Hydration Meets Regulation: Insights into Bicarbonate Mineral Water and Acid–Base Balance
Abstract
1. Introduction
2. Literature Search Strategy
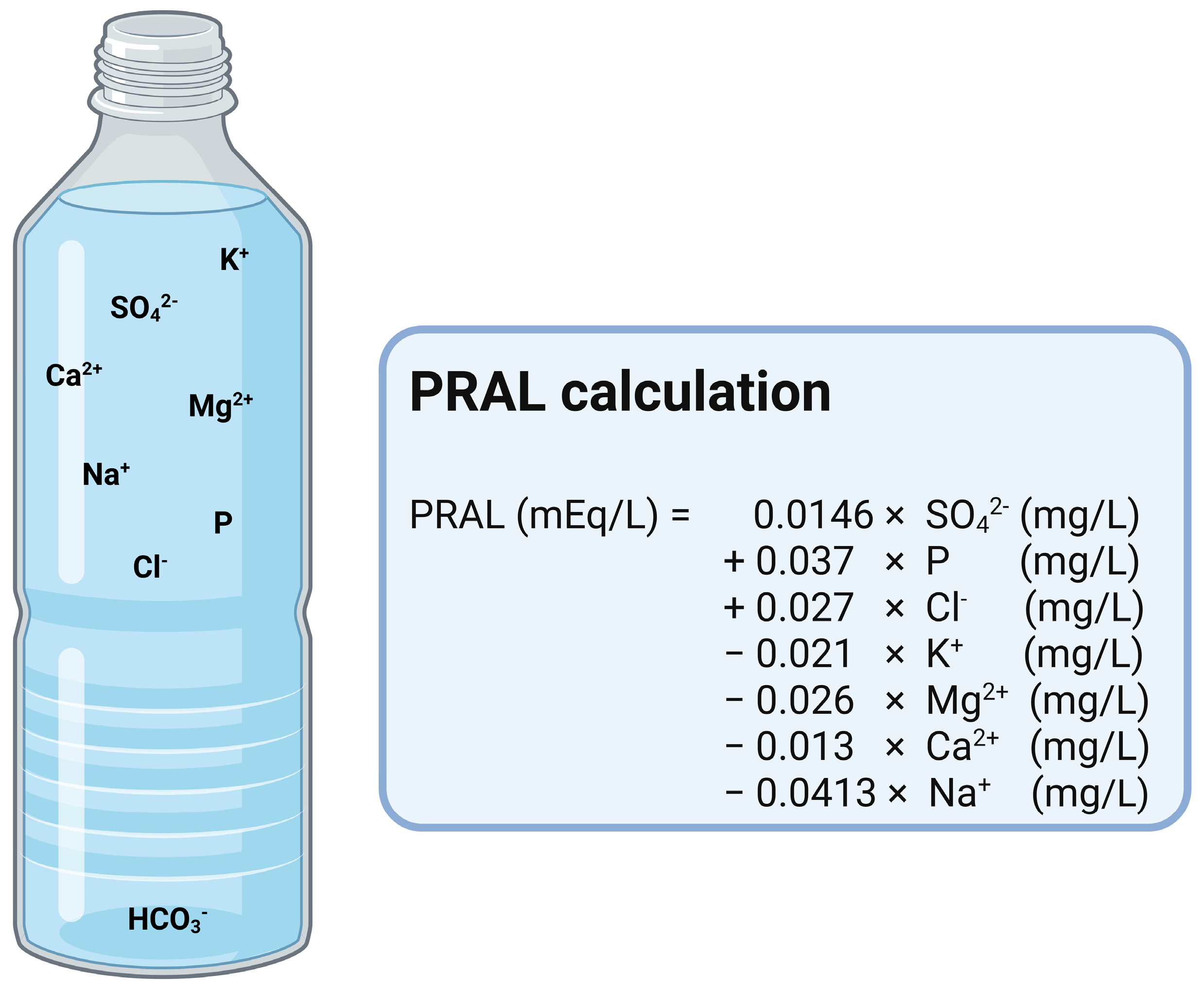
3. Intake of Acid Precursors and Acid Excretion
| Name of the Equation | Equation/Formula | Reference |
|---|---|---|
| NEAPR (mEq/d) | PRALR (mEq/d) * + OAanthro (mEq/d) | [14] |
| OAantho (mEq/d) | BSA ** (m2) × 41 (mEq/d)/1.73 (m2) | [11] |
| NEAPL (mEq/d) OAdiet (mEq/d) | PRALR (mEq/d) * + OAdiet (mEq/d) 32.9 + (0.15 × [{potassium} + {calcium × 2} + {magnesium × 2} − {phosphorus × 1.8}]) (all in mmol/d) | [16] |
| NEAPS (mEq/d) OAdiet (mEq/d) | PRALS (mEq/d) * + OAdiet (mEq/d) 32.9 + (0.15 × [{potassium} + {calcium × 2} + {magnesium × 2} − {phosphorus × 1.8}]) (all in mmol/d) | [15] |
| NEAPF (mEq/d) | Equation (1): [54.4 × protein (g/d)/potassium (mEq/d)] − 10.2 Equation (2): [0.91 × protein (g/d)] − [0.57 × potassium (mEq/d)] + 21 | [17] |
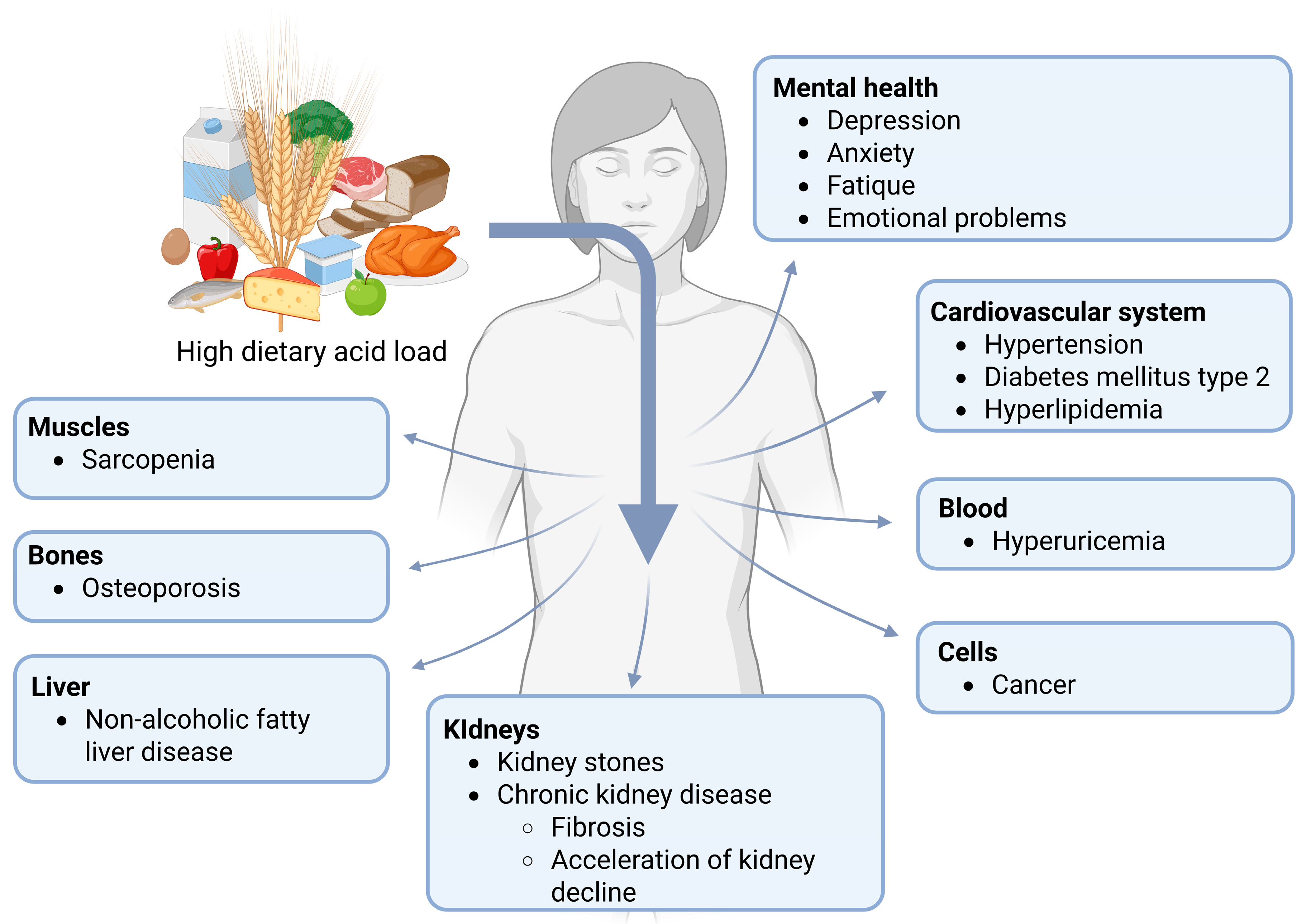
4. Associations Between Dietary Acid Load and Metabolic Alterations or Diseases
5. Mineral Water
6. Bicarbonate-Rich Mineral Water
7. Bicarbonate-Rich Mineral Water and Human Health
7.1. Urinary Parameters
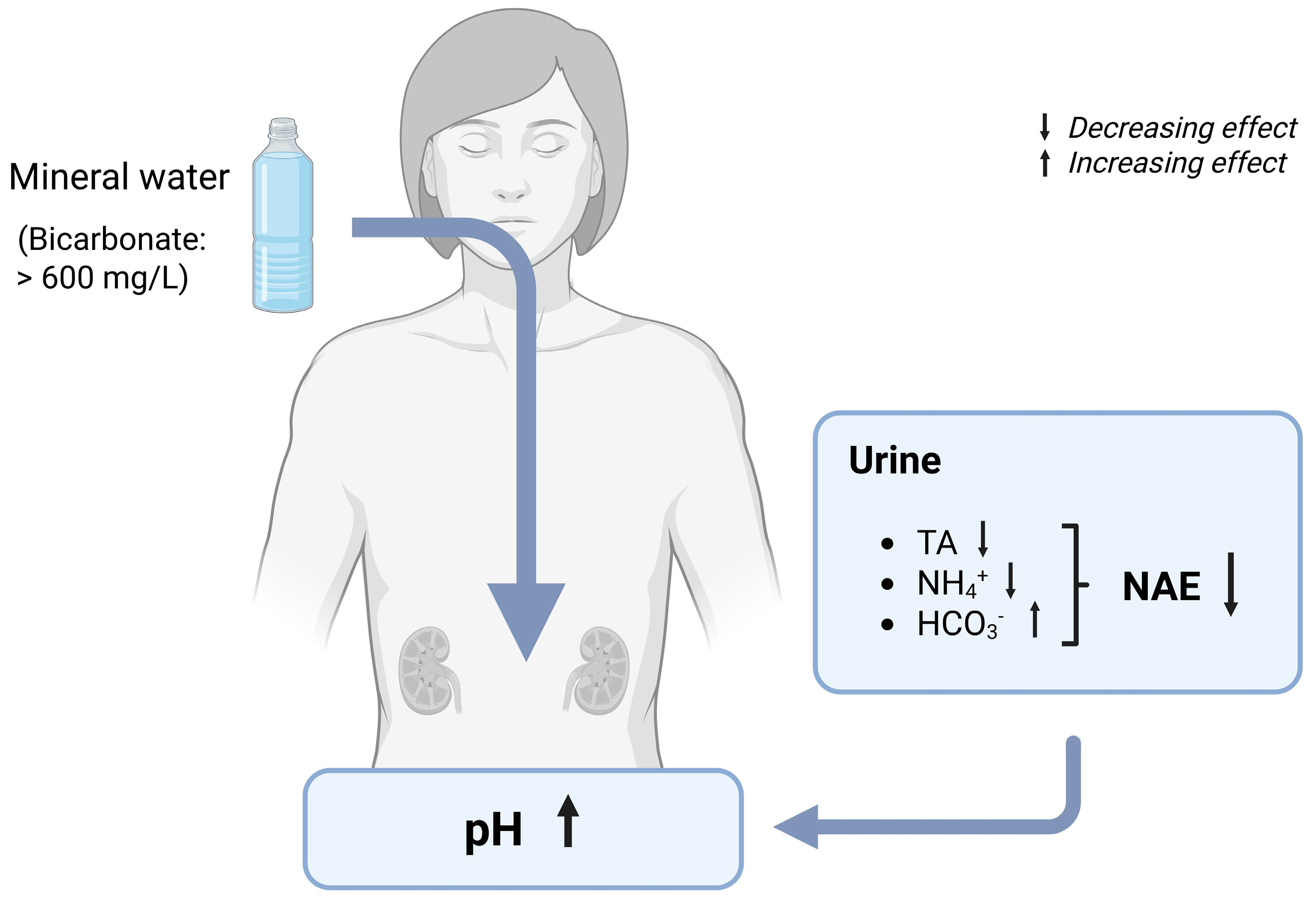
7.1.1. Urine pH and Net Acid Excretion (NAE)
7.1.2. Effects of Bicarbonate-Rich Mineral Water on Urine pH and NAE
7.2. Mechanisms of Changed Urinary Composition by Bicarbonate-Rich Mineral Water
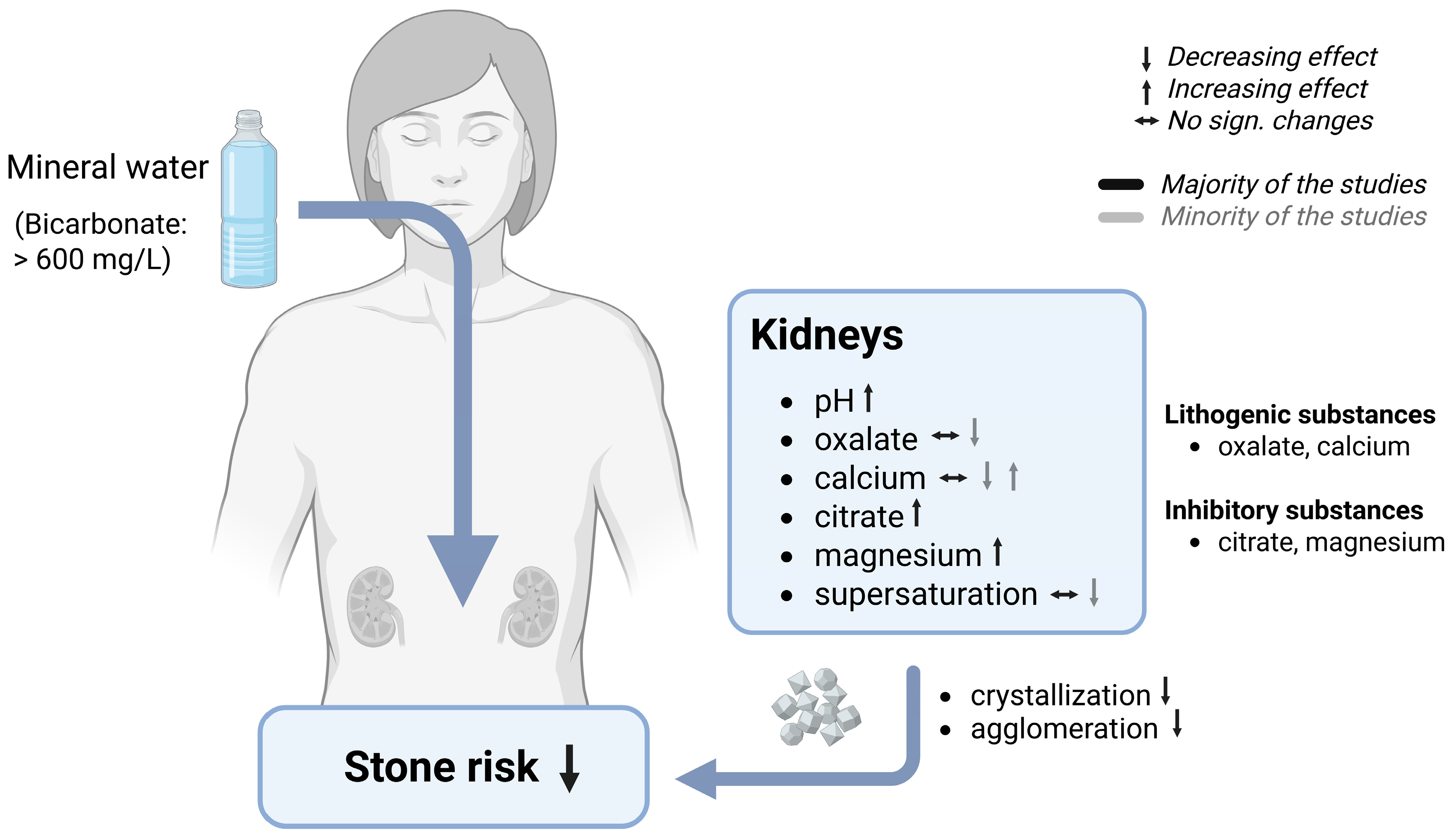
7.2.1. Renal Stones
7.2.2. Effects of Bicarbonate-Rich Mineral Water on Stone Risk
7.3. Mechanisms of Stone Risk Reduction by Bicarbonate-Rich Mineral Water
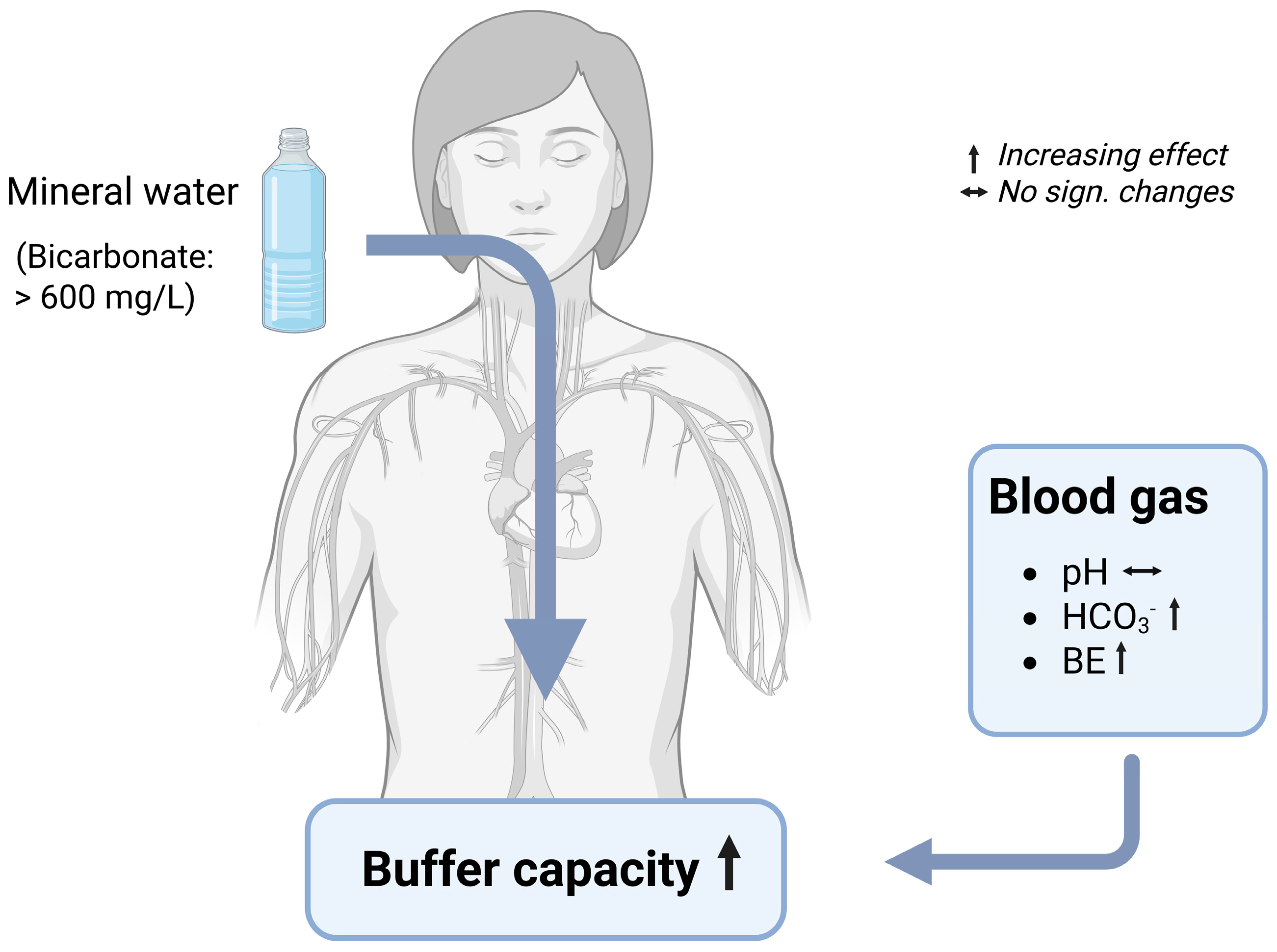
Blood Gas Parameters
7.4. Mechanisms of Changes in Blood Gas Parameters by Bicarbonate-Rich Mineral Water
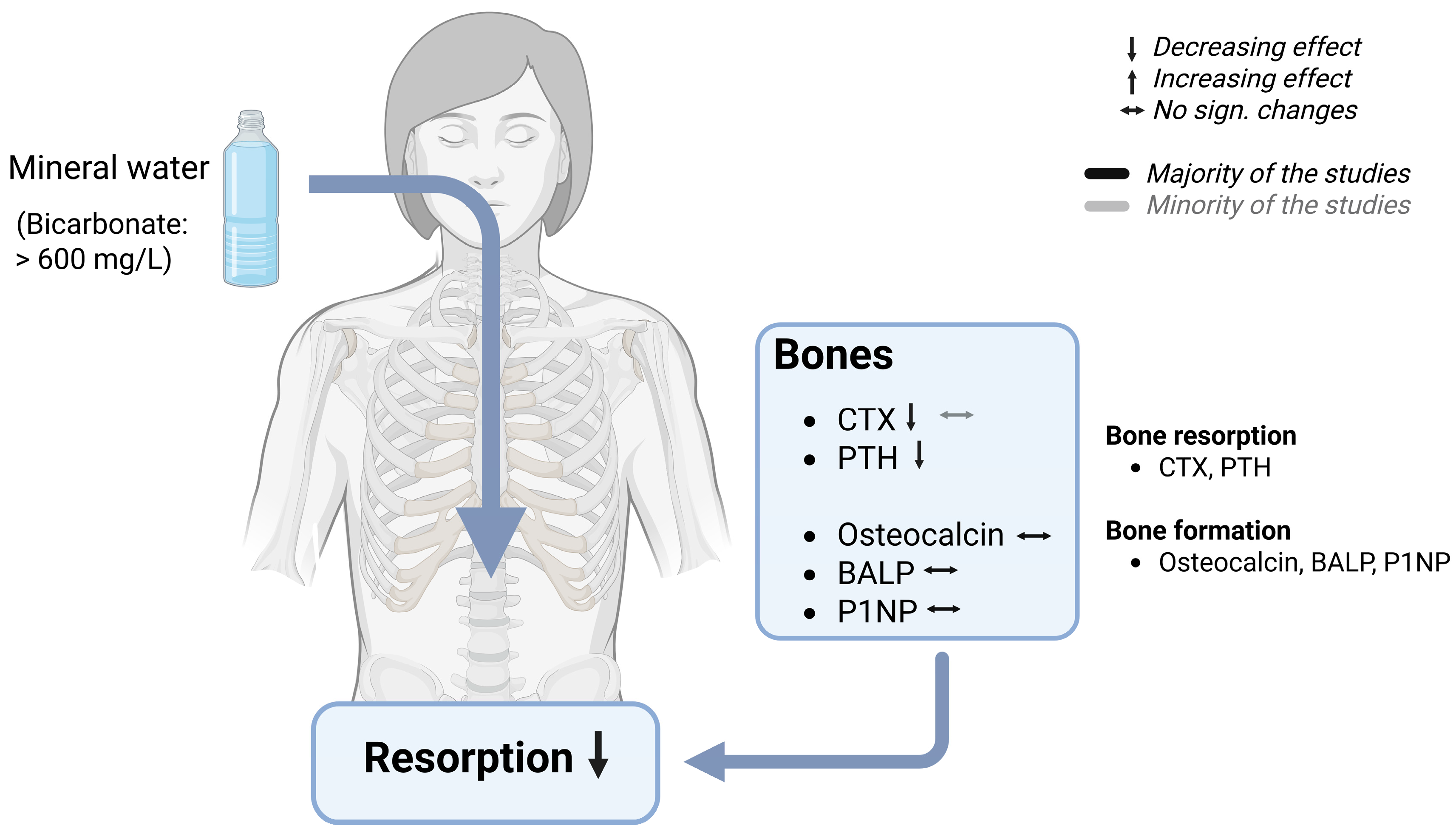
7.4.1. Bone Health
7.4.2. Effects of Bicarbonate-Rich Mineral Water on Bone Turnover
7.5. Mechanism of Bone Protection by Bicarbonate-Rich Mineral Water
7.5.1. Effects of Bicarbonate-Rich Mineral Water on Bone Density and Fracture Risk
7.5.2. Bicarbonate-Rich Mineral Water and the Complexity with Other Nutrients Regarding Bone Health
8. Summary Bicarbonate-Rich Mineral Water
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PRAL | Potential Renal Acid Load |
| NEAP | Net Endogenous Acid Production |
| NAE | Net Acid Excretion |
| OA | Organic acids |
| TA | Titrable acids |
| NH4+ | Ammonium |
| HCO3- | Bicarbonate |
| Ca | Calcium |
| Mg | Magnesium |
| Na | Sodium |
| K | Potassium |
| Cl | Chloride |
| SO4 | Sulfate |
| P | Phosphorus |
| BE | Base Excess |
| CaOx | Calcium oxalate |
| CaP | Calcium phosphate |
| RS | Relative supersaturation |
| UA | Uric acid |
| Cr | Creatinine |
| CTX | C-terminal fragment of the type I collagen (CTX) |
| iCa | Ionized calcium |
| P1NP | Procollagen type 1 N-terminal propeptide |
| PTH | Parathyroid hormone |
References
- Osuna-Padilla, I.A.; Leal-Escobar, G.; Garza-García, C.A.; Rodríguez-Castellanos, F.E. Dietary acid load: Mechanisms and evidence of its health repercussions. Nefrol. Engl. Ed. 2019, 39, 343–354. [Google Scholar] [CrossRef]
- Ströhle, A.; Remer, T. Ernährung und Säure-Basen-Haushalt. Physiologie und Prävention. Ernähr. Im Fokus 2014, 14, 314–324. [Google Scholar]
- Gholami, F.; Naghshi, S.; Samadi, M.; Rasaei, N.; Mirzaei, K. Dietary Acid Load and Bone Health: A Systematic Review and Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 869132. [Google Scholar] [CrossRef]
- Wieërs, M.L.A.J.; Beynon-Cobb, B.; Visser, W.J.; Attaye, I. Dietary acid load in health and disease. Pflüg. Arch.-Eur. J. Physiol. 2024, 476, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Remer, T. Influence of nutrition on acid-base balance—Metabolic aspects. Eur. J. Nutr. 2001, 40, 214–220. [Google Scholar] [CrossRef]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef]
- Betz, M.V.; Penniston, K.L. Primary Contributors to Dietary Acid Load in Patients With Urolithiasis. J. Ren. Nutr. 2023, 33, 53–58. [Google Scholar] [CrossRef]
- Dawson-Hughes, B. Acid–base balance of the diet—Implications for bone and muscle. Eur. J. Clin. Nutr. 2020, 74, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A.; Müller, A.; Ronco, A.L. Nutrient Intake and Dietary Acid Load of Special Diets in the NHANES: A Descriptive Analysis (2009–2018). Int. J. Environ. Res. Public. Health 2022, 19, 5748. [Google Scholar] [CrossRef]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid Balance, Dietary Acid Load, and Bone Effects—A Controversial Subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am. J. Clin. Nutr. 1994, 59, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Carnauba, R.; Baptistella, A.; Paschoal, V.; Hübscher, G. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Wasserfurth, P.; Schneider, I.; Ströhle, A.; Nebl, J.; Bitterlich, N.; Hahn, A. Effects of mineral waters on acid-base status in healthy adults: Results of a randomized trial. Food Nutr. Res. 2019, 63, 3515. [Google Scholar] [CrossRef]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef]
- Sebastian, A.; Frassetto, L.A.; Sellmeyer, D.E.; Merriam, R.L.; Morris, R.C. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am. J. Clin. Nutr. 2002, 76, 1308–1316. [Google Scholar] [CrossRef]
- Frassetto, L.; Shi, L.; Schloetter, M.; Sebastian, A.; Remer, T. Established dietary estimates of net acid production do not predict measured net acid excretion in patients with Type 2 diabetes on Paleolithic–Hunter–Gatherer-type diets. Eur. J. Clin. Nutr. 2013, 67, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Lanham-New, S.A.; Macdonald, H.M.; Remer, T.; Sebastian, A.; Tucker, K.L.; Tylavsky, F.A. Standardizing Terminology for Estimating the Diet-Dependent Net Acid Load to the Metabolic System. J. Nutr. 2007, 137, 1491–1492. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents123. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Dymock, M.; Banerjee, T.; Sebastian, A.; Slater, G.J.; Frassetto, L.A. Performance of Predictive Equations and Biochemical Measures Quantifying Net Endogenous Acid Production and the Potential Renal Acid Load. Kidney Int. Rep. 2020, 5, 1738–1745. [Google Scholar] [CrossRef]
- Nagami, G.T.; Kraut, J.A. The Role of the Endocrine System in the Regulation of Acid–Base Balance by the Kidney and the Progression of Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 2420. [Google Scholar] [CrossRef]
- Rylander, R.; Remer, T.; Berkemeyer, S.; Vormann, J. Acid-Base Status Affects Renal Magnesium Losses in Healthy, Elderly Persons. J. Nutr. 2006, 136, 2374–2377. [Google Scholar] [CrossRef] [PubMed]
- Gannon, R.H.T.; Millward, D.J.; Brown, J.E.; Macdonald, H.M.; Lovell, D.P.; Frassetto, L.A.; Remer, T.; Lanham-New, S.A. Estimates of daily net endogenous acid production in the elderly UK population: Analysis of the National Diet and Nutrition Survey (NDNS) of British adults aged 65 years and over. Br. J. Nutr. 2008, 100, 615–623. [Google Scholar] [CrossRef]
