Preventive Effects of Medium-Chain Fatty Acid Intake on Muscle Atrophy
Highlights
- Coconut oil-derived medium-chain fatty acids (MCFAs) attenuated muscle atrophy induced by a high-fat diet in mice.
- Mice fed a coconut oil diet exhibited lower body weight gain, reduced blood glucose and total cholesterol levels, and improved relative grip strength compared with lard-fed mice.
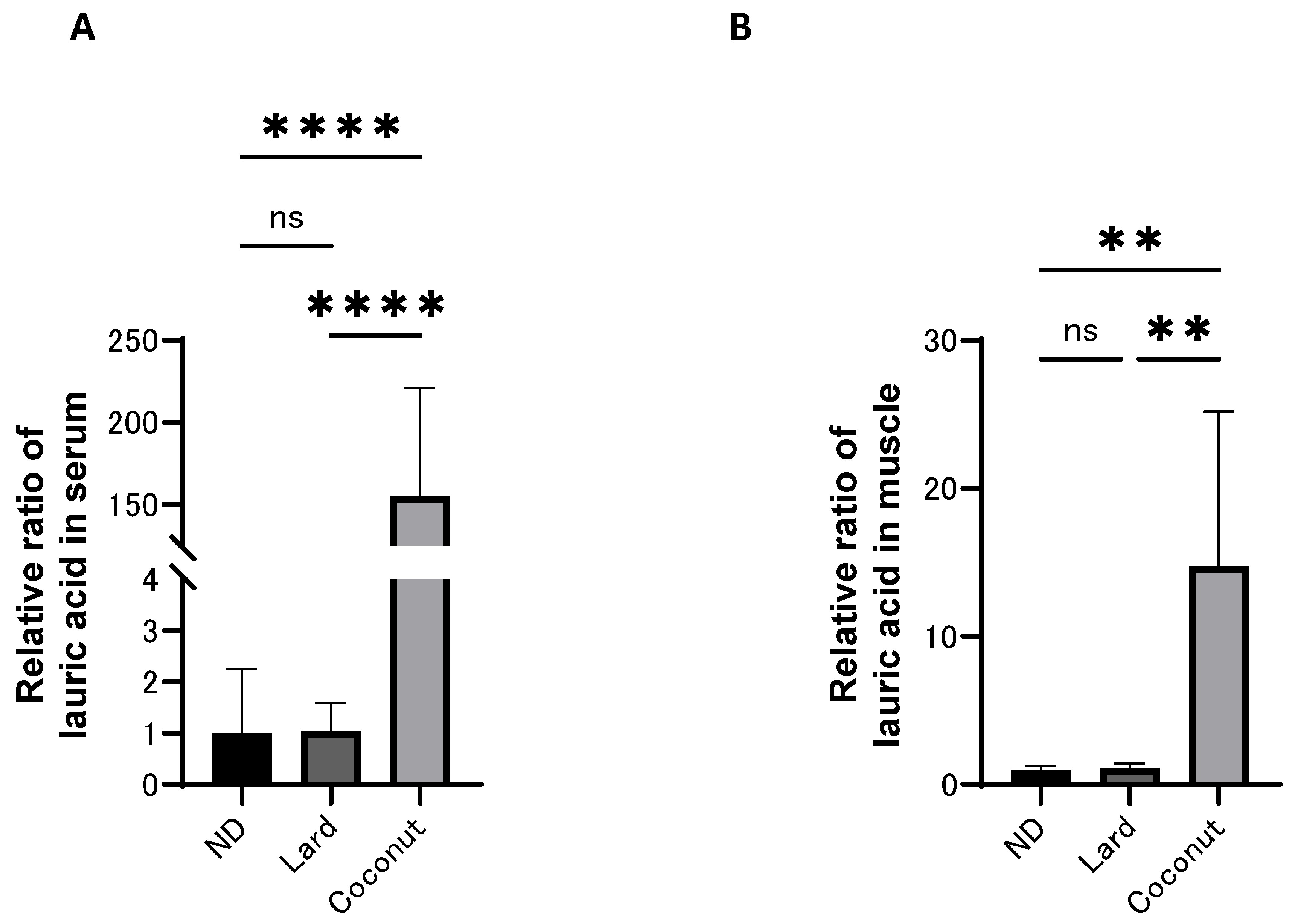
- MCFA concentrations, especially lauric acid (C12), were significantly increased in both serum and muscle tissues of coconut oil-fed mice.
