Identification, Antioxidant and Immunomodulatory Activities of a Neutral Exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Exopolysaccharides Production
2.2. Isolation and Purification of EPS
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. Ultraviolet Spectral Analysis and Molecular Weight Analysis
2.5. Thermogravimetric (TG), and FT-IR Spectroscopy
2.6. Analysis of Monosaccharide Composition
2.7. Methylation Analysis and Nuclear Magnetic Resonance (NMR) Analysis
2.8. Cellular Antioxidant Activity
2.8.1. Cell Culture and Cell Viability Assay
2.8.2. Intracellular Reactive Oxygen Species
2.8.3. Determination of Cellular Antioxidant Status
2.9. Immunoregulatory Activity
2.9.1. Cell Survival Analysis
2.9.2. Nitric Oxide (NO) and Cytokines Analysis
2.9.3. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
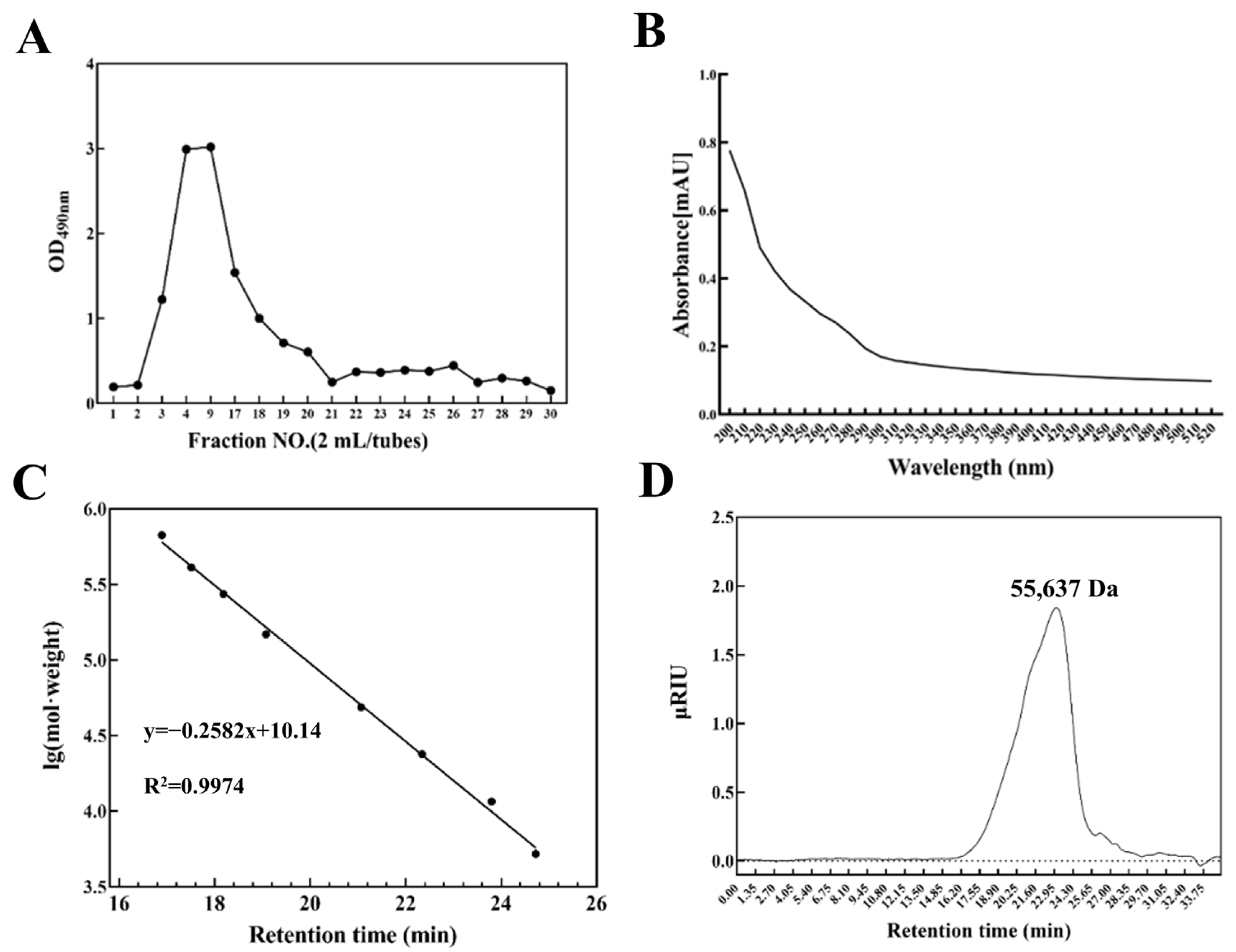
3.1. Production, Isolation, and Purification of Exopolysaccharide
3.2. Ultraviolet Spectral Analysis and Molecular Weight Analysis
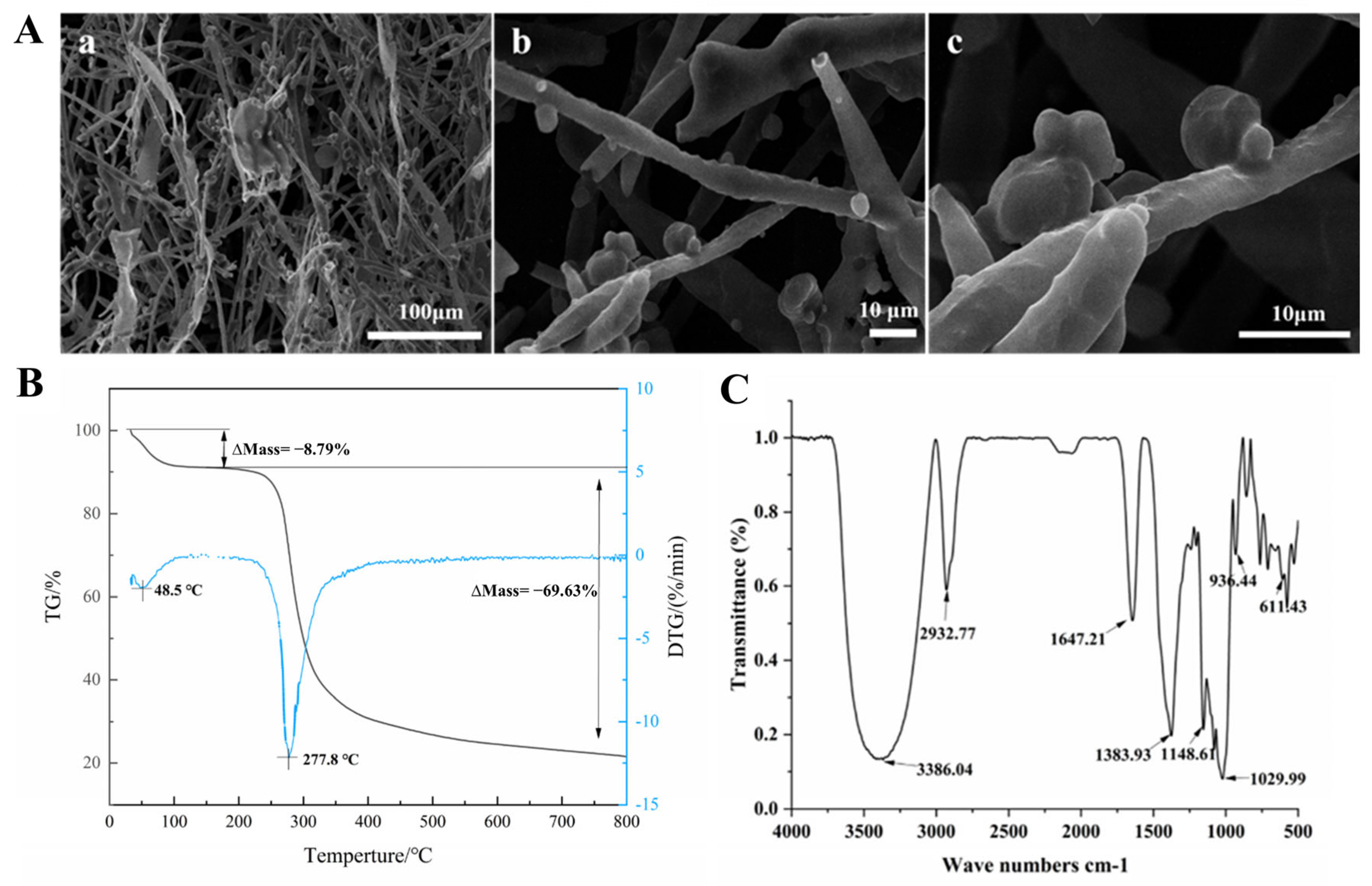
3.3. Scanning Electron Microscopy (SEM) Analysis
3.4. Thermogravimetric (TG) Analysis and FT-IR Spectroscopy
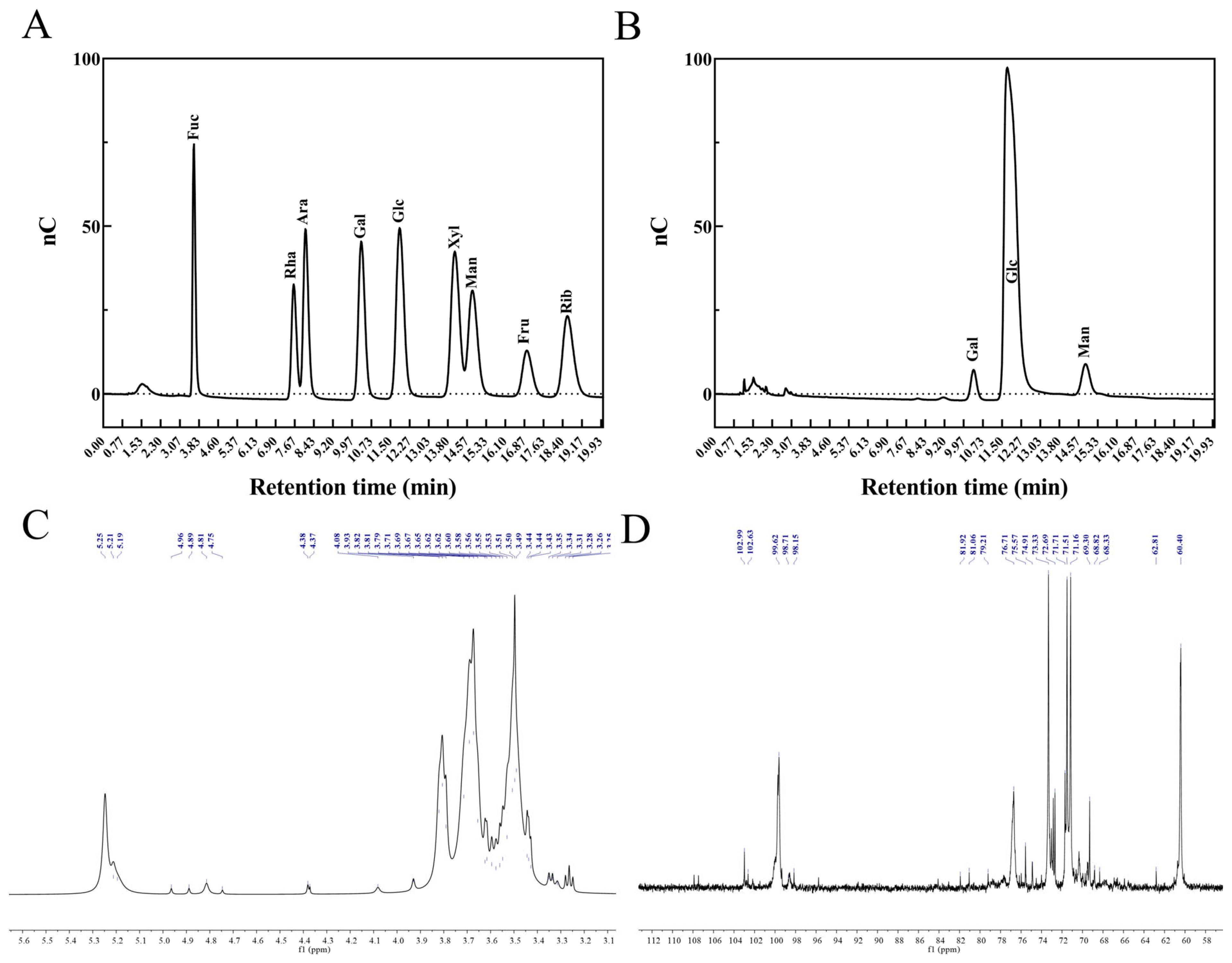
3.5. Monosaccharide Composition, Methylation, and Nuclear Magnetic Resonance Analysis of EPS-LP1
3.6. Antioxidant Activity of EPS-LP1
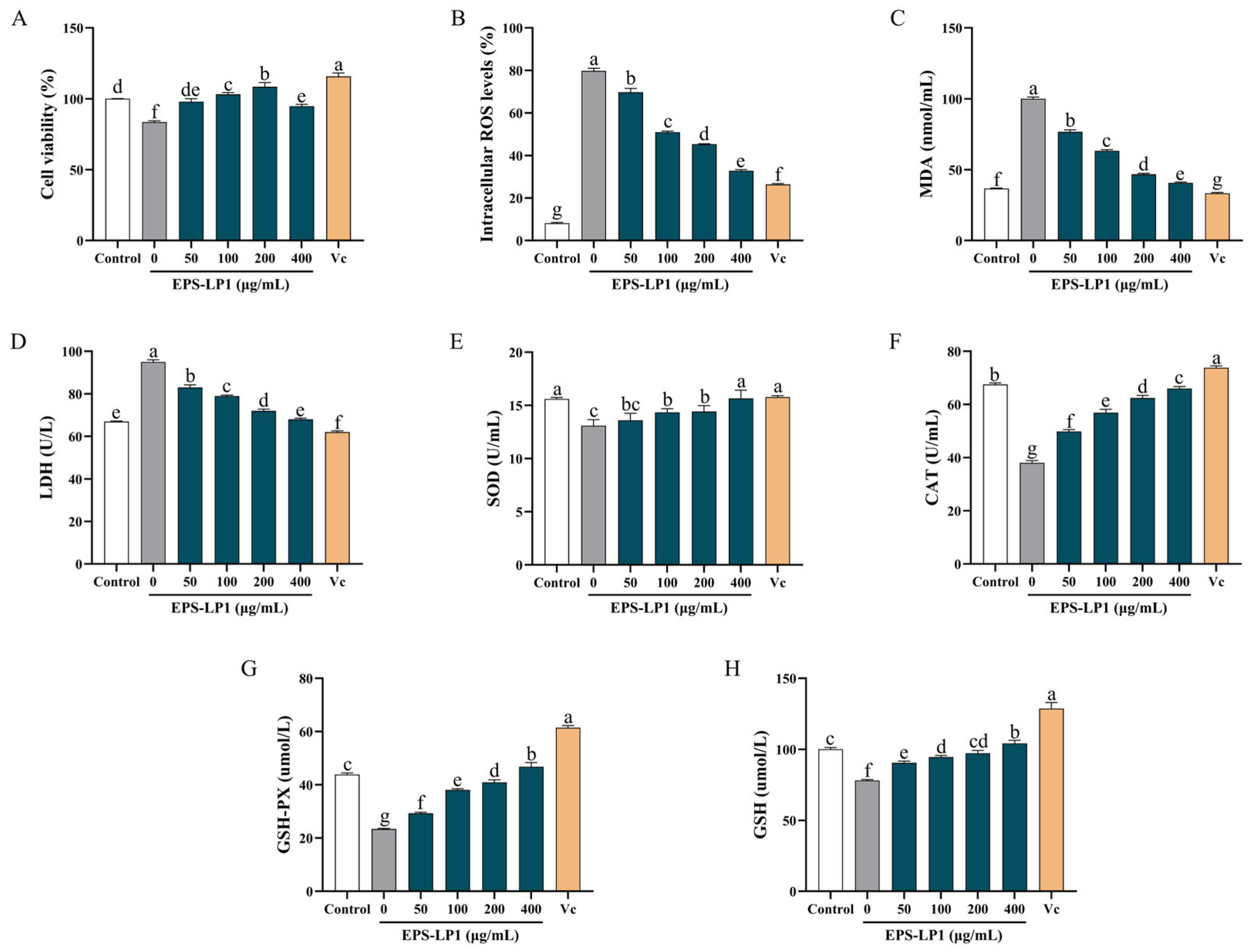
3.6.1. Effect of EPS-LP1 on ROS, MDA, and LDH Generation
