Vegetarian Diet and Dietary Intake, Health, and Nutritional Status in Infants, Children, and Adolescents: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Subsection Reporting and Registration
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria and Study Selection
2.4. Selection Process and Data Collection
2.5. Study Risk of Bias Assessment
2.6. Statistics
3. Results
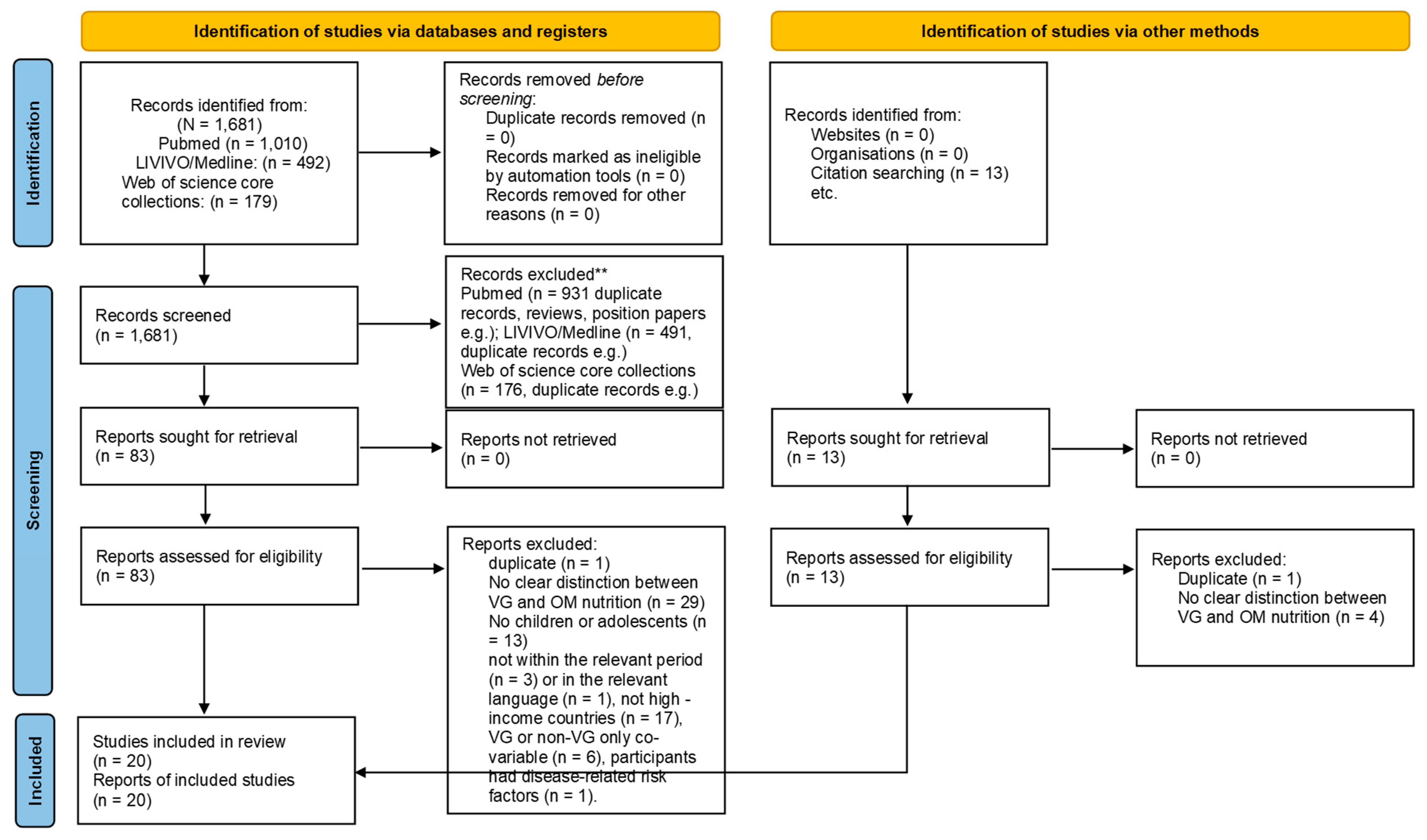
3.1. Study Selection
3.2. Study Characteristics
3.3. Summary of Study Results
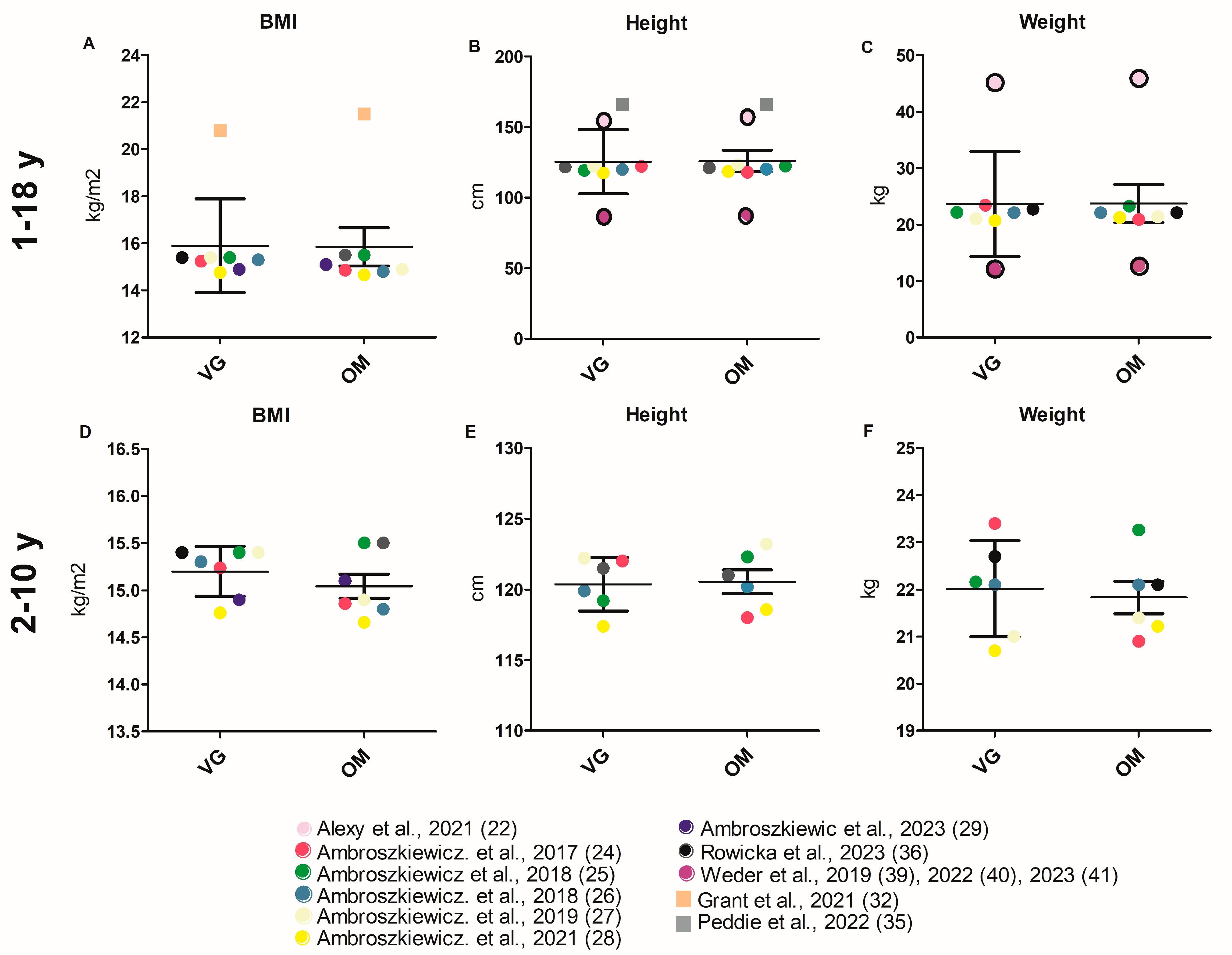
3.3.1. Anthropometry
3.3.2. Biomarkers
Nutritional Status (Vitamins, Minerals Including Iron)
Inflammation Markers
Blood Lipids
Bone Metabolism
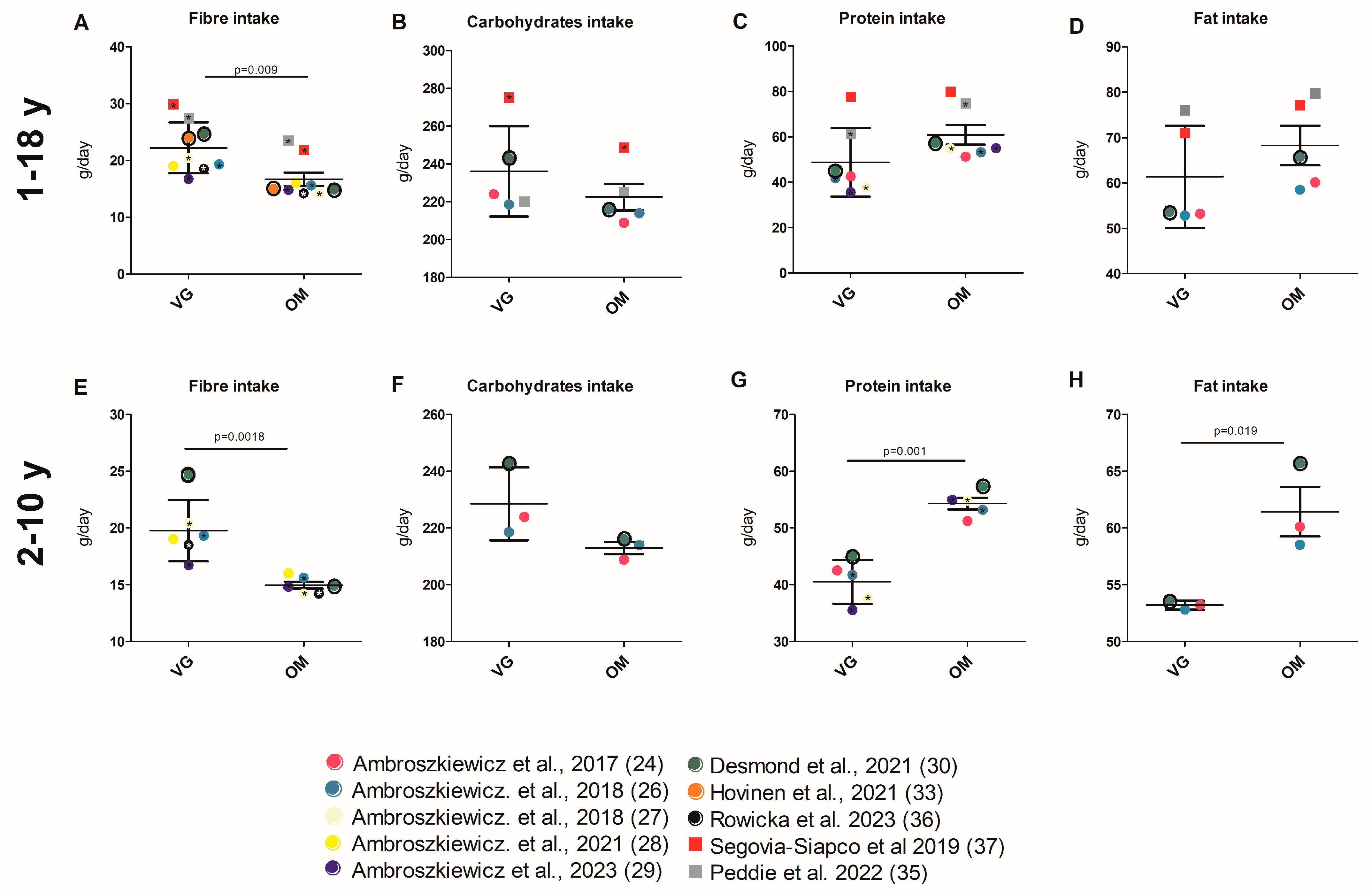
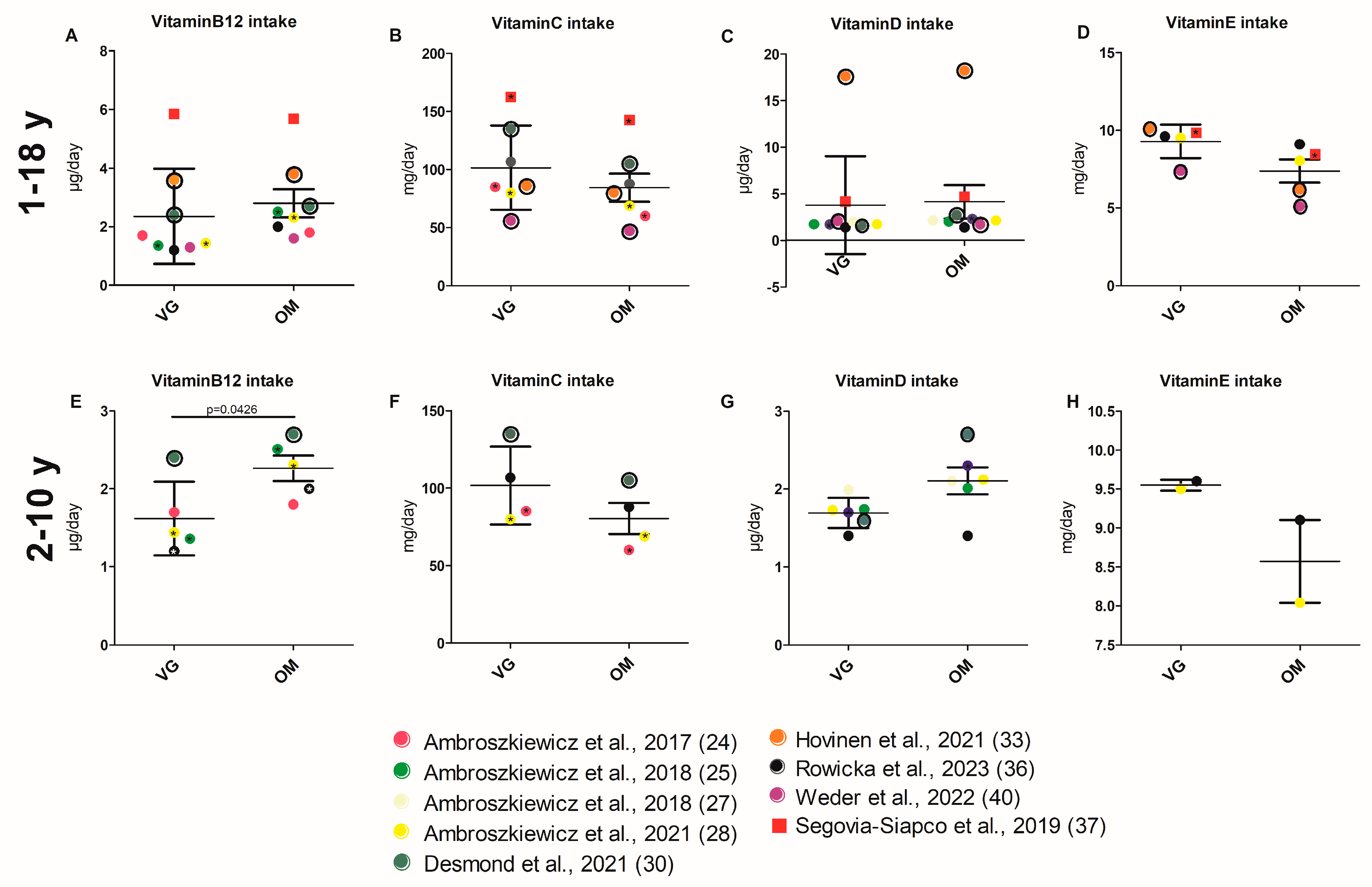
3.3.3. Energy and Nutrient Intake
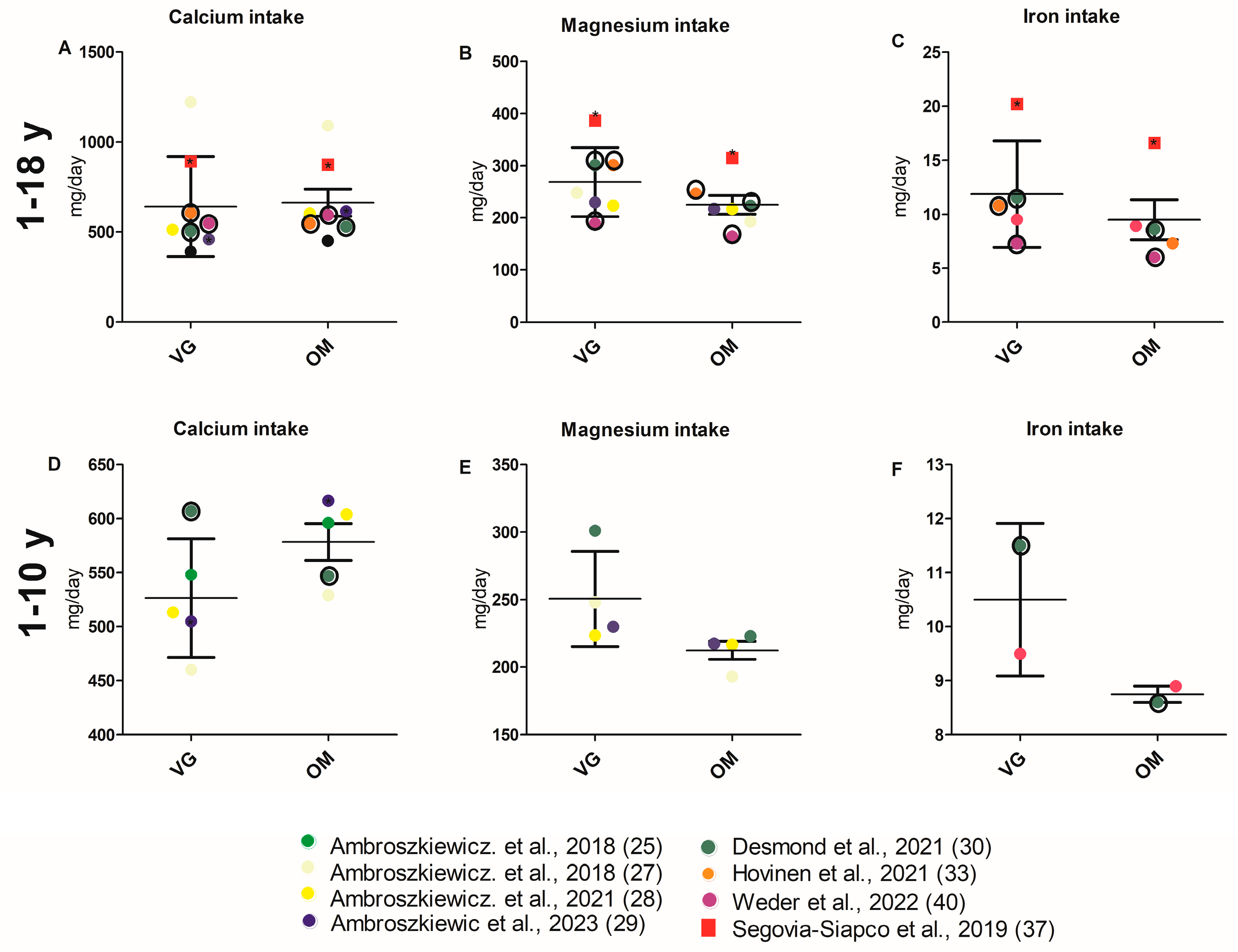
3.3.4. Intake of Micronutrients
3.3.5. Intake of Vitamins