- Expression of muscle atrophy-associated genes and proteins was downregulated by MCFAs in both in vivo and C2C12 cell models.
- These findings highlight the potential of coconut oil as a dietary intervention for preventing long-chain fatty acid-induced muscle atrophy.
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.1.1. Sample Size Estimation
2.1.2. Analytical Procedures and Glucose and Insulin Tolerance Tests
2.1.3. Biochemistry
2.1.4. Grip Strength
2.1.5. Histology of the Plantaris and Soleus Muscle
2.1.6. Determination of MCFAs Levels in the Serum and Muscle
2.1.7. Protein Extraction and Western Blot Analysis
2.1.8. Gene Expression Analysis in the Muscle
2.1.9. Culturing of Murine Myocytes of Skeletal Muscle
2.1.10. Protein Extraction and Western Blot Analysis of C2C12 Myotube Cells
2.1.11. Gene Expression Analysis in C2C12 Myotube Cells
2.2. Statistical Analysis
3. Results
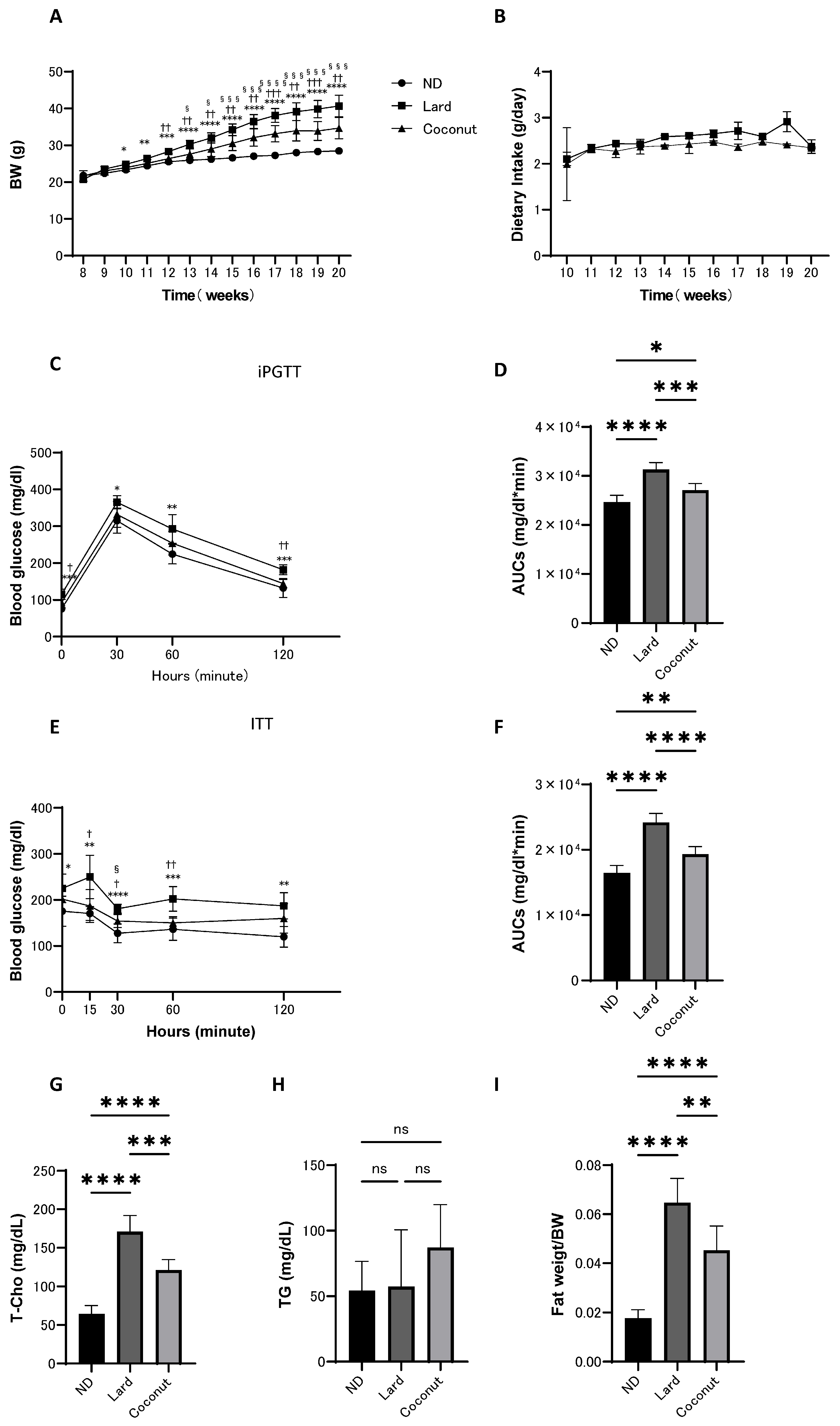
3.1. The Coconut Oil Diet Group Exhibited Reduced Body Weight Gain and Lower Glucose Levels Compared to the Lard Diet Group
3.2. The Coconut Oil Diet Group Exhibited a Decrease in T-Cho Levels and a Reduction in Fat Mass Compared to the Lard Diet Group
3.3. The Coconut Oil Diet Group Exhibited Higher Grip Strength than the Lard Diet Group
3.4. The Coconut Oil Diet Group Exhibited Higher Concentrations of MCFAs in Both Serum and Muscle Compared to the Lard Diet Group
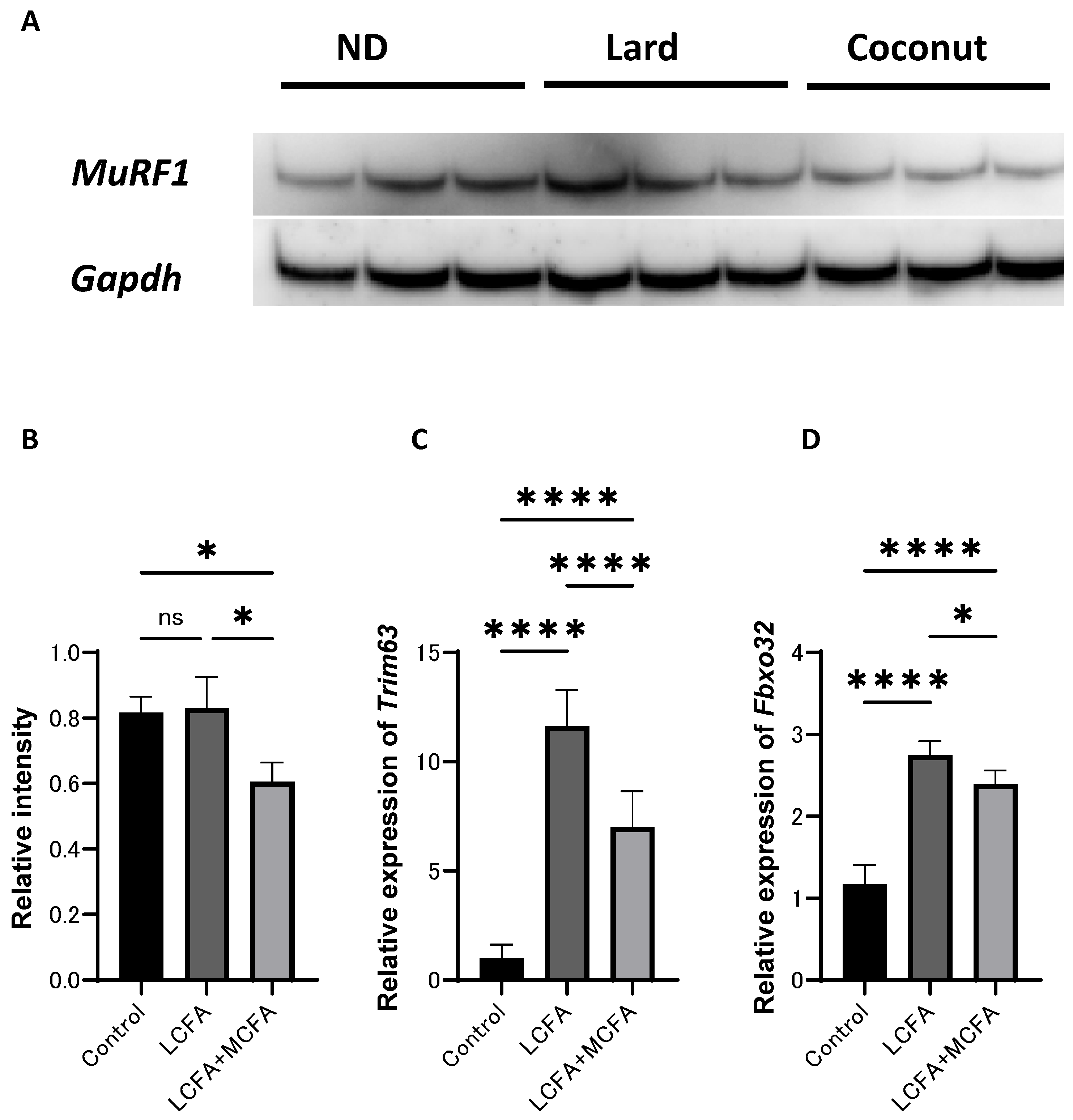
3.5. The Coconut Oil Diet Group Exhibited Lower Expression Levels of a Protein Implicated in Muscle Atrophy and Gene in Muscle Tissue Compared to the Lard Diet Group
