Novel Preoperative Carbohydrate Drinks Versus Commercial Syrup-Based Drinks on Gastric Emptying, Glycemic Responses, and Fasting Discomfort: A Pilot Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Participant Inclusion and Exclusion Criteria
2.3. Test Beverages
- Novel carbohydrate drink 400 mL (C400)
- Novel carbohydrate drink 250 mL (C250)
- Syrup concentrates 250 mL (SYR)
2.4. Patient and Public Involvement
2.5. Randomization and Blinding
2.6. Study Protocol
2.7. Primary Outcome
2.8. Secondary Outcomes
2.9. Sample Size Calculation
2.10. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Effect on CSA of the Gastric Antrum
