Anti-Inflammatory and Joint-Protective Effects of Blueberries in a Monosodium Iodoacetate (MIA)-Induced Rat Model of Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatment
2.3. Behavioral and Edema Analysis
2.4. Tissue Collection and Analysis
2.5. Serum Inflammatory and Cartilage Markers
2.6. Western Blot Analysis
2.7. Gene Expression Analysis
2.8. Histopathological Analysis
2.9. Statistical Analysis
3. Results
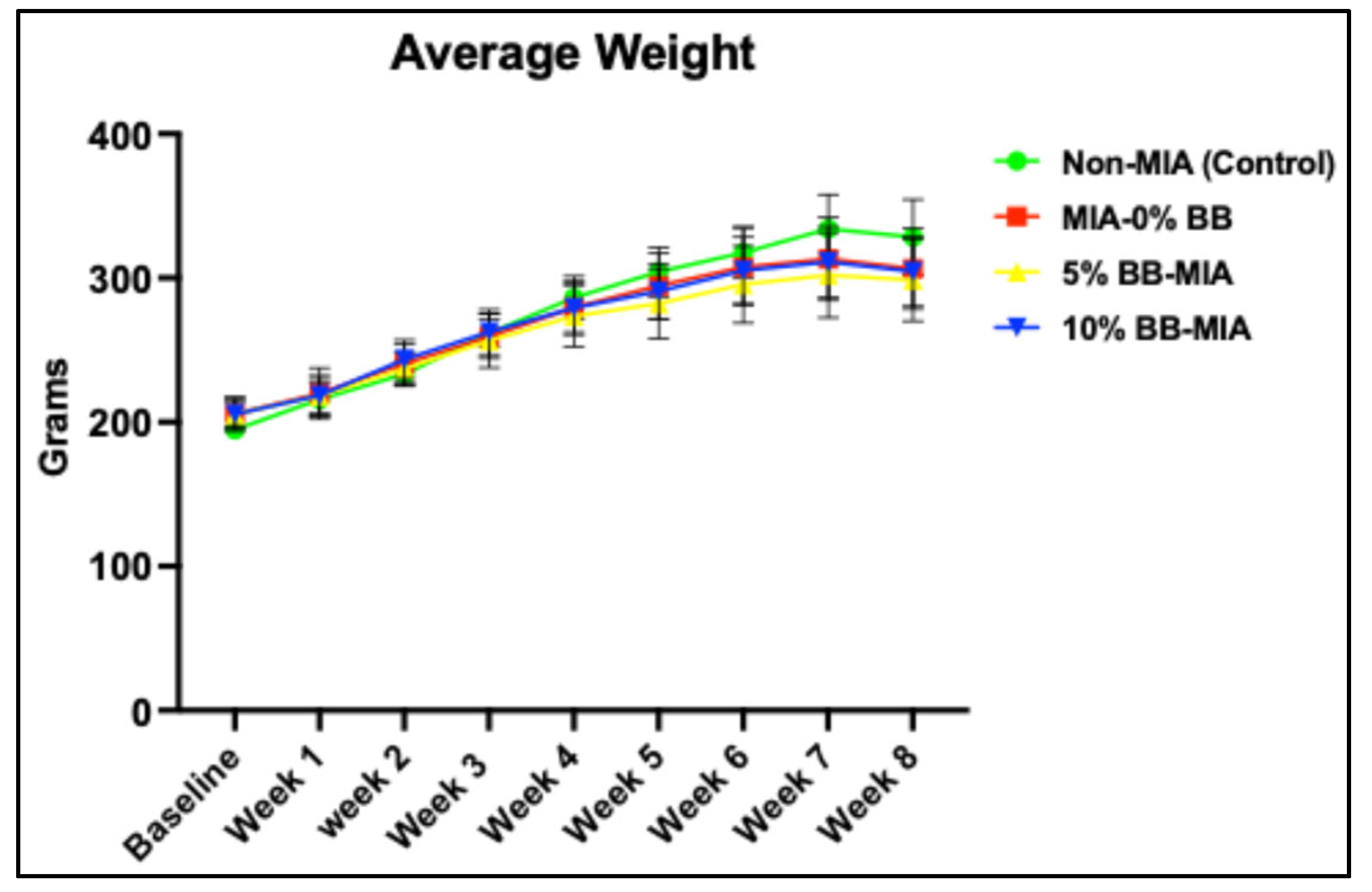
3.1. Effect of Blueberry Diet on Body Weight
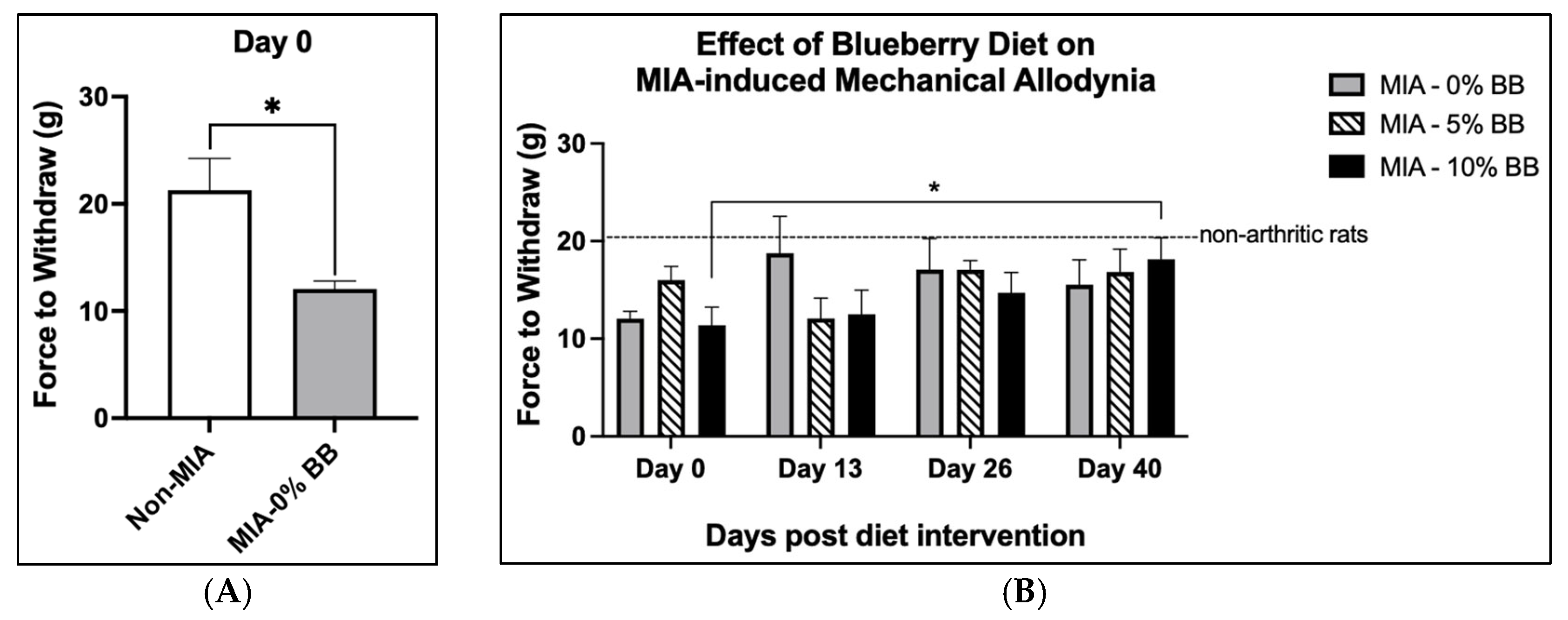
3.2. Pain and Edema
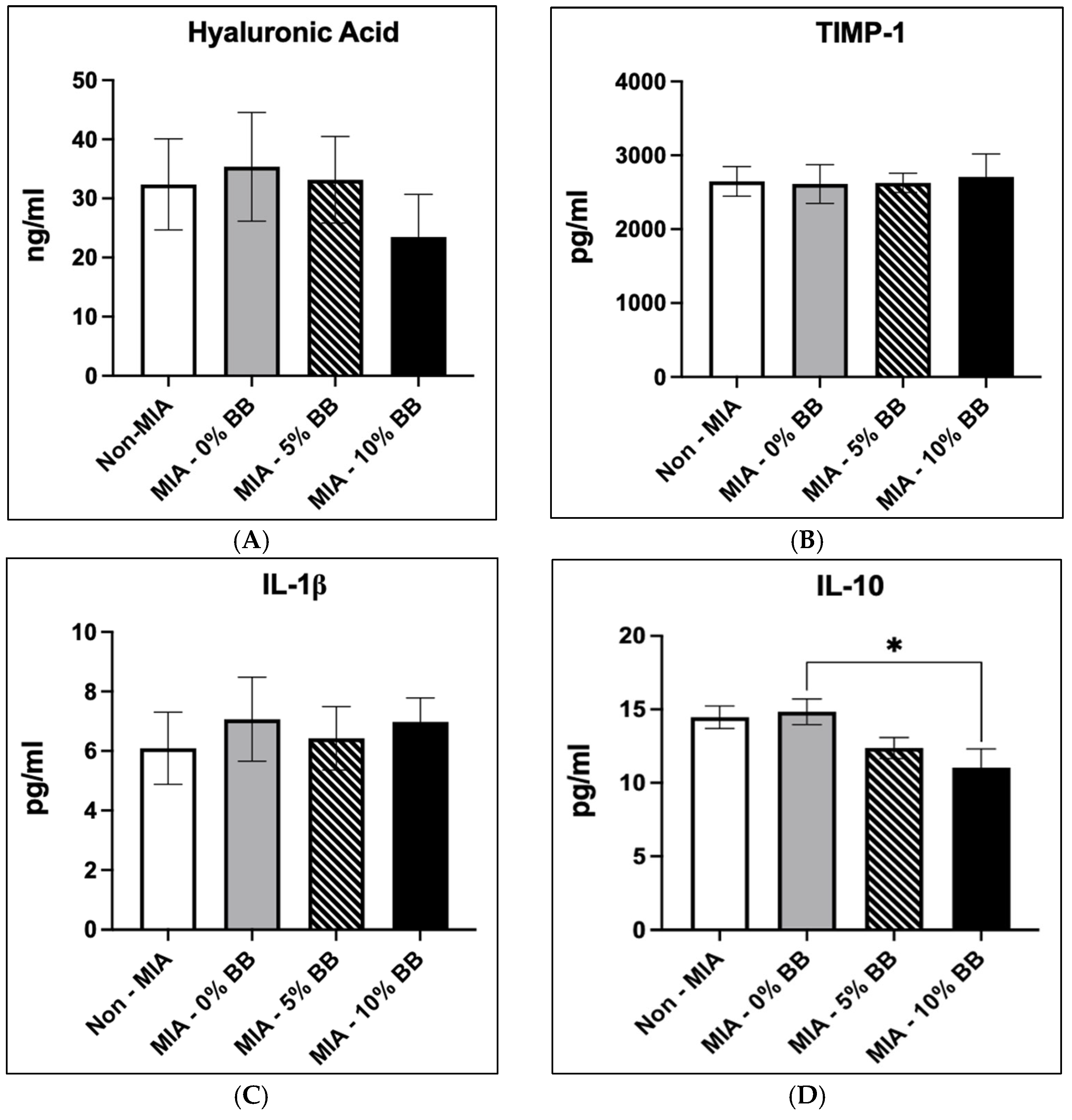
3.3. Effects of Blueberry Diet on Serum Inflammatory and Cartilage Markers
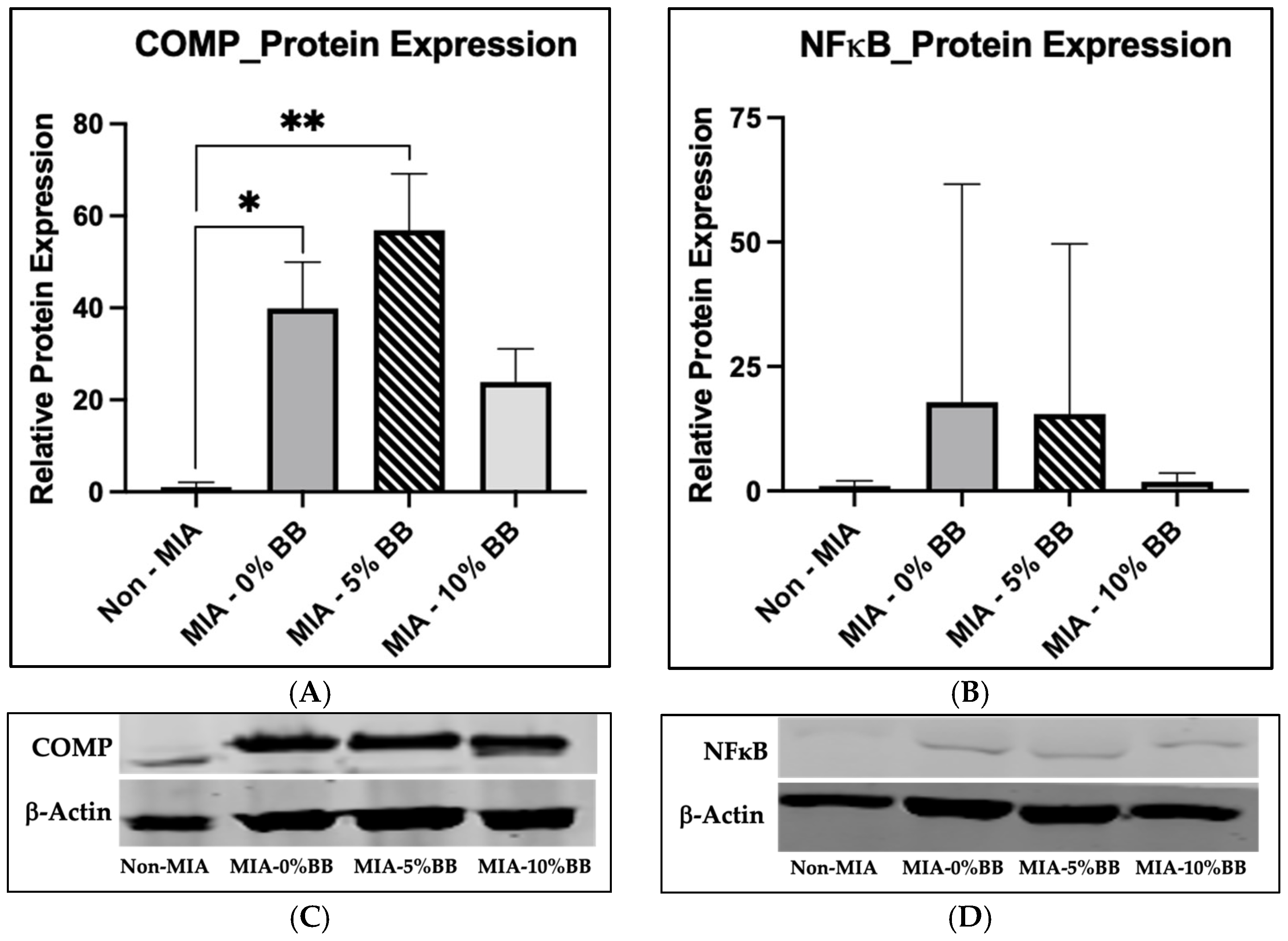
3.4. Protein Expression
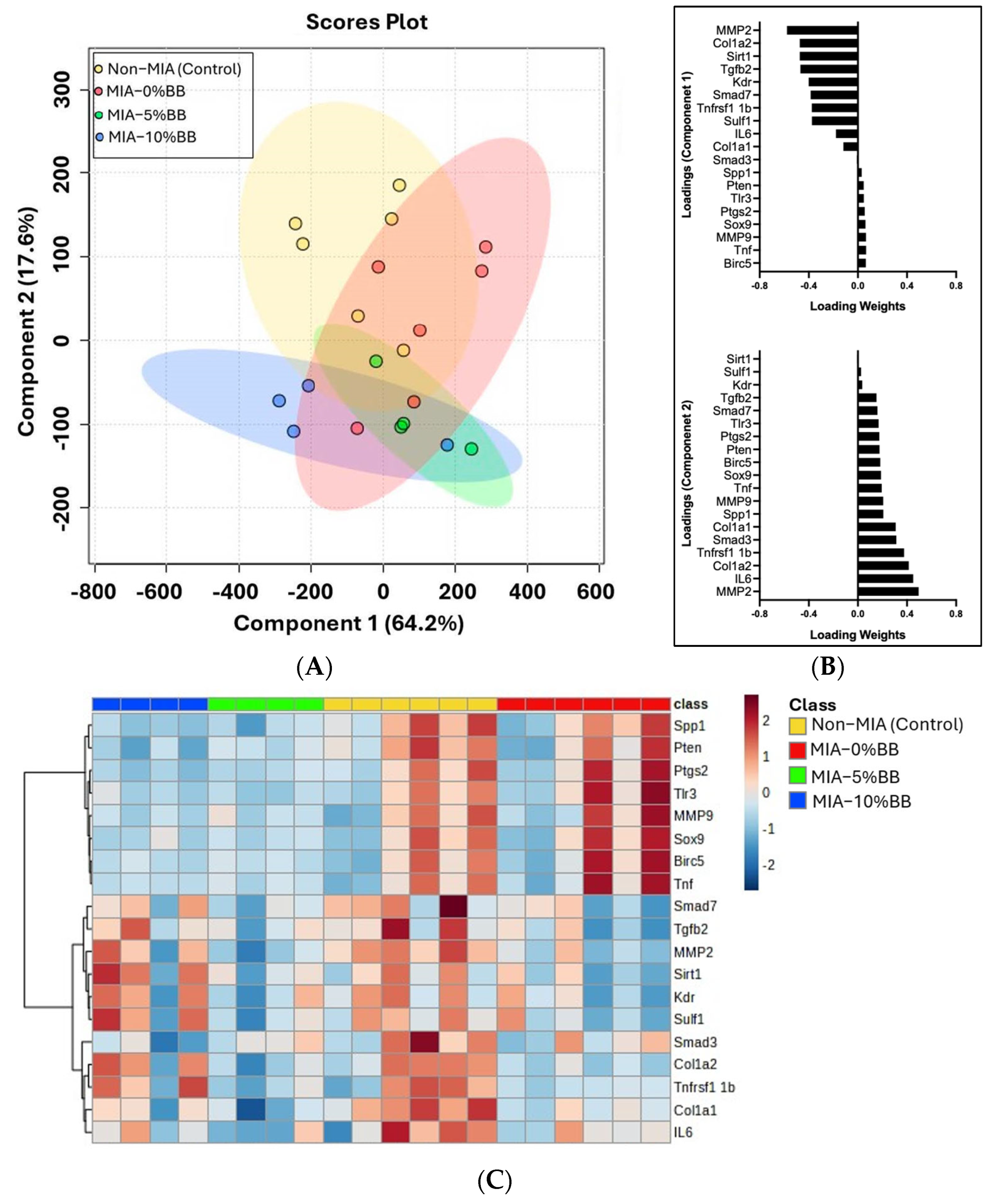
3.5. Effect of Blueberry Diet on Gene Expression
3.6. Effects of Blueberry Diet on Joint Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Schell, J.; Scofield, R.H. Dietary fruits and arthritis. Food Funct. 2018, 9, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of Posttraumatic Osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Mandl, L.A. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef]
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995.e1. [Google Scholar] [CrossRef]
- Bortoluzzi, A.; Furini, F.; Scirè, C.A. Osteoarthritis and its management—Epidemiology, nutritional aspects and environmental factors. Autoimmun. Rev. 2018, 17, 1097–1104. [Google Scholar] [CrossRef]