3.6.2. Effects of EPS-LP1 on Enzymatic Antioxidant System in Cells
3.6.3. Effects of EPS-LP1 on Non-Enzymatic Antioxidant System in Cells
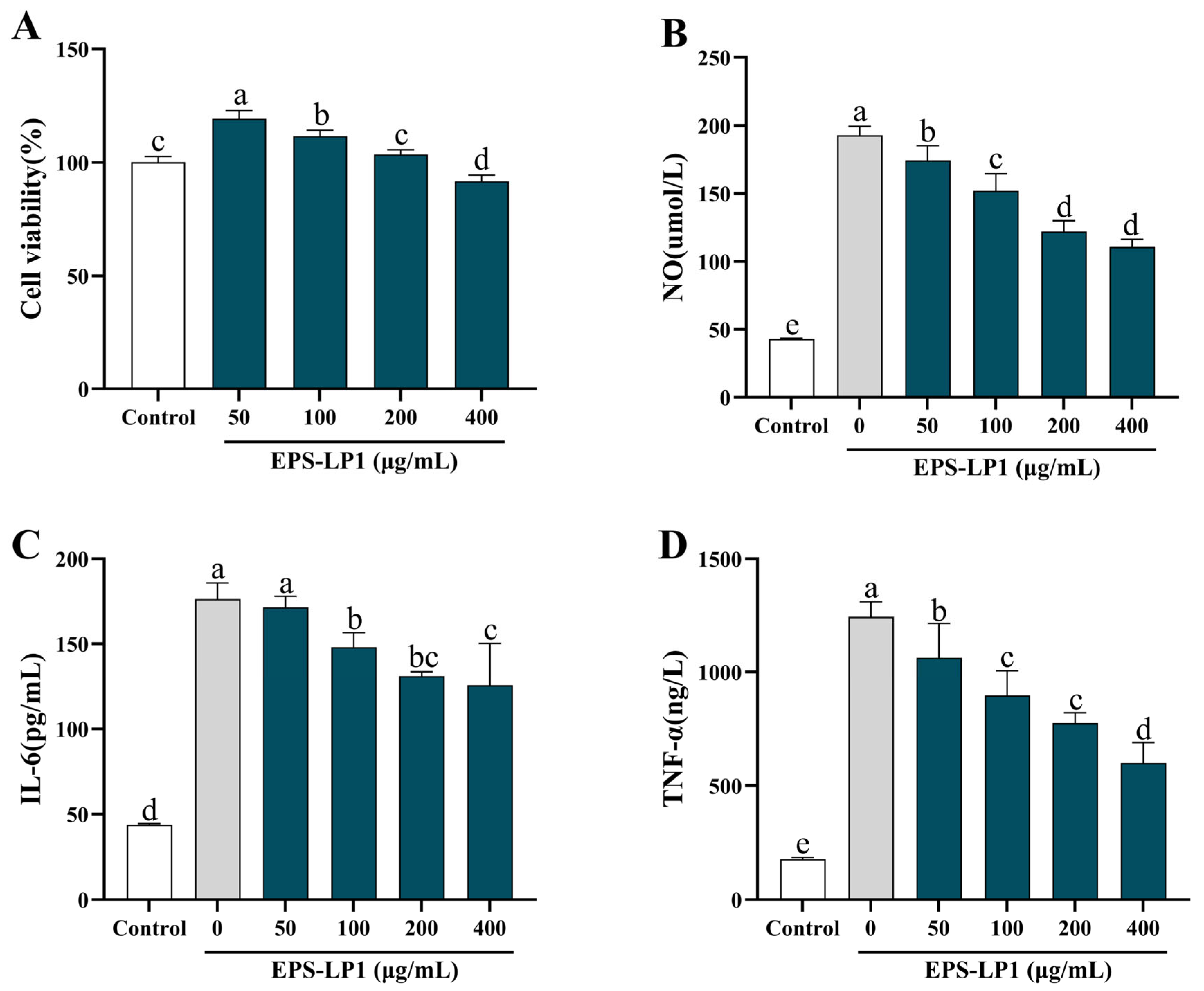
3.7. Immunomodulatory Activity of EPS-LP1
3.7.1. Effects of EPS-LP1 on NO Production and Cytokines
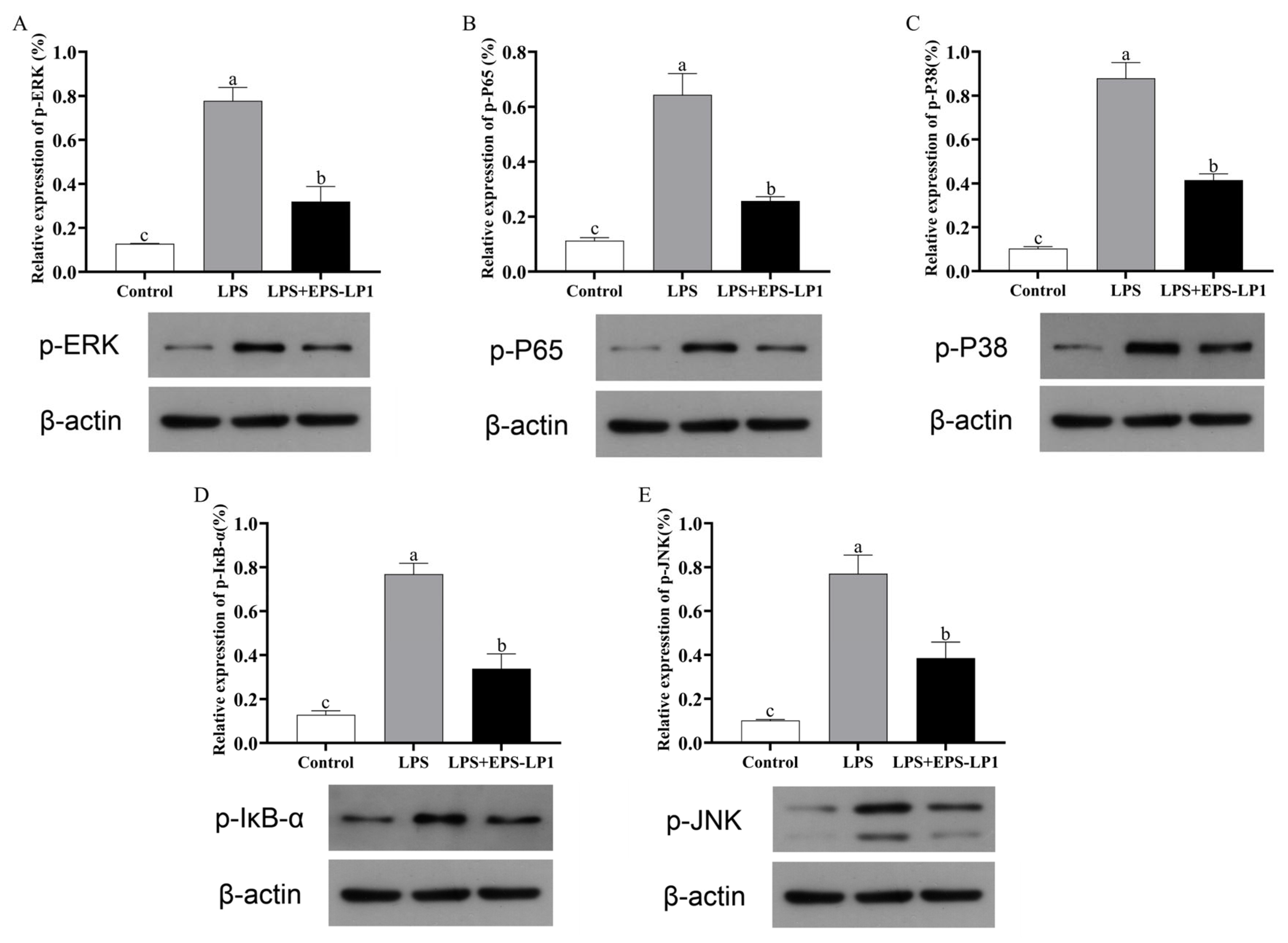
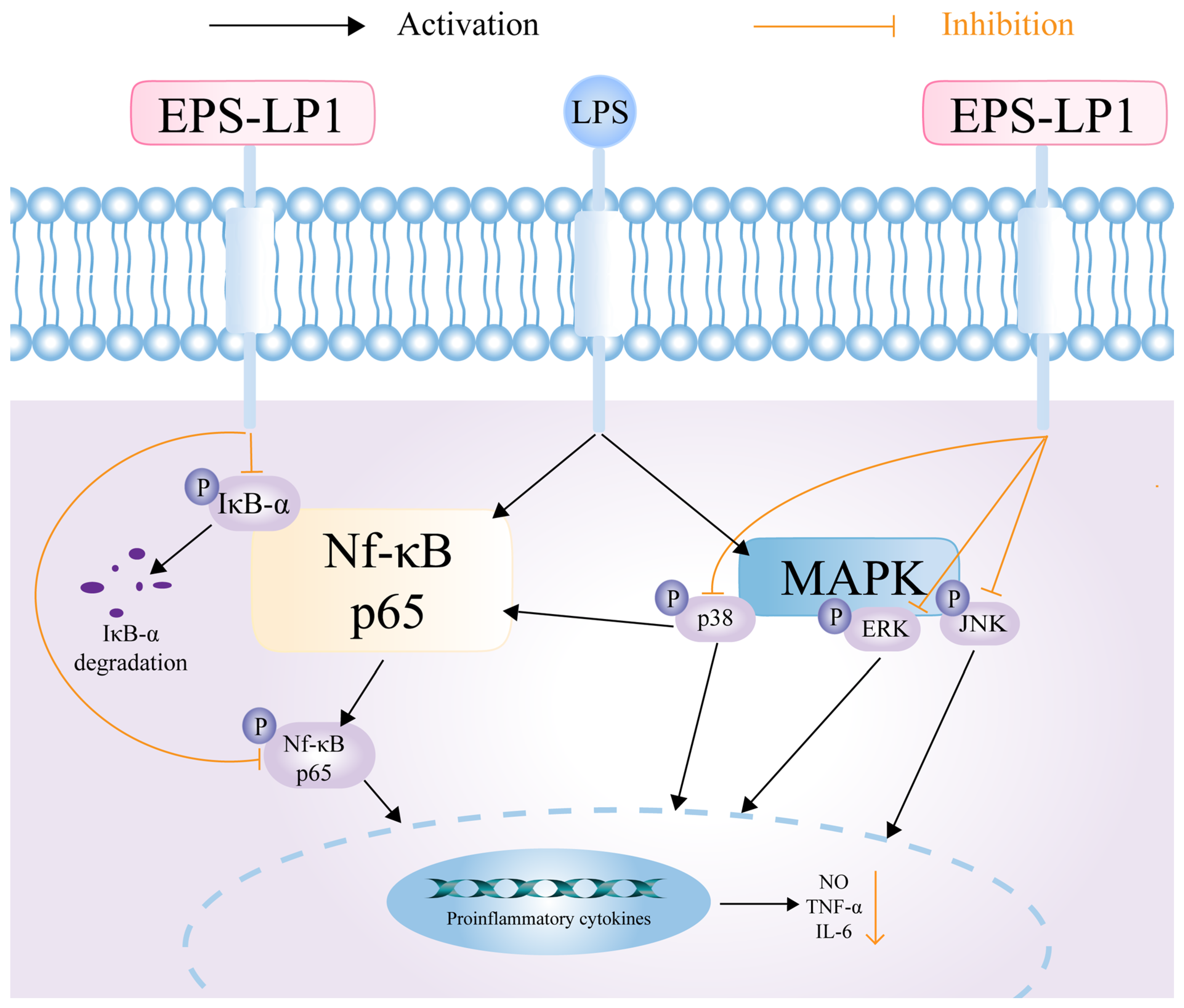
3.7.2. Effect of EPS-LP1 on MAPK Signaling Pathway
3.7.3. Effect of EPS-LP1 on NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, F.; Li, Q.; Qin, L.; Du, Z.; Li, Q.; Liu, G. Structural characterization, antioxidant activities and physicochemical properties analysis of a galactose-rich exopolysaccharide produced by Limosilactobacillus fermentum YL-11. LWT 2024, 211, 116893. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Silva, L.A.; Lopes Neto, J.H.P.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar] [CrossRef]
- Rosenfeldt, F.; Wilson, M.; Lee, G.; Kure, C.; Ou, R.; Braun, L.; de Haan, J. Oxidative stress in surgery in an ageing population: Pathophysiology and therapy. Exp. Gerontol. 2013, 48, 45–54. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Xia, W.; Han, J.; Zhu, S.; Wang, Y.; Zhang, W.; Wu, Z. Structural elucidation of the exopolysaccharide from Streptococcus thermophilus XJ53 and the effect of its molecular weight on immune activity. Int. J. Biol. Macromol. 