Examination of Genetic and Epigenetic Characteristics of Patients with Hyperhomocysteinemia Following High-Dose Folic Acid Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Folic Acid and Homocysteine Level Measurements
2.3. Cell-Free and Genomic DNA Isolation
2.4. Bisulfite Conversion
2.5. Bisulfite Pyrosequencing
2.6. Biological Age Determination
2.7. Whole-Exome Sequencing
3. Results
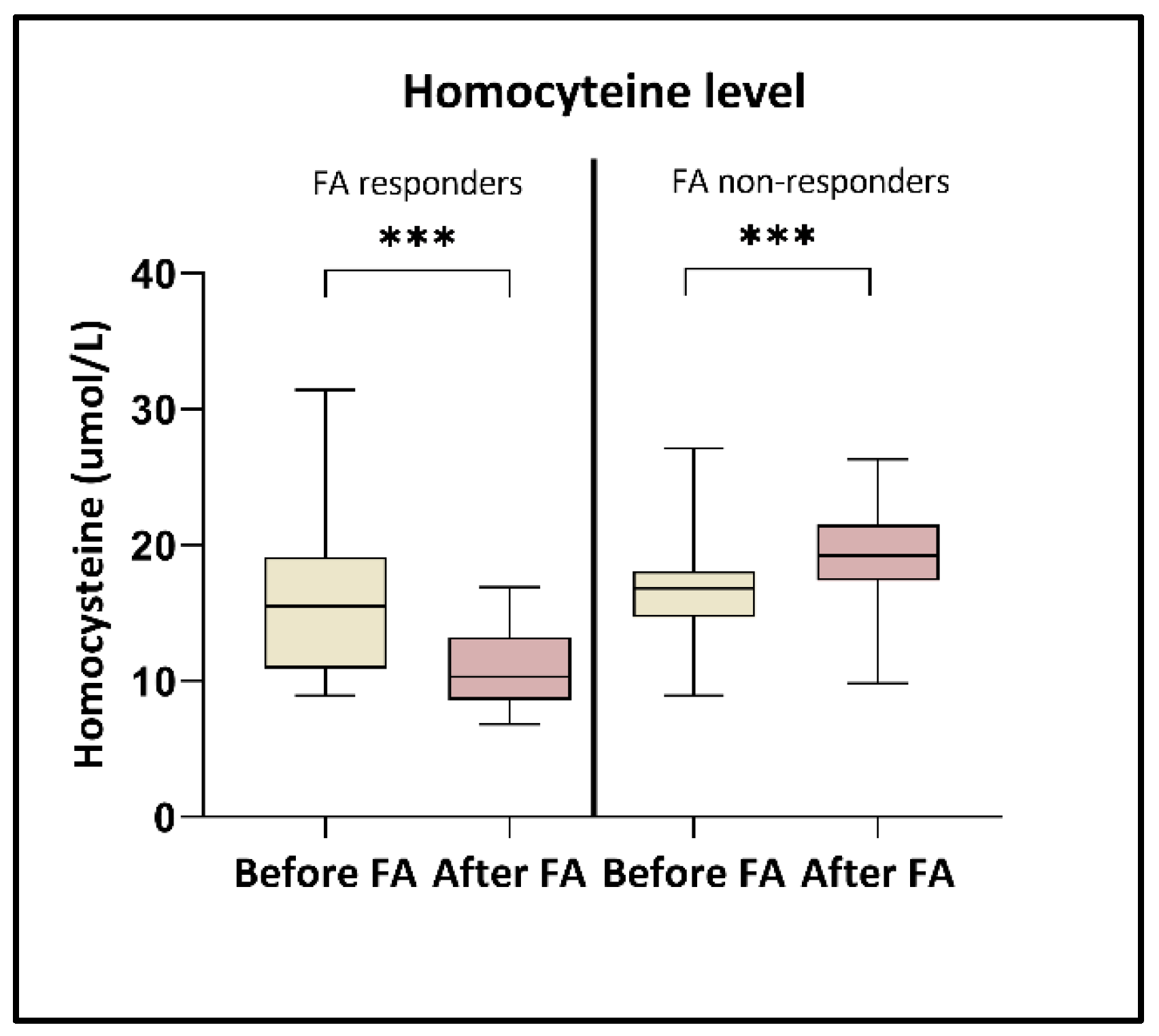
3.1. Alterations of Folic Acid and Homocysteine Level
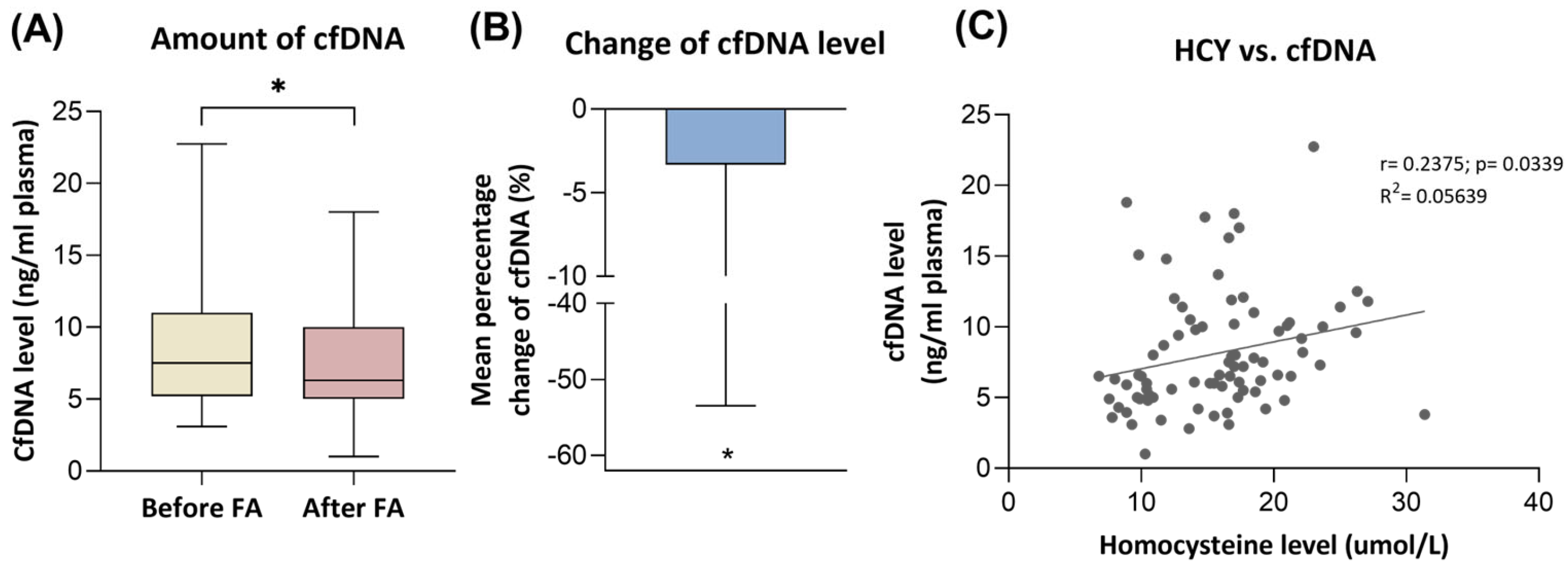
3.2. Cell-Free DNA Level Changes
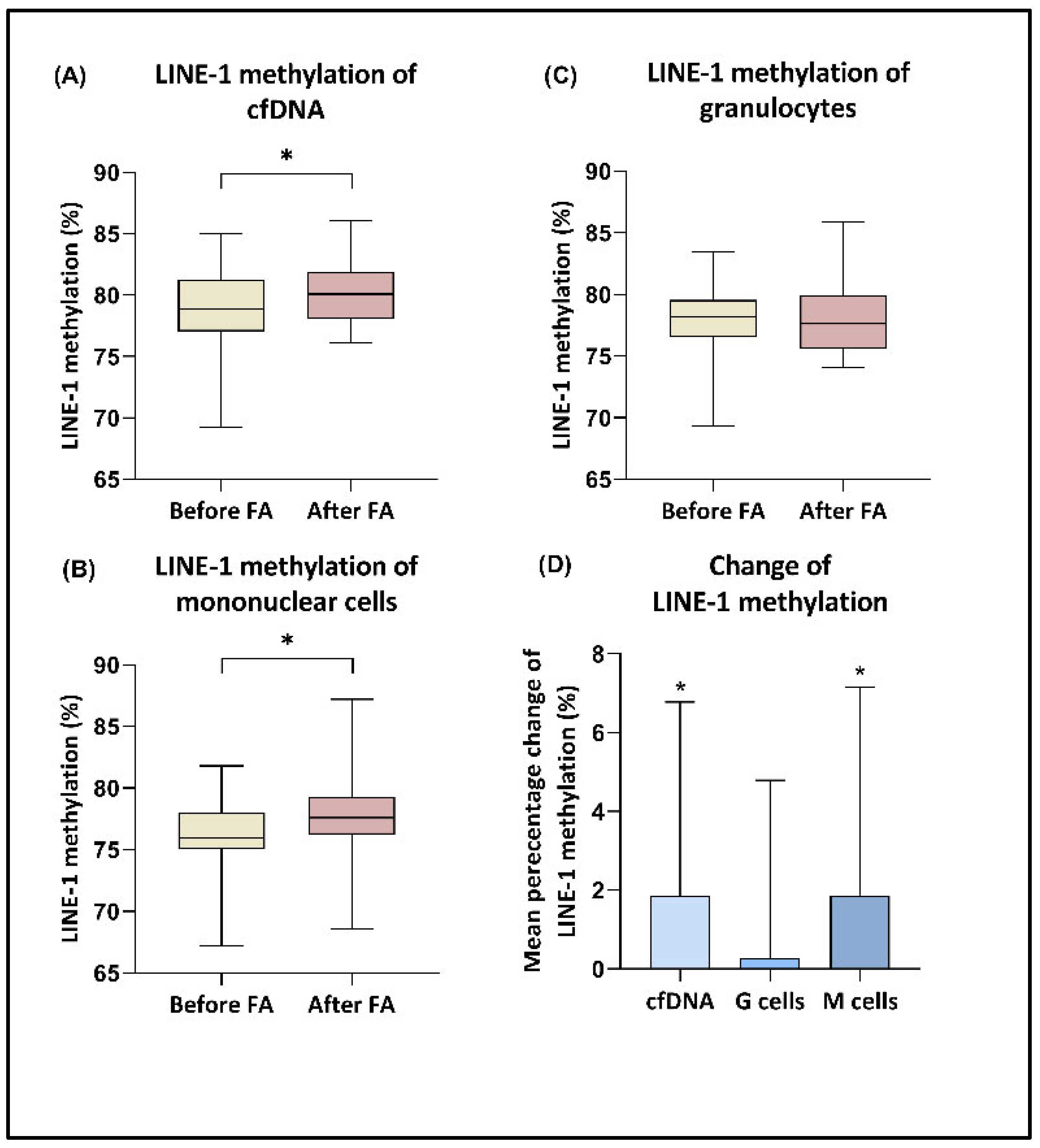
3.3. LINE-1 Methylation
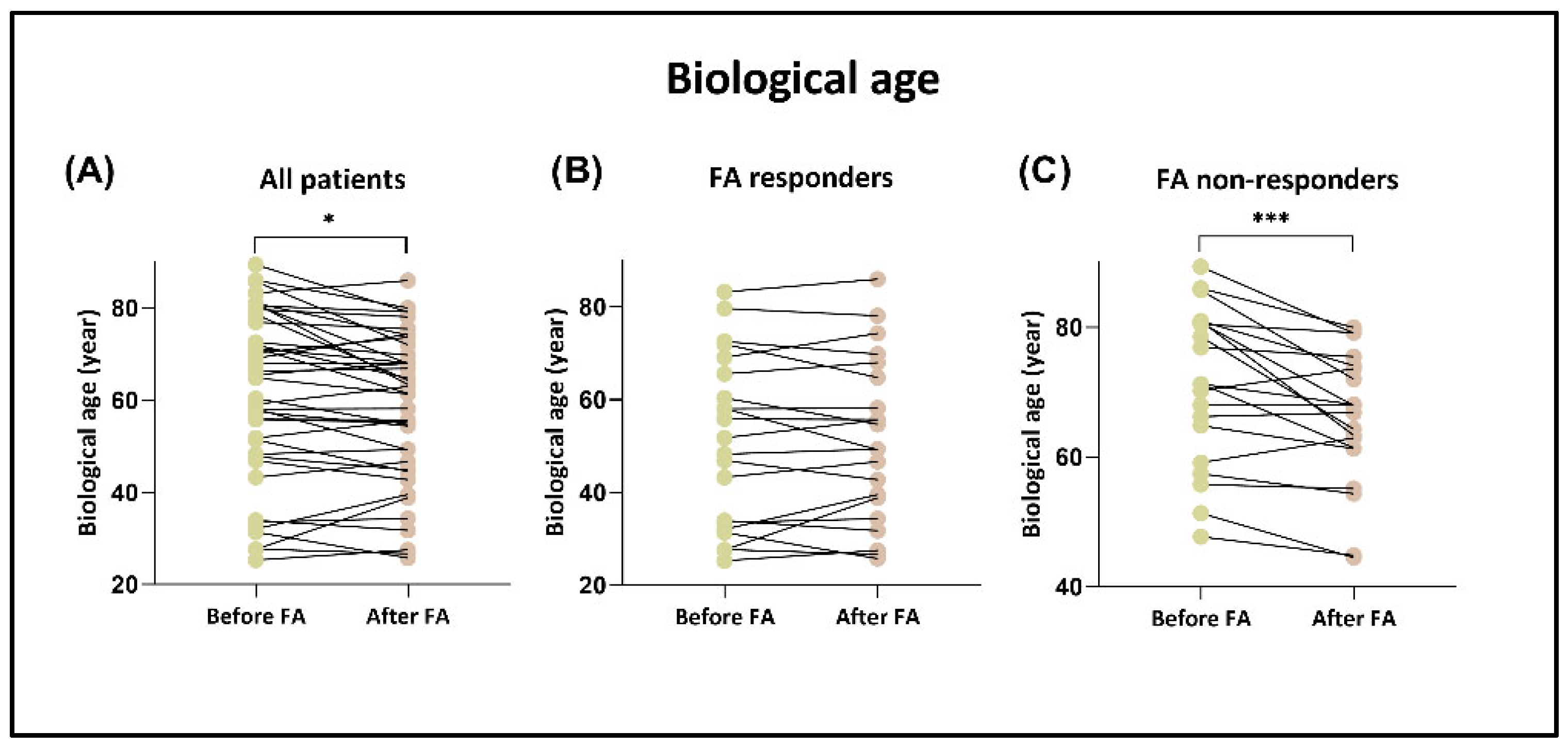
3.4. Biological Age
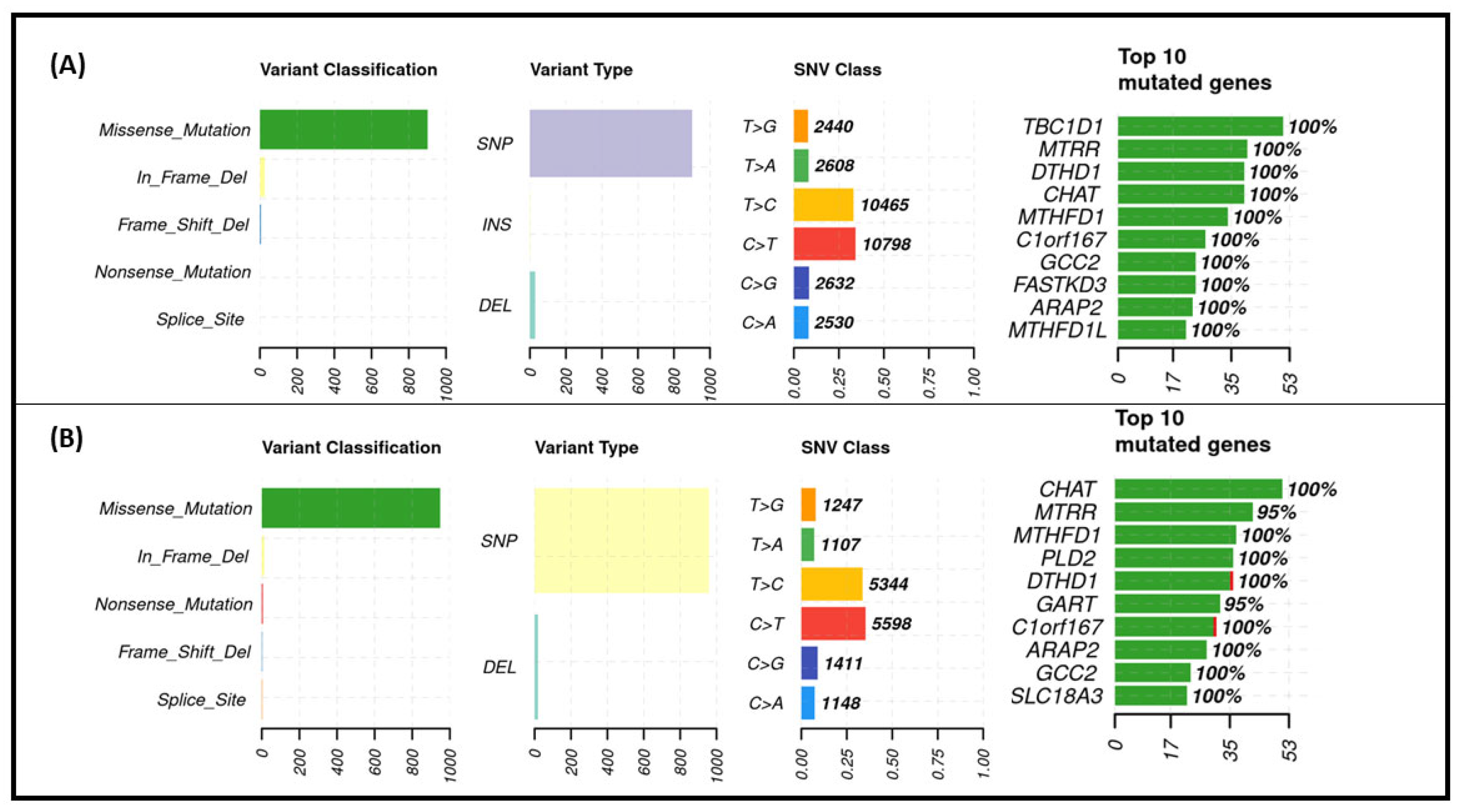
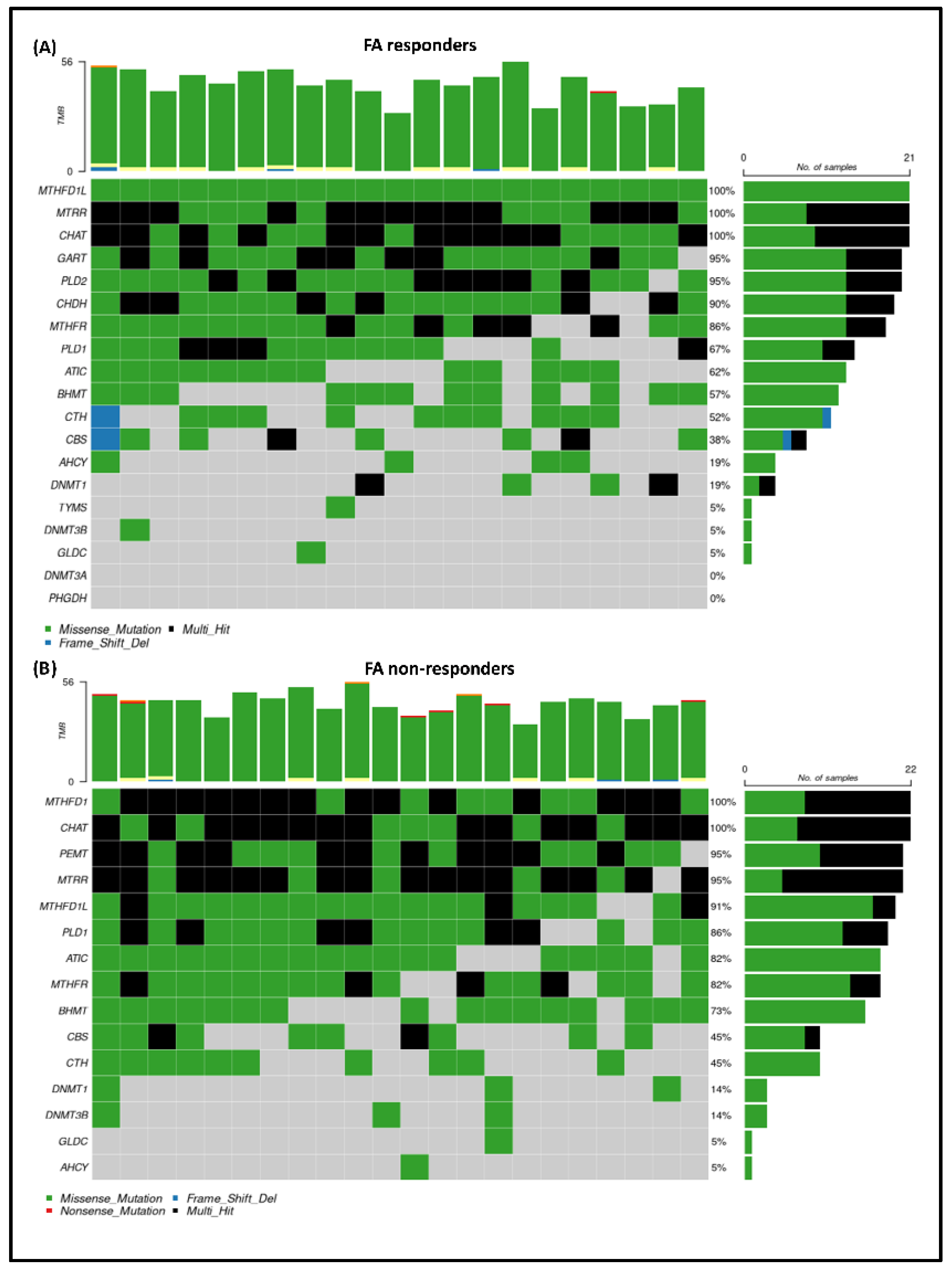
3.5. Exome Sequencing Results
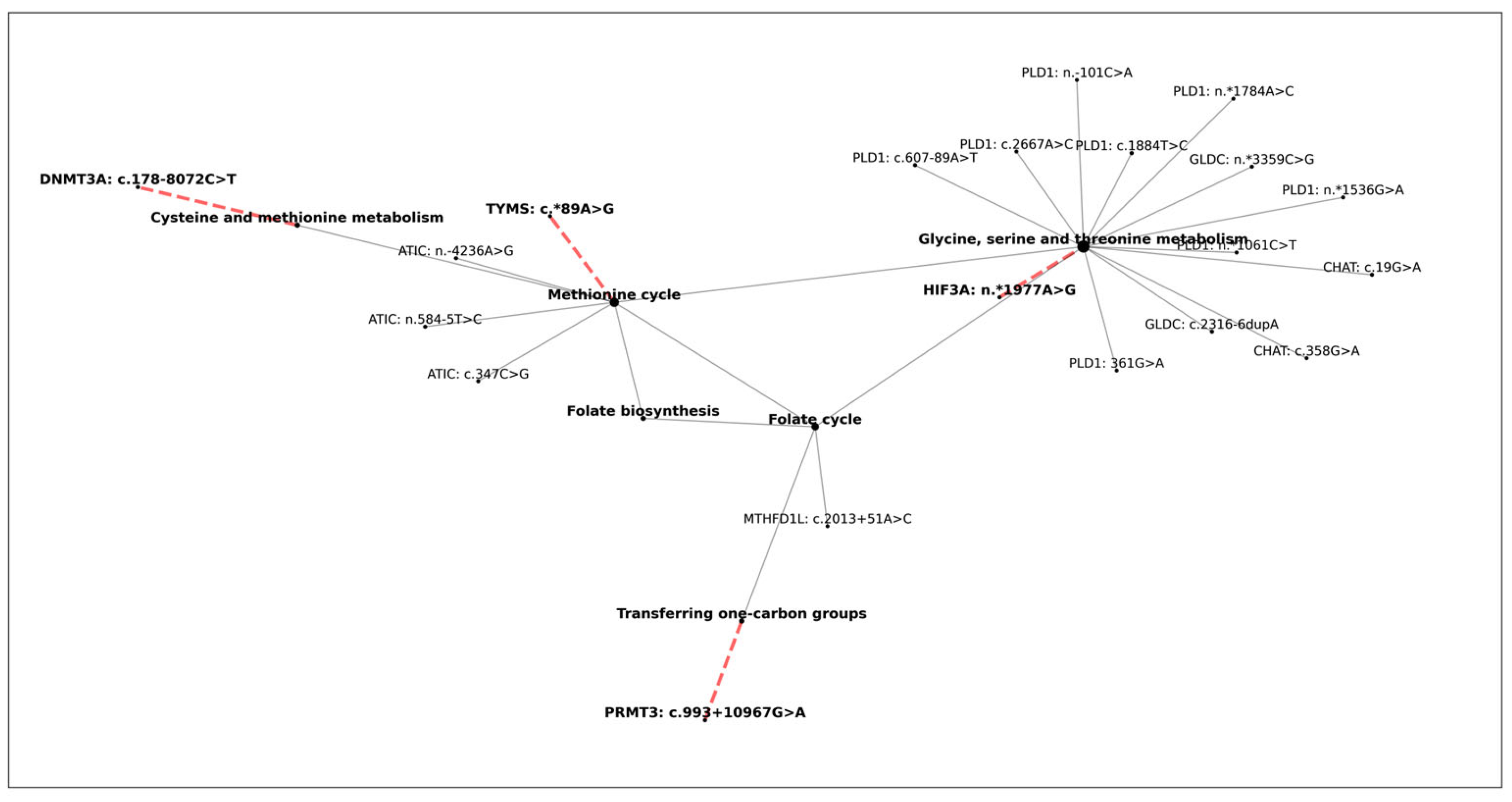
3.6. The Effect of One-Carbon Cycle Genes on Biological Aging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, W.; Gao, C.; Cueto, R.; Liu, L.; Fu, H.; Shao, Y.; Yang, W.Y.; Fang, P.; Choi, E.T.; Wu, Q.; et al. Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signaling. Redox Biol. 2020, 28, 101322. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.; Mottola, G.; Fromonot, J.; Marlinge, M.; Deharo, P.; Guieu, R.; Ruf, J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? Int. J. Mol. Sci. 2021, 22, 1690. [Google Scholar] [CrossRef] [PubMed]
- Zsigrai, S.; Kalmár, A.; Nagy, Z.B.; Barták, B.K.; Valcz, G.; Szigeti, K.A.; Galamb, O.; Dankó, T.; Sebestyén, A.; Barna, G.; et al. S-Adenosylmethionine Treatment of Colorectal Cancer Cell Lines Alters DNA Methylation, DNA Repair and Tumor Progression-Related Gene Expression. Cells 2020, 9, 1864. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.F.; Yin, R.X.; Deng, J.L. Homocysteine, hyperhomocysteinemia, and H-type hypertension. Eur. J. Prev. Cardiol. 2024, 31, 1092–1103. [Google Scholar] [CrossRef]
- Kaye, A.D.; Jeha, G.M.; Pham, A.D.; Fuller, M.C.; Lerner, Z.I.; Sibley, G.T.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kevil, C.G. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv. Ther. 2020, 37, 4149–4164. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Kim, Y.I. Folate and colorectal cancer: An evidence-based critical review. Mol. Nutr. Food Res. 2007, 51, 267–292. [Google Scholar] [CrossRef]
- Steluti, J.; Reginaldo, C.; Selhub, J.; Paul, L.; Fisberg, R.M.; Marchioni, D.M. Presence of circulating folic acid in plasma and its relation with dietary intake, vitamin B complex concentrations and genetic variants. Eur. J. Nutr. 2019, 58, 3069–3077. [Google Scholar] [CrossRef]