- Nees, T.A.; Rosshirt, N.; Zhang, J.A.; Reiner, T.; Sorbi, R.; Tripel, E.; Walker, T.; Schiltenwolf, M.; Hagmann, S.; Moradi, B. Synovial Cytokines Significantly Correlate with Osteoarthritis-Related Knee Pain and Disability: Inflammatory Mediators of Potential Clinical Relevance. J. Clin. Med. 2019, 8, 1343. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, S.I.; Choi, B.R.; Lee, Y.S.; Lee, D.Y.; Kim, G.S. Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats. BMC Complement. Altern. Med. 2019, 19, 325. [Google Scholar] [CrossRef]
- Mandelbaum, B.; Waddell, D. Etiology and pathophysiology of osteoarthritis. Orthopedics 2005, 28 (Suppl. S2), s207–s214. [Google Scholar] [CrossRef]
- Fajardo, M.; Di Cesare, P.E. Disease-modifying therapies for osteoarthritis: Current status. Drugs Aging 2005, 22, 141–161. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: Destruction or repair? Nat. Clin. Pract. Rheumatol. 2008, 4, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2016, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Melbard, E. The use of dietary supplements in the treatment of osteoarthritis. Physician Assist. 2003, 27, 40. [Google Scholar]
- Singh, R.; Akhtar, N.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate: Inflammation and arthritis. Life Sci. 2010, 86, 907–918, Erratum in Life Sci. 2010, 87, 196. [Google Scholar] [CrossRef]