3.3.6. Food Groups
4. Discussion
4.1. Anthropometry
4.2. Nutrient Intake and Biomarker
4.2.1. Health Risk Markers (Inflammation Markers, Bone Markers)
4.2.2. Energy and Nutrient Intake
4.2.3. Protein Intake and Amino Acid Status
4.2.4. Fat, Cholesterol Intake, and Status
4.2.5. Vitamin and Mineral Intake and Status
4.2.6. Iron Intake and Status
4.3. Food Groups
4.4. Practical Implications
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OM | Omnivore, omnivorous |
| RCT | Randomized controlled trial |
| VE | Vegan |
| VG | Vegetarian |
References
- Schürmann, S.; Kersting, M.; Alexy, U. Vegetarian diets in children: A systematic review. Eur. J. Nutr. 2017, 56, 1797–1817. [Google Scholar] [CrossRef] [PubMed]
- FranceAgriMer Etablissement National des Produits de l’agriculture et de la Mer et l’OCHA. Combien de Végétariens en Europe?: Synthèse des Résultats à Partir de l’Étude « Panorama de la Consommation Végétarienne en Europe », Réalisée par le CREDOC pour FranceAgriMer et l’OCHA en 2018. Available online: https://www.franceagrimer.fr/sites/default/files/rdd/documents/11_Synth%C3%A8se%20Panorama%20v%C3%A9g%C3%A9tarisme%20en%20Europe_2.pdf (accessed on 27 May 2025).
- Eurispes. Rapporto Italia 2018. Vegani e Vegetariani Sono il 7% della Popolazione dai 18 Anni in su.—Eurispes. Available online: https://eurispes.eu/news/eurispes-rapporto-italia-2018-vegani-e-vegetariani-sono-il-7-della-popolazione-dai-18-anni-in-su/ (accessed on 15 June 2023).
- Smith, M.; Prescott Smith, S. Meet Britain’s Vegans and Vegetarians. YouGov, 20 January 2022. Available online: https://yougov.co.uk/society/articles/40517-meet-britains-vegans-and-vegetarians?redirect_from=%2Ftopics%2Fsociety%2Farticles-reports%2F2022%2F01%2F20%2Fmeet-britains-vegans-and-vegetarians (accessed on 21 September 2023).
