Efficacy of Probiotic VITA-PB2 from Fermented Foods on Alcohol Consumption and Hangover Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Inclusion/Exclusion Criteria
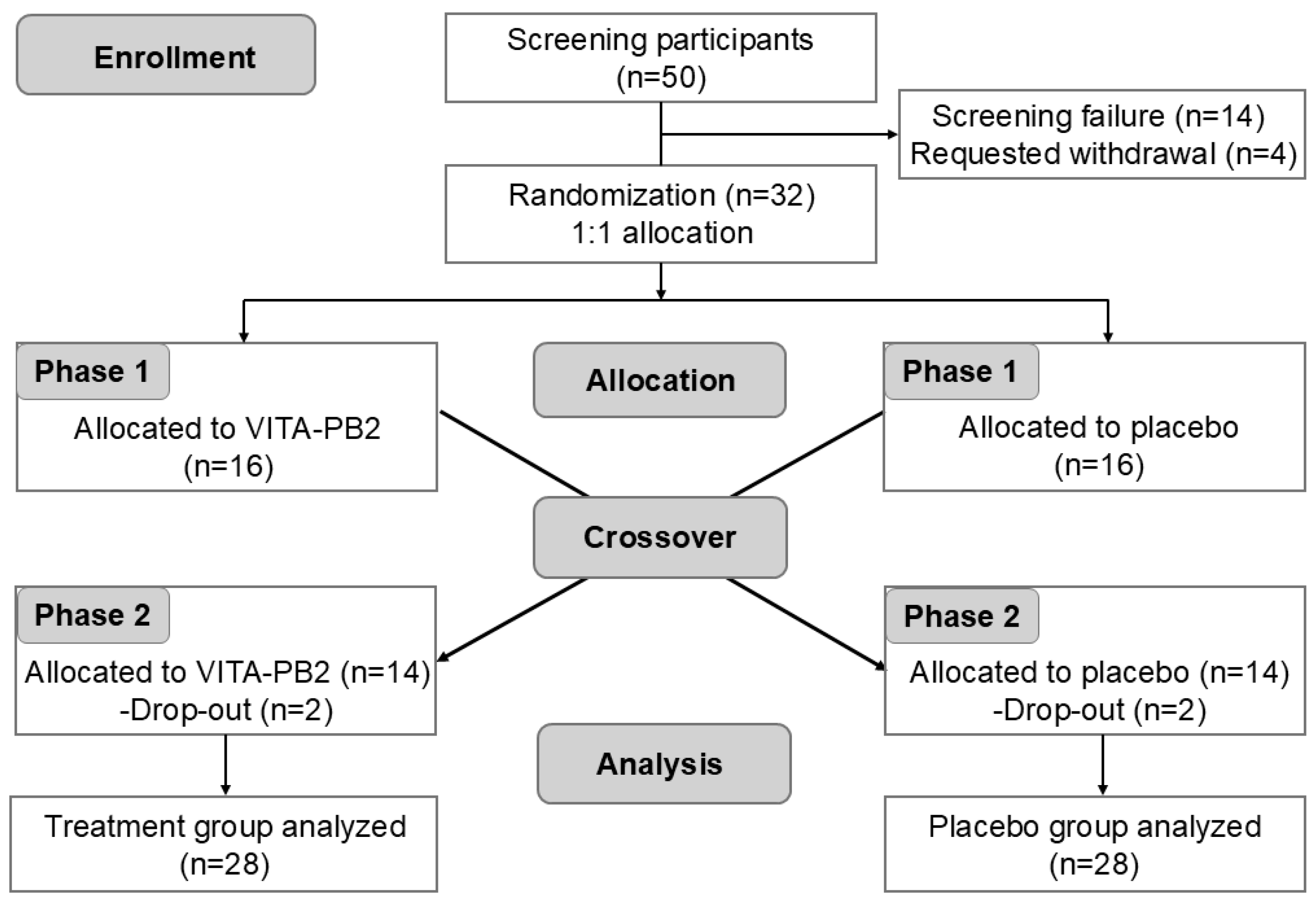
2.2. Study Design
2.3. Interventions
2.4. Outcome Measurements
2.4.1. Primary Outcomes
2.4.2. Secondary Outcomes
2.5. Liver Enzyme Measurements
2.6. Oxidative Stress and Antioxidant Defense Assessments
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Effect of VITA-PB2 on Blood Alcohol and Acetaldehyde Levels
3.3. Effect of VITA-PB2 on Blood ALDH Activity
3.4. Effect of VITA-PB2 on AHS Scores After Alcohol Consumption
3.5. Effect of VITA-PB2 on Liver Enzyme Activity
3.6. Effect of VITA-PB2 on ROS and NO Levels
3.7. Effect of VITA-PB2 on Antioxidant Enzyme Activities and Free Radical Scavenging Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | Alcohol dehydrogenase |
| ALDH | Acetaldehyde dehydrogenase |
| CFUs | Colony-forming units |
| BMI | Body mass index |
| CBC | Complete blood count |
| AHS | Acute Hangover Scale |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| GGT | Gamma-glutamyl transferase |
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| DCF-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| GPx | Glutathione peroxidase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| WBC | White blood cell count |
| RBC | Red blood cell count |
| Hb | Hemoglobin |
| PLT | Platelet count |
| H | Hour |
| NO2− | Nitrite |
References
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Alcohol consumption. In Our World Data; Global Change Data Lab: Oxford, UK, 2022; Available online: https://ourworldindata.org/alcohol-consumption (accessed on 3 July 2025).
- Schweitzer, S.O.; Intriligator, M.D.; Salehi, H. Alcoholism: An econometric model of its causes, its effects and its control. In Economics and Alcohol; Routledge: London, UK, 2023; pp. 107–127. [Google Scholar]
- Barbería-Latasa, M.; Gea, A.; Martínez-González, M.A. Alcohol, Drinking Pattern, and Chronic Disease. Nutrients 2022, 14, 1954. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W. Alcohol, its absorption, distribution, metabolism, and excretion in the body and pharmacokinetic calculations. Wiley Interdiscip. Rev. Forensic Sci. 2019, 1, e1340. [Google Scholar]
- Mueller, S. Ethanol Metabolism. In Alcohol and Alcohol-Related Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 929–951. [Google Scholar]
