Associations of Dietary Protein Intake and Amino Acid Patterns with the Risk of Diabetic Kidney Disease in Adults with Type 2 Diabetes: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
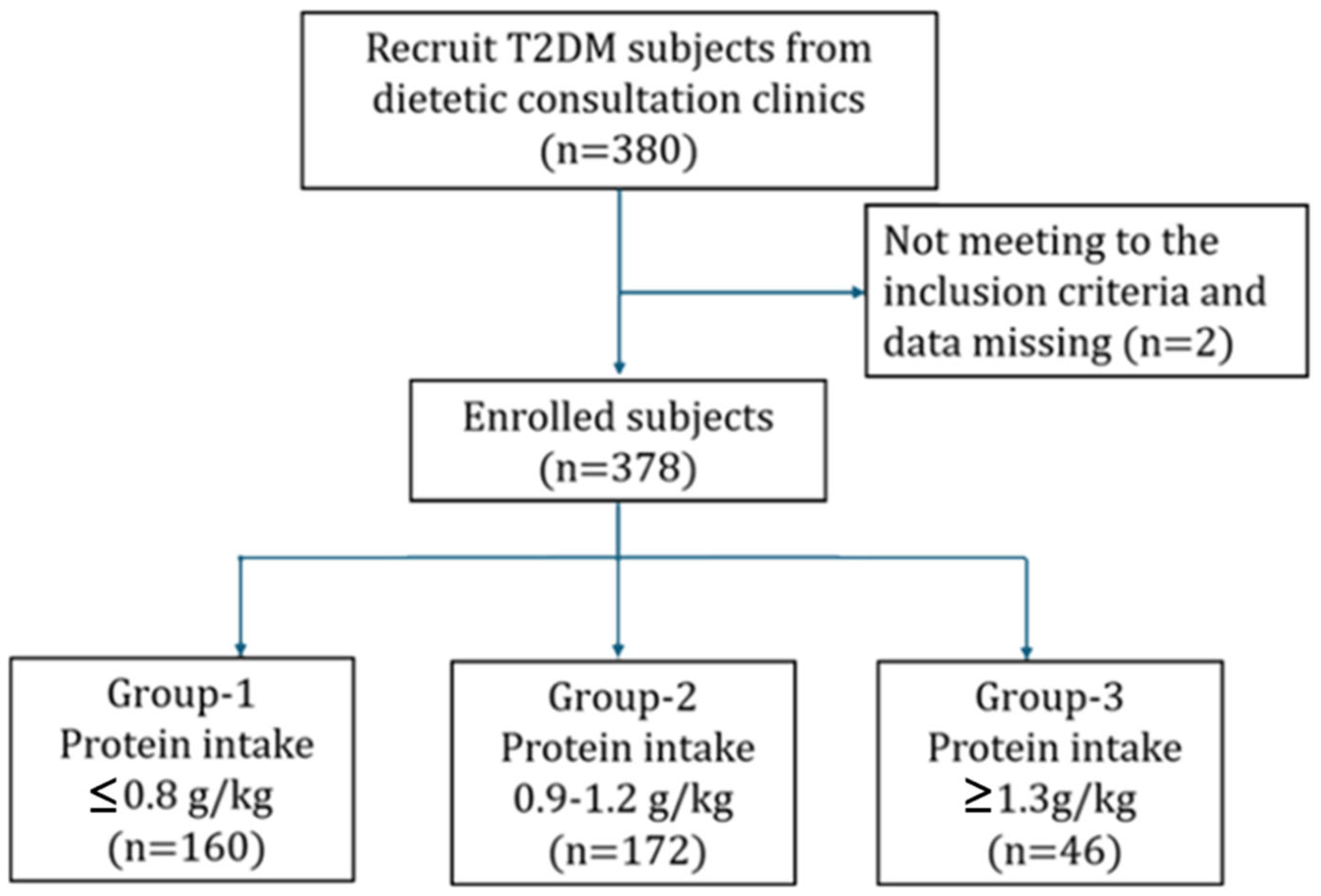
2.1. Study Participants and Data Collection
2.2. Assessment of Habitual Dietary Intake Using a Semi-Quantitative Food Frequency Questionnaire
2.3. Nutrient Computation and Data Quality Assurance
2.4. Anthropometry and Blood Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Comparison of Demographic, Anthropometric, and Biochemical Characteristics Among Different Protein Intake Groups in Patients with Type 2 Diabetes
3.2. Comparison of Total Protein and Individual Amino Acid Intake Across Protein Intake Groups
3.3. Association of Protein and Amino Acid Intake with DKD Risk
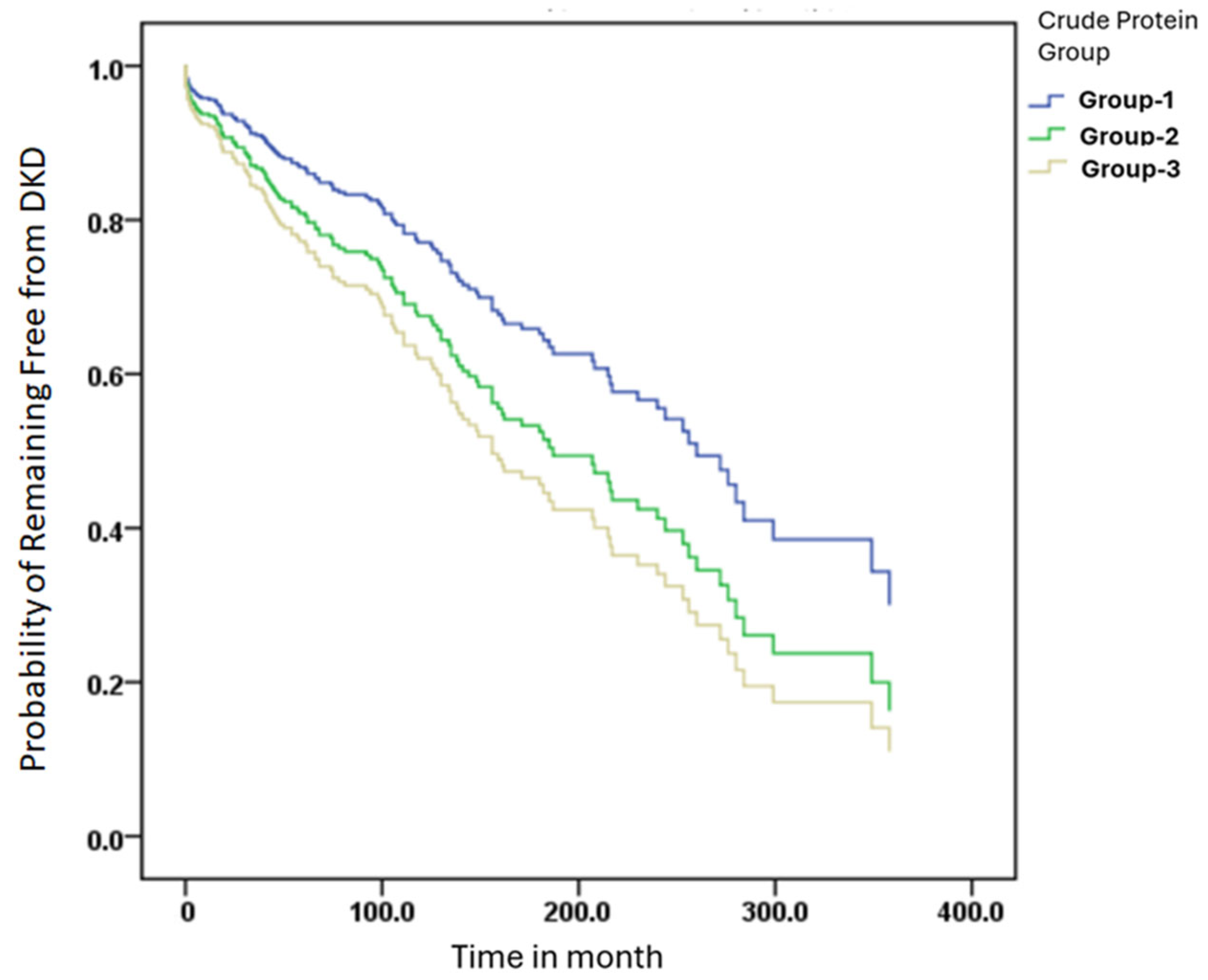
3.4. Kaplan–Meier Curves for DKD-Free Survival Across Different Crude Protein Intake
3.5. Adjusted Cox Models Suggest an Inverse Association Between Ketogenic Amino Acid Intake and DKD Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | aromatic amino acids |
| ANOVA | analysis of variance |
| BCAA | branched-chain amino acids |
| BCAA/AAA | branched-chain to aromatic amino acids ratio |
| BCKA | branched-chain keto acids |
| BMI | body mass index |
| BUN | blood urea nitrogen |
| DKD | diabetic kidney disease |
| eGFR | estimated glomerular filtration rate |
| ESRD | end-stage renal disease |
| FFQ | Food Frequency Questionnaire |
| FPG | fasting plasma glucose |
| HbA1c | glycated hemoglobin |
| HR | hazard ratio |
| IS | indoxyl sulfate |
| K/DOQI | Kidney Disease Outcomes Quality Initiative |
| LPD-KA | low protein diet with ketoacid supplementation |
| MDRD | modification of diet in renal disease |
| PCS | p-cresyl sulfate |
| T2DM | type 2 diabetes mellitus |
| UACR | urine albumin-to-creatinine ratio |
| VLPD-KA | very low protein diet with ketoacid supplementation |
References
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Yen, F.S.; Lin, K.D.; Shin, S.J.; Hsu, Y.H.; Hsu, C.C.; Diabetes Kidney Disease Research Committee of the Diabetes Association of the Republic of China. Epidemsiological characteristics of diabetic kidney disease in Taiwan. J. Diabetes Investig. 