- Leitzmann, C. Vegetarian nutrition: Past, present, future. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 496S–502S. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.B.M.; Barbosa, C.L.; Brettschneider, A.-K. Prevalence of persons following a vegetarian diet in Germany. J. Health Monit. 2016, 1, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Patelakis, E.; Barbosa, C.L.; Haftenberger, M.; Brettschneider, A.-K.; Lehmann, F.; Heide, K.; Frank, M.; Perlitz, H.; Richter, A.; Mensink, G.B.M. Prevalence of vegetarian diet among children and adolescents in Germany: Results from EsKiMo II. Ernährungs Umsch. 2019, 66, 85–91. [Google Scholar]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Goggi, S.; Metro, D.; Papa, M.; Sbarbati, R.; Scarino, M.L.; et al. A comprehensive review of healthy effects of vegetarian diets. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1308–1315. [Google Scholar] [CrossRef]
- Oussalah, A.; Levy, J.; Berthezène, C.; Alpers, D.H.; Guéant, J.-L. Health outcomes associated with vegetarian diets: An umbrella review of systematic reviews and meta-analyses. Clin. Nutr. 2020, 39, 3283–3307. [Google Scholar] [CrossRef]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef]
- Tucker, K.L. Vegetarian diets and bone status. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 329S–335S. [Google Scholar] [CrossRef]
- Rosell, M.S.; Lloyd-Wright, Z.; Appleby, P.N.; Sanders, T.A.B.; Allen, N.E.; Key, T.J. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am. J. Clin. Nutr. 2005, 82, 327–334. [Google Scholar] [CrossRef]
- Niklewicz, A.; Hannibal, L.; Warren, M.; Ahmadi, K.R. A systematic review and meta-analysis of functional vitamin B12 status among adult vegans. Nutr. Bull. 2024, 49, 463–479. [Google Scholar] [CrossRef]
- Alexy, U. Diet and growth of vegetarian and vegan children. BMJ Nutr. Prev. Health 2023, 6, s3–s11. [Google Scholar] [CrossRef]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, S.; Bührer, C.; Jochum, F.; Kauth, T.; Kersting, M.; Körner, A.; Koletzko, B.; Mihatsch, W.; Prell, C.; Reinehr, T.; et al. Vegetarian diets in childhood and adolescence: Position paper of the nutrition committee, German Society for Paediatric and Adolescent Medicine (DGKJ). Mol. Cell. Pediatr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cochrane Deutschland. Literaturbewertung. Available online: https://www.cochrane.de/literaturbewertung#erg (accessed on 27 September 2023).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 7 August 2023).
- Alexy, U.; Fischer, M.; Weder, S.; Längler, A.; Michalsen, A.; Sputtek, A.; Keller, M. Nutrient Intake and Status of German Children and Adolescents Consuming Vegetarian, Vegan or Omnivore Diets: Results of the VeChi Youth Study. Nutrients 2021, 13, 1707. [Google Scholar] [CrossRef]
- Alexy, U.; Fischer, M.; Weder, S.; Längler, A.; Michalsen, A.; Keller, M. Food group intake of children and adolescents (6–18 years) on a vegetarian, vegan or omnivore diet: Results of the VeChi Youth Study. Br. J. Nutr. 2022, 128, 851–862. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Klemarczyk, W.; Mazur, J.; Gajewska, J.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Serum Hepcidin and Soluble Transferrin Receptor in the Assessment of Iron Metabolism in Children on a Vegetarian Diet. Biol. Trace Elem. Res. 2017, 180, 182–190. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Chełchowska, M.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. The Assessment of Bone Regulatory Pathways, Bone Turnover, and Bone Mineral Density in Vegetarian and Omnivorous Children. Nutrients 2018, 10, 183. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Chełchowska, M.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. Anti-Inflammatory and Pro-Inflammatory Adipokine Profiles in Children on Vegetarian and Omnivorous Diets. Nutrients 2018, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Ambroszkiewicz, J.; Chełchowska, M.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. Bone status and adipokine levels in children on vegetarian and omnivorous diets. Clin. Nutr. 2019, 38, 730–737. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Klemarczyk, W.; Rowicka, G.; Ołtarzewski, M.; Strucińska, M.; Chełchowska, M. Does a Vegetarian Diet Affect the Levels of Myokine and Adipokine in Prepubertal Children? J. Clin. Med. 2021, 10, 3995. [Google Scholar] [CrossRef] [PubMed]
- Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Kusmierska, K.; Klemarczyk, W.; Rowicka, G.; Strucinska, M.; Chelchowska, M. Dietary Intake and Circulating Amino Acid Concentrations in Relation with Bone Metabolism Markers in Children Following Vegetarian and Omnivorous Diets. Nutrients 2023, 15, 1376. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.A.; Sobiecki, J.G.; Jaworski, M.; Płudowski, P.; Antoniewicz, J.; Shirley, M.K.; Eaton, S.; Książyk, J.; Cortina-Borja, M.; Stavola, B.d.; et al. Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am. J. Clin. Nutr. 2021, 113, 1565–1577. [Google Scholar] [CrossRef]
- Elliott, L.J.; Keown-Stoneman, C.D.G.; Birken, C.S.; Jenkins, D.J.A.; Borkhoff, C.M.; Maguire, J.L. Vegetarian Diet, Growth, and Nutrition in Early Childhood: A Longitudinal Cohort Study. Pediatrics 2022, 149, e2021052598. [Google Scholar] [CrossRef]
- Grant, R.; Pawlak, R.; Vos, P.; Bilgin, A.A.; Berg, J.; Pearce, R.; Morris, M. Cardiovascular Disease Risk Factors Profile Among Australian Vegetarian and Nonvegetarian Teenagers. Am. J. Lifestyle Med. 2021, 15, 313–321. [Google Scholar] [CrossRef]
- Hovinen, T.; Korkalo, L.; Freese, R.; Skaffari, E.; Isohanni, P.; Niemi, M.; Nevalainen, J.; Gylling, H.; Zamboni, N.; Erkkola, M.; et al. Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO Mol. Med. 2021, 13, e13492. [Google Scholar] [CrossRef]
- Nieczuja-Dwojacka, J.; Klemarczyk, W.; Siniarska, A.; Kozieł, S.; Szysz, T. Socio-economic determinants of the somatic development and reaction time of vegetarian and non-vegetarian children. Anthropol. Anz. 2020, 77, 137–146. [Google Scholar] [CrossRef]