- Figueira, M.E.; Oliveira, M.; Direito, R.; Rocha, J.; Alves, P.; Serra, A.T.; Duarte, C.; Bronze, R.; Fernandes, A.; Brites, D.; et al. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed. Pharmacother. 2016, 83, 1191–1202. [Google Scholar] [CrossRef]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef]
- Carey, A.N.; Gomes, S.M.; Shukitt-Hale, B. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J. Agric. Food Chem. 2014, 62, 3972–3978. [Google Scholar] [CrossRef]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef]
- Shukitt-Hale, B. Blueberries and neuronal aging. Gerontology 2012, 58, 518–523. [Google Scholar] [CrossRef]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, S.M.; Xu, Z.Y.; Ou-Yang, S. Rabbiteye blueberry prevents osteoporosis in ovariectomized rats. J. Orthop. Surg. Res. 2014, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Benton, W.L.; Tongkhuya, S.A.; Lopez, C.M.C.; Uphouse, L.; Averitt, D.L. Sex Differences and Estrous Cycle Effects of Peripheral Serotonin-Evoked Rodent Pain Behaviors. Neuroscience 2018, 384, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.; Ma, H.; Yuan, T.; Chichester, C.O., 3rd; Seeram, N.P. Anti-inflammatory effects of polyphenolic-enriched red raspberry extract in an antigen-induced arthritis rat model. J. Agric. Food Chem. 2012, 60, 5755–5762. [Google Scholar] [CrossRef]
- Lerna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef]