2021, 12, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Petrazzuolo, A.; Sabiu, G.; Assi, E.; Maestroni, A.; Pastore, I.; Lunati, M.E.; Montefusco, L.; Loretelli, C.; Rossi, G.; Ben Nasr, M.; et al. Broadening horizons in mechanisms, management, and treatment of diabetic kidney disease. Pharmacol. Res. 2023, 190, 106710. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Bernier-Jean, A.; Prince, R.L.; Lewis, J.R.; Craig, J.C.; Hodgson, J.M.; Lim, W.H.; Teixeira-Pinto, A.; Wong, G. Dietary plant and animal protein intake and decline in estimated glomerular filtration rate among elderly women: A 10-year longitudinal cohort study. Nephrol. Dial. Transpl. 2021, 36, 1640–1647. [Google Scholar] [CrossRef]
- Michail, A.; Andreou, E. A Plant-Dominant Low-Protein Diet in Chronic Kidney Disease Management: A Narrative Review with Considerations for Cyprus. Nutrients 2025, 17, 970. [Google Scholar] [CrossRef]
- Oosterwijk, M.M.; Soedamah-Muthu, S.S.; Geleijnse, J.M.; Bakker, S.J.L.; Navis, G.; Binnenmars, S.H.; Gant, C.M.; Laverman, G.D. High Dietary Intake of Vegetable Protein Is Associated with Lower Prevalence of Renal Function Impairment: Results of the Dutch DIALECT-1 Cohort. Kidney Int. Rep. 2019, 4, 710–719. [Google Scholar] [CrossRef]
- Rossing, P.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- Oosterwijk, M.M.; Groothof, D.; Navis, G.; Bakker, S.J.; Laverman, G.D. High-normal protein intake is not associated with faster renal function deterioration in patients with type 2 diabetes: A prospective analysis in the DIALECT cohort. Diabetes Care 2022, 45, 35–41. [Google Scholar] [CrossRef]
- Heyman, S.N.; Raz, I.; Dwyer, J.P.; Weinberg Sibony, R.; Lewis, J.B.; Abassi, Z. Diabetic proteinuria revisited: Updated physiologic perspectives. Cells 2022, 11, 2917. [Google Scholar] [CrossRef]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 2467. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, I.; Ardavani, A.; Vanweert, F.; Mellett, A.; Atherton, P.J.; Idris, I. The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients 2022, 14, 4411. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Chen, S.; Li, Y.; Han, H.; Gao, J.; Liu, G.; Wu, X.; Li, T.; Woo Kim, S.; et al. Metabolic Regulation of Methionine Restriction in Diabetes. Mol. Nutr. Food Res. 2018, 62, e1700951. [Google Scholar] [CrossRef]