- Ströhle, A.; Waldmann, A.; Koschizke, J.; Leitzmann, C.; Hahn, A. Diet-Dependent Net Endogenous Acid Load of Vegan Diets in Relation to Food Groups and Bone Health-Related Nutrients: Results from the German Vegan Study. Ann. Nutr. Metab. 2011, 59, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Moss, J.; Thisted, R. Predictors of body surface area. J. Clin. Anesth. 1992, 4, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Manz, F. History of nutrition and acid-base physiology. Eur. J. Nutr. 2001, 40, 189–199. [Google Scholar] [CrossRef]
- Jehle, S.; Zanetti, A.; Muser, J.; Hulter, H.N.; Krapf, R. Partial Neutralization of the Acidogenic Western Diet with Potassium Citrate Increases Bone Mass in Postmenopausal Women with Osteopenia. J. Am. Soc. Nephrol. 2006, 17, 3213–3222. [Google Scholar] [CrossRef]
- Naude, D.F. Chronic Sub-Clinical Systemic Metabolic Acidosis—A Review with Implications for Clinical Practice. J. Evid.-Based Integr. Med. 2022, 27, 2515690X221142352. [Google Scholar] [CrossRef]
- Raphael, K.L. Metabolic Acidosis and Subclinical Metabolic Acidosis in CKD. J. Am. Soc. Nephrol. JASN 2018, 29, 376–382. [Google Scholar] [CrossRef]
- DuBose, T.D. Urine Ammonium and Preclinical Acidosis in CKD. J. Am. Soc. Nephrol. JASN 2017, 28, 2258–2260. [Google Scholar] [CrossRef]
- Alpern, R.J.; Sakhaee, K. The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1997, 29, 291–302. [Google Scholar] [CrossRef]
- Maurer, M.; Riesen, W.; Muser, J.; Hulter, H.N.; Krapf, R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am. J. Physiol.-Ren. Physiol. 2003, 284, F32–F40. [Google Scholar] [CrossRef] [PubMed]
- Esche, J. Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int. 2016, 90, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Esche, J.; Krupp, D.; Mensink, G.B.; Remer, T. Dietary Potential Renal Acid Load Is Positively Associated with Serum Uric Acid and Odds of Hyperuricemia in the German Adult Population. J. Nutr. 2018, 148, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, M.; Przysławski, J. The relationship between serum uric acid concentration and cardiovascular risk factors in normotensivepostmenopausal women with dyslipidemia. Acta Sci. Pol. Technol. Aliment. 2020, 19, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ye, C.; Wang, R.; Zhang, Z.; Huang, X.; Halimulati, M.; Sun, M.; Ma, Y.; Zhang, Z. Association between Dietary Acid Load and Hyperuricemia in Chinese Adults: Analysis of the China Health and Nutrition Survey (2009). Nutrients 2023, 15, 1806. [Google Scholar] [CrossRef]
- Han, Y.; An, M.; Yang, L.; Li, L.; Rao, S.; Cheng, Y. Effect of Acid or Base Interventions on Bone Health: A Systematic Review, Meta-Analysis, and Meta-Regression. Adv. Nutr. 2021, 12, 1540–1557. [Google Scholar] [CrossRef]
- Passey, C. Reducing the Dietary Acid Load: How a More Alkaline Diet Benefits Patients With Chronic Kidney Disease. J. Ren. Nutr. 2017, 27, 151–160. [Google Scholar] [CrossRef]
- Trinchieri, A.; Lizzano, R.; Marchesotti, F.; Zanetti, G. Effect of potential renal acid load of foods on urinary citrate excretion in calcium renal stone formers. Urol. Res. 2006, 34, 1–7. [Google Scholar] [CrossRef]
- Vezzoli, G.; Dogliotti, E.; Terranegra, A.; Arcidiacono, T.; Macrina, L.; Tavecchia, M.; Pivari, F.; Mingione, A.; Brasacchio, C.; Nouvenne, A.; et al. Dietary style and acid load in an Italian population of calcium kidney stone formers. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 588–593. [Google Scholar] [CrossRef]
- Miki, A.; Hashimoto, Y.; Tanaka, M.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Kido, Y.; Yamazaki, M.; Fukui, M. Urinary pH reflects dietary acid load in patients with type 2 diabetes. J. Clin. Biochem. Nutr. 2017, 61, 74–77. [Google Scholar] [CrossRef]
- Mofrad, M.D.; Daneshzad, E.; Azadbakht, L. Dietary acid load, kidney function and risk of chronic kidney disease: A systematic review and meta-analysis of observational studies. Int. J. Vitam. Nutr. Res. 2021, 91, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Siener, R. Nutrition and Kidney Stone Disease. Nutrients 2021, 13, 1917. [Google Scholar] [CrossRef]
- Ströhle, A.; Hahn, A. Ossäre Bedeutung von Mineral- und Heilwässern: Physiologie und epidemiologische Evidenz. Osteologie 2023, 32, 278–294. [Google Scholar] [CrossRef]
- Costa-Vieira, D.; Monteiro, R.; Martins, M.J. Metabolic Syndrome Features: Is There a Modulation Role by Mineral Water Consumption? A Review. Nutrients 2019, 11, 1141. [Google Scholar] [CrossRef]
- Dolati, S.; Razmjouei, S.; Alizadeh, M.; Faghfouri, A.H.; Moridpour, A.H. A high dietary acid load can potentially exacerbate cardiometabolic risk factors: An updated systematic review and meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 569–580. [Google Scholar] [CrossRef]
- Dupont, C.; Hébert, G. Magnesium Sulfate-Rich Natural Mineral Waters in the Treatment of Functional Constipation—A Review. Nutrients 2020, 12, 2052. [Google Scholar] [CrossRef]
- Harris, P.R.; Keen, D.A.; Constantopoulos, E.; Weninger, S.N.; Hines, E.; Koppinger, M.P.; Khalpey, Z.I.; Konhilas, J.P. Fluid type influences acute hydration and muscle performance recovery in human subjects. J. Int. Soc. Sports Nutr. 2019, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Chycki, J.; Kostrzewa, M.; Maszczyk, A.; Zajac, A. Chronic Ingestion of Bicarbonate-Rich Water Improves Anaerobic Performance in Hypohydrated Elite Judo Athletes: A Pilot Study. Int. J. Environ. Res. Public. Health 2021, 18, 4948. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Rettura, F.; Pancetti, A.; Ceccarelli, L.; Ricchiuti, A.; Costa, F.; De Bortoli, N.; Marchi, S.; et al. Chronic Constipation: Is a Nutritional Approach Reasonable? Nutrients 2021, 13, 3386. [Google Scholar] [CrossRef]
- Pampaloni, B.; Brandi, M.L. Mineral water as food for bone: An overview. Int. J. Bone Fragility 2022, 2, 48–55. [Google Scholar] [CrossRef]
- Wynn, E.; Raetz, E.; Burckhardt, P. The composition of mineral waters sourced from Europe and North America in respect to bone health: Composition of mineral water optimal for bone. Br. J. Nutr. 2009, 101, 1195–1199. [Google Scholar] [CrossRef]
- Gundermann, G.; Hoffmann, H.; Gutenbrunner, C. Drinking—But what?—Medicinal waters in urinary stone metaphylaxis. Ernähr. Med. 2004, 19, 178–183. [Google Scholar] [CrossRef]
- Burckhardt, P. Der Einfluss von Mineralwasser auf die Knochengesundheit. Schweiz. Z. Für ERnährungsmedizin 2011, 2, 15–19. [Google Scholar]
- Stoots, S.J.M.; Geraghty, R.; Kamphuis, G.M.; Jamnadass, E.; Henderickx, M.M.E.L.; Ventimiglia, E.; Traxer, O.; Keller, E.X.; DeConinck, V.; Talso, M.; et al. Variations in the Mineral Content of Bottled “Still” Water Across Europe: Comparison of 182 Brands Across 10 Countries. J. Endourol. 2021, 35, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Stoots, S.J.M.; Geraghty, R.; Kamphuis, G.M.; Jamnadass, E.; Henderickx, M.M.E.L.; Ventimiglia, E.; Traxer, O.; Keller, E.X.; De Coninck, V.; Talso, M.; et al. Variations in the mineral content of bottled ‘carbonated or sparkling’ water across Europe: A comparison of 126 brands across 10 countries. Cent. Eur. J. Urol. 2021, 74, 71–75. [Google Scholar] [CrossRef]
- Wynn, E.; Krieg, M.-A.; Aeschlimann, J.-M.; Burckhardt, P. Alkaline mineral water lowers bone resorption even in calcium sufficiency. Bone 2009, 44, 120–124. [Google Scholar] [CrossRef]
- Burckhardt, P. The Effect of the Alkali Load of Mineral Water on Bone Metabolism: Interventional Studies. J. Nutr. 2008, 138, 435S–437S. [Google Scholar] [CrossRef]