- Ahmed Laskar, A.; Younus, H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar]
- Barile, F.A. Alcohols and Aldehydes. In Barile’s Clinical Toxicology; CRC Press: Boca Raton, FL, USA, 2019; pp. 361–379. [Google Scholar]
- Palmer, E.; Tyacke, R.; Sastre, M.; Lingford-Hughes, A.; Nutt, D.; Ward, R.J. Alcohol hangover: Underlying biochemical, inflammatory and neurochemical mechanisms. Alcohol Alcohol. 2019, 54, 196–203. [Google Scholar]
- Royle, S.; Owen, L.; Roberts, D.; Marrow, L. Pain catastrophising predicts alcohol hangover severity and symptoms. J. Clin. Med. 2020, 9, 280. [Google Scholar] [PubMed]
- Turner, B.R.; Jenkinson, P.I.; Huttman, M.; Mullish, B.H. Inflammation, oxidative stress and gut microbiome perturbation: A narrative review of mechanisms and treatment of the alcohol hangover. Alcohol Clin. Exp. Res. 2024, 48, 1451–1465. [Google Scholar]
- Ballway, J.W.; Song, B.-J. Translational approaches with antioxidant phytochemicals against alcohol-mediated oxidative stress, gut dysbiosis, intestinal barrier dysfunction, and fatty liver disease. Antioxidants 2021, 10, 384. [Google Scholar] [CrossRef]
- Irwin, C.; Khalesi, S.; Cox, A.J.; Grant, G.; Davey, A.K.; Bulmer, A.C.; Desbrow, B. Effect of 8-weeks prebiotics/probiotics supplementation on alcohol metabolism and blood biomarkers of healthy adults: A pilot study. Eur. J. Nutr. 2018, 57, 1523–1534. [Google Scholar]
- Gwak, J.-H.; Choi, Y.J.; Ayub, H.; Seol, M.K.; Kim, H.; Jung, M.-Y. Comprehensive genomic and functional analysis of Leuconostoc lactic acid bacteria in alcohol and acetaldehyde metabolism. J. Microbiol. 2025, 63, e2410026. [Google Scholar]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar]
- Yu, H.; Jeong, H.; Yang, K.-Y.; Cho, J.-Y.; Hong, I.K.; Nam, S.-H. Synthesis of ellagic acid glucoside using glucansucrase from Leuconostoc and characterization of this glucoside as a functional neuroprotective agent. AMB Express 2021, 11, 108. [Google Scholar] [PubMed]
- Zikmanis, P.; Brants, K.; Kolesovs, S.; Semjonovs, P. Extracellular polysaccharides produced by bacteria of the Leuconostoc genus. World J. Microbiol. Biotechnol. 2020, 36, 161. [Google Scholar]
- Yun, M.; Jo, H.E.; Kim, N.; Park, H.K.; Jang, Y.S.; Choi, G.H.; Jo, H.E.; Seo, J.H.; Mok, J.Y.; Park, S.M. Oral Administration of Alcohol-Tolerant Lactic Acid Bacteria Alleviates Blood Alcohol Concentration and Ethanol-Induced Liver Damage in Rodents. J. Microbiol. Biotechnol. 2024, 34, 838. [Google Scholar]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Cantatore, V.; Cavoski, I.; Gobbetti, M. Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury. Nutrients 2019, 11, 248. [Google Scholar] [CrossRef]