- Lin, S.-P.; Chen, C.-M.; Wang, K.-L.; Wu, K.-L.; Li, S.-C. Association of Dietary Fish and n-3 Unsaturated Fatty Acid Consumption with Diabetic Nephropathy from a District Hospital in Northern Taiwan. Nutrients 2022, 14, 2148. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S17–S38. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Zhang, Z.; Ren, D.; Wu, Y.; Wang, D.; Zhang, Y.; Zhao, S.; Chen, Q.; Wang, T. Metabolic homeostasis of amino acids and diabetic kidney disease. Nutrients 2022, 15, 184. [Google Scholar] [CrossRef]
- Leto, G.; Tartaglione, L.; Rotondi, S.; Pasquali, M.; Maddaloni, E.; Mignogna, C.; D’Onofrio, L.; Zampetti, S.; Carlone, A.; Muci, M.L. Diastolic Pressure and ACR Are Modifiable Risk Factors of Arterial Stiffness in T2DM Without Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2022, 107, e3857–e3865. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of care in diabetes—2024. Diabetes Care 2024, 47, S179–S218. [Google Scholar] [CrossRef]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014, 37, S120–S143. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Yeh, C.-Y.; Yen, T.-Y.; Chen, C.-C.; Chen, J.-F.; Chu, C.-H.; Huang, C.-N.; Lin, C.-L.; Lin, S.-Y.; Liu, F.-H. The expert consensus on care and education for patients with diabetic kidney disease in Taiwan. Prim. Care Diabetes 2024, 18, 284–290. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S219–S230. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Soulage, C.O. The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef]
- Mi, N.; Zhang, X.J.; Ding, Y.; Li, G.H.; Wang, W.D.; Xian, H.X.; Xu, J. Branched-chain amino acids attenuate early kidney injury in diabetic rats. Biochem. Biophys. Res. Commun. 2015, 466, 240–246. [Google Scholar] [CrossRef]
- Koppe, L.; Soulage, C.O. The impact of dietary nutrient intake on gut microbiota in the progression and complications of chronic kidney disease. Kidney Int. 2022, 102, 728–739. [Google Scholar] [CrossRef]
- Menni, C.; Zhu, J.; Le Roy, C.I.; Mompeo, O.; Young, K.; Rebholz, C.M.; Selvin, E.; North, K.E.; Mohney, R.P.; Bell, J.T. Serum metabolites reflecting gut microbiome alpha diversity predict type 2 diabetes. Gut Microbes 2020, 11, 1632–1642. [Google Scholar] [CrossRef]
- Gonzalez, P.; Lozano, P.; Solano, F. Unraveling the metabolic hallmarks for the optimization of protein intake in pre-dialysis chronic kidney disease patients. Nutrients 2022, 14, 1182. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Lu, L.; Wang, J.; Liu, D.; Liu, Z. Metabolomic profiling of amino acids in human plasma distinguishes diabetic kidney disease from type 2 diabetes mellitus. Front. Med. 2021, 8, 765873. [Google Scholar] [CrossRef]