- Peddie, M.; Scott, T.; Ranasinghe, C.; Fleming, E.; Webster, K.; Brown, R.; Houghton, L.; Haszard, J. A Sample of Female Adolescent Self-Identified Vegetarians in New Zealand Consume Less Protein and Saturated Fat, but More Fiber than Their Omnivorous Peers. Nutrients 2022, 14, 711. [Google Scholar] [CrossRef]
- Rowicka, G.; Klemarczyk, W.; Ambroszkiewicz, J.; Strucinska, M.; Kawiak-Jawor, E.; Weker, H.; Chelchowska, M. Assessment of Oxidant and Antioxidant Status in Prepubertal Children following Vegetarian and Omnivorous Diets. Antioxidants 2023, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Siapco, G.; Burkholder-Cooley, N.; Haddad Tabrizi, S.; Sabaté, J. Beyond Meat: A Comparison of the Dietary Intakes of Vegetarian and Non-vegetarian Adolescents. Front. Nutr. 2019, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Světnička, M.; Sigal, A.; Selinger, E.; Heniková, M.; El-Lababidi, E.; Gojda, J. Cross-Sectional Study of the Prevalence of Cobalamin Deficiency and Vitamin B12 Supplementation Habits among Vegetarian and Vegan Children in the Czech Republic. Nutrients 2022, 14, 535. [Google Scholar] [CrossRef] [PubMed]
- Weder, S.; Hoffmann, M.; Becker, K.; Alexy, U.; Keller, M. Energy, Macronutrient Intake, and Anthropometrics of Vegetarian, Vegan, and Omnivorous Children (1–3 Years) in Germany (VeChi Diet Study). Nutrients 2019, 11, 832. [Google Scholar] [CrossRef]
- Weder, S.; Keller, M.; Fischer, M.; Becker, K.; Alexy, U. Intake of micronutrients and fatty acids of vegetarian, vegan, and omnivorous children (1–3 years) in Germany (VeChi Diet Study). Eur. J. Nutr. 2022, 61, 1507–1520. [Google Scholar] [CrossRef]
- Weder, S.; Zerback, E.H.; Wagener, S.M.; Koeder, C.; Fischer, M.; Alexy, U.; Keller, M. How Does Selenium Intake Differ among Children (1–3 Years) on Vegetarian, Vegan, and Omnivorous Diets? Results of the VeChi Diet Study. Nutrients 2023, 15, 34. [Google Scholar] [CrossRef]
- Craig, B.A.; Morton, D.P.; Morey, P.J.; Kent, L.M.; Gane, A.B.; Butler, T.L.; Rankin, P.M.; Price, K.R. The association between self-rated health and social environments, health behaviors and health outcomes: A structural equation analysis. BMC Public Health 2018, 18, 440. [Google Scholar] [CrossRef]
- Wirnitzer, K.C.; Drenowatz, C.; Cocca, A.; Tanous, D.R.; Motevalli, M.; Wirnitzer, G.; Schätzer, M.; Ruedl, G.; Kirschner, W. Health Behaviors of Austrian Secondary Level Pupils at a Glance: First Results of the From Science 2 School Study Focusing on Sports Linked to Mixed, Vegetarian, and Vegan Diets. Int. J. Environ. Res. Public Health 2021, 18, 12782. [Google Scholar] [CrossRef]
- Kostecka, M.; Kostecka, J.; Jackowska, I.; Iłowiecka, K. Parental Nutritional Knowledge and Type of Diet as the Key Factors Influencing the Safety of Vegetarian Diets for Children Aged 12–36 Months. Nutrients 2023, 15, 2244. [Google Scholar] [CrossRef]
- Latimer, K.R.; Peddie, M.C.; Scott, T.; Haszard, J.J. Rationalisation of meat consumption in New Zealand adolescents. Public Health Nutr. 2022, 25, 904–912. [Google Scholar] [CrossRef]
- Lemke, N.; Murawski, A.; Schmied-Tobies, M.I.H.; Rucic, E.; Hoppe, H.-W.; Conrad, A.; Kolossa-Gehring, M. Glyphosate and aminomethylphosphonic acid (AMPA) in urine of children and adolescents in Germany—Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Environ. Int. 2021, 156, 106769. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Siapco, G.; Khayef, G.; Pribis, P.; Oda, K.; Haddad, E.; Sabaté, J. Animal Protein Intake Is Associated with General Adiposity in Adolescents: The Teen Food and Development Study. Nutrients 2019, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Macknin, M.; Kong, T.; Weier, A.; Worley, S.; Tang, A.S.; Alkhouri, N.; Golubic, M. Plant-based, no-added-fat or American Heart Association diets: Impact on cardiovascular risk in obese children with hypercholesterolemia and their parents. J. Pediatr. 2015, 166, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.C.; Blaauwendraad, S.M.; Koletzko, B.; Oei, E.H.G.; Jaddoe, V.W.V.; Gaillard, R. Associations of childhood BMI, general and visceral fat mass with metabolite profiles at school-age. Int. J. Obes. 2024, 48, 1307–1317. [Google Scholar] [CrossRef]
- Sutter, D.O.; Bender, N. Nutrient status and growth in vegan children. Nutr. Res. 2021, 91, 13–25. [Google Scholar] [CrossRef]
- Hoppe, C.; Mølgaard, C.; Michaelsen, K.F. Cow′s milk and linear growth in industrialized and developing countries. Annu. Rev. Nutr. 2006, 26, 131–173. [Google Scholar] [CrossRef]
- Thompson, A.L. What is normal, healthy growth? Global health, human biology, and parental perspectives. Am. J. Hum. Biol. 2021, 33, e23597. [Google Scholar] [CrossRef]
- Hua, Y.; Remer, T. Adult Stature and Protein Intake During Childhood and Adolescence From 3 Years Onward. J. Clin. Endocrinol. Metab. 2022, 107, e2833–e2842. [Google Scholar] [CrossRef]
- Grenov, B.; Larnkjær, A.; Ritz, C.; Michaelsen, K.F.; Damsgaard, C.T.; Mølgaard, C. The effect of milk and rapeseed protein on growth factors in 7–8 year-old healthy children—A randomized controlled trial. Growth Horm. IGF Res. 2021, 60–61, 101418. [Google Scholar] [CrossRef]
- Koch, C.A.; Kjeldsen, E.W.; Frikke-Schmidt, R. Vegetarian or vegan diets and blood lipids: A meta-analysis of randomized trials. Eur. Heart J. 2023, 44, 2609–2622. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.O.; Doerfer, J. Atherosklerose im Kindesalter. In Pädiatrische Fettstoffwechselstörungen und Atheroskleroserisiko—Kompakt; Schwab, K.O., Doerfer, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 23–41. ISBN 978-3-662-63319-9. [Google Scholar]
- Selinger, E.; Neuenschwander, M.; Koller, A.; Gojda, J.; Kühn, T.; Schwingshackl, L.; Barbaresko, J.; Schlesinger, S. Evidence of a vegan diet for health benefits and risks—An umbrella review of meta-analyses of observational and clinical studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 9926–9936. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-T.; Lee, J.-I.; Lu, C.-C.; Huang, S.-P.; Chen, S.-C.; Geng, J.-H. The association between body mass index and osteoporosis in a Taiwanese population: A cross-sectional and longitudinal study. Sci. Rep. 2024, 14, 8509. [Google Scholar] [CrossRef]