- Khan, S.A.; Liu, L.; Lai, T.; Zhang, R.; Wei, Z.; Xiao, J.; Deng, Y.; Zhang, M. Phenolic profile, free amino acids composition and antioxidant potential of dried longan fermented by lactic acid bacteria. J. Food Sci. Technol. 2018, 55, 4782–4791. [Google Scholar]
- Lee, N.-K.; Lim, S.-M.; Cheon, M.-J.; Paik, H.-D. Physicochemical analysis of yogurt produced by Leuconostoc mesenteroides H40 and its effects on oxidative stress in neuronal cells. Food Sci. Anim. Resour. 2021, 41, 261. [Google Scholar]
- Giles-Gómez, M.; Sandoval García, J.G.; Matus, V.; Campos Quintana, I.; Bolívar, F.; Escalante, A. In vitro and in vivo probiotic assessment of Leuconostoc mesenteroides P45 isolated from pulque, a Mexican traditional alcoholic beverage. SpringerPlus 2016, 5, 708. [Google Scholar]
- Yang, X.; Teng, K.; Li, L.; Su, R.; Zhang, J.; Ai, G.; Zhong, J. Transcriptional Regulator AcrR Increases Ethanol Tolerance through Regulation of Fatty Acid Synthesis in Lactobacillus plantarum. Appl. Environ. Microbiol. 2019, 85, e01690-19. [Google Scholar] [CrossRef]
- Chen, S.; Jia, S.; Suo, K.; Kang, Q.; Hao, L.; Lu, L.; Liu, X.; Huang, J.; Lu, J. Positive effect of ethanol-induced Lactococcus lactis on alcohol metabolism in mice. Food Sci. Hum. Wellness 2022, 11, 1183–1190. [Google Scholar] [CrossRef]
- Wang, Y.; Kirpich, I.; Liu, Y.; Ma, Z.; Barve, S.; McClain, C.J.; Feng, W. Lactobacillus rhamnosus GG Treatment Potentiates Intestinal Hypoxia-Inducible Factor, Promotes Intestinal Integrity and Ameliorates Alcohol-Induced Liver Injury. Am. J. Pathol. 2011, 179, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Zhang, Y.J.; Zhou, Y.; Li, S.; Li, H.B. Natural Products for the Prevention and Treatment of Hangover and Alcohol Use Disorder. Molecules 2016, 21, 64. [Google Scholar] [CrossRef]
- Verster, J.C.; Vermeulen, S.A.; van de Loo, A.J.; Balikji, S.; Kraneveld, A.D.; Garssen, J.; Scholey, A. Dietary nutrient intake, alcohol metabolism, and hangover severity. J. Clin. Med. 2019, 8, 1316. [Google Scholar]
- Wang, F.; Zhang, Y.-J.; Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Zhang, J.-J.; Li, S.; Xu, D.-P.; Li, H.-B. Effects of Beverages on Alcohol Metabolism: Potential Health Benefits and Harmful Impacts. Int. J. Mol. Sci. 2016, 17, 354. [Google Scholar] [PubMed]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245. [Google Scholar] [PubMed]