- Miyamoto, S.; Miyamoto, S.; Nakamura, J.; Ohtori, S.; Orita, S.; Omae, T.; Nakajima, T.; Suzuki, T.; Takahashi, K. Intra-articular injection of mono-iodoacetate induces osteoarthritis of the hip in rats. BMC Musculoskelet. Disord. 2016, 17, 132. [Google Scholar] [CrossRef]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Wang, Z.M.; Chen, Y.C.; Wang, D.P. Resveratrol, a natural antioxidant, protects monosodium iodoacetate-induced osteoarthritic pain in rats. Biomed. Pharmacother. 2016, 83, 763–770. [Google Scholar] [CrossRef]
- Perrot, S. Osteoarthritis pain. Best Pr. Res. Clin. Rheumatol. 2015, 29, 90–97. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Investig. 2019, 129, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Wenham, C.Y.; Conaghan, P.G. New horizons in osteoarthritis. Age Ageing 2013, 42, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lu, B.; Bathon, J.M.; Haythornthwaite, J.A.; Smith, M.T.; Page, G.G.; Edwards, R.R. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res. 2011, 63, 320–327. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Schaible, H.G. Mechanisms of chronic pain in osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 549–556. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; Jiang, H.; Zhou, J.; Tang, Y. Inhibition of interleukin-6 function attenuates the central sensitization and pain behavior induced by osteoarthritis. Eur. J. Pharmacol. 2017, 811, 260–267. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Kraus, V.B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Zhao, X.W.; Zhang, Y.G.; Kong, Y.; Niu, S.S.; Ma, L.F.; Zhang, Y.M. Effects of miR-145 on the inhibition of chondrocyte proliferation and fibrosis by targeting TNFRSF11B in human osteoarthritis. Mol. Med. Rep. 2017, 15, 75–80. [Google Scholar] [CrossRef]
- Shen, J.; Abu-Amer, Y.; O’Keefe, R.J.; McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2017, 58, 49–63. [Google Scholar] [CrossRef]
- Adams, L.S.; Kanaya, N.; Phung, S.; Liu, Z.; Chen, S. Whole blueberry powder modulates the growth and metastasis of MDA-MB-231 triple negative breast tumors in nude mice. J. Nutr. 2011, 141, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Hasnat, M.A.; Lim, J.H.; Lee, Y.M.; Kim, E.O.; Um, B.H.; Lim, B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016, 28, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.H.; Kuliwaba, J.S.; Tsangari, H.; Fazzalari, N.L. Differential gene expression of bone anabolic factors and trabecular bone architectural changes in the proximal femoral shaft of primary hip osteoarthritis patients. Arthritis Res. Ther. 2006, 8, R188. [Google Scholar] [CrossRef]
- Couchourel, D.; Aubry, I.; Delalandre, A.; Lavigne, M.; Martel-Pelletier, J.; Pelletier, J.P.; Lajeunesse, D. Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production. Arthritis Rheum. 2009, 60, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Ager, D.M.; Redman, K.A.; Mitzner, M.A.; Benson, K.F.; Schauss, A.G. Pain reduction and improvement in range of motion after daily consumption of an açai (Euterpe oleracea Mart.) pulp-fortified polyphenolic-rich fruit and berry juice blend. J. Med. Food 2011, 14, 702–711. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef]