2023, 230, 123177. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, Z.; Zhang, Z.; Shi, X. Characterization on structure and bioactivities of an exopolysaccharide from Lactobacillus curvatus SJTUF 62116. Int. J. Biol. Macromol. 2022, 210, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.T.; Liu, D.M.; Luo, T.H.; Chen, G.; Wu, H.; Li, L.; Yu, Y.G. Molecular characterization of Lactobacillus plantarum DMDL 9010, a strain with efficient nitrite degradation capacity. PLoS ONE 2014, 9, e113792. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Liu, D.M.; Liang, M.H.; Brennan, C.S.; Brennan, M. Detection of nitrite degradation by Lactobacillus plantarum DMDL9010 through the anaerobic respiration electron transport chain using proteomic analysis. Int. J. Food Sci. Technol. 2020, 56, 1608–1622. [Google Scholar] [CrossRef]
- Huang, Y.y.; Liang, M.h.; Sun, L.n.; Brennan, C.S.; Liu, D.m. Effect of microencapsulation on morphology, physicochemical properties and flavour profiles of solid yoghurt-flavoured bases. Int. J. Food Sci. Technol. 2020, 56, 2565–2578. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Liu, D.-M.; Jia, X.-Z.; Liang, M.-H.; Lu, Y.; Liu, J. Whole genome sequencing of Lactobacillus plantarum DMDL 9010 and its effect on growth phenotype under nitrite stress. LWT 2021, 149, 111778. [Google Scholar] [CrossRef]