- Huang, X.; Bao, H.; Ding, C.; Li, J.; Cao, T.; Liu, L.; Wei, Y.; Zhou, Z.; Zhang, N.; Song, Y.; et al. Optimal folic acid dosage in lowering homocysteine: Precision Folic Acid Trial to lower homocysteine (PFAT-Hcy). Eur. J. Nutr. 2024, 63, 1513–1528. [Google Scholar] [CrossRef]
- Goyette, P.; Pai, A.; Milos, R.; Frosst, P.; Tran, P.; Chen, Z.; Chan, M.; Rozen, R. Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm. Genome 1998, 9, 652–656. [Google Scholar] [CrossRef]
- Malinow, M.R.; Nieto, F.J.; Kruger, W.D.; Duell, P.B.; Hess, D.L.; Gluckman, R.A.; Block, P.C.; Holzgang, C.R.; Anderson, P.H.; Seltzer, D.; et al. The effects of folic acid supplementation on plasma total homocysteine are modulated by multivitamin use and methylenetetrahydrofolate reductase genotypes. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.C.; Gupta, E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Markan, S.; Sachdeva, M.; Sehrawat, B.S.; Kumari, S.; Jain, S.; Khullar, M. MTHFR 677 CT/MTHFR 1298 CC genotypes are associated with increased risk of hypertension in Indians. Mol. Cell Biochem. 2007, 302, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ashfield-Watt, P.A.; Pullin, C.H.; Whiting, J.M.; Clark, Z.E.; Moat, S.J.; Newcombe, R.G.; Burr, M.L.; Lewis, M.J.; Powers, H.J.; McDowell, I.F. Methylenetetrahydrofolate reductase 677C → T genotype modulates homocysteine responses to a folate-rich diet or a low-dose folic acid supplement: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 180–186. [Google Scholar] [CrossRef]
- Lu, X.T.; Wang, Y.N.; Mo, Q.W.; Huang, B.X.; Wang, Y.F.; Huang, Z.H.; Luo, Y.; Maierhaba, W.; He, T.T.; Li, S.Y.; et al. Effects of low-dose B vitamins plus betaine supplementation on lowering homocysteine concentrations among Chinese adults with hyperhomocysteinemia: A randomized, double-blind, controlled preliminary clinical trial. Eur. J. Nutr. 2023, 62, 1599–1610. [Google Scholar] [CrossRef]
- Sobral, A.F.; Cunha, A.; Silva, V.; Gil-Martins, E.; Silva, R.; Barbosa, D.J. Unveiling the Therapeutic Potential of Folate-Dependent One-Carbon Metabolism in Cancer and Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 9339. [Google Scholar] [CrossRef]
- Platt, D.E.; Hariri, E.; Salameh, P.; Merhi, M.; Sabbah, N.; Helou, M.; Mouzaya, F.; Nemer, R.; Al-Sarraj, Y.; El-Shanti, H.; et al. Type II diabetes mellitus and hyperhomocysteinemia: A complex interaction. Diabetol. Metab. Syndr. 2017, 9, 19. [Google Scholar] [CrossRef]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed homocysteine metabolism is associated with cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Tabibzadeh, S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2019, 49, 144–164. [Google Scholar] [CrossRef]