3.6. The Coconut Oil Diet Group Exhibited Lower Expression Levels of Proteins and Genes Implicated in Muscle Atrophy in C2C12 Myotube Cells Compared to the Lard Diet Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, S.; Tsujino, S. Applications of Medium-Chain Triglycerides in Foods. Front. Nutr. 2022, 9, 802805. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Hernández, E.; Chávez-Tapia, N.C.; Uribe, M.; Barbero-Becerra, V.J. Role of bioactive fatty acids in nonalcoholic fatty liver disease. Nutr. J. 2016, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Paul, H.A.; Hart, D.A.; Reimer, R.A.; Smith, I.C.; Rios, J.L.; Seerattan, R.A.; Herzog, W. A High-Fat High-Sucrose Diet Rapidly Alters Muscle Integrity, Inflammation and Gut Microbiota in Male Rats. Sci. Rep. 2016, 6, 37278. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Du, N.; Hu, J.; Ning, F.; Mei, X.; Li, Q.; Peng, L. Intramuscular accumulation of pentadecanoic acid activates AKT1 to phosphorylate NCOR1 and triggers FOXM1-mediated apoptosis in the pathogenesis of sarcopenia. Am. J. Transl. Res. 2020, 12, 5064–5079. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7540105/ (accessed on 17 March 2025). [PubMed]
- Tan, Y.; Liu, X.; Yang, Y.; Li, B.; Yu, F.; Zhao, W.; Fu, C.; Yu, X.; Han, Z.; Cheng, M. Metabolomics analysis reveals serum biomarkers in patients with diabetic sarcopenia. Front. Endocrinol. 2023, 14, 1119782. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-chain triglycerides (8:0 and 10:0) are promising nutrients for sarcopenia: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ohmori, H.; Luo, Y.; Mori, S.; Miyagawa, Y.; Nukaga, S.; Goto, K.