- Lew, Q.-L.J.; Jafar, T.H.; Koh, H.W.L.; Jin, A.; Chow, K.Y.; Yuan, J.-M.; Koh, W.-P. Red meat intake and risk of ESRD. J. Am. Soc. Nephrol. 2017, 28, 304–312. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Patterson, R.E.; Kalhorn, T.F.; Skor, H.E.; Howald, W.N.; Lampe, J.W. Validation of a soy food frequency questionnaire with plasma concentrations of isoflavones in US adults. J. Am. Diet. Assoc. 2002, 102, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Deibert, P.; Lutz, L.; Konig, D.; Zitta, S.; Meinitzer, A.; Vitolins, M.Z.; Becker, G.; Berg, A. Acute effect of a soy protein-rich meal-replacement application on renal parameters in patients with the metabolic syndrome. Asia Pac. J. Clin. Nutr. 2011, 20, 527–534. [Google Scholar] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-supplemented vegetarian very low–protein diet and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Li, Q.; Wen, F.; Wang, Y.; Li, S.; Lin, S.; Qi, C.; Chen, Z.; Qiu, X.; Zhang, Y.; Zhang, S. Diabetic kidney disease benefits from intensive low-protein diet: Updated systematic review and meta-analysis. Diabetes Ther. 2021, 12, 21–36. [Google Scholar] [CrossRef]
- Asghari, G.; Teymoori, F.; Farhadnejad, H.; Mirmiran, P.; Azizi, F. Dietary amino acid patterns are associated with incidence of chronic kidney disease. J. Ren. Nutr. 2022, 32, 312–318. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Gu, L.; Bao, J.; Yin, J.; Tang, Z.; Wang, L.; Yuan, W. Effect of a low-protein diet supplemented with ketoacids on skeletal muscle atrophy and autophagy in rats with type 2 diabetic nephropathy. PLoS ONE 2013, 8, e81464. [Google Scholar] [CrossRef][Green Version]
- Shah, A.P.; Kalantar-Zadeh, K.; Kopple, J.D. Is there a role for ketoacid supplements in the management of CKD? Am. J. Kidney Dis. 2015, 65, 659–673. [Google Scholar] [CrossRef]
- Wang, M.; Xu, H.; Chong Lee Shin, O.L.-S.; Li, L.; Gao, H.; Zhao, Z.; Zhu, F.; Zhu, H.; Liang, W.; Qian, K.; et al. Compound α-keto acid tablet supplementation alleviates chronic kidney disease progression via inhibition of the NF-kB and MAPK pathways. J. Transl. Med. 2019, 17, 122. [Google Scholar] [CrossRef]