- Mansouri, K.; Greupner, T.; van de Flierdt, E.; Schneider, I.; Hahn, A. Acid-Base Balance in Healthy Adults: Beneficial Effects of Bicarbonate and Sodium-Rich Mineral Water in a Randomized Controlled Trial: The BicarboWater Study. J. Nutr. Metab. 2024, 2024, 3905500. [Google Scholar] [CrossRef]
- Chiron, F.; Thomas, C.; Bardin, J.; Mullie, F.; Bennett, S.; Chéradame, J.; Caliz, L.; Hanon, C.; Tiollier, E. Influence of Ingestion of Bicarbonate-Rich Water Combined with an Alkalizing or Acidizing Diet on Acid-Base Balance and Anaerobic Performance. J. Hum. Kinet. 2024, 93, 105–117. [Google Scholar] [CrossRef]
- He, F.J.; Marciniak, M.; Carney, C.; Markandu, N.D.; Anand, V.; Fraser, W.D.; Dalton, R.N.; Kaski, J.C.; MacGregor, G.A. Effects of Potassium Chloride and Potassium Bicarbonate on Endothelial Function, Cardiovascular Risk Factors, and Bone Turnover in Mild Hypertensives. Hypertension 2010, 55, 681–688. [Google Scholar] [CrossRef]
- Limmer, M.; De Marées, M.; Platen, P. Effects of daily ingestion of sodium bicarbonate on acid-base status and anaerobic performance during an altitude sojourn at high altitude: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; You, Z.; Andrews, E.; Farmer-Bailey, H.; Moreau, K.; Chonchol, M.; Steele, C.; Wang, W.; Nowak, K.L.; Patel, N. Sodium Bicarbonate Treatment and Vascular Function in CKD: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Soc. Nephrol. 2023, 34, 1433. [Google Scholar] [CrossRef]
- Tyson, C.C.; Luciano, A.; Modliszewski, J.L.; Corcoran, D.L.; Bain, J.R.; Muehlbauer, M.; Ilkayeva, O.; Pourafshar, S.; Allen, J.; Bowman, C.; et al. Effect of Bicarbonate on Net Acid Excretion, Blood Pressure, and Metabolism in Patients With and Without CKD: The Acid Base Compensation in CKD Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, 78, 38–47. [Google Scholar] [CrossRef]
- Lemann, J.; Gray, R.W.; Pleuss, J.A. Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int. 1989, 35, 688–695. [Google Scholar] [CrossRef]
- Sebastian, A.; Harris, S.T.; Ottaway, J.H.; Todd, K.M.; Morris, R.C. Improved Mineral Balance and Skeletal Metabolism in Postmenopausal Women Treated with Potassium Bicarbonate. N. Engl. J. Med. 1994, 330, 1776–1781. [Google Scholar] [CrossRef]
- Ceglia, L.; Harris, S.S.; Abrams, S.A.; Rasmussen, H.M.; Dallal, G.E.; Dawson-Hughes, B. Potassium Bicarbonate Attenuates the Urinary Nitrogen Excretion That Accompanies an Increase in Dietary Protein and May Promote Calcium Absorption. J. Clin. Endocrinol. Metab. 2009, 94, 645–653. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Palermo, N.J.; Castaneda-Sceppa, C.; Rasmussen, H.M.; Dallal, G.E. Treatment with Potassium Bicarbonate Lowers Calcium Excretion and Bone Resorption in Older Men and Women. J. Clin. Endocrinol. Metab. 2009, 94, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Ceglia, L.; Rivas, D.; Dawson-Hughes, B.; Fielding, R. Pilot Study Examining the Influence of Potassium Bicarbonate Supplementation on Nitrogen Balance and Whole-Body Ammonia and Urea Turnover Following Short-Term Energy Restriction in Older Men. Nutrients 2018, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Nash, E.; Morris, R.C.; Sebastian, A. Comparative effects of potassium chloride and bicarbonate on thiazide-induced reduction in urinary calcium excretion. Kidney Int. 2000, 58, 748–752. [Google Scholar] [CrossRef]
- Schorr, U.; Distler, A.; Sharma, A.M. Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: A randomized double-blind crossover trial. J. Hypertens. 1996, 14, 131–135. [Google Scholar]
- Marangella, M.; Vitale, C.; Petrarulo, M.; Rovera, L.; Dutto, F. Effects of Mineral Composition of Drinking Water on Risk for Stone Formation and Bone Metabolism in Idiopathic Calcium Nephrolithiasis. Clin. Sci. 1996, 91, 313–318. [Google Scholar] [CrossRef]
- Caudarella, R.; Rizzoli, E.; Buffa, A. Comparative study of the influence of 3 types of mineral water in patients with idiopathic calcium lithiasis. J. Urol. 1998, 159, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Keßler, T.; Hesse, A. Cross-over study of the influence of bicarbonate-rich mineral water on urinary composition in comparison with sodium potassium citrate in healthy male subjects. Br. J. Nutr. 2000, 84, 865–871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coen, G.; Sardella, D.; Barbera, G.; Ferrannini, M.; Comegna, C.; Ferazzoli, F.; Dinnella, A.; D’Anello, E.; Simeoni, P. Urinary Composition and Lithogenic Risk in Normal Subjects following Oligomineral versus Bicarbonate-Alkaline High Calcium Mineral Water Intake. Urol. Int. 2001, 67, 49–53. [Google Scholar] [CrossRef]
- Siener, R.; Jahnen, A.; Hesse, A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. Eur. J. Clin. Nutr. 2004, 58, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Schoppen, S.; Pérez-Granados, A.M.; Carbajal, Á.; Piedra, C.D.L.; Vaquero, M.P. Bone remodelling is not affected by consumption of a sodium-rich carbonated mineral water in healthy postmenopausal women. Br. J. Nutr. 2005, 93, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Karagülle, O.; Smorag, U.; Candir, F.; Gundermann, G.; Jonas, U.; Becker, A.J.; Gehrke, A.; Gutenbrunner, C. Clinical study on the effect of mineral waters containing bicarbonate on the risk of urinary stone formation in patients with multiple episodes of CaOx-urolithiasis. World J. Urol. 2007, 25, 315–323. [Google Scholar] [CrossRef]
- Pérez-Granados, A.M.; Navas-Carretero, S.; Schoppen, S.; Vaquero, M.P. Reduction in cardiovascular risk by sodium-bicarbonated mineral water in moderately hypercholesterolemic young adults. J. Nutr. Biochem. 2010, 21, 948–953. [Google Scholar] [CrossRef]
- Chiron, F.; Erblang, M.; Gulören, B.; Bredariol, F.; Hamri, I.; Leger, D.; Hanon, C.; Tiollier, E.; Thomas, C. Exploring the Influence of Acid-Base Status on Athletic Performance during Simulated Three-Day 400 m Race. Nutrients 2024, 16, 1987. [Google Scholar] [CrossRef] [PubMed]
- Schoppen, S.; Pérez-Granados, A.M.; Carbajal, Á.; Sarriá, B.; Navas-Carretero, S.; Pilar Vaquero, M. Sodium-bicarbonated mineral water decreases aldosterone levels without affecting urinary excretion of bone minerals. Int. J. Food Sci. Nutr. 2008, 59, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Keßler, T.; Hesse, A. Harnsäure- und Kalzium-oxalatsteinmetaphylaxe mit natriumbikarbonathaltigem Heilwasser. Urol. B 1998, 38, 363–369. [Google Scholar] [CrossRef]
- Roux, S.; Baudoin, C.; Boute, D.; Brazier, M.; De La Guéronniere, V.; De Vernejoul, M.C. Biological effects of drinking-water mineral composition on calcium balance and bone remodeling markers. J. Nutr. Health Aging 2004, 8, 380–384. [Google Scholar]
- Brancaccio, P. Influence of Acqua Lete® (Bicarbonate Calcic Natural Mineral Water) Hydration on Blood Lactate After Exercise. Open Sports Med. J. 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Brancaccio, P.; Limongelli, F.M.; Paolillo, I.; D’Aponte, A.; Donnarumma, V.; Rastrelli, L. Supplementation of Acqua Lete® (Bicarbonate Calcic Mineral Water) improves hydration status in athletes after short term anaerobic exercise. J. Int. Soc. Sports Nutr. 2012, 9, 35. [Google Scholar] [CrossRef]