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; et al. Giving combined medium-chain fatty acids and glucose protects against cancer-associated skeletal muscle atrophy. Cancer Sci. 2019, 10, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of Three Types of Mixed Anesthetic Agents Alternate to Ketamine in Mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Claustre, A.; Malige, M.; Macheton, M.; Combaret, L.; Lefai, E.; Fafournoux, P.; Taillandier, D.; Henri, J.; Polge, C. Structure predictions of MuRF1-UBE2 complexes identify amino acid residues governing interaction selectivity for each MuRF1-E2 pair. FEBS J. 2025, 292, 2559–2577. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, E.; He, L.; Wang, Y.; Xiang, Y.; Zhang, P.; Liu, X.; Yin, J. Dietary Supplementation with Lauric Acid Improves Aerobic Endurance in Sedentary Mice via Enhancing Fat Mobilization and Glyconeogenesis. J. Nutr. 2023, 153, 3207–3219. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Han, X.; Zhou, F.; Guo, J.; Huang, W.; Zhan, J.; You, Y. Coconut oil and medium-chain fatty acids attenuate high-fat diet-induced obesity in mice through increased thermogenesis by activating brown adipose tissue. Front. Nutr. 2022, 9, 896021. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, H.; Ohue-Kitano, R.; Masujima, Y.; Igarashi, M.; Kimura, I. Dietary Medium-Chain Triglyceride Decanoate Affects Glucose Homeostasis Through GPR84-Mediated GLP-1 Secretion in Mice. Front. Nutr. 2022, 9, 848450. [Google Scholar] [CrossRef] [PubMed]
- Kötter, S.; Andresen, C.; Krüger, M. Titin: Central player of hypertrophic signaling and sarcomeric protein quality control. Biol. Chem. 2014, 395, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Inai, M.; Takagi, T.; Nonaka, Y.; Urashima, S.; Honda, K.; Aoyama, T.; Terada, S. Preventive Effects of the Dietary Intake of Medium-chain Triacylglycerols on Immobilization-induced Muscle Atrophy in Rats. J. Oleo Sci. 2017, 66, 917–924. [Google Scholar] [CrossRef] [PubMed]