- Van de Loo, A.; Mackus, M.; Korte-Bouws, G.; Brookhuis, K.; Garssen, J.; Verster, J. Urine ethanol concentration and alcohol hangover severity. Psychopharmacology 2017, 234, 73–77. [Google Scholar]
- Scholey, A.; Benson, S.; Kaufman, J.; Terpstra, C.; Ayre, E.; Verster, J.C.; Allen, C.; Devilly, G.J. Effects of alcohol hangover on cognitive performance: Findings from a field/internet mixed methodology study. J. Clin. Med. 2019, 8, 440. [Google Scholar]
- Penning, R.; van Nuland, M.; AL Fliervoet, L.; Olivier, B.; C Verster, J. The pathology of alcohol hangover. Curr. Drug Abus. Rev. 2010, 3, 68–75. [Google Scholar]
- Mackus, M.; Loo, A.J.v.d.; Garssen, J.; Kraneveld, A.D.; Scholey, A.; Verster, J.C. The role of alcohol metabolism in the pathology of alcohol hangover. J. Clin. Med. 2020, 9, 3421. [Google Scholar]
- Zimatkin, S.M.; Deitrich, R.A. Ethanol metabolism in the brain. Addict. Biol. 1997, 2, 387–400. [Google Scholar]
- Sládek, N.E. Human aldehyde dehydrogenases: Potential pathological, pharmacological, and toxicological impact. J. Biochem. Mol. Toxicol. 2003, 17, 7–23. [Google Scholar]
- Rodríguez-Zavala, J.S.; Calleja, L.F.; Moreno-Sánchez, R.; Yoval-Sánchez, B. Role of aldehyde dehydrogenases in physiopathological processes. Chem. Res. Toxicol. 2019, 32, 405–420. [Google Scholar]
- Deitrich, R.A.; Petersen, D.; Vasiliou, V. Removal of acetaldehyde from the body. In Acetaldehyde-Related Pathology: Bridging the Trans-Disciplinary Divide: Novartis Foundation Symposium 285; Chadwick, D.J., Goode, J., Eds.; John Wiley: Hoboken, NJ, USA, 2007; pp. 23–51. [Google Scholar]
- Chen, Y.-C.; Peng, G.-S.; Tsao, T.-P.; Wang, M.-F.; Lu, R.-B.; Yin, S.-J. Pharmacokinetic and pharmacodynamic basis for overcoming acetaldehyde-induced adverse reaction in Asian alcoholics, heterozygous for the variant ALDH2* 2 gene allele. Pharmacogenetics Genom. 2009, 19, 588–599. [Google Scholar]
- Eng, M.Y.; Luczak, S.E.; Wall, T.L. ALDH2, ADH1B, and ADH1C genotypes in Asians: A literature review. Alcohol Res. Health 2007, 30, 22. [Google Scholar] [PubMed]
- Inenaga, K.; Ono, K.; Hitomi, S.; Kuroki, A.; Ujihara, I. Thirst sensation and oral dryness following alcohol intake. Jpn. Dent. Sci. Rev. 2017, 53, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, S.; Huo, J.; Dong, K.; Ding, Y.; Man, C.; Zhang, Y.; Jiang, Y. Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways. Nutrients 2023, 15, 190. [Google Scholar]