- Li, J.H.; Tong, D.X.; Wang, Y.; Gao, L.; Liu, Y.; Zhang, X.H.; Chen, W.J.; Chi, J.Y.; Liu, N.; Yang, K.; et al. Neutrophil extracellular traps exacerbate coagulation and endothelial damage in patients with essential hypertension and hyperhomocysteinemia. Thromb. Res. 2021, 197, 36–43. [Google Scholar] [CrossRef]
- Joshi, M.B.; Baipadithaya, G.; Balakrishnan, A.; Hegde, M.; Vohra, M.; Ahamed, R.; Nagri, S.K.; Ramachandra, L.; Satyamoorthy, K. Elevated homocysteine levels in type 2 diabetes induce constitutive neutrophil extracellular traps. Sci. Rep. 2016, 6, 36362. [Google Scholar] [CrossRef] [PubMed]
- Grabuschnig, S.; Bronkhorst, A.J.; Holdenrieder, S.; Rosales Rodriguez, I.; Schliep, K.P.; Schwendenwein, D.; Ungerer, V.; Sensen, C.W. Putative Origins of Cell-Free DNA in Humans: A Review of Active and Passive Nucleic Acid Release Mechanisms. Int. J. Mol. Sci. 2020, 21, 8062. [Google Scholar] [CrossRef] [PubMed]
- Bartak, B.K.; Fodor, T.; Kalmar, A.; Nagy, Z.B.; Zsigrai, S.; Szigeti, K.A.; Valcz, G.; Igaz, P.; Dank, M.; Takacs, I.; et al. A Liquid Biopsy-Based Approach for Monitoring Treatment Response in Post-Operative Colorectal Cancer Patients. Int. J. Mol. Sci. 2022, 23, 3774. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Ulrich, C.M.; Bailey, L.B.; Malysheva, O.; Brown, E.C.; Maneval, D.R.; Neuhouser, M.L.; Cheng, T.Y.; Miller, J.W.; Zheng, Y.; et al. Impact of folic acid fortification on global DNA methylation and one-carbon biomarkers in the Women’s Health Initiative Observational Study cohort. Epigenetics 2014, 9, 396–403. [Google Scholar] [CrossRef]
- Kazazian, H.H., Jr. Mobile elements: Drivers of genome evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef]
- Pusceddu, I.; Herrmann, M.; Kirsch, S.H.; Werner, C.; Hübner, U.; Bodis, M.; Laufs, U.; Wagenpfeil, S.; Geisel, J.; Herrmann, W. Prospective study of telomere length and LINE-1 methylation in peripheral blood cells: The role of B vitamins supplementation. Eur. J. Nutr. 2016, 55, 1863–1873. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H., Jr. Roles for retrotransposon insertions in human disease. Mob. DNA 2016, 7, 9. [Google Scholar] [CrossRef]
- Hoffman, R.M. Is DNA methylation the new guardian of the genome? Mol. Cytogenet. 2017, 10, 11. [Google Scholar] [CrossRef]
- Fenech, M. The role of folic acid and Vitamin B12 in genomic stability of human cells. Mutat. Res. 2001, 475, 57–67. [Google Scholar] [CrossRef]
- Badiga, S.; Siddiqui, N.R.; Macaluso, M.; Johanning, G.L.; Piyathilake, C.J. Homocysteinemia is Associated with a Lower Degree of PBMC LINE-1 Methylation and a Higher Risk of CIN 2C in the U.S. Post-Folic Acid Fortification Era. Nutr. Cancer 2016, 68, 446–455. [Google Scholar] [CrossRef]