- Toxqui, L.; Vaquero, M.P. An Intervention with Mineral Water Decreases Cardiometabolic Risk Biomarkers. A Crossover, Randomised, Controlled Trial with Two Mineral Waters in Moderately Hypercholesterolaemic Adults. Nutrients 2016, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sundaram, P.; Li, H.; Chong, T.W. The effects of drinking bicarbonate-rich mineral water in calcium oxalate stone formers: An open label prospective randomized controlled study in an Asian cohort. Int. Urol. Nephrol. 2022, 54, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Greupner, T.; Hahn, A. Blood Pressure Stability and Plasma Aldosterone Reduction: The Effects of a Sodium and Bicarbonate-Rich Water—A Randomized Controlled Intervention Study. Blood Press. 2023, 33, 2291411. [Google Scholar] [CrossRef]
- Fenton, T.R.; Lyon, A.W.; Eliasziw, M.; Tough, S.C.; Hanley, D.A. Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 1835–1840. [Google Scholar] [CrossRef]
- Grgic, J.; Pedisic, Z.; Saunders, B.; Artioli, G.G.; Schoenfeld, B.J.; McKenna, M.J.; Bishop, D.J.; Kreider, R.B.; Stout, J.R.; Kalman, D.S.; et al. International Society of Sports Nutrition position stand: Sodium bicarbonate and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 61. [Google Scholar] [CrossRef]
- Kavanagh, O.N. Alkalising agents in urinary tract infections: Theoretical contraindications, interactions and synergy. Ther. Adv. Drug Saf. 2022, 13, 204209862210807. [Google Scholar] [CrossRef]
- Horn, F. Biochemie des Menschen: Das Lehrbuch für das Medizinstudium, 6th ed.; Thieme: Stuttgart, Germany, 2015; ISBN 978-3-13-130886-3. [Google Scholar]
- Meyers, A.M.; Naicker, S. Nephrolithiasis (part 1): Epidemiology, causes and pathogenesis of recurrent nephrolithiasis. S. Afr. Med. J. 2021, 111, 930–933. [Google Scholar] [CrossRef]
- Shabani, E.; Khorshidi, A.; Sayehmiri, K.; Moradi, K.; Abdolyousefi, E.N. The effect of nutritional factors on urolithiasis: A case-control study. J. Med. Life 2023, 16, 1062–1069. [Google Scholar] [CrossRef]
- Lieske, J.C.; Rule, A.D.; Krambeck, A.E.; Williams, J.C.; Bergstralh, E.J.; Mehta, R.A.; Moyer, T.P. Stone composition as a function of age and sex. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 2141–2146. [Google Scholar] [CrossRef]
- Luft, F.C.; Zemel, M.B.; Sowers, J.A.; Fineberg, N.S.; Weinberger, M.H. Sodium bicarbonate and sodium chloride: Effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J. Hypertens. 1990, 8, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Von Unruh, G.E.; Voss, S.; Sauerbruch, T.; Hesse, A. Dependence of Oxalate Absorption on the Daily Calcium Intake. J. Am. Soc. Nephrol. 2004, 15, 1567–1573. [Google Scholar] [CrossRef]
- Rodgers, A.L. Effect of Mineral Water Containing Calcium and Magnesium on Calcium Oxalate Urolithiasis Risk Factors. Urol. Int. 1997, 58, 93–99. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N. Engl. J. Med. 1993, 328, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.G. Methods for diagnosing the risk factors of stone formation. Arab. J. Urol. 2012, 10, 250–257. [Google Scholar] [CrossRef]
- Sulaiman, S.K.; Enakshee, J.; Traxer, O.; Somani, B.K. Which Type of Water Is Recommended for Patients with Stone Disease (Hard or Soft Water, Tap or Bottled Water): Evidence from a Systematic Review over the Last 3 Decades. Curr. Urol. Rep. 2020, 21, 6. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Kidney Stone Prevention. Adv. Nutr. 2023, 14, 555–569. [Google Scholar] [CrossRef]
- Fink, H.A.; Akornor, J.W.; Garimella, P.S.; MacDonald, R.; Cutting, A.; Rutks, I.R.; Monga, M.; Wilt, T.J. Diet, Fluid, or Supplements for Secondary Prevention of Nephrolithiasis: A Systematic Review and Meta-Analysis of Randomized Trials. Eur. Urol. 2009, 56, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gutenbrunner, C. Kontrollierte Studie über die Wirkung einer Haustrinkkur mit einem Natrium-Hydrogencarbonat-Säuerling auf die Blutzuckerregulation bei gesunden Versuchspersonen. Phys. Med. Rehabil. Kurortmed. 1993, 03, 108–110. [Google Scholar] [CrossRef]
- Hamm, L.L. Renal handling of citrate. Kidney Int. 1990, 38, 728–735. [Google Scholar] [CrossRef]
- Kok, D.J.; Papapoulos, S.E.; Bijvoet, O.L.M. Excessive Crystal Agglomeration With Low Citrate Excretion in Recurrent Stone Formers. Lancet 1986, 327, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Liebman, M.; Costa, G. Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J. Urol. 2000, 163, 1565–1569. [Google Scholar] [CrossRef]
- Hagele, A.M.; Boring, J.L.; Moon, J.M.; Sunderland, K.L.; Mumford, P.W.; Kerksick, C.M. Naturally Bicarbonated Water Supplementation Does Not Improve Anaerobic Cycling Performance or Blood Gas Parameters in Active Men and Women. Nutrients 2023, 15, 5052. [Google Scholar] [CrossRef] [PubMed]
- Richard, R.; Jimenez, L.; Duvallet, A.; Rieu, M. Effet d’une boisson bicarbonatée sodée sur les adaptations physiologiques à l’effort. Sci. Sports 2000, 15, 18–25. [Google Scholar] [CrossRef]
- Fordtran, J.S.; Morawski, S.G.; Santa Ana, C.A.; Rector, F.C. Gas production after reaction of sodium bicarbonate and hydrochloric acid. Gastroenterology 1984, 87, 1014–1021. [Google Scholar] [CrossRef]
- Turnberg, L.A.; Fordtran, J.S.; Carter, N.W.; Rector, F.C. Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J. Clin. Investig. 1970, 49, 548–556. [Google Scholar] [CrossRef]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 2232–2242. [Google Scholar] [CrossRef]
- Cormick, G.; Betran, A.; Romero, I.; Cormick, M.; Belizán, J.; Bardach, A.; Ciapponi, A. Effect of Calcium Fortified Foods on Health Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kuang, X.; Li, K.; Guo, X.; Deng, Q.; Li, D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 10817–10827. [Google Scholar] [CrossRef]
- Hidayat, K.; Zhang, L.-L.; Rizzoli, R.; Guo, Y.-X.; Zhou, Y.; Shi, Y.-J.; Su, H.-W.; Liu, B.; Qin, L.-Q. The Effects of Dairy Product Supplementation on Bone Health Indices in Children Aged 3 to 18 Years: A Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, H.; Müller, H.; Resch, K.-L. Calcium Supplementation with Calcium-Rich Mineral Waters: A Systematic Review and Meta-analysis of its Bioavailability. Osteoporos. Int. 2000, 11, 938–943. [Google Scholar] [CrossRef]
- Greupner, T.; Schneider, I.; Hahn, A. Calcium Bioavailability from Mineral Waters with Different Mineralization in Comparison to Milk and a Supplement. J. Am. Coll. Nutr. 2017, 36, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Naumann, J.; Sadaghiani, C.; Alt, F.; Huber, R. Effects of Sulfate-Rich Mineral Water on Functional Constipation: A Double-Blind, Randomized, Placebo-Controlled Study. Complement. Med. Res. 2016, 23, 356–363. [Google Scholar] [CrossRef]
- Arnett, T.R.; Dempster, D.W. Effect of pH on Bone Resorption by Rat Osteoclasts in Vitro. Endocrinology 1986, 119, 119–124. [Google Scholar] [CrossRef]
- Frick, K.K.; Bushinsky, D.A. Chronic metabolic acidosis reversibly inhibits extracellular matrix gene expression in mouse osteoblasts. Am. J. Physiol. 1998, 275, F840–F847. [Google Scholar] [CrossRef]