- Jensen, G.S.; Attridge, V.L.; Benson, K.F.; Beaman, J.L.; Carter, S.G.; Ager, D. Consumption of dried apple peel powder increases joint function and range of motion. J. Med. Food 2014, 17, 1204–1213. [Google Scholar] [CrossRef]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; Cho, M.L.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef]
- Vasconcelos, C.C.; Lopes, A.J.O.; Sousa, E.L.F.; Camelo, D.S.; Lima, F.C.V.M.; Rocha, C.Q.D.; Silva, G.E.B.; Garcia, J.B.S.; Cartágenes, M.D.S.S. Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4717. [Google Scholar] [CrossRef]
- Yan, B.; Zhou, L.; Wang, C.; Wang, R.; Yan, L.; Yu, L.; Liu, F.; Du, W.; Yu, G.; Yuan, Q.; et al. Intra-Articular Injection of Fructus Ligustri Lucidi Extract Attenuates Pain Behavior and Cartilage Degeneration in Mono-Iodoacetate Induced Osteoarthritic Rats. Front. Pharmacol. 2018, 9, 1360. [Google Scholar] [CrossRef]
- Tiernan, C.; Imrhan, V.; Prasad, C.; Vijayagopal, P.; Juma, S. Tart cherry in amelioration of pain in the elderly. Nutr. Aging 2015, 3, 203–217. [Google Scholar] [CrossRef]

| Groups | Treatment Group (n = 10) | MIA Injection | Protein Source | Blueberry (BB) (g/kg Diet) |
|---|---|---|---|---|
| 1 | Non-MIA (Control) | No | Casein-Based | 0 |
| 2 | MIA | Yes | Casein-Based | 0 |