- Chen, N.; Jin, Y.; Ren, H.; Xu, J.; Shen, P.; Huang, X. Anti-inflammatory effects of low protein diet supplemented with keto-amino acid in the treatment of type 2 diabetic nephropathy. Kidney Res. Clin. Pract. 2012, 31, A24. [Google Scholar] [CrossRef][Green Version]
- Lobel, L.; Cao, Y.G.; Fenn, K.; Glickman, J.N.; Garrett, W.S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 2020, 369, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, M.; Li, L.; Gao, X.; Yang, B.; Mei, S.; Fu, L.; Mei, C. Low-protein diet supplemented with ketoacids delays the progression of diabetic nephropathy by inhibiting oxidative stress in the KKAy mice model. Br. J. Nutr. 2018, 119, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Benoit, B.; Bres, E.; Chanon, S.; Vieille-Marchiset, A.; Pinteur, C.; Pesenti, S.; Glorieux, G.; Picard, C.; Fouque, D.; et al. A low aromatic amino-acid diet improves renal function and prevent kidney fibrosis in mice with chronic kidney disease. Sci. Rep. 2021, 11, 19184. [Google Scholar] [CrossRef]
- Letourneau, P.; Bataille, S.; Chauveau, P.; Fouque, D.; Koppe, L. Source and Composition in Amino Acid of Dietary Proteins in the Primary Prevention and Treatment of CKD. Nutrients 2020, 12, 3892. [Google Scholar] [CrossRef]
- Li, M.R.; Sun, Z.J.; Chang, D.Y.; Yu, X.J.; Wang, S.X.; Chen, M.; Zhao, M.H. C3c deposition predicts worse renal outcomes in patients with biopsy-proven diabetic kidney disease in type 2 diabetes mellitus. J. Diabetes 2022, 14, 291–297. [Google Scholar] [CrossRef]
- Zhao, Z.; Huo, L.; Wang, L.; Wang, L.; Fu, Z.; Li, Y.; Wu, X. Survival of Chinese people with type 2 diabetes and diabetic kidney disease: A cohort of 12 -year follow-up. BMC Public Health 2019, 19, 1498. [Google Scholar] [CrossRef]
| All n = 378 | Group-1 | Group-2 | Group-3 | p-Value | |
|---|---|---|---|---|---|
| ≤0.8 g/kg n = 160 | 0.9–1.2 g/kg n = 172 | ≥1.3 g/kg n = 46 | |||
| Number (%) | 378 (100) | 160 (42.8) | 172 (45.5) | 46 (12.2) | |
| DM | 237 (62.7) | 90 (37.9) | 114 (48.1) | 33 (13.9) | 0.068 |
| DKD | 141 (37.3) | 70 (49.6) | 58 (41.1) | 13 (9.2) | |
| Male | 189 (50) | 23 (12.2) | 128 (67.7) | 38 (20.1) | 0.390 |
| Female | 189 (50) | 37 (19.5) | 127 (67.2) | 25 (13.2) | |
| Age (years) | 63.4 ± 11.6 | 62.9 ± 12.6 | 63.9 ± 10.8 | 63.6 ± 11.1 | 0.716 |
| Body height (cm) | 160.7 ± 9.0 | 161.7 ± 9.0 | 160.5 ± 9.1 | 158.3 ± 8.6 | 0.061 |