- Tong, T.Y.N.; Perez-Cornago, A.; Bradbury, K.E.; Key, T.J. Biomarker Concentrations in White and British Indian Vegetarians and Nonvegetarians in the UK Biobank. J. Nutr. 2021, 151, 3168–3179. [Google Scholar] [CrossRef]
- Tran, E.; Dale, H.F.; Jensen, C.; Lied, G.A. Effects of Plant-Based Diets on Weight Status: A Systematic Review. Diabetes Metab. Syndr. Obes. 2020, 13, 3433–3448. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef]
- Harris, W.S. Achieving optimal n-3 fatty acid status: The vegetarian’s challenge or not. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 449S–452S. [Google Scholar] [CrossRef]
- Lane, K.E.; Wilson, M.; Hellon, T.G.; Davies, I.G. Bioavailability and conversion of plant based sources of omega-3 fatty acids—A scoping review to update supplementation options for vegetarians and vegans. Crit. Rev. Food Sci. Nutr. 2022, 62, 4982–4997. [Google Scholar] [CrossRef]
- Lütjohann, D.; Meyer, S.; von Bergmann, K.; Stellaard, F. Cholesterol Absorption and Synthesis in Vegetarians and Omnivores. Mol. Nutr. Food Res. 2018, 62, e1700689. [Google Scholar] [CrossRef]
- Jensen, C.F. Vitamin B12 levels in children and adolescents on plant-based diets: A systematic review and meta-analysis. Nutr. Rev. 2023, 81, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Rohrmann, S.; Wakolbinger, M.; Gojda, J.; Selinger, E.; Cahova, M.; Světnička, M.; Haider, S.; Schlesinger, S.; Kühn, T.; et al. Health aspects of vegan diets among children and adolescents: A systematic review and meta-analyses. Crit. Rev. Food Sci. Nutr. 2024, 64, 13247–13258. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, K.Z.; Seljak, B.K.; Gregorič, M.; Jurak, G.; Golja, P. Questionable advisability of vitamin and mineral dietary supplement use in adolescents. BMC Nutr. 2023, 9, 110. [Google Scholar] [CrossRef]
- Burgard, L.; Spiegler, C.; Jansen, S.; Brettschneider, A.-K.; Straßburg, A.; Alexy, U.; Storcksdieck Genannt Bonsmann, S.; Ensenauer, R.; Heuer, T. Critical vitamin D and iron intakes in infants aged 6–11 months: Results from the nationwide German KiESEL study. Front. Nutr. 2025, 12, 1472685. [Google Scholar] [CrossRef]
- Bickelmann, F.V.; Leitzmann, M.F.; Keller, M.; Baurecht, H.; Jochem, C. Calcium intake in vegan and vegetarian diets: A systematic review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 10659–10677. [Google Scholar] [CrossRef]
- Proia, P.; Amato, A.; Drid, P.; Korovljev, D.; Vasto, S.; Baldassano, S. The Impact of Diet and Physical Activity on Bone Health in Children and Adolescents. Front. Endocrinol. 2021, 12, 704647. [Google Scholar] [CrossRef]
- Iguacel, I.; Miguel-Berges, M.L.; Gómez-Bruton, A.; Moreno, L.A.; Julián, C. Veganism, vegetarianism, bone mineral density, and fracture risk: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Moscheo, C.; Licciardello, M.; Samperi, P.; La Spina, M.; Di Cataldo, A.; Russo, G. New Insights into Iron Deficiency Anemia in Children: A Practical Review. Metabolites 2022, 12, 289. [Google Scholar] [CrossRef]
- Hansch, L.; Fischer, M.; Längler, A.; Michalsen, A.; Weder, S.; Keller, M.; Alexy, U.; Perrar, I. Dietary patterns among children and adolescents in Germany consuming vegetarian, vegan or omnivore diets: Results of the VeChi Youth Study. Eur. J. Nutr. 2024, 63, 3161–3174. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Iqbal, K.; Schwedhelm, C.; Boeing, H. Food groups and intermediate disease markers: A systematic review and network meta-analysis of randomized trials. Am. J. Clin. Nutr. 2018, 108, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883.e31. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A.; Müller, A.; Lombardo, M. Diet and Consumer Behavior in U.S. Vegetarians: A National Health and Nutrition Examination Survey (NHANES) Data Report. Int. J. Environ. Res. Public Health 2021, 19, 67. [Google Scholar] [CrossRef]
- Orlich, M.J.; Jaceldo-Siegl, K.; Sabaté, J.; Fan, J.; Singh, P.N.; Fraser, G.E. Patterns of food consumption among vegetarians and non-vegetarians. Br. J. Nutr. 2014, 112, 1644–1653. [Google Scholar] [CrossRef]
- Newman, J.C.; Malek, A.M.; Hunt, K.J.; Marriott, B.P. Nutrients in the US Diet: Naturally Occurring or Enriched/Fortified Food and Beverage Sources, Plus Dietary Supplements: NHANES 2009–2012. J. Nutr. 2019, 149, 1404–1412. [Google Scholar] [CrossRef]

| Reference; Study Location (Implementation Period) | Study Design/ Study Quality 1 (Scale: 0–9) | Participant Characteristics | Assessed Dietary Intake Parameters | Assessed Biomarkers | Further Assessed Parameters | Sign. Diff. Between VG and OM/Main Outcome |
|---|---|---|---|---|---|---|
| Alexy et al., 2021 [22], Germany (2017–2018) | Cross-sectional study Subanalysis of the VeChi Youth Study ****** | Total: N = 401 (229 f/172 m) Age group: 5–19 y VG: n = 150 (91 f/59 m) OM: n = 137 (62 f/75 m) Other groups: VE |
|
|
| VG ↑ Energy from carbohydrates (p = 0.0002) 2 ↑ Tocopherol-Equivalents intake (Vit.E) (p = 0.0015) 2 ↓ Intake of Vit.B1 (p = 0.0413) 2, Vit.B2 (p = 0.0149) 2, Vit.B12 (p = 0.0002) 2 ↓ Protein intake (p = 0.0011) 2 ↓ Fat intake (p = 0.0002) ↑ Fiber intake (p = 0.0006) ↓ Ferritin level (p = 0.0134) 2 ↓ Holotranscobalamin level (p = 0.0042) 2 ↑ Methylmalonic acid level (p = 0.0253) 2 Data show no nutritional risks for VG adolescents. |
| Alexy et al., 2022 [23], Germany, (2017–2018) | Cross-sectional study Subanalysis of the VeChi Youth Study ***** | Total N = 390 (221 f/169 m) Age group: 5–19 y VG: n = 145 (87 f/58 m) OM: n = 135 (61 f/74 m) Other groups: VE |
| N/A |
| VG ↓ Dairy intake (p = 0.0003) ↑ Grain intake (p = 0.0065) The lower intake of dairy products in VG children suggests the need for vitamin B12 supplementation. |
| Ambroszkiewicz et al., 2017 [24], Poland (2015–2016) | Cross-sectional study ****** | Total: N = 89 (44 f/45 m) Age group: 4–9 y VG: n = 43 (25 f/18 m; mean age: 6.5 y) OM: n = 46 (19 f/27 m; mean age: 6.3 y) |