- Brandao-Burch, A.; Utting, J.C.; Orriss, I.R.; Arnett, T.R. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif. Tissue Int. 2005, 77, 167–174. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Frick, K.K. The effects of acid on bone. Curr. Opin. Nephrol. Hypertens. 2000, 9, 369–379. [Google Scholar] [CrossRef]
- García-Gavilán, J.F.; Martínez, A.; Konieczna, J.; Mico-Perez, R.; García-Arellano, A.; Basora, J.; Barrubés, L.; Goday, A.; Canudas, S.; Salas-Salvadó, J.; et al. U-Shaped Association between Dietary Acid Load and Risk of Osteoporotic Fractures in 2 Populations at High Cardiovascular Risk. J. Nutr. 2021, 151, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Buhari, A.M.; Murphy, P.A.; Riley, T.J. Effects of fluoridated drinking water on bone mass and fractures: The study of osteoporotic fractures. J. Bone Miner. Res. 1995, 10, 1076–1086. [Google Scholar] [CrossRef]
- Helte, E.; Donat Vargas, C.; Kippler, M.; Wolk, A.; Michaëlsson, K.; Åkesson, A. Fluoride in Drinking Water, Diet, and Urine in Relation to Bone Mineral Density and Fracture Incidence in Postmenopausal Women. Environ. Health Perspect. 2021, 129, 047005. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.; Cooper, C.; Kellingray, S.; Russell, G.; Hughes, H.; Coggon, D. Fluoride in drinking water and risk of hip fracture in the UK: A case-control study. Lancet 2000, 355, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Näsman, P.; Ekstrand, J.; Granath, F.; Ekbom, A.; Fored, C.M. Estimated drinking water fluoride exposure and risk of hip fracture: A cohort study. J. Dent. Res. 2013, 92, 1029–1034. [Google Scholar] [CrossRef]
- Yin, X.-H.; Huang, G.-L.; Lin, D.-R.; Wan, C.-C.; Wang, Y.-D.; Song, J.-K.; Xu, P. Exposure to Fluoride in Drinking Water and Hip Fracture Risk: A Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0126488. [Google Scholar] [CrossRef]
| Brand | Country | Na | K | Ca | Mg | Cl | SO42− | P | HCO3− | PRAL |
|---|---|---|---|---|---|---|---|---|---|---|
| Rogaska Donat | Slovenia | 1500 | 13 | 380 | 1030 | 59 | 2400 | --- | 7700 | −57.3 |
| St-Yorre | France | 1708 | 110 | 90 | 11 | 322 | 174 | 0 | 4368 | −63.1 |
| Borjomi | Georgia | 1478 | 30 | 85 | 85 | 393 | 50 | 3965 | −53.6 | |
| Adelheidquelle, Adelholzener | Germany | 950 | 47.2 | 152 | 102 | 112 | 317 | 0 | 2999 | −37.2 |
| Vichy Celestins | France | 1172 | 66 | 103 | 10 | 235 | 138 | --- | 2989 | −43.0 |
| Heppinger Extra Medicinal Water | Germany | 481 | 27.1 | 150 | 199 | 118 | 60 | 0.06 | 2495 | −23.5 |
| Jamnica | Croatia | 921 | 32 | 115 | 34 | 252 | 109 | --- | 2247 | −32.7 |
| Ardesy (Arvie) | France | 650 | 130 | 170 | 92 | 387 | 31 | --- | 2195 | −23.3 |
| Radenska | Slovenia | 400 | 70 | 220 | 95 | 44 | 72 | --- | 2000 | −21.1 |
| Rozana | France | 493 | 52 | 301 | 160 | 649 | 230 | --- | 1837 | −8.6 |
| Kryniczanka | Poland | 43 | 5 | 436 | 68 | 19 | 8 | --- | 1818 | −8.7 |
| Gerolsteiner | Germany | 118 | 11 | 348 | 108 | 40 | 38 | --- | 1816 | −10.8 |
| Apollinaris | Germany | 470 | 30 | 90 | 120 | 130 | 100 | 0 | 1800 | −19.4 |
| Rhodius | Germany | 137 | 33 | 143 | 151 | 22 | 37 | --- | 1562 | −11.0 |
| Ferrarelle | Italy | 50 | 50 | 392 | 22 | 20 | 4 | --- | 1433 | −8.2 |
| Kalnicka | Croatia | 650 | 8 | 62 | 23 | 350 | 0 | 1410 | −19.0 | |
| Badoit | France | 165 | 10 | 190 | 85 | 44 | 38 | 0 | 1300 | −10.0 |
| Rhäzünser | Switzerland | 149 | 7 | 224 | 46 | 18 | 131 | --- | 1120 | −8.0 |
| Verniere | France | 110 | 40 | 180 | 173 | 14 | 140 | 1100 | −9.8 | |
| Quezac | France | 110 | 0 | 170 | 69 | 0 | 0 | --- | 1100 | −8.5 |
| Sangemini | Italy | 19.6 | 3.9 | 323 | 16.5 | 18.5 | 61 | 0 | 996 | −4.1 |
| Lete Acqua minerale | Italy | 4.9 | 2.1 | 313 | 15.1 | 8.2 | 6.6 | 0 | 981 | −4.4 |
| Author | Design Target Group | Intervention | Characteristics of Mineral Water/Treatment (Rich in) Bicarbonate/Day | Main Results | |
|---|---|---|---|---|---|
| Time Effects * (Bicarbonate Group) | Group Differences Time × Group Interaction | ||||
| Sub-chronic studies | |||||
| Schorr et al., 1996 [70] | Cross-over, randomized, double-blind 21 healthy older (60–72 years) subjects | NaCl reduction (<100 mmol/d) + 4 weeks (each) 3 different mineral water brands 1.5 L/d | Water A: HCO3, Na, Mg Water B: HCO3, Na, Cl Water C: low mineralized HCO3 Water A: 2975 mg/d Water B: 1318 mg/d Water C: 18 mg/d | 24-h urine | |
| NAE: (water A) ↔ (water B) ↑ | Week 4 NAE: n.s. group differences | ||||
| Marangella et al., 1996 [71] | Cross-over, randomized 21 subjects with idiopathic calcium nephrolithiasis | 1 month (each) 3 different mineral water brands Standardized diet 2 L/d | Water A: HCO3, Ca Water B: SO4 Water C: low mineralized HCO3 Water A: 3051 mg/d Water B: 610 mg/d Water C: 31 mg/d | 24-h urine | |
| pH: ↑ NAE: ↓ | pH: water A > water B NAE: water A < water B and C | ||||
| Caudarella et al., 1998 [72] | Cross-over 22 subjects with idiopathic calcium nephrolithiasis | 20 days (each) 3 different mineral water brands Standardized diet 2 L/d | Water A: HCO3, Ca Water B: SO4−rich Water C: low mineralized HCO3 Water A: 2794 mg/d Water B: 610 mg/d Water C: 100 mg/d | Fasting morning urine | |
| pH: ↔ | pH: n.s. group differences | ||||
| Keßler and Hesse, 1998, 2000 [73,81] | Cross-over, randomized 24 kidney-healthy younger (23–38 years) men | Run-In (standardized diet) + 2 days treatment (standardized diet) 4 weeks follow-up, same as cross-over week 2 (usual diet) Mineral water (”Staatl. Fachingen”) vs. supplement 2 L/d | Water: HCO3, Na, Mg Supplement: K citrate HCO3 Water 3430 mg/d K citrate: not reported | 24-h urine | |
| Standardized diet pH: ↑ Usual diet pH: ↑ | Not reported | ||||
| Coen et al., 2001 [74] | Parallel-group 21 healthy subjects | 2 weeks 2 different mineral water brands Standardized diet 2 L/d | Water A: HCO3, Ca Water B: low mineralized HCO3 Water A: 2780 mg/d Water B: 121 mg/d | Spot urine | |
| pH: ↔ | pH: n.s. time × group interaction | ||||
| Siener et al., 2004 [75] | Cross-over (first control, later mineral water) + single-arm 12 young healthy male subjects | 2 weeks baseline (usual diet/beverages) 5 days cross-over (standardized diet); Mineral water vs. fruit tea (control) 4 weeks follow-up with mineral water (usual diet/beverages) 1.4 L/d | Water: HCO3, Ca, Mg, Na HCO3 Water: 4743 mg/d | 24-h urine | |
| Standardized diet pH: ↑ NH4: ↓ Usual diet pH: ↑ NH4: ↓ | Standardized diet pH: water > control NH4: water < control ---- | ||||
| Roux et al., 2004 [82] | Cross-over, randomized 60 postmenopausal women | 4 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Ca Water B: Ca, Mg, SO4 HCO3 Water A: 2179 mg/d Water B: 292 mg/d | 2-h fasting urine | |
| pH: ↑ TA- HCO3: ↓ NH4: ↓ | pH: water A > B TA- HCO3: water A < water B NH4: water A < water B | ||||
| Schoppen et al., 2005 [76] | Cross-over 18 postmenopausal women | 8 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 2094 mg/d Water B: 71 mg/d | 24-h urine | |
| Not reported | pH: water A > water B | ||||
| Karagülle et al., 2007 [77] | Cross-over, double-blind 34 subjects with multiepisodic CaOx stone formation | 3 days (each) 2 different mineral water brands (water A: “Heppinger”, water B: “Bad Harzburger Urquell”) 1.5 L/d | Water A: HCO3, Mg, Na, Cl Water B: low mineralized HCO3 Water A: 4010 mg/d Water B: 149 mg/d | 24-h urine | |
| pH: ↑ | pH: water A > water B | ||||