| 3 | MIA-5% BB | Yes | Casein-Based | 50 |
| 4 | MIA-10% BB | Yes | Casein-Based | 100 |
| Parameters | Descriptions | Score |
|---|---|---|
| Inflammatory Infiltration | Normal, no infiltrate present. | 0 |
| Minimal infiltrate detected. | 1 | |
| Mild infiltrate present. | 2 | |
| Moderate infiltrate present with loss of synovial structure. | 3 | |
| Severe infiltrate present with loss of synovial and articular structure. | 4 | |
| Synovial Hyperplasia | Normal, none present. | 0 |
| Minimal synovial hyperplasia detected. | 1 | |
| Mild synovial hyperplasia. | 2 | |
| Moderate synovial hyperplasia. | 3 | |
| Severe synovial hyperplasia present with loss of synovial structure. Expansion of synovial lining. | 4 | |
| Erosion of the Cartilage and Bone | Normal, no erosion detected. | 0 |
| Minimal erosion detected in fibrillation of the cartilage. | 1 | |
| Mild erosion cartilage loss and mild erosion of the subchondral bone. | 2 | |
| Moderate cartilage fibrillation and loss with infiltration of the subchondral bone. | 3 | |
| Severe cartilage and bone erosion present with loss of cartilage and infiltration of the bone. | 4 |
| Diameters (mm) of the Joint | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Day 0 | Day 13 | Day 26 | Day 40 | ||||
| Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | |
| Control | 6.0 ± 0.3 | 4.8 ± 0.3 | 5.7 ± 0.3 | 5.3 ± 0.3 | 4.5 ± 0.2 | 4.5 ± 0.2 | 5.7 ± 0.2 | 5.3 ± 0.2 |