|
|
| VG ↓ Energy from protein (p = 0.03) ↑ Energy from carbohydrates (p = 0.001) ↑ Vit.C intake (p < 0.05) ↓ Ferritin level (p < 0.01) ↓ Hepcidin level (p < 0.05) ↑ sTfR level (p < 0.001) ↓ CRP (p = 0.011) sTfR and hepcidin are possible markers for the detection of subclinical iron deficiency in VG children. |
| Ambroszkiewicz, et al., 2018 [25], Poland (2014–2017) | Cross-sectional study ****** | Total N = 130 Age group: 5–10 y VG: n = 70 (mean age: 6.6 y) OM: n = 60 (mean age: 6.9 y) |
|
|
| VG ↓ Energy from protein (p < 0.001) ↑ Energy from carbohydrates (p = 0.004) ↓Vit.B12 intake (p < 0.001) ↑ BALP level (p = 0.002) ↑ CTX-I level (p = 0.027), positive correlation of CTX-I with BMC, total BMD, and lumbar spine BMD VG children do not have a lower bone mass; CTX-I might be an important marker for the protection of bone abnormalities in VG children. |
| Ambroszkiewicz et al., 2018 [26]; Poland (2017–2018) | Cross-sectional study ****** | Total: N = 117 (56 f/61 m) Age group: 5–10 y VG: n = 62 (28 f/34 m; mean age: 6 y) OM: n = 55 (28 f/27 m; mean age: 6.5 y) |
|
|
| VG ↓ Fat free mass (p = 0.044) ↓ Energy from protein (p < 0.001) ↓ Protein intake (p = 0.002) ↑ Fiber intake (p = 0.011) ↓ Leptin/sOB-R ratio (p = 0.017) ↑ Ratios of anti-inflammatory to pro-inflammatory adipokines: adiponectin/leptin (p = 0.005); omentin/leptin (p = 0.011) The adipokine profile and inflammatory status of prepubertal children might be beneficially affected by VG diet. |
| Ambroszkiewicz et al., 2019 [27], Poland (2014–2016) | Cross-sectional study ****** | Total: N = 106 Age group: 5–10 y VG: N = 53 (median age: 7.0 y) OM: n = 53 (median age: 7.0 y) |
|
|
| VG ↓ Fat mass (p = 0.018) ↓ TBLH-BMD z-score (p = 0.009); BMDL2-L4 z-score (p = 0.019) ↓ Leptin level (p < 0.001); Leptin/adiponectin ratio (p < 0.001) ↓ OC/CTX ratio 0.039 ↑ c-OC/uc-OC ratio (p = 0.010); c-OC/OC ratio (p = 0.048) ↑ Parathormone level (p = 0.015) ↓ Energy from protein (p = 0.001), protein intake (p = 0.002) ↓ Energy from fat (p = 0.043) ↑ Energy from carbohydrates (p = 0.001) ↑ Fiber intake (p = 0.015) Values of bone mineral density are similar in VG and OM children. Significantly lower total and lumbar spine BMD z-scores were seen in VG children. |
| Ambroszkiewicz et al., 2021 [28], Poland (2018–2020) | Cross-sectional study ****** | Total N = 105 (52 f/53 m) Age group: 5–9 y VG: n = 55 (26 f/29 m; mean age: 5.5 y) OM n = 50 (26 f/24 m; mean age: 6.0 y) |
|
|
| VG ↓ Fat mass (p = 0.018) ↓ Leptin level (p = 0.003) ↓ Energy from protein (p < 0.001) ↑ Energy from carbohydrates (p = 0.002) ↑ Fiber intake (p = 0.063) ↑ Manganese intake (p = 0.020) ↓ Vit.B12 intake (p < 0.001) ↑ Vit.C intake (p = 0.019) A lacto-ovo vegetarian diet has no impact on myokines and adipokines levels in prepubertal children. |
| Ambroszkiewicz et al., 2023 [29], Poland (2020–2021) | Cross-sectional study ****** | Total N = 76 (37 f/39 m) Age group: 5–9 y VG: n = 51 (25 f/26 m; mean age: 6 y) OM: n = 25 (12 f/13 m; mean age: 5.5 y) |
|
|
| VG ↓ Energy from protein (p = 0.0002); protein intake (p = 0.0009) ↑ Energy from carbohydrates (p = 0.0125) ↑ Fiber intake (p = 0.0034) ↓ Calcium intake (p = 0.0149) ↓ Vit.D intake (p = 0.0116) ↓ Amino acids intake (p < 0.001) ↓ Valine level (p = 0.0253), Lysine level (p = 0.0297), Leucine level (p= 0.0315), Isoleucine level (p = 0.0231) ↓ Albumin level (p = 0.0001) ↑ CTX-I level (p = 0.0343) Significant relationships of osteoprotegerin with alanine, ornithine, and aspartate might suggest an impact of diet on the bone regulatory pathway. |
| Desmond et al., 2021 [30], Poland (2014–2016) | Cross-sectional study ****** | Total: N = 187 (100 f/87 m) Age group: 5–10 y VG: n = 63 (32 f/31 m; mean age: 7.6 y) OM: n = 72 (38 f/34 m; mean age: 7.7 y) Other groups: VE |
|
|
| ↓ Protein intake, sucrose intake, fat intake, cholesterol intake, Vit.B12 intake (without supplementation), Vit.D intake (with and without supplementation) (p < 0.001) 3 ↑ Carbohydrate intake, starch intake, fiber intake, folate intake, beta-carotene intake, Vit.C intake, magnesium intake, calcium intake, iron intake (p < 0.001) 3 ↓ Height z score (p < 0.05) 3 ↓ Thigh girth z score (p < 0.05) 3 ↓ Total body less head BMC (p < 0.01) 3 ↑ Fasting glucose level (p < 0.01) 3 ↓ HDL cholesterol level (p < 0.05) 3 ↓ VLDL cholesterol level (p < 0.05) 3 ↑ Triglycerides level (p < 0.01) 3 ↓ Hematocrit (p < 0.05) 3 ↓ Ferritin level (p < 0.05) 3 VGs showed fewer nutritional deficiencies but a more unfavorable cardiometabolic risk profile than OMs. |
| Elliott et al., 2022 [31] Canada (2008–2019) | Longitudinal cohort study ******** | Total: N = 8907 (4242 f/4665 m) Age group: 0.5–8 y VG(+VE): n = 248 (111 f/137 m; mean age: 2.3 y) OM: n = 8659 (4131 f/4528 m; mean age: 2.2 y) |
|
|
| VG: ↓ Height z score (p < 0.02) ↑ Underweight (p = 0.008) Growth or biochemical measures of nutrition do not meaningfully differ between VG children and OM children. However, VG diet is associated with a higher odd of underweight. |
| Grant et al., 2021 [32], Australia (2008) | Cross-sectional study ****** | Total: N = 688 (602 f/83 m) Age group: 14–17 y VG: n = 49 (39 f/9 m) OM: n = 639 (563 f/74 m) |
|
|
| VG: ↓ Total and LDL-cholesterol level (p = 0.001) ↑ Diastolic BP (p = 0.038) The prevalence rate of participants with 3 or more risk factors was similar among VG and OM participants. Abnormal cholesterol values were detected in both diet groups. |
| Hovinen et al., 2021 [33], Finland (2017) | Cross-sectional study ****** | Total: 40 (19 f/21 m) Age group: 1–7 y VG: n = 10 (4 f/6 m; mean age: 3.37 y) OM: n = 24 (12 f/12 m; mean age: 3.89 y) Other groups: VE |
|
|