- Kuang, J.-H.; Huang, Y.-Y.; Hu, J.-S.; Yu, J.-J.; Zhou, Q.-Y.; Liu, D.-M. Exopolysaccharides from Bacillus amyloliquefaciens DMBA-K4 ameliorate dextran sodium sulfate-induced colitis via gut microbiota modulation. J. Funct. Foods 2020, 75, 104212. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wu, J.M.; Wu, W.T.; Lin, J.W.; Liang, Y.T.; Hong, Z.Z.; Jia, X.Z.; Liu, D.M. Structural, antioxidant, and immunomodulatory activities of an acidic exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010. Front. Nutr. 2022, 9, 1073071. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K.; Ojediran, J.O.; Ogunsakin, A.O.; Banwo, K. Extracellular polysaccharide from Weissella confusa OF126: Production, optimization, and characterization. Int. J. Biol. Macromol. 2018, 111, 514–525. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; Senthil, S.L.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zhang, M.; Lai, T.; Yang, Z. Characterization and In Vitro Fecal Microbiota Regulatory Activity of a Low-Molecular-Weight Exopolysaccharide Produced by Lactiplantibacillus plantarum NMGL2. Foods 2022, 11, 393. [Google Scholar] [CrossRef]
- Abid, Y.; Casillo, A.; Gharsallah, H.; Joulak, I.; Lanzetta, R.; Corsaro, M.M.; Attia, H.; Azabou, S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int. J. Biol. Macromol. 2018, 108, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, J.; Ho, C.T.; Li, B.; Mu, J.; Zhang, Y.; Hu, H.; Mo, W.; Chen, Z.; Xie, Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohydr. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Yu, J.J.; Huang, J.; Liu, D.M.; Liang, M.H. Structural characterization of a novel Lactarius volemus Fr. polysaccharide and its immunity activity in BALB/c mice. RSC Adv. 2020, 10, 30254–30264. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, Q.; Zhang, H.; Wu, Y.; Hang, X.; Ai, L. Exopolysaccharide produced by Streptococcus thermophiles S-3: Molecular, partial structural and rheological properties. Carbohydr. Polym. 2018, 194, 132–138. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Xiang, X.W.; Du, M.; Zhang, L.F.; Cheng, N.X.; Liu, X.L.; Zheng, B.; Wen, Z.S. Protective effect of polysaccharides of sea cucumber Acaudina leucoprocta on hydrogen peroxide-induced oxidative injury in RAW264.7 cells. Int. J. Biol. Macromol. 2019, 139, 1133–1140. [Google Scholar] [CrossRef]
- Yang, L.C.; Chang, Y.C.; Yeh, K.L.; Huang, F.M.; Su, N.Y.; Kuan, Y.H. Protective Effect of Rutin on Triethylene Glycol Dimethacrylate-Induced Toxicity through the Inhibition of Caspase Activation and Reactive Oxygen Species Generation in Macrophages. Int. J. Mol. Sci. 2022, 23, 11773. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Zhong, Q.; Wu, Y.; Zhuang, J.; Qu, F.; Xu, C. Suppressing ROS generation by apocynin inhibited cyclic stretch-induced inflammatory reaction in HPDLCs via a caspase-1 dependent pathway. Int. Immunopharmacol. 2021, 90, 107129. [Google Scholar] [CrossRef]
