.; Willson, T.M.; Rosenfeld, M.G. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 2005, 437, 759–763. [Google Scholar] [CrossRef]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Zhao, J.; Martin, B.; Zepp, J.A.; Ko, J.S.; Gu, C.; Cai, G.; Ouyang, W.; Sen, G.; et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 2015, 212, 1571–1587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, M.; Gaffen, S.L. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010, 21, 449–453. [Google Scholar] [CrossRef]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001, 194, 519–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trinchieri, G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012, 30, 677–706. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; De Medina, F.S. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Abu Zaid, M.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.-H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, P.F.; Samuels, D.C.; Elson, J.; Turnbull, D.M. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: Is there a common mechanism? Lancet 2002, 360, 1323–1325. [Google Scholar] [CrossRef]
- Sharebiani, H.; Keramat, S.; Chavoshan, A.; Fazeli, B.; Stanek, A. The influence of antioxidants on oxidative stress-induced vascular aging in obesity. Antioxidants 2023, 12, 1295. [Google Scholar] [CrossRef]
- Keramat, S.; Sharebiani, H.; Patel, M.; Fazeli, B.; Stanek, A. The potential role of antioxidants in the treatment of peripheral arterial disease: A systematic review. Antioxidants 2022, 11, 2126. [Google Scholar] [CrossRef] [PubMed]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef] [PubMed]
- Abdou, K.H.; Moselhy, W.A.; Mohamed, H.M.; El-Nahass, E.-S.; Khalifa, A.G. Moringa oleifera leaves extract protects titanium dioxide nanoparticles-induced nephrotoxicity via Nrf2/HO-1 signaling and amelioration of oxidative stress. Biol. Trace Elem. Res. 2019, 187, 181–191. [Google Scholar] [CrossRef]
- Ma, L.; Wu, F.; Shao, Q.; Chen, G.; Xu, L.; Lu, F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des. Dev. Ther. 2021, 15, 3207–3221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z.; Peluso, I. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxidative Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Courtaut, F.; Aires, V.; Acar, N.; Bretillon, L.; Guerrera, I.C.; Chhuon, C.; de Barros, J.-P.P.; Olmiere, C.; Delmas, D. RESVEGA, a Nutraceutical Omega-3/Resveratrol Supplementation, Reduces Angiogenesis in a Preclinical Mouse Model of Choroidal Neovascularization. Int. J. Mol. Sci. 2021, 22, 11023. [Google Scholar] [CrossRef]
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric Syndromes: Clinical, Research, and Policy Implications of a Core Geriatric Concept: (See Editorial Comments by Dr. William Hazzard on pp 794–796). J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef]
- Gray, S.R.; Mittendorfer, B. Fish oil-derived n-3 polyunsaturated fatty acids for the prevention and treatment of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 104–109. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The effect of Omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2021, 18, 9. [Google Scholar] [CrossRef]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Echeverri, J.H.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Labonté, M.; Dewailly, É.; Lucas, M.; Couture, P.; Lamarche, B. Association of red blood cell n-3 polyunsaturated fatty acids with plasma inflammatory biomarkers among the Quebec Cree population. Eur. J. Clin. Nutr. 2014, 68, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARγ as a molecular target of EPA anti-inflammatory activity during TNF-α-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef]
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int. J. Mol. Sci. 2019, 20, 4025. [Google Scholar] [CrossRef]
- Wang, T.; He, X.; Liu, X.; Liu, Y.; Zhang, W.; Huang, Q.; Liu, W.; Xiong, L.; Tan, R.; Wang, H.; et al. Weighted Gene Co-expression Network Analysis Identifies FKBP11 as a Key Regulator in Acute Aortic Dissection through a NF-kB Dependent Pathway. Front. Physiol. 2017, 8, 1010. [Google Scholar] [CrossRef]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, S884–S890. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Inagi, R. Mitochondria: A therapeutic target in acute kidney injury. Nephrol. Dial. Transplant. 2016, 31, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n−3 fatty acids: Molecular mechanisms and potential clinical applications. Clin. Sci. 2013, 124, 1–15. [Google Scholar] [CrossRef]