| VG: ↓ Energy from saturated fatty acids (p = 0.029) 4 ↑ Energy from polyunsaturated fatty acid (p = 0.0027) 4 ↑ Linoleic acid (p = 0.0037) 4, alpha-linolenic acid intake (p = 0.0057) 4 ↓ Cholesterol intake (p = 0.034) 4 ↑ Fiber intake (p = 0.0036) 4 ↑ Thiamine (B1) intake (p = 0.034) 4; Folate (B9) 4 intake (p = 0.0034) 4 ↑ Magnesium intake (p = 0.034) 4, iron intake (p = 0.0038) 4 ↓ Zinc level (p = 0.039) 4 ↑ Sitosterol level (p = 0.021) 4 ↑ Avenasterol level (p = 0.021) 4 ↑ Cholestenol level (p = 0.021) 4 ↑ Lathosterol level (p = 0.021) 4 The risk of nutrient deficiency in children can be reduced by part-time consumption of lacto-ovo-vegetarian products in an otherwise strict vegan diet. |
| Nieczuja-Dwojacka et al., 2020 [34], Poland (2015–2016) | Cross-sectional study ****** | Total: N = 218 (100 f/118 m) Age group: 3–15 y VG: n = 47 (22 f/25 m) OM: n = 171 (78 f/93 m) |
|
|
| VG: ↓ Height (p < 0.05) ↓ BMI (p < 0.01) ↓ Sum of three skinfolds (p < 0.01) ↑ Reaction time (p < 0.05) VG diet affects the height, BMI, and body fatness, as well as the reaction time. |
| Peddie et al., 2022 [35], New Zealand (2019) | Cross-sectional study ***** | Total: N = 254 f, Age group: 15–18 y VG(+VE): n = 38 f (mean age 17.1 y) OM: n = 216 f (mean age 16.8 y) |
|
|
| VG: ↓ BMI z-score (p = 0.003) ↓ Consumers of poultry, sausages and processed meat; red meat; eggs and egg-based dishes; pies and pasties (p < 0.05) ↑ Consumers of vegetarian meat alternatives (p < 0.05) ↑ Energy intake from legumes (p = 0.011) and vegetables (p = 0.012) ↓ Protein intake (p < 0.001) ↓ Saturated fat intake (p = 0.014) ↑ Energy from polysaturated fat (p < 0.001) ↑ Energy from fiber intake (p = 0.019) VG children consume more fiber, more polyunsaturated fat, and less protein than children with an OM diet. Some VGs consume food groups associated with poorer dietary quality. |
| Rowicka et al., 2023 [36] Poland (2020–2021) | Cross-sectional study ****** | Total N = 72 (39 f/33 m) Age group: 2–10 y VG: n = 32 (17 f/15 m) OM: n = 40 (22 f/18 m) |
|
|
| VG: ↓ Energy from protein (p = 0.010) ↑ Energy from carbohydrates (p = 0.005) ↑ Fiber intake (p = 0.012) ↓ Vit.B12 (p < 0.0001) ↓ TOC (p = 0.001), GSH level (p = 0.001), GSSG level (p = 0.002) ↑ TAC (p < 0.001) ↓ Oxidative stress index (p < 0.001) Maintaining the oxidant–antioxidant balance in prepubertal VG children is possible. |
| Segovia-Siapco et al., 2019 [37], USA (-) | Cross-sectional study; Subanalysis of the Teen Food and Development Study **** | Total N = 534 (302 f/323 m) Age group: 12–18 y; VG(+VE): n = 137 (90 f/47 m) OM: n = 397 (212 f/185 m) |
| N/A |
| VG: ↑ Consumption of breads/grains/pastas/cereals (p = 0.022), fruits (p = 0.001), vegetables (p < 0.0001), nuts, nut butters, meat alternatives (p < 0.0001), dairy substitutes (p < 0.0001) ↓ Consumption of meat, poultry, eggs (p < 0.0001), cheese, dairy (p = 0.003), milk, dairy (p < 0.0001), dairy desserts (p = 0.007), sugar-sweetened beverages (p <0.0001), coffee/tea (p = 0.002) ↑ Intake of carbohydrates, fiber, thiamin, Vit.E, folate, calcium, iron, potassium, magnesium (p < 0.0001), sodium (p = 0.022), Vit.C (p = 0.029) ↓ Intakes of fat, zinc (p < 0.0001) VG adolescents have a more favorable dietary intake profile. |
| Světnička et al., 2022 [38] Czech Republic (2019–2021) | Cross-sectional study ****** | Total N = 200 (100 f/100 m) Age group: 0–18 y VG n = 79 (44 f/35 m; median age: 4.5 y) OM: n = 52 (25 f/27 m; median age: 4.5 y) Other groups: VE |
|
|
| ↑ Cyanocobalamin (B12) level (p = 0.019) 5 VG diet does not cause severe vitamin B12 deficiency but rather supplementation seems to lead to vitamin B12 hypervitaminosis in several of the investigated children. |
| Weder et al., 2019 [39], Germany (2016 –2018) | Cross-sectional study Subanalyses of the VeChi-Diet-Study ****** | Total: N = 430 (223 f/207 m) Age group: 1–3 y VG: n = 127 (64 f/63 m, mean age: 2 y) OM: n = 164 (83 f/81 m; mean age: 2 y) Other groups: VE |
| N/A |
| ↓ Protein intake (p ≤ 0.001) 6 ↑ Fiber intake (p ≤ 0.001) 7 Children with VG and OM diet in early childhood have the same amount of energy and macronutrients intake, leading to a normal growth in comparison to OM children. |
| Weder et al., 2022 [40], Germany (2016–2018) | Cross-sectional study Subanalyses of the VeChi-Diet-Study ****** | Total: N = 430 (223 f/207 m) Age group: 1–3 y VG: n = 127 (64 f/63 m, mean age: 2 y) OM: n = 164 (83 f/81 m; mean age: 2 y) Other groups: VE |
| N/A |
| ↓ Protein intake, Energy from protein (p ≤ 0.001) ↑ Vit.E intake (p ≤ 0.001) 8 ↓ Vit.B2 intake (p ≤ 0.001) 9 ↑ Magnesium 10, iron 11 intake (p ≤ 0.001) ↓ DHA, EPA intake (p ≤ 0.001) 12 VG diets can provide most micronutrients in desirable amounts and a preferable fat quality in young children (1–3 y) compared to an OM diet. |
| Weder et al., 2023 [41] Germany (2016–2018) | Cross-sectional study Subanalyses of the VeChi Diet Study ****** | Total: N = 430 (223 f/207 m) Age group: 1–3 y VG: n = 127 (64 f/63 m, mean age: 2 y) OM: n = 164 (83 f/81 m; mean age: 2 y) Other groups: VE |
| N/A | N/A | VGs have a lower selenium consumption (not significant) than OMs. A total of 39% of VG and 16% of OM children consume less than the recommended amount of selenium. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, D.; Schwermer, M.; Nowak, L.; Naami, N.; Zuzak, T.J.; Längler, A. Vegetarian Diet and Dietary Intake, Health, and Nutritional Status in Infants, Children, and Adolescents: A Systematic Review. Nutrients 2025, 17, 2183. https://doi.org/10.3390/nu17132183
Reis D, Schwermer M, Nowak L, Naami N, Zuzak TJ, Längler A. Vegetarian Diet and Dietary Intake, Health, and Nutritional Status in Infants, Children, and Adolescents: A Systematic Review. Nutrients. 2025; 17(13):2183. https://doi.org/10.3390/nu17132183
Chicago/Turabian StyleReis, Daniela, Melanie Schwermer, Lara Nowak, Nibras Naami, Tycho Jan Zuzak, and Alfred Längler. 2025. "Vegetarian Diet and Dietary Intake, Health, and Nutritional Status in Infants, Children, and Adolescents: A Systematic Review" Nutrients 17, no. 13: 2183. https://doi.org/10.3390/nu17132183
APA StyleReis, D., Schwermer, M., Nowak, L., Naami, N., Zuzak, T. J., & Längler, A. (2025). Vegetarian Diet and Dietary Intake, Health, and Nutritional Status in Infants, Children, and Adolescents: A Systematic Review. Nutrients, 17(13), 2183. https://doi.org/10.3390/nu17132183