| Body weight (kg) | 68.3 ± 15.6 | 74.4 ± 16.5 a | 65.9 ± 13.3 b | 56.6 ± 10.6 c | <0.001 * |
| Body mass index (kg/m2) | 26.2 ± 5.0 | 28.3 ± 5.2 a | 25.3 ± 4.4 b | 22.5 ± 2.9 c | <0.001 * |
| Diastolic blood pressure (mm Hg) | 131.2 ± 14.5 | 132.9 ± 13.8 | 130.3 ± 14.8 | 128.9 ± 15.8 | 0.151 |
| Systolic blood pressure (mm Hg) | 77.3 ± 10.5 | 78.3 ± 10.9 | 76.4 ± 10.4 | 77.8 ± 9.3 | 0.278 |
| Creatinine (mg/dL) | 1.0 ± 0.7 | 1.1 ± 0.8 a | 1.0 ± 0.6 ab | 0.9 ± 0.8 b | 0.035 * |
| eGFR (mL/min/1.73 m2) | 82.4 ± 29.4 | 78.0 ± 30.7 a | 84.9 ± 29.0 ab | 88.6 ± 23.8 b | 0.033 * |
| UACR (mg/dL) | 208.5 ± 738.7 | 306.9 ± 959.6 | 146.3 ± 548.7 | 98.71 ± 336.1 | 0.079 |
| Microalbumin (mg/dL) | 17.3 ± 58.7 | 26.4 ± 77.4 a | 10.8 ± 39.6 b | 9.8 ± 35.0 b | 0.034 * |
| Spot urine creatine (mg/dL) | 96.7 ± 61.6 | 103.0 ± 60.7 | 93.0 ± 62.2 | 88.9 ± 61.9 | 0.219 |
| HbA1c (%) | 7.4 ± 1.4 | 7.6 ± 1.5 a | 7.2 ± 1.3 b | 7.4 ± 1.2 ab | 0.037 * |
| Fasting plasma glucose (mg/dL) | 132.8 ± 39.9 | 136.1 ± 48.0 | 130.3 ± 33.4 | 130.5 ± 29.6 | 0.380 |
| Triglyceride (mg/dL) | 143.4 ± 146.7 | 148.0 ± 88.1 | 145.5 ± 197.6 | 119.4 ± 61.4 | 0.491 |
| Cholesterol (mg/dL) | 154.6 ± 38.7 | 154.0 ± 41.8 | 152.1 ± 35.1 | 165.6 ± 39.4 | 0.108 |
| All | Group-1 | Group-2 | Group-3 | |
|---|---|---|---|---|
| ≤0.8 g/kg | 0.9–1.2 g/kg | ≥1.3 g/kg | ||
| Number % | 378 | 160 (15.8) | 172 (67.5) | 46 (16.7) |
| Crude protein (g) | 61.2 ± 17.5 | 49.1 ± 13.1 | 66.9 ± 13.7 | 82.3 ± 12.3 |
| Total hydrolyzed amino acids (g) | 58.9 ± 16.7 | 47.4 ± 12.7 | 64.3 ± 13.1 | 78.8 ± 11.7 |
| Aspartic acid (Asp) (g) | 5.4 ± 1.5 | 4.3 ± 1.1 | 5.9 ± 1.2 | 7.2 ± 1.1 |
| Threonine (Thr) (g) | 2.4 ± 0.7 | 1.9 ± 0.5 | 2.6 ± 0.6 | 3.6 ± 0.5 |
| Serine (Ser) (g) | 2.7 ± 0.7 | 2.2 ± 0.6 | 2.9 ± 0.6 | 3.4 ± 0.6 |
| Glutamic acid (Glu) (g) | 10.8 ± 3.1 | 8.7 ± 2.4 | 11.8 ± 2.4 | 14.4 ± 2.3 |
| Proline (Pro) (g) | 3.4 ± 1.0 | 2.7 ± 0.9 | 3.7 ± 0.8 | 4.5 ± 0.8 |
| Glycine (Gly) (g) | 2.7 ± 0.8 | 2.2 ± 0.6 | 3.0 ± 0.6 | 3.7 ± 0.6 |
| Alanine (Ala) (g) | 3.1 ± 0.9 | 2.5 ± 0.7 | 3.4 ± 0.7 | 4.2 ± 0.7 |
| Cystine (Cys) (g) | 1.8 ± 0.5 | 1.5 ± 0.5 | 1.9 ± 0.4 | 2.3 ± 0.4 |
| Valine (Val) (g) | 2.9 ± 0.8 | 2.4 ± 0.6 | 3.2 ± 0.7 | 3.9 ± 0.6 |
| Methionine (Met) (g) | 1.3 ± 0.4 | 1.1 ± 0.3 | 1.4 ± 0.3 | 1.8 ± 0.3 |
| Isoleucine (Ile) (g) | 2.6 ± 0.7 | 2.1 ± 0.6 | 2.8 ± 0.6 | 3.5 ± 0.5 |