- Alonso-Bouzón, C.; Carcaillon, L.; García-García, F.J.; Amor-Andrés, M.S.; El Assar, M.; Rodríguez-Mañas, L. Association between endothelial dysfunction and frailty: The Toledo Study for Healthy Aging. AGE 2014, 36, 495–505. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Madonna, R.; Novo, G.; Balistreri, C.R. Cellular and molecular basis of the imbalance between vascular damage and repair in ageing and age-related diseases: As biomarkers and targets for new treatments. Mech. Ageing Dev. 2016, 159, 22–30. [Google Scholar] [CrossRef]
- Amarasekera, A.T.; Chang, D.; Schwarz, P.; Tan, T.C. Does vascular endothelial dysfunction play a role in physical frailty and sarcopenia? A systematic review. Age Ageing 2021, 50, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Haß, U.; Kochlik, B.; Herpich, C.; Rudloff, S.; Norman, K. Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial. Nutrients 2022, 14, 4274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chong, Z.Z.; Shang, Y.C.; Maiese, K. Cardiovascular disease and mTOR signaling. Trends Cardiovasc. Med. 2011, 21, 151–155. [Google Scholar] [CrossRef]
- Jing, K.; Song, K.-S.; Shin, S.; Kim, N.; Jeong, S.; Oh, H.-R.; Park, J.-H.; Seo, K.-S.; Heo, J.-Y.; Han, J.; et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy 2011, 7, 1348–1358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konagai, C.; Yanagimoto, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Koga, Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin. Interv. Aging 2013, 8, 1247–1257. [Google Scholar] [CrossRef]
- Yoshino, J.; Smith, G.I.; Kelly, S.C.; Julliand, S.; Reeds, D.N.; Mittendorfer, B. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol. Rep. 2016, 4, e12785. [Google Scholar] [CrossRef] [PubMed]
- Ojaroodi, A.F.; Jafarnezhad, F.; Eskandari, Z.; Keramat, S.; Stanek, A. Recent Updates and Advances in the Association Between Vitamin D Deficiency and Risk of Thrombotic Disease. Nutrients 2024, 17, 90. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. N-3 PUFAs fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Cabo, J.; Alonso, R.; Mata, P. N-3 PUFAs fatty acids and blood pressure. Br. J. Nutr. 2012, 107 (Suppl. S2), S195–S200. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, M.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; Li, J. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 2007, 89, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gousset-Dupont, A.; Robert, V.; Grynberg, A.; Lacour, B.; Tardivel, S. The effect of n-3 PUFA on eNOS activity and expression in Ea hy 926 cells. Prostaglandins Leukot Essent. Fat. Acids 2007, 76, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, C.; Dong, Y.; Wang, S.; Song, P.; Viollet, B.; Zou, M.-H.; Huang, Y. Activation of the AMP-Activated Protein Kinase by Eicosapentaenoic Acid (EPA, 20:5 n-3) Improves Endothelial Function In Vivo. PLoS ONE 2012, 7, e35508. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.; Cassidy, A.; Kay, C. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, L.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Chandra, P.; Kay, C.; Edirisinghe, I.; Burton-Freeman, B. Strawberry Consumption, Cardiometabolic Risk Factors, and Vascular Function: A Randomized Controlled Trial in Adults with Moderate Hypercholesterolemia. J. Nutr. 2021, 151, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545, Erratum in Am. J. Clin. Nutr. 2019, 110, 1262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rabelo, C.A.S.; Taarji, N.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A. Formulation and characterization of water-in-oil nanoemulsions loaded with açaí berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Res. Int. 2018, 106, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, B.E.; Saroglu, O.; Karakas, C.Y.; Karadag, A. Encapsulation of purple basil leaf extract by electrospraying in double emulsion (W/O/W) filled alginate-carrageenan beads to improve the bioaccessibility of anthocyanins. Int. J. Biol. Macromol. 2023, 250, 126207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, M.; Piližota, V. Prevention of thermal degradation of anthocyanins in blackberry juice with addition of different sugars. Cyta J. Food 2011, 9, 237–242. [Google Scholar] [CrossRef]
- Wu, J.; Guan, Y.; Zhong, Q. Yeast mannoproteins improve thermal stability of anthocyanins at pH 7.0. Food Chem. 2015, 172, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of nanoliposomal carriers to improve the stability of antho-cyanins. LWT 2019, 109, 101–107. [Google Scholar] [CrossRef]