- Hübner, U.; Geisel, J.; Kirsch, S.H.; Kruse, V.; Bodis, M.; Klein, C.; Herrmann, W.; Obeid, R. Effect of 1 year B and D vitamin supplementation on LINE-1 repetitive element methylation in older subjects. Clin. Chem. Lab. Med. 2013, 51, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Gezer, U.; Bronkhorst, A.J.; Holdenrieder, S. The Utility of Repetitive Cell-Free DNA in Cancer Liquid Biopsies. Diagnostics 2022, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, T.; Vavourakis, C.D.; Jones, A.; Evans, I.; Schreiberhuber, L.; Kastner, C.; Ishaq-Parveen, I.; Redl, E.; Watson, A.W.; Brandt, K.; et al. Technical and biological sources of unreliability of Infinium probes on Illumina methylation microarrays. Clin. Epigenetics 2024, 16, 131. [Google Scholar] [CrossRef]
- Martínez-Magaña, J.J.; Hurtado-Soriano, J.; Rivero-Segura, N.A.; Montalvo-Ortiz, J.L.; Garcia-delaTorre, P.; Becerril-Rojas, K.; Gomez-Verjan, J.C. Towards a Novel Frontier in the Use of Epigenetic Clocks in Epidemiology. Arch. Med. Res. 2024, 55, 103033. [Google Scholar] [CrossRef]
- Alsaleh, H.; Haddrill, P.R. Identifying blood-specific age-related DNA methylation markers on the Illumina MethylationEPIC® BeadChip. Forensic Sci. Int. 2019, 303, 109944. [Google Scholar] [CrossRef]
- Gems, D.; Virk, R.S.; de Magalhães, J.P. Epigenetic clocks and programmatic aging. Ageing Res. Rev. 2024, 101, 102546. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Lee, Y.; Haftorn, K.L.; Denault, W.R.P.; Nustad, H.E.; Page, C.M.; Lyle, R.; Lee-Ødegård, S.; Moen, G.H.; Prasad, R.B.; Groop, L.C.; et al. Blood-based epigenetic estimators of chronological age in human adults using DNA methylation data from the Illumina MethylationEPIC array. BMC Genom. 2020, 21, 747. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Zhang, Q.; Vallerga, C.L.; Walker, R.M.; Lin, T.; Henders, A.K.; Montgomery, G.W.; He, J.; Fan, D.; Fowdar, J.; Kennedy, M.; et al. Improved precision of epigenetic clock estimates across tissues and its implication for biological ageing. Genome Med. 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Pipek, O.A.; Csabai, I. A revised multi-tissue, multi-platform epigenetic clock model for methylation array data. J. Math. Chem. 2023, 61, 376–388. [Google Scholar] [CrossRef]
- Czeizel, E.; Timar, L.; Botto, L. Prevalence of methylenetetrahydrofolate reductase (MTHFR) gene polymorphism (C677T) in the Hungarian population. Orv. Hetil. 2001, 142, 1227–1229. [Google Scholar]
- Verhoef, H.; Veenemans, J.; Mwangi, M.N.; Prentice, A.M. Safety and benefits of interventions to increase folate status in malaria-endemic areas. Br. J. Haematol. 2017, 177, 905–918. [Google Scholar] [CrossRef]