| Leucine (Leu) (g) | 4.8 ± 1.4 | 3.8 ± 1.0 | 5.2 ± 1.1 | 6.4 ± 0.9 |
| Tyrosine (Tyr) (g) | 2.2 ± 0.6 | 1.8 ± 0.5 | 2.4 ± 0.5 | 3.0 ± 0.4 |
| Phenyalanine (Phe) (g) | 2.7 ± 0.8 | 2.2 ± 0.6 | 3.0 ± 0.6 | 3.6 ± 0.5 |
| Lysine (Lys) (g) | 3.9 ± 1.2 | 3.1 ± 0.9 | 4.3 ± 1.0 | 5.3 ± 0.9 |
| Histidine (His) (g) | 1.7 ± 0.5 | 1.4 ± 0.4 | 1.9 ± 0.4 | 2.3 ± 0.3 |
| Arginine (Arg) (g) | 3.8 ± 1.1 | 3.1 ± 0.8 | 4.2 ± 0.9 | 5.1 ± 0.8 |
| Tryptophan (Trp) (g) | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 |
| Branched-chain amino acids (g) | 10.3 ± 2.9 | 8.3 ± 2.2 | 11.2 ± 2.3 | 13.8 ± 2.0 |
| Aromatic amino acids (g) | 5.5 ± 1.5 | 4.5 ± 1.2 | 6.0 ± 1.2 | 7.4 ± 1.1 |
| BCAA/AAA | 1.9 ± 0.0 | 1.8 ± 0.0 | 1.9 ± 0.0 | 1.9 ± 0.0 |
| Ketogenic amino acids (g) | 8.7 ± 2.6 | 7.0 ± 1.9 | 9.5 ± 2.1 | 11.7 ± 1.8 |
| Covariate | β | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|
| Crude protein (g/kg) a | −0.001 | 0.999 | 0.998–1.000 | 0.001 * |
| BCAA (g/kg) | −0.002 | 0.994 | 0.990–0.997 | 0.001 * |
| AAA (g/kg) | −0.012 | 0.988 | 0.981–0.995 | 0.001 * |
| Ketogenic amino acids (g/kg) b | −0.008 | 0.992 | 0.988–0.997 | 0.001 * |
| Covariate | β | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|
| Group-1 (≤0.8 g/kg) | ||||
| BCAA (g/kg) | ||||
| Unadjusted | −0.015 | 0.985 | 0.974–0.996 | 0.006 * |
| Model-1 | −0.018 | 0.982 | 0.971–0.993 | 0.001 * |
| Model-2 | −0.018 | 0.982 | 0.972–0.993 | 0.001 * |
| Model-3 | −0.013 | 0.987 | 0.976–0.999 | 0.031 * |
| AAA (g/kg) | ||||
| Unadjusted | −0.027 | 0.973 | 0.953–0.993 | 0.009 * |
| Model-1 | −0.033 | 0.967 | 0.948–0.987 | 0.002 * |
| Model-2 | −0.032 | 0.969 | 0.949–0.988 | 0.002 * |
| Model-3 | −0.023 | 0.977 | 0.956–0.999 | 0.042 * |
| Ketogenic Amino Acids (g/kg) | ||||
| Unadjusted | −0.018 | 0.982 | 0.970–0.994 | 0.004 * |
| Model-1 | −0.021 | 0.979 | 0.967–0.991 | 0.001 * |
| Model-2 | −0.021 | 0.979 | 0.967–0.992 | 0.001 * |
| Model-3 | −0.016 | 0.984 | 0.971–0.997 | 0.018 * |
| Group-2 (0.9–1.2 g/kg) | ||||
| BCAA (g/kg) | ||||
| Unadjusted | −0.014 | 0.986 | 0.973–1.000 | 0.043 * |
| Model-1 | −0.018 | 0.982 | 0.971–0.993 | 0.001 * |
| Model-2 | −0.015 | 0.985 | 0.972–0.999 | 0.038 * |
| Model-3 | −0.010 | 0.990 | 0.976–1.005 | 0.198 |
| AAA (g/kg) | ||||
| Unadjusted | −0.026 | 0.974 | 0.949–0.999 | 0.044 * |
| Model-1 | −0.033 | 0.967 | 0.948–0.987 | 0.002 * |