- Mone, P.; Varzideh, F.; Kansakar, U.; Infante, C.; Lombardi, A.; de Donato, A.; Frullone, S.; Santulli, G. Omega-3 fatty acids coordinate glucose and lipid metabolism in diabetic patients. Lipids Health Dis. 2022, 21, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Wang, F.; Liu, X.; Xie, Y.; Xia, H.; Wang, S.; Sun, G. Effects of marine-derived and plant-derived omega-3 polyunsaturated fatty acids on erythrocyte fatty acid composition in type 2 diabetic patients. Lipids Health Dis. 2022, 21, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, A.; McDermott, M.M.; Joshi, J.K.; Sturgis, J.C.; Zhang, D.; Ho, K.J.; Sufit, R.; Ferrucci, L.; Peterson, C.A.; Kosmac, K. Cocoa flavanols, Nrf2 activation, and oxidative stress in peripheral artery disease: Mechanistic findings in muscle based on outcomes from a randomized trial. Am. J. Physiol. Cell Physiol. 2024, 326, C589–C605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDermott, M.M.; Spring, B.; Tian, L.; Treat-Jacobson, D.; Ferrucci, L.; Lloyd-Jones, D.; Zhao, L.; Polonsky, T.; Kibbe, M.R.; Bazzano, L.; et al. Effect of Low-Intensity vs High-Intensity Home-Based Walking Exercise on Walk Distance in Patients with Peripheral Artery Disease: The LITE Randomized Clinical Trial. JAMA 2021, 325, 1266–1276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lo, H.-Y.; Lin, Y.-S.; Lin, D.S.-H.; Lee, J.-K.; Chen, W.-J. Association of Statin Therapy with Major Adverse Cardiovascular and Limb Outcomes in Patients with End-stage Kidney Disease and Peripheral Artery Disease Receiving Maintenance Dialysis. JAMA Netw. Open 2022, 5, e2229706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2020, 96, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Bharat, D.; Cavalcanti, R.R.M.; Petersen, C.; Begaye, N.; Cutler, B.R.; Costa, M.M.A.; Ramos, R.K.L.G.; Ferreira, M.R.; Li, Y.; Bharath, L.P.; et al. Blueberry Metabolites Attenuate Lipotoxicity-Induced Endothelial Dysfunction. Mol. Nutr. Food Res. 2018, 62, 1700601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riso, P.; Klimis-Zacas, D.; DEL Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Olinic, D.M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.A.; Liew, A.; Schernthaner, G.H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loffredo, L.; Perri, L.; Catasca, E.; Pignatelli, P.; Brancorsini, M.; Nocella, C.; De Falco, E.; Bartimoccia, S.; Frati, G.; Carnevale, R.; et al. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J. Am. Heart Assoc. 2014, 3, e001072, Erratum in J. Am. Heart Assoc. 2014, 3, e000456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDermott, M.M.; Criqui, M.H.; Domanchuk, K.; Ferrucci, L.; Guralnik, J.M.; Kibbe, M.R.; Kosmac, K.; Kramer, C.M.; Leeuwenburgh, C.; Li, L.; et al. Cocoa to Improve Walking Performance in Older People with Peripheral Artery Disease: The COCOA-PAD Pilot Randomized Clinical Trial. Circ. Res. 2020, 126, 589–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yannakoulia, M.; Ntanasi, E.; Anastasiou, C.A.; Scarmeas, N. Frailty and nutrition: From epidemiological and clinical evidence to potential mechanisms. Metabolism 2017, 68, 64–76. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamshidi, S.; Eskandari, Z.; Ojaroodi, A.F.; Keramat, S.; Stanek, A. The Impact of Flavonoids and Omega-3 in Mitigating Frailty Syndrome to Improve Treatment Outcomes in Peripheral Artery Disease (PAD) Patients. Nutrients 2025, 17, 2303. https://doi.org/10.3390/nu17142303
Jamshidi S, Eskandari Z, Ojaroodi AF, Keramat S, Stanek A. The Impact of Flavonoids and Omega-3 in Mitigating Frailty Syndrome to Improve Treatment Outcomes in Peripheral Artery Disease (PAD) Patients. Nutrients. 2025; 17(14):2303. https://doi.org/10.3390/nu17142303
Chicago/Turabian StyleJamshidi, Sanaz, Zahra Eskandari, Amirhossein Faghih Ojaroodi, Shayan Keramat, and Agata Stanek. 2025. "The Impact of Flavonoids and Omega-3 in Mitigating Frailty Syndrome to Improve Treatment Outcomes in Peripheral Artery Disease (PAD) Patients" Nutrients 17, no. 14: 2303. https://doi.org/10.3390/nu17142303
APA StyleJamshidi, S., Eskandari, Z., Ojaroodi, A. F., Keramat, S., & Stanek, A. (2025). The Impact of Flavonoids and Omega-3 in Mitigating Frailty Syndrome to Improve Treatment Outcomes in Peripheral Artery Disease (PAD) Patients. Nutrients, 17(14), 2303. https://doi.org/10.3390/nu17142303