- Sun, X.; Shi, J.; Kong, L.; Shen, Q.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Recent insights into the hepatoprotective effects of lactic acid bacteria in alcoholic liver disease. Trends Food Sci. Technol. 2022, 125, 91–99. [Google Scholar] [CrossRef]
- Mishra, G.; Singh, P.; Molla, M.; Yimer, Y.S.; Dinda, S.C.; Chandra, P.; Singh, B.K.; Dagnew, S.B.; Assefa, A.N.; Ewunetie, A. Harnessing the potential of probiotics in the treatment of alcoholic liver disorders. Front. Pharmacol. 2023, 14, 1212742. [Google Scholar] [CrossRef]
- Bailey, S.M. Review A Review of the Role of Reactive Oxygen and Nitrogen Species in Alcohol-induced Mitochondrial Dysfunction. Free Radic. Res. 2003, 37, 585–596. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Contreras-Zentella, M.L.; Villalobos-García, D.; Hernández-Muñoz, R. Ethanol Metabolism in the Liver, the Induction of Oxidant Stress, and the Antioxidant Defense System. Antioxidants 2022, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Kang, C.-H.; Kim, J.-S.; Park, H.M.; Kim, S.; Paek, N.-S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech 2021, 11, 217. [Google Scholar] [CrossRef]
- Mukherjee, A.; Breselge, S.; Dimidi, E.; Marco, M.L.; Cotter, P.D. Fermented foods and gastrointestinal health: Underlying mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering probiotic microorganisms: In vitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Variables | Total Participants (n = 28) | |
|---|---|---|
| Age (years) | 44.11 ± 6.91 | |
| Sex | Male | 14 |
| Female | 14 | |
| Hematological parameters | WBC (103/µL) | 5.83 ± 1.25 |
| RBC (106/µL) | 4.75 ± 0.31 | |
| Hb (g/dL) | 14.50 ± 1.21 | |
| PLT (103/µL) | 269.07 ± 68.26 | |
| Body composition metrics | Height (cm) | 167.46 ± 8.76 |
| Weight (kg) | 69.51 ± 13.78 | |
| BMI (kg/m2) | 24.61 ± 3.32 | |
| Body Fat (%) | 27.95 ± 8.09 | |
| Basal Metabolic Rate (kcal) | 1452.89 ± 244.21 | |
| Vital signs | Systolic Blood Pressure (mm/Hg) | 109.29 ± 10.16 |
| Diastolic Blood Pressure (mm/Hg) | 70.71 ± 7.16 | |
| Pulse (beats per min) | 74.43 ± 4.12 | |
| Temperature (°C) | 36.44 ± 0.15 | |
| Parameter | Time Post-Alcohol Consumption (h) | Placebo (n = 28) | VITA-PB2 (n = 28) | p-Value 1 |
|---|---|---|---|---|
| Blood alcohol level (mg/dL) | 0 | 0.12 ± 0.33 | 0.72 ± 1.79 | 1.00 |