| Model-2 | −0.027 | 0.973 | 0.948–0.999 | 0.041 * |
| Model-3 | −0.018 | 0.982 | 0.955–1.010 | 0.211 |
| Ketogenic Amino Acids (g/kg) | ||||
| Unadjusted | −0.015 | 0.985 | 0.970–0.999 | 0.042 * |
| Model-1 | −0.017 | 0.984 | 0.968–0.999 | 0.038 * |
| Model-2 | −0.017 | 0.983 | 0.968–0.999 | 0.036 * |
| Model-3 | −0.011 | 0.989 | 0.972–1.006 | 0.191 |
| Group-3 (≥1.3 g/kg) | ||||
| BCAA (g/kg) | ||||
| Unadjusted | −0.015 | 0.985 | 0.962–1.009 | 0.214 |
| Model-1 | −0.042 | 0.959 | 0.913–1.007 | 0.093 |
| Model-2 | −0.015 | 0.985 | 0.959–1.012 | 0.270 |
| Model-3 | −0.016 | 0.984 | 0.953–1.017 | 0.343 |
| AAA (g/kg) | ||||
| Unadjusted | −0.030 | 0.970 | 0.927–1.015 | 0.189 |
| Model-1 | −0.041 | 0.960 | 0.911–1.011 | 0.121 |
| Model-2 | −0.035 | 0.966 | 0.915–1.020 | 0.210 |
| Model-3 | −0.032 | 0.968 | 0.910–1.030 | 0.311 |
| Ketogenic Amino Acids (g/kg) | ||||
| Unadjusted | −0.016 | 0.984 | 0.959–1.010 | 0.221 |
| Model-1 | −0.021 | 0.980 | 0.953–1.007 | 0.145 |
| Model-2 | −0.016 | 0.984 | 0.955–1.014 | 0.281 |
| Model-3 | −0.017 | 0.984 | 0.951–1.018 | 0.343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-P.; Chen, C.-M.; Chiu, S.-H.; Hsiao, P.-J.; Liu, K.-T.; Li, S.-C. Associations of Dietary Protein Intake and Amino Acid Patterns with the Risk of Diabetic Kidney Disease in Adults with Type 2 Diabetes: A Cross-Sectional Study. Nutrients 2025, 17, 2168. https://doi.org/10.3390/nu17132168
Lin S-P, Chen C-M, Chiu S-H, Hsiao P-J, Liu K-T, Li S-C. Associations of Dietary Protein Intake and Amino Acid Patterns with the Risk of Diabetic Kidney Disease in Adults with Type 2 Diabetes: A Cross-Sectional Study. Nutrients. 2025; 17(13):2168. https://doi.org/10.3390/nu17132168
Chicago/Turabian StyleLin, Shih-Ping, Chiao-Ming Chen, Szu-Han Chiu, Po-Jen Hsiao, Kuang-Ting Liu, and Sing-Chung Li. 2025. "Associations of Dietary Protein Intake and Amino Acid Patterns with the Risk of Diabetic Kidney Disease in Adults with Type 2 Diabetes: A Cross-Sectional Study" Nutrients 17, no. 13: 2168. https://doi.org/10.3390/nu17132168
APA StyleLin, S.-P., Chen, C.-M., Chiu, S.-H., Hsiao, P.-J., Liu, K.-T., & Li, S.-C. (2025). Associations of Dietary Protein Intake and Amino Acid Patterns with the Risk of Diabetic Kidney Disease in Adults with Type 2 Diabetes: A Cross-Sectional Study. Nutrients, 17(13), 2168. https://doi.org/10.3390/nu17132168