- Morellato, A.E.; Umansky, C.; Pontel, L.B. The toxic side of one-carbon metabolism and epigenetics. Redox Biol. 2021, 40, 101850. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Hoepner, C.T.; McIntyre, R.S.; Papakostas, G.I. Impact of Supplementation and Nutritional Interventions on Pathogenic Processes of Mood Disorders: A Review of the Evidence. Nutrients 2021, 13, 767. [Google Scholar] [CrossRef]
- Martin-Masot, R.; Mota-Martorell, N.; Jove, M.; Maldonado, J.; Pamplona, R.; Nestares, T. Alterations in One-Carbon Metabolism in Celiac Disease. Nutrients 2020, 12, 3723. [Google Scholar] [CrossRef]
- Chen, W.Y.; Bertone-Johnson, E.R.; Hunter, D.J.; Willett, W.C.; Hankinson, S.E. Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2335–2339. [Google Scholar] [CrossRef]
- Fernandez-Ramos, D.; Lopitz-Otsoa, F.; Millet, O.; Alonso, C.; Lu, S.C.; Mato, J.M. One Carbon Metabolism and S-Adenosylmethionine in Non-Alcoholic Fatty Liver Disease Pathogenesis and Subtypes. Livers 2022, 2, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Steluti, J.; Carvalho, A.M.; Carioca, A.A.F.; Miranda, A.; Gattas, G.J.F.; Fisberg, R.M.; Marchioni, D.M. Genetic Variants Involved in One-Carbon Metabolism: Polymorphism Frequencies and Differences in Homocysteine Concentrations in the Folic Acid Fortification Era. Nutrients 2017, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zheng, Y.; Qi, Q.; Xu, M.; Ley, S.H.; Li, Y.; Kang, J.H.; Wiggs, J.; Pasquale, L.R.; Chan, A.T.; et al. DNA Methylation Variants at HIF3A Locus, B-Vitamin Intake, and Long-term Weight Change: Gene-Diet Interactions in Two U.S. Cohorts. Diabetes 2015, 64, 3146–3154. [Google Scholar] [CrossRef]
- Onono, F.O.; Morris, A.J. Phospholipase D and Choline Metabolism. Handb. Exp. Pharmacol. 2020, 259, 205–218. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Zikopoulos, A.; Grigoriadis, S.; Seretis, N.; Maziotis, E.; Anifandis, G.; Xystra, P.; Kostoulas, C.; Giougli, U.; Pantos, K.; et al. The Role of One-Carbon Metabolism and Methyl Donors in Medically Assisted Reproduction: A Narrative Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4977. [Google Scholar] [CrossRef]
- Kandoth, C.; Gao, J.; Mattioni, M.; Struck, A.; Boursin, Y.; Penson, A.; Chavan, S. mskcc/vcf2maf: vcf2maf v1.6.16 [Software]. Zenodo. [CrossRef]
- Mayakonda, A.; Lin, D.C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef]
- Hagberg, A.A.; Swart, P.J.; Schult, D.A. Exploring network structure, dynamics, and function using NetworkX. In Proceedings of the 7th Python in Science Conference (SciPy2008), Pasadena, CA, USA, 19–24 August 2008. [Google Scholar]
- González-Lamuño, D.; Arrieta-Blanco, F.J.; Fuentes, E.D.; Forga-Visa, M.T.; Morales-Conejo, M.; Peña-Quintana, L.; Vitoria-Miñana, I. Hyperhomocysteinemia in Adult Patients: A Treatable Metabolic Condition. Nutrients 2023, 16, 135. [Google Scholar] [CrossRef]