| Wynn et al., 2009 [56] | Parallel-group, randomized 30 young (18–45 years) women | 4 weeks 2 different mineral water brands (water A: “Adelbodner”; water B: “Kryniczanka”) Standardized diet 1.5 L/d | Water A: HCO3, Ca, Mg Water B: Ca, SO4 HCO3 Water A: 3258 mg/d Water B: 437 mg/d | 24-h urine | |
| pH: ↑ HCO3: ↑ | pH: water A > water B HCO3: water A > water B | ||||
| Perez-Granados et al., 2010 [78] | Cross-over (first water B, later water A), single-blind 18 young (>18–<40 years) hypercholesterolemic subjects | 8 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 2120 mg/d Water B: 104 mg/d | 24-h urine | |
| Not reported | pH: water A > water B | ||||
| Brancaccio et al., 2012/12 [83,84] | Parallel-group 88 amateur athletes | 7 days 2 different mineral water brands (water A “Aqua Lete”, water B) Repeated Wingate Tests (cycling) 1.5 L/d + 750 mL 1 h before exercise + 250 mL after exercise | Water A: HCO3, Ca Water B: low mineralized HCO3 (1.5 L) Water A: 1472 mg/d Water B: 5 mg/d | Urine (mixture of several time points) | |
| Not reported During the day at the end of the intervention pH: ↑ | pH: no changes over time with water B ‡ | ||||
| Toxqui and Vaquero, 2016 [85] | Cross-over, randomized, single-blind 64 moderately hypercholesterolemic men and women | 8 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 2050 mg/d Water B: 75 mg/d | Fasting morning urine | |
| pH: ↑ | pH: sign. time × group interaction | ||||
| Wasserfurth et al., 2019 [13] | Parallel-group, randomized 129 healthy subjects | 4 weeks 4 different mineral water brands 1.5–2 L/d | Water A: HCO3, Ca, Mg Water B: HCO3, Ca, Mg, Na Water C: HCO3, Mg, Na Water D: Ca, Mg, SO4 HCO3 (1.5 L) Water A: 2724 mg/d Water B: 3677 mg/d Water C: 2769 mg/d Water D: 605 mg/d | 24-h urine | |
| pH: ↔ (water A) (p = 0.068) ↑ (water B and C) TA: ↓ (water A-C) HCO3: ↑ (water A and B) ↔ (water C) NH4: ↓ (water A-C) NAE: ↓ (water A-C) | Week 4 pH: sign. group differences TA: n.s. group differences HCO3: sign. group differences NH4: n.s. group differences (p = 0.052) NAE: sign. group differences | ||||
| Spontaneous urine | |||||
| pH: ↑ (water A and C) | pH: sign. group differences | ||||
| Chycki et al., 2021 [48] | Cross-over (first table water, then HCO3 water), single-blind 8 elite judo athletes | 3 weeks (each) Mineral water (water A) vs. table water (water B) Standardized meals Tests under hydrated conditions, treadmill to induce hypohydration, anaerobic Wingate tests under dehydrated conditions, later rehydration Amount individualized, approx. 3.2–3.4 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 (3.3 L) Water A: 13,207 mg/d Water B: 12 mg/d | 24-h urine | |
| Not reported | Post-supplementation time point Hydrated + dehydrated condition pH: n.s. group differences (trend: water A > water B) | ||||
| Lu et al., 2022 [86] | Parallel-group, randomized 58 subjects with Ca stones | 12 weeks Mineral water (water A: “Ardesy”) vs. tap water (water B) 1.25 L/d | Water A: HCO3, Ca, Mg, Na, Cl Water B: low mineralized HCO3 Water A: 2744 mg/d Water B: not reported | 24-h urine | |
| (no p-values reported) pH: ↑ (trend) † | Week 12 § pH: n.s. group differences (p = 0.071) | ||||
| Chiron et al., 2024 [59] | Parallel group (diet) Cross-over (water, within a diet group), randomized, double-blind 24 recreationally active men | 7 days 2 different mineral water brands (water A: “St-Yorre”, water B) Dietary restrictions (alkalizing diet vs. acidifying diet) 1-min supra-maximal rowing Wingate Test 1.5–2 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 (2 L) Water A: 8736 mg/d Water B: 612 mg/d | 24-h urine | |
| Not reported | Post-supplementation time point Water effects (whole group) # pH: water A > water B Water effects (alkalizing diet) pH: water A > water B Water effects (acidifying diet) pH: water A > water B | ||||
| Mansouri et al., 2023, 2024 [58,87] | Parallel-group, randomized 94 healthy subjects | 4 weeks 2 different mineral water brands 1.5–2 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 (1.5 L) Water A: 6552 mg/d Water B: 342 mg/d | 24-h urine | |
| pH: ↑ TA: ↓ NH4: ↓ HCO3: ↑ NAE: ↓ | pH: sign. time × water interaction (water A ↑, water B ↔) TA: sign. time × water interaction (water A < water B) NH4: sign. time × water interaction (water A < water B) HCO3: sign. time × water interaction (water A ↑; water B ↓) NAE: sign. time × water interaction (water A < water B) | ||||
| Chiron et al., 2024 [79] | Parallel-group, randomized, double-blind 22 highly trained athletes | 6 days 2 different mineral water brands (water A: “St-Yorre”, water B) Dietary restrictions (alkalizing diet) Last 3 days: 400 m race + handgrip strength + squat jumps (each day) 4 × 500 mL /d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 8736 mg/d Water B: 612 mg/d | 24-h urine | |
| pH: ↑ | pH: water A > water B | ||||
| Acute studies | |||||
| Schoppen et al., 2008 [80] | Cross-over, randomized 18 postmenopausal women | --- 3 different mineral water brands 500 mL | Water A: HCO3, Na (higher than water B), Cl Water B: HCO3, Na, Cl Water C: low mineralized HCO3 Water A: 1047 mg Water B: 1007 mg Water C: 36 mg | Postprandial urine | |
| Not reported | pH: n.s. group differences | ||||
| Author | Design Target Group | Intervention | Characteristics of Mineral Water/ Treatment Bicarbonate/Day | Main Results | |
|---|---|---|---|---|---|
| Time Effects * (Bicarbonate Group) | Group Differences Time × Water Interaction | ||||
| Sub-chronic studies | |||||
| Luft et al., 1990 [95] | Cross-over, randomized, single-blind 10 subjects (hypertensive + normotensive) | 4 days Run-In + 7 days (each) Mineral water (“Staatl. Fachingen”) vs. control solution (NaCl) Standardized diet (low sodium, low calcium) 3 L/d | Water: HCO3, Na, Mg Control solution: Na, Cl, Mg HCO3 Water A: 6046 mg/d Water B: 0 mg/d | Urine (not specified) | |
| Not reported | Ca: sign. group differences water < NaCl normotensives < hypertensives blacks < whites | ||||
| Schorr et al., 1996 [70] | Cross-over, randomized, double-blind 21 healthy older (60–72 years) subjects | NaCl reduction (<100 mmol/d) + 4 weeks (each) 3 different mineral water brands 1.5 L/d | Water A: HCO3, Na, Mg Water B: HCO3, Na, Cl Water C: low mineralized HCO3 Water A: 2975 mg/d Water B: 1318 mg/d Water C: 18 mg/d | 24-h urine | |
| NAE: (water A) ↔ (water B) ↑ Ca: (water A) ↓ (water B) ↔ | Week 4 NAE: (water A): n.s. group differences (water B): n.s. group differences Ca: (water A): n.s. group differences (water B): n.s. group differences | ||||
| Marangella et al., 1996 [71] | Cross-over, randomized 21 subjects with idiopathic calcium nephrolithiasis | 1 month (each) 3 different mineral water brands Standardized diet 2 L/d | Water A: HCO3, Ca Water B: SO4 Water C: low mineralized HCO3 Water A: 3051 mg/d Water B: 610 mg/d Water C: 31 mg/d | 24-h urine | |
| pH: ↑ Ca: ↑ Oxalate: ↓ Citrate: ↑ Mg: ↑ RS CaOx: ↓ | pH: water A > water B Ca: water A > water B Oxalate: water A < water B and C Citrate: water A > water B and C Mg: water A > water B and C RS CaOx: n.s. group differences | ||||