- Li, J.Y.; Jin, M.M.; Meng, J.; Gao, S.M.; Lu, R.R. Exopolysaccharide from Lactobacillus planterum LP6: Antioxidation and the effect on oxidative stress. Carbohydr. Polym. 2013, 98, 1147–1152. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Wu, T.; Fang, L.; Liu, C.; Min, W. In vitro immunomodulatory effects of acidic exopolysaccharide produced by Lactobacillus planetarium JLAU103 on RAW264.7 macrophages. Int. J. Biol. Macromol. 2020, 156, 1308–1315. [Google Scholar] [CrossRef]
- Liao, Y.; Gao, M.; Wang, Y.; Liu, X.; Zhong, C.; Jia, S. Structural characterization and immunomodulatory activity of exopolysaccharide from Aureobasidium pullulans CGMCC 23063. Carbohydr. Polym. 2022, 288, 119366. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Xu, D.; Ma, K.; Zhang, C.; Wang, G.; Dong, M.; Li, W. Structural and prebiotic activity analysis of the polysaccharide produced by Lactobacillus helveticus SNA12. Carbohydr. Polym. 2022, 296, 119971. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, purification and characterization of exopolysaccharide produced by Leuconostoc pseudomesenteroides YF32 from soybean paste. Int. J. Biol. Macromol. 2018, 114, 529–535. [Google Scholar] [CrossRef]