- Guieu, R.; Ruf, J.; Mottola, G. Hyperhomocysteinemia and cardiovascular diseases. Ann. Biol. Clin. 2022, 80, 7–14. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Baracos, V.E.; Madsen, K.L. Hyperhomocysteinemia as a potential contributor of colorectal cancer development in inflammatory bowel diseases: A review. World J. Gastroenterol. 2015, 21, 1081–1090. [Google Scholar] [CrossRef]
- Ortiz-Salguero, C.; Romero-Bernal, M.; González-Díaz, Á.; Doush, E.S.; Del Río, C.; Echevarría, M.; Montaner, J. Hyperhomocysteinemia: Underlying Links to Stroke and Hydrocephalus, with a Focus on Polyphenol-Based Therapeutic Approaches. Nutrients 2024, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of hyperhomocysteinemia and its pathological significance. Arch. Pharm. Res. 2018, 41, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Givvimani, S.; Brown, K.; Metreveli, N.; Tyagi, S.C.; Tyagi, N. Nutri-epigenetics ameliorates blood-brain barrier damage and neurodegeneration in hyperhomocysteinemia: Role of folic acid. J. Mol. Neurosci. 2014, 52, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Malinow, M.R.; Duell, P.B.; Williams, M.A.; Kruger, W.D.; Evans, A.A.; Anderson, P.H.; Block, P.C.; Hess, D.L.; Upson, B.M.; Graf, E.E.; et al. Short-term folic acid supplementation induces variable and paradoxical changes in plasma homocyst(e)ine concentrations. Lipids 2001, 36 (Suppl. S1), S27–S32. [Google Scholar] [CrossRef]
- Liu, C.S.; Chiang, H.C.; Chen, H.W. Methylenetetrahydrofolate reductase polymorphism determines the plasma homocysteine-lowering effect of large-dose folic acid supplementation in patients with cardiovascular disease. Nutrition 2004, 20, 974–978. [Google Scholar] [CrossRef]
- Huang, X.; Qin, X.; Yang, W.; Liu, L.; Jiang, C.; Zhang, X.; Jiang, S.; Bao, H.; Su, H.; Li, P.; et al. MTHFR Gene and Serum Folate Interaction on Serum Homocysteine Lowering: Prospect for Precision Folic Acid Treatment. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 679–685. [Google Scholar] [CrossRef]
- Austin, R.C.; Lentz, S.R.; Werstuck, G.H. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004, 11 (Suppl. S1), S56–S64. [Google Scholar] [CrossRef]
- Kruger, W.D.; Evans, A.A.; Wang, L.; Malinow, M.R.; Duell, P.B.; Anderson, P.H.; Block, P.C.; Hess, D.L.; Graf, E.E.; Upson, B. Polymorphisms in the CBS gene associated with decreased risk of coronary artery disease and increased responsiveness to total homocysteine lowering by folic acid. Mol. Genet. Metab. 2000, 70, 53–60. [Google Scholar] [CrossRef]