| Caudarella et al., 1998 [72] | Cross-over 22 subjects with idiopathic calcium nephrolithiasis | 20 days (each) 3 different mineral water brands Standardized diet 2 L/d | Water A: HCO3, Ca Water B: SO4-rich Water C: low mineralized HCO3 Water A: 2794 mg/d Water B: 610 mg/d Water C: 100 mg/d | Fasting morning urine | |
| pH: ↔ RS CaOx: ↔ | pH: n.s. group differences RS CaOx: n.s. group differences | ||||
| 24-h urine | |||||
| Ca: ↔ (tendency ↑) † Oxalate: ↓ Mg: ↑ | Ca: n.s. group differences Oxalate: n.s. group differences Mg: water A > water B | ||||
| Urine (not specified) | |||||
| Citrate: ↑ | Citrate: n.s. group differences | ||||
| Keßler and Hesse, 1998, 2000 [73,81] | Cross-over, randomized 24 kidney-healthy younger (23–38 years) men | Run-In (standardized diet) + 2 days treatment (standardized diet) 4 weeks follow-up, same as cross-over week 2 (usual diet) Mineral water (”Staatl. Fachingen”) vs. supplement 2 L/d | Water: HCO3, Na, Mg Supplement: K Citrate HCO3 Water 3430 mg/d K Citrate: not reported | 24-h urine | |
Standardized diet pH: ↑ Ca: ↔ (tendency ↓) † Oxalate: ↓ Citrate: ↑ Mg: ↔ RS CaOx: ↓ RS UA: ↓ Usual diet pH: ↑ Ca: ↔ (tendency ↓) † Oxalate: ↔ (tendency ↓) † Citrate: ↑ Mg: ↔ (tendency ↑) † RS CaOx: ↓ RS UA: ↓ | Day 5 Standardized diet n.s. group differences Week 4 Usual diet n.s. group differences | ||||
| Coen et al., 2001 [74] | Parallel-group 21 healthy subjects | 2 weeks 2 different mineral water brands Standardized diet 2 L/d | Water A: HCO3, Ca Water B: low mineralized HCO3 Water A: 2780 mg/d Water B: 121 mg/d | Spot urine | |
| pH: ↔ Ca: ↔ RS CaOx (Tiselius Index): ↔ | pH: n.s. time × group interaction Ca: sign. time × group interaction (water A > water B) RS CaOx (Tiselius Index): n.s. time × group interaction | ||||
| 24-h urine | |||||
| Oxalate: ↔ (tendency: ↑ p = 0.068) † Citrate: ↑ Mg: ↔ (tendency: ↑ p = 0.058) † | Oxalate: n.s. time × group interaction Citrate: n.s. time × group interaction Mg: n.s. time × group interaction | ||||
| Siener et al., 2004 [75] | Cross-over (first control, later mineral water) + single-arm 12 young healthy male subjects | 2 weeks baseline (usual diet/beverages) 5 days cross-over (standardized diet); Mineral water vs. fruit tea (control) 4 weeks follow-up with mineral water (usual diet/beverages) 1.4 L/d | Water: HCO3, Ca, Mg, Na HCO3 Water: 4743 mg/d | 24-h urine | |
| Standardized diet pH: ↑ Ca: ↑ Oxalate: ↔ Citrate: ↑ Mg: ↑ RS CaOx: ↔ Usual diet pH: ↑ Ca: ↔ Oxalate: ↔ Citrate: ↑ Mg: ↑ RS CaOx: ↓ | Standardized diet pH: water A > control Ca: water A > control Oxalate: n.s. group differences Citrate: water A > control Mg: water A > control RS CaOx: n.s. group differences ---- | ||||
| Schoppen et al., 2005 [76] | Cross-over 18 postmenopausal women | 8 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 2094 mg/d Water B: 71 mg/d | 24-h urine | |
| Not reported | pH: water A > water B Ca: water A < water B | ||||
| Karagülle et al., 2007 [77] | Cross-over, double-blind 34 subjects with multiepisodic CaOx stone formation | 3 days (each) 2 different mineral water brands (water A: “Heppinger”, water B: “Bad Harzburger Urquell”) 1.5 L/d | Water A: HCO3, Mg, Na, Cl Water B: low mineralized HCO3 Water A: 4010 mg/d Water B: 149 mg/d | 24-h urine | |
| pH: ↑ Ca: ↑ Oxalate: ↑ Citrate: ↑ Mg: ↑ RS CaOx: ↓ | pH: water A > water B Ca: n.s. group differences Oxalate: n.s. group differences Citrate: water A > water B Mg: water A > water B RS CaOx: n.s. group differences | ||||
| Wynn et al., 2009 [56] | Parallel-group, randomized 30 young (18–45 years) women | 4 weeks 2 different mineral water brands (water A: “Adelbodner”, water B: “Kryniczanka”) Standardized diet 1.5 L/d | Water A: HCO3, Ca, Mg Water B: Ca, SO4 HCO3 Water A: 3258 mg/d Water B: 437 mg/d | 24-h urine | |
| pH: ↑ Ca: ↑ | pH: water A > water B Ca: water A < water B | ||||
| Toxqui and Vaquero, 2016 [85] | Cross-over, randomized, single-blind 64 moderately hypercholesteremic men and women | 8 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 2050 mg/d Water B: 75 mg/d | Fasting morning urine | |
| pH: ↑ Ca/creatinine: ↓ | pH: sign. time × group interaction Ca/creatinine: sign. time × group interaction | ||||
| Wasserfurth et al., 2019 [13] | Parallel-group, randomized 129 healthy subjects | 4 weeks 4 different mineral water brands 1.5–2 L/d | Water A: HCO3, Ca, Mg Water B: HCO3, Ca, Mg, Na Water C: HCO3, Mg, Na Water D: Ca, Mg, SO4 HCO3 (1.5 L) Water A: 2724 mg/d Water B: 3677 mg/d Water C: 2769 mg/d Water D: 605 mg/d | 24-h urine | |
| pH: ↔ (water A) (p = 0.068) ↑ (water B and C) Ca: ↑ (water A, B and D) ↔ (water C) | pH: sign. group differences Ca: sign group differences | ||||
| Lu et al., 2022 [86] | Parallel-group, randomized 58 subjects with Ca stones | 12 weeks Mineral water (water A: “Ardesy”) vs. tap water (water B) 1.25 L/d | Water A: HCO3, Ca, Mg, Na, Cl Water B: low mineralized HCO3 Water A: 2744 mg/d Water B: not reported | 24-h urine | |
| pH: ↑ (trend) † Ca: ↑ Oxalate: ↓ Citrate: ↑ (trend) † Mg: ↑ (trend) † RS CaOx (Tiselius Index): ↑ (No p-values reported) | Week 12 ‡ pH: n.s. group differences (p = 0.071) Ca: n.s. group differences Oxalate: n.s. group differences Citrate: n.s. group differences (p = 0.084) † Mg: water A > water B RS CaOx (Tiselius Index): n.s. group differences (p = 0.060) | ||||
| Mansouri et al., 2023, 2024 [58,87] | Parallel-group, randomized 94 healthy subjects | 4 weeks 2 different mineral water brands 1.5–2 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 (1.5 L) Water A: 6552 mg/d Water B: 342 mg/d | 24-h urine | |
| pH: ↑ Ca: ↓ | pH: sign. time × water interaction (water A ↑, water B ↔) Ca: n.s. time × water interaction (p = 0.060) | ||||
| Acute studies | |||||
| Schoppen et al., 2008 [80] | Cross-over, randomized 18 postmenopausal women | --- 3 different mineral water brands 500 mL | Water A: HCO3, Na (higher than water B), Cl Water B: HCO3, Na, Cl Water C: low mineralized HCO3 Water A: 1047 mg Water B: 1007 mg Water C: 36 mg | Postprandial urine | |
| Not reported | pH: n.s. group differences Ca: n.s. group differences | ||||
| Author | Design Target Group | Intervention | Characteristics of Mineral Water/Treatment Bicarbonate/day | Main Results | |
|---|---|---|---|---|---|
| Time Effects * (Bicarbonate Group) | Group Differences Time × Water Interaction ** | ||||
| Sub-chronic studies | |||||
| Wasserfurth et al., 2019 [13] | Parallel-group, randomized 129 healthy subjects | 4 weeks 4 different mineral water brands 1.5–2 L/d | Water A: HCO3, Ca, Mg Water B: HCO3, Ca, Mg, Na Water C: HCO3, Mg, Na Water D: Ca, Mg, SO4 HCO3 (1.5 L) Water A: 2724 mg/d Water B: 3677 mg/d Water C: 2769 mg/d Water D: 605 mg/d | 12-h fasting blood (venous) | |
| pH: ↔ (water B and C) ↓ (water A) HCO3: ↑ (water C) ↔ (water A and B) (water B p = 0.057) BE: ↑ (water C) ↔ (water A and B) | pH: sign. group differences HCO3: n.s. group differences BE: n.s. group differences | ||||
| Chycki et al., 2021 [48] | Cross-over (first table water, later HCO3 water), single-blind 8 elite judo athletes | 21 days (each) Mineral water (water A) vs. table water (water B) Standardized diet Anaerobic Wingate tests (high intensity) under hydrated + dehydrated conditions, treadmill to induce hypohydration, later rehydration Amount individualized, approx. 3.2–3.4 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 (3.3 L) Water A: 13,207 mg/d Water B: 12 mg/d | Blood (capillary: fingertip) | |