- Prasanna, P.H.; Bell, A.; Grandison, A.S.; Charalampopoulos, D. Emulsifying, rheological and physicochemical properties of exopolysaccharide produced by Bifidobacterium longum subsp. infantis CCUG 52486 and Bifidobacterium infantis NCIMB 702205. Carbohydr. Polym. 2012, 90, 533–540. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Lu, L.; Liu, Y.; Wang, F.; Xiao, M. Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresour. Technol. 2010, 101, 5528–5533. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, M.; Du, Z.; Wang, H.; Wu, Y.; Yang, L. Purification, characterization and promoting effect on wound healing of an exopolysaccharide from Lachnum YM405. Carbohydr. Polym. 2014, 105, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kavita, K.; Singh, V.K.; Mishra, A.; Jha, B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014, 101, 29–35. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, R.; Huang, J.; Fei, Z.; Wang, L.; Zhao, J.; Si, J.; Jin, P. Efficient production, structural characterization and bioactivity of an extracellular polysaccharide from Grifola frondosa endophytic Burkholderia sp. Int. J. Biol. Macromol. 2025, 309, 143090. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Tian, H.; Ling, N.; Guo, C.; Gao, M.; Wang, Z.; Liu, B.; Sun, Y.; Chen, Y.; Ji, C.; Li, W. Immunostimulatory activity of sea buckthorn polysaccharides via TLR2/4-mediated MAPK and NF-kappaB signaling pathways in vitro and in vivo. Int. J. Biol. Macromol. 