| 0.5 | 54.20 ± 31.82 | 46.71 ± 18.81 | 0.82 | |
| 1 | 56.22 ± 23.49 | 49.18 ± 18.25 | 0.85 | |
| 2 | 53.72 ± 20.92 | 49.70 ± 17.11 | 0.99 | |
| 4 | 35.11 ± 16.62 | 30.95 ± 12.74 | 0.98 | |
| Blood acetaldehyde level (mg/dL) | 0 | 3.23 ± 1.19 | 3.24 ± 1.64 | 1.00 |
| 0.5 | 3.25 ± 1.19 | 3.47 ± 1.44 | 1.00 | |
| 1 | 5.08 ± 1.56 | 3.42 ± 0.38 | 0.04 * | |
| 2 | 4.63 ± 0.83 | 4.11 ± 0.53 | 0.97 | |
| 4 | 4.67 ± 1.50 | 3.42 ± 0.78 | 0.31 |
| Parameter | Time Post-Alcohol Consumption (h) | Placebo (n = 28) | VITA-PB2 (n = 28) | p-Value 1 |
|---|---|---|---|---|
| ALDH (mU/mL) | 0 | 3.14 ± 0.85 | 3.73 ± 0.57 | 0.94 |
| 0.5 | 4.27 ± 2.40 | 6.08 ± 2.48 | 0.03 * | |
| 1 | 5.30 ± 1.35 | 6.16 ± 0.88 | 0.87 | |
| 2 | 4.58 ± 0.92 | 4.97 ± 1.81 | 0.99 | |
| 4 | 4.12 ± 1.42 | 4.60 ± 1.13 | 0.95 |
| Indicators | Placebo (n = 28) | VITA-PB2 (n = 28) | p-Value 1 |
|---|---|---|---|
| Hangover | 1.43 ± 2.17 | 0.86 ± 1.10 | 0.99 |
| Thirst | 2.71 ± 2.30 | 0.50 ± 0.65 | 0.048 * |
| Fatigue | 3.07 ± 2.59 | 1.50 ± 1.40 | 0.64 |
| Headache | 0.50 ± 1.09 | 0.14 ± 0.36 | 0.97 |
| Dizziness | 0.36 ± 0.84 | 0.21 ± 0.58 | 1.00 |
| Loss of appetite | 0.29 ± 0.73 | 0.36 ± 0.84 | 1.00 |
| Gastrointestinal disturbances | 0.43 ± 1.34 | 0.14 ± 0.36 | 1.00 |
| Nausea | 0.43 ± 1.09 | 0.14 ± 0.36 | 1.00 |
| Heart palpitations | 0.57 ± 1.09 | 0.07 ± 0.27 | 0.77 |
| Mean AHS score | 1.09 ± 1.18 | 0.44 ± 0.37 | 0.31 |
| Liver Enzyme | Time Post-Alcohol Consumption (h) | Placebo (n = 28) | VITA-PB2 (n = 28) | p-Value 1 |
|---|---|---|---|---|
| AST (U/L) | 0 | 23.07 ± 5.73 | 20.86 ± 6.80 | 0.80 |
| 0.5 | 27.86 ± 6.36 | 27.67 ± 11.00 | 1.00 | |
| 4 | 27.36 ± 5.89 | 25.43 ± 6.12 | 0.86 | |
| ALT (U/L) | 0 | 17.40 ± 4.84 | 17.21 ± 8.75 | 1.00 |
| 0.5 | 16.80 ± 5.03 | 18.71 ± 8.02 | 0.87 | |
| 4 | 16.40 ± 4.45 | 16.79 ± 6.44 | 1.00 | |
| GGT (U/L) | 0 | 18.90 ± 6.37 | 17.67 ± 8.64 | 0.97 |
| 0.5 | 18.50 ± 5.08 | 19.50 ± 9.81 | 0.98 | |
| 4 | 18.40 ± 5.30 | 16.75 ± 5.75 | 0.93 |
| Parameter | Time Post-Alcohol Consumption (h) | Placebo (n = 28) | VITA-PB2 (n = 28) | p-Value 1 |
|---|---|---|---|---|
| ROS level (Fluorescence Intensity) | 0 | 718.05 ± 31.66 | 706.98 ± 97.46 | 1.00 |
| 0.5 | 763.71 ± 96.11 | 782.59 ± 72.54 | 0.98 | |
| 1 | 797.44 ± 91.46 | 704.17 ± 107.90 | 0.02 * | |
| 2 | 681.48 ± 20.43 | 659.18 ± 61.25 | 0.99 | |
| 4 | 758.66 ± 48.10 | 759.70 ± 30.56 | 1.00 | |
| NO2− (μM) | 0 | 0.23 ± 0.13 | 0.20 ± 0.11 | 1.00 |