| Not reported During post-supplementation time point (no p-values reported) pH (resting): ↓ HCO3 (resting): ↓ | Post-supplementation time point Hydrated and dehydrated condition pH (resting): n.s. group differences HCO3 (resting): water A > water B | ||||
| Hagele et al., 2023 [107] | Parallel-group, randomized, double-blind 39 recreationally active men and women | 7 days Mineral water (water A: “Borjomi”) vs. spring water (water B) Same diet before each visit Anaerobic cycling 10 mL/kg, 40–60 min prior to exercise tests | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: approx. 3000 mg/d Water B: not reported | 8-h fasting blood (venous) | |
| Not reported During post-supplementation time point pH: ↓ HCO3: ↓ BE: ↓ | pH: n.s. time × group interaction (water A > water B at immediate post + 10 min post exercise) HCO3: n.s. time × group interaction BE: n.s. time × group interaction | ||||
| Chiron et al., 2024 [59] | Parallel-group (diet) Cross-over (water, within a diet group), randomized, double-blind 24 recreationally active men | 7 days 2 different mineral water brands (water A: “St-Yorre”, water B) Dietary restrictions (alkalizing diet vs. acidifying diet) 1-min supra-maximal rowing Wingate Test 1.5–2 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 (2 L) Water A: 8736 mg/d Water B: 612 mg/d | Blood (capillary: earlobe) | |
| Not reported During post-supplementation time point (no p-values reported) Water effect pH: ↓ HCO3: ↓ Water effect (alkalizing diet) Not reported Water effects (acidifying diet) Not reported | Water effects (whole group) † pH: water A > water B (immediately after + 3 min + 5 min post exercise) HCO3: n.s. group difference Water effects (alkalizing diet) pH: water A > water B (immediately after + 5 min post exercise) HCO3: water A > water B (warm-up + immediately post exercise) HCO3 (peak): water A > water B Water effects (acidifying diet) pH: n.s. group differences HCO3: n.s. group differences | ||||
| Mansouri et al., 2024 [58] | Parallel-group, randomized 94 healthy subjects | 4 weeks 2 different mineral water brands 1.5–2 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 (1.5 L) Water A: 6552 mg/d Water B: 342 mg/d | 12-h fasting blood (venous) | |
| pH: ↔ HCO3: ↑ BE: ↑ | pH: n.s. time × group interaction HCO3: sign. time × group interaction (water A ↑, water B ↔) BE: sign. time × group interaction (water A ↑, water B ↔) | ||||
| Chiron et al., 2024 [79] | Parallel-group, randomized, double-blind 22 highly trained athletes | 6 days 2 different mineral water brands (water A: “St-Yorre”, water B) Dietary restrictions (alkalizing diet) Last 3 days: 400 m run + handgrip strength + squat jumps (each day) 4 × 500 mL/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 8736 mg/d Water B: 612 mg/d | Blood (capillary: fingertip) | |
| Not reported During post-supplementation time point (no p-values reported) pH: ↓ HCO3: ↓ BE: ↓ | pH: water A > water B (pre 400 m run) HCO3: water A > water B (HCO3 max) BE: water A > water B (1 h post 400 m run) | ||||
| Acute Studies | |||||
| Richard et al., 2000 [108] | Cross-over, randomized, single-blind 12 regularly trained athletes | --- 3 different mineral water brands (water A: “St-Yorre”, water B, water C) Standardized meal Anaerobic cycling + isokinetic endurance test (after recovery) 3 L (1.5 l before exercise + 0.5 L during exercise + 1 L during recovery) | Water A: HCO3, Na Water B: Na water C: low mineralized HCO3 Water A: 13,104 mg/d Water B: not reported Water C: not reported | Blood (capillary) | |
| (no p-values reported) pH: ↑ (pre—post cycling) ↓ (until end of isokinetic test) HCO3: ↓ (pre- post cycling) ↑ (recovery) ↓ (slightly, until end of isokinetic test) | pH: water A > water C (immediately after exercise + after isokinetic test) HCO3: water A > water C | ||||
| Author | Design Target Group | Intervention | Characteristics of Mineral Water/Treatment Bicarbonate/Day | Main Results | |
|---|---|---|---|---|---|
| Time Effects * (Bicarbonate Group) | Group Differences Time × Water Interaction | ||||
| Sub-chronic studies | |||||
| Marangella et al., 1996 [71] | Cross-over, randomized 21 subjects with idiopathic calcium nephrolithiasis | 1 month (each) 3 different mineral water brands Standardized diet 2 L/d | Water A: HCO3, Ca Water B: SO4 Water C: low mineralized HCO3 Water A: 3051 mg/d Water B: 610 mg/d Water C: 31 mg/d | Fasting urine | |
| Not reported | Hydroxyproline: water A < water C Cross-linked N-telopeptide type I: water A < water B and C | ||||
| Blood (not specified) | |||||
| Not reported | PTH: water A and B < water C Osteocalcin: n.s. group differences | ||||
| Roux et al., 2004 [82] | Cross-over, randomized 60 postmenopausal women | 4 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Ca Water B: Ca, Mg, SO4 HCO3 Water A: 2179 mg/d Water B: 292 mg/d | 2-h fasting urine | |
| pH: ↑ CTX/Cr: ↓ Pyr/Cr: ↓ | pH: water A > water B CTX/Cr: n.s. group differences Pyr/Cr: n.s. group differences | ||||
| Fasting blood | |||||
| iCa (serum): ↑ total Ca (serum): ↔ P (serum): ↔ iPTH (plasma): ↓ Osteocalcin (serum): ↔ BALP (serum): ↔ | No sign. group differences | ||||
| Schoppen et al., 2005 [76] | Cross-over 18 postmenopausal women | 8 weeks (each) 2 different mineral water brands 1 L/d | Water A: HCO3, Na, Cl Water B: low mineralized HCO3 Water A: 2094 mg/d Water B: 71 mg/d | 24-h urine | |
| Not reported | pH: water A > water B Ca: water A < water B | ||||
| 12-h fasting blood | |||||
| Not reported | CTX (serum): n.s. group differences P1NP (serum): n.s. group differences | ||||
| Wynn et al., 2009 [56] | Parallel-group, randomized 30 young (18–45 years) women | 4 weeks 2 different mineral water brands (water A: “Adelbodner”, water B: “Kryniczanka”) Standardized diet 1.5 L/d | Water A: HCO3, Ca, Mg Water B: Ca, SO4 HCO3 Water A: 3258 mg/d Water B: 437 mg/d | 24-h urine | |
| pH: ↑ Ca: ↑ CTX: ↔ (slightly ↓) † | pH: water A > water B Ca: water A < water B CTX: n.s. group differences | ||||
| Blood (not specified) | |||||
| iCa: ↔ total Ca: ↔ P: ↔ CTX (serum): ↓ PTH (plasma): ↓ BALP: ↔ | iCa: n.s. group differences total Ca: n.s. group differences P: n.s. group differences CTX (serum): changes water A > water B PTH (plasma): changes water A > water B BALP: n.s. group differences | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansouri, K.; Hanh, T.; Hahn, A. Hydration Meets Regulation: Insights into Bicarbonate Mineral Water and Acid–Base Balance. Nutrients 2025, 17, 2291. https://doi.org/10.3390/nu17142291
Mansouri K, Hanh T, Hahn A. Hydration Meets Regulation: Insights into Bicarbonate Mineral Water and Acid–Base Balance. Nutrients. 2025; 17(14):2291. https://doi.org/10.3390/nu17142291
Chicago/Turabian StyleMansouri, Katharina, Thierry Hanh, and Andreas Hahn. 2025. "Hydration Meets Regulation: Insights into Bicarbonate Mineral Water and Acid–Base Balance" Nutrients 17, no. 14: 2291. https://doi.org/10.3390/nu17142291
APA StyleMansouri, K., Hanh, T., & Hahn, A. (2025). Hydration Meets Regulation: Insights into Bicarbonate Mineral Water and Acid–Base Balance. Nutrients, 17(14), 2291. https://doi.org/10.3390/nu17142291