2024, 283, 137678. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Shi, P.; Zhang, L.; Yan, X.; Xu, J.; Liao, K. Trans-cinnamic acid alleviates high-fat diet-induced renal injury via JNK/ERK/P38 MAPK pathway. J. Nutr. Biochem. 2025, 135, 109769. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Liang, W.; Lu, Y.; Xiong, J.; Liu, D.; Jia, X. Identification, Antioxidant and Immunomodulatory Activities of a Neutral Exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010. Nutrients 2025, 17, 2265. https://doi.org/10.3390/nu17142265
Huang Y, Liang W, Lu Y, Xiong J, Liu D, Jia X. Identification, Antioxidant and Immunomodulatory Activities of a Neutral Exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010. Nutrients. 2025; 17(14):2265. https://doi.org/10.3390/nu17142265
Chicago/Turabian StyleHuang, Yanyan, Weiting Liang, Yunhui Lu, Jie Xiong, Dongmei Liu, and Xiangze Jia. 2025. "Identification, Antioxidant and Immunomodulatory Activities of a Neutral Exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010" Nutrients 17, no. 14: 2265. https://doi.org/10.3390/nu17142265
APA StyleHuang, Y., Liang, W., Lu, Y., Xiong, J., Liu, D., & Jia, X. (2025). Identification, Antioxidant and Immunomodulatory Activities of a Neutral Exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010. Nutrients, 17(14), 2265. https://doi.org/10.3390/nu17142265