| Lard | Coconut Oil | |
|---|---|---|
| (%) | (%) | |
| Saturated | 40 | 90 |
| Caprylic acid (C8:0) | — | 8 |
| Capric acid (C10:0) | — | 7 |
| Lauric acid (C12:0) | — | 48 |
| Myristic acid (C14:0) | 2 | 16 |
| Palmitic acid (C16:0) | 27 | 9 |
| Stearic acid (C18:0) | 11 | 2 |
| Unsaturated | 59 | 9 |
| Oleic acid (C18:1 (n − 9)) | 44 | 7 |
| Linoleic acid (C18:2 (n − 6)) | 11 | 2 |
| Linolenic acid (C18:3) | — | — |
| Palmitoleic acid (C16:1 (n − 7)) | 4 | — |
| Other | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumi, M.; Okamura, T.; Matsuyama, T.; Miyoshi, T.; Nakajima, H.; Nakanishi, N.; Sasano, R.; Hamaguchi, M.; Fukui, M. Preventive Effects of Medium-Chain Fatty Acid Intake on Muscle Atrophy. Nutrients 2025, 17, 2154. https://doi.org/10.3390/nu17132154
Sumi M, Okamura T, Matsuyama T, Miyoshi T, Nakajima H, Nakanishi N, Sasano R, Hamaguchi M, Fukui M. Preventive Effects of Medium-Chain Fatty Acid Intake on Muscle Atrophy. Nutrients. 2025; 17(13):2154. https://doi.org/10.3390/nu17132154
Chicago/Turabian StyleSumi, Madoka, Takuro Okamura, Tomoyuki Matsuyama, Tomoki Miyoshi, Hanako Nakajima, Naoko Nakanishi, Ryoichi Sasano, Masahide Hamaguchi, and Michiaki Fukui. 2025. "Preventive Effects of Medium-Chain Fatty Acid Intake on Muscle Atrophy" Nutrients 17, no. 13: 2154. https://doi.org/10.3390/nu17132154
APA StyleSumi, M., Okamura, T., Matsuyama, T., Miyoshi, T., Nakajima, H., Nakanishi, N., Sasano, R., Hamaguchi, M., & Fukui, M. (2025). Preventive Effects of Medium-Chain Fatty Acid Intake on Muscle Atrophy. Nutrients, 17(13), 2154. https://doi.org/10.3390/nu17132154