- Liu, Y.X.; Ding, M.H.; Sheng, Y.; Sun, M.F.; Liu, L.; Zhang, Y. Doubly bi-allelic variants of MTHFR and MTHFD1 in a Chinese patient with hyperhomocysteinemia and failure of folic acid therapy. Front. Genet. 2022, 13, 964990. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Seremak-Mrozikiewicz, A.; Malinowska, A.M.; Rozycka, A.; Radziejewska, A.; KurzawiNska, G.; Barlik, M.; Wolski, H.; Drews, K. Associations between folate and choline intake, homocysteine metabolism, and genetic polymorphism of MTHFR, BHMT and PEMT in healthy pregnant Polish women. Nutr. Diet. 2020, 77, 368–372. [Google Scholar] [CrossRef]
- Ma, J.; Song, P.; Liu, X.; Ma, C.; Zheng, M.; Ren, X.; Wang, R.; Liu, W.; Lu, Z.; Li, J. Insights into the roles and driving forces of CCT3 in human tumors. Front. Pharmacol. 2022, 13, 1005855. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.H.; Kasembeli, M.; Bakthavatsalam, D.; Chiu, W.; Tweardy, D.J. Contribution of the Type II Chaperonin, TRiC/CCT, to Oncogenesis. Int. J. Mol. Sci. 2015, 16, 26706–26720. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Zhong, F.L.; Venteicher, A.S.; Meng, Z.; Veenstra, T.D.; Frydman, J.; Artandi, S.E. Proteostatic control of telomerase function through TRiC-mediated folding of TCAB1. Cell 2014, 159, 1389–1403. [Google Scholar] [CrossRef]
- Kananen, L.; Hurme, M.; Bürkle, A.; Moreno-Villanueva, M.; Bernhardt, J.; Debacq-Chainiaux, F.; Grubeck-Loebenstein, B.; Malavolta, M.; Basso, A.; Piacenza, F.; et al. Circulating cell-free DNA in health and disease—The relationship to health behaviours, ageing phenotypes and metabolomics. Geroscience 2023, 45, 85–103. [Google Scholar] [CrossRef]
- Sheng, W.; Wang, H.; Ma, X.; Qian, Y.; Zhang, P.; Wu, Y.; Zheng, F.; Chen, L.; Huang, G.; Ma, D. LINE-1 methylation status and its association with tetralogy of fallot in infants. BMC Med. Genom. 2012, 5, 20. [Google Scholar] [CrossRef]
- Fryer, A.A.; Nafee, T.M.; Ismail, K.M.; Carroll, W.D.; Emes, R.D.; Farrell, W.E. LINE-1 DNA methylation is inversely correlated with cord plasma homocysteine in man: A preliminary study. Epigenetics 2009, 4, 394–398. [Google Scholar] [CrossRef]
- Pizzolo, F.; Blom, H.J.; Choi, S.W.; Girelli, D.; Guarini, P.; Martinelli, N.; Stanzial, A.M.; Corrocher, R.; Olivieri, O.; Friso, S. Folic acid effects on s-adenosylmethionine, s-adenosylhomocysteine, and DNA methylation in patients with intermediate hyperhomocysteinemia. J. Am. Coll. Nutr. 2011, 30, 11–18. [Google Scholar] [CrossRef]
- Jung, A.Y.; Smulders, Y.; Verhoef, P.; Kok, F.J.; Blom, H.; Kok, R.M.; Kampman, E.; Durga, J. No effect of folic acid supplementation on global DNA methylation in men and women with moderately elevated homocysteine. PLoS ONE 2011, 6, e24976. [Google Scholar] [CrossRef]