| MIA | 6.0 ± 0.4 | 4.3 ± 0.2 | 5.7 ± 0.3 | 5.2 ± 0.5 | 5.5 ± 0.2 | 5.2 ± 0.4 | 6.0 ± 0.3 | 5.0 ± 0.3 |
| MIA + 5% BB | 5.8 ± 0.3 | 5.0 ± 0.4 | 5.8 ± 0.3 | 5.2 ± 0.3 | 5.0 ± 0.4 | 4.5 ± 0.2 | 5.8 ± 0.2 | 5.0 ± 0.3 |
| MIA + 10% BB | 6.0 ± 0.4 | 5.2 ± 0.6 | 6.2 ± 0.4 | 5.0 ± 0.4 | 6.0 ± 0.4 | 5.2 ± 0.4 | 5.7 ± 0.2 | 5.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
South, S.M.; Crabtree, K.; Averitt, D.L.; Vijayagopal, P.; Juma, S. Anti-Inflammatory and Joint-Protective Effects of Blueberries in a Monosodium Iodoacetate (MIA)-Induced Rat Model of Osteoarthritis. Nutrients 2025, 17, 2134. https://doi.org/10.3390/nu17132134
South SM, Crabtree K, Averitt DL, Vijayagopal P, Juma S. Anti-Inflammatory and Joint-Protective Effects of Blueberries in a Monosodium Iodoacetate (MIA)-Induced Rat Model of Osteoarthritis. Nutrients. 2025; 17(13):2134. https://doi.org/10.3390/nu17132134
Chicago/Turabian StyleSouth, Sanique M., Keith Crabtree, Dayna L. Averitt, Parakat Vijayagopal, and Shanil Juma. 2025. "Anti-Inflammatory and Joint-Protective Effects of Blueberries in a Monosodium Iodoacetate (MIA)-Induced Rat Model of Osteoarthritis" Nutrients 17, no. 13: 2134. https://doi.org/10.3390/nu17132134
APA StyleSouth, S. M., Crabtree, K., Averitt, D. L., Vijayagopal, P., & Juma, S. (2025). Anti-Inflammatory and Joint-Protective Effects of Blueberries in a Monosodium Iodoacetate (MIA)-Induced Rat Model of Osteoarthritis. Nutrients, 17(13), 2134. https://doi.org/10.3390/nu17132134