| 0.5 | 0.28 ± 0.24 | 0.22 ± 0.14 | 0.95 | |
| 1 | 0.34 ± 0.17 | 0.22 ± 0.17 | 0.38 | |
| 2 | 0.27 ± 0.14 | 0.21 ± 0.15 | 0.97 | |
| 4 | 0.25 ± 0.21 | 0.19 ± 0.13 | 0.96 |
| Parameter | Time Post-Alcohol Consumption (h) | Placebo (n = 28) | VITA-PB2 (n = 28) | p-Value 1 |
|---|---|---|---|---|
| Catalase (mU/mL) | 0 | 32.07 ± 4.54 | 29.60 ± 3.91 | 0.94 |
| 0.5 | 26.70 ± 6.90 | 25.79 ± 7.56 | 1.00 | |
| 1 | 22.98 ± 6.10 | 28.59 ± 4.13 | 0.36 | |
| 2 | 24.14 ± 7.02 | 24.60 ± 8.00 | 1.00 | |
| 4 | 26.63 ± 5.32 | 25.93 ± 4.38 | 1.00 | |
| GPx (mU/mL) | 0 | 246.36 ± 33.31 | 281.02 ± 68.76 | 0.66 |
| 0.5 | 274.60 ± 39.82 | 269.04 ± 31.70 | 1.00 | |
| 1 | 259.12 ± 40.07 | 263.60 ± 46.24 | 1.00 | |
| 2 | 315.19 ± 39.81 | 431.58 ± 149.50 | 0.002 ** | |
| 4 | 348.15 ± 49.45 | 391.93 ± 132.11 | 0.70 | |
| DPPH (% inhibition) | 0 | 55.75 ± 9.13 | 53.84 ± 16.54 | 1.00 |
| 0.5 | 53.69 ± 10.65 | 53.55 ± 6.75 | 1.00 | |
| 1 | 52.45 ± 7.32 | 60.26 ± 15.83 | 0.45 | |
| 2 | 50.92 ± 10.87 | 59.26 ± 8.23 | 0.44 | |
| 4 | 56.51 ± 9.59 | 63.30 ± 7.64 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, C.; Bajgai, J.; Rahman, M.H.; Abdul-Nasir, S.; Ma, H.; Pham, T.T.; Zhang, H.; Cao, B.; Goh, S.H.; Kim, B.; et al. Efficacy of Probiotic VITA-PB2 from Fermented Foods on Alcohol Consumption and Hangover Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2025, 17, 2276. https://doi.org/10.3390/nu17142276
Mo C, Bajgai J, Rahman MH, Abdul-Nasir S, Ma H, Pham TT, Zhang H, Cao B, Goh SH, Kim B, et al. Efficacy of Probiotic VITA-PB2 from Fermented Foods on Alcohol Consumption and Hangover Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2025; 17(14):2276. https://doi.org/10.3390/nu17142276
Chicago/Turabian StyleMo, Chaodeng, Johny Bajgai, Md. Habibur Rahman, Sofian Abdul-Nasir, Hui Ma, Thu Thao Pham, Haiyang Zhang, Buchan Cao, Seong Hoon Goh, Bomi Kim, and et al. 2025. "Efficacy of Probiotic VITA-PB2 from Fermented Foods on Alcohol Consumption and Hangover Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 17, no. 14: 2276. https://doi.org/10.3390/nu17142276
APA StyleMo, C., Bajgai, J., Rahman, M. H., Abdul-Nasir, S., Ma, H., Pham, T. T., Zhang, H., Cao, B., Goh, S. H., Kim, B., Kim, H., Seol, M. K., Yu, Y. G., Kim, C.-S., Lee, K.-J., & Lim, S.-T. (2025). Efficacy of Probiotic VITA-PB2 from Fermented Foods on Alcohol Consumption and Hangover Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 17(14), 2276. https://doi.org/10.3390/nu17142276