- Sae-Lee, C.; Corsi, S.; Barrow, T.M.; Kuhnle, G.G.C.; Bollati, V.; Mathers, J.C.; Byun, H.M. Dietary Intervention Modifies DNA Methylation Age Assessed by the Epigenetic Clock. Mol. Nutr. Food Res. 2018, 62, e1800092. [Google Scholar] [CrossRef]
- Michels, K.B.; Binder, A.M. Impact of folic acid supplementation on the epigenetic profile in healthy unfortified individuals—A randomized intervention trial. Epigenetics 2024, 19, 2293410. [Google Scholar] [CrossRef]
- White, L.K.; Hesselberth, J.R. Modification mapping by nanopore sequencing. Front. Genet. 2022, 13, 1037134. [Google Scholar] [CrossRef] [PubMed]
| Samples Type | CpG Position | Baseline (%) | After FA Supplementation (%) |
|---|---|---|---|
| CfDNA | LINE-1 CpG1 | 85.0 ± 3.5 | 86.2 ± 3.7 |
| LINE-1 CpG2 * | 75.4 ± 4 | 77.2 ± 2.4 | |
| LINE-1 CpG3 | 75.9 ± 3.6 | 77.1 ± 3.6 | |
| LINE-1 CpG av * | 78.8 ± 3 | 80.2 ± 2.5 | |
| Granulocytes | LINE-1 CpG1 | 84.2 ± 3.4 | 84.4 ± 4.2 |
| LINE-1 CpG2 | 74.5 ± 2.5 | 74.8 ± 3.7 | |
| LINE-1 CpG3 | 74.8 ± 3.8 | 74.5 ± 3.0 | |
| LINE-1 CpG av | 77.9 ± 2.6 | 78.1 ± 2.8 | |
| Mononuclear cells | LINE-1 CpG1 | 81.4 ± 3.8 | 82.1 ± 4.2 |
| LINE-1 CpG2 | 74.0 ± 2.7 | 74.5 ± 4.3 | |
| LINE-1 CpG3 * | 73.5 ± 3.0 | 75.3 ± 3.7 | |
| LINE-1 CpG av * | 76.2 ± 2.7 | 77.5 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartak, B.K.; Nagy, Z.B.; Szakallas, N.; Kalmar, A.; Farkas, E.; Banyai, F.; Pipek, O.; Csabai, I.; Sydo, N.; Csulak, E.; et al. Examination of Genetic and Epigenetic Characteristics of Patients with Hyperhomocysteinemia Following High-Dose Folic Acid Consumption. Nutrients 2025, 17, 2133. https://doi.org/10.3390/nu17132133
Bartak BK, Nagy ZB, Szakallas N, Kalmar A, Farkas E, Banyai F, Pipek O, Csabai I, Sydo N, Csulak E, et al. Examination of Genetic and Epigenetic Characteristics of Patients with Hyperhomocysteinemia Following High-Dose Folic Acid Consumption. Nutrients. 2025; 17(13):2133. https://doi.org/10.3390/nu17132133
Chicago/Turabian StyleBartak, Barbara K., Zsofia B. Nagy, Nikolett Szakallas, Alexandra Kalmar, Eszter Farkas, Fruzsina Banyai, Orsolya Pipek, Istvan Csabai, Nora Sydo, Emese Csulak, and et al. 2025. "Examination of Genetic and Epigenetic Characteristics of Patients with Hyperhomocysteinemia Following High-Dose Folic Acid Consumption" Nutrients 17, no. 13: 2133. https://doi.org/10.3390/nu17132133
APA StyleBartak, B. K., Nagy, Z. B., Szakallas, N., Kalmar, A., Farkas, E., Banyai, F., Pipek, O., Csabai, I., Sydo, N., Csulak, E., Merkely, B., Takacs, I., & Molnar, B. (2025). Examination of Genetic and Epigenetic Characteristics of Patients with Hyperhomocysteinemia Following High-Dose Folic Acid Consumption. Nutrients, 17(13), 2133. https://doi.org/10.3390/nu17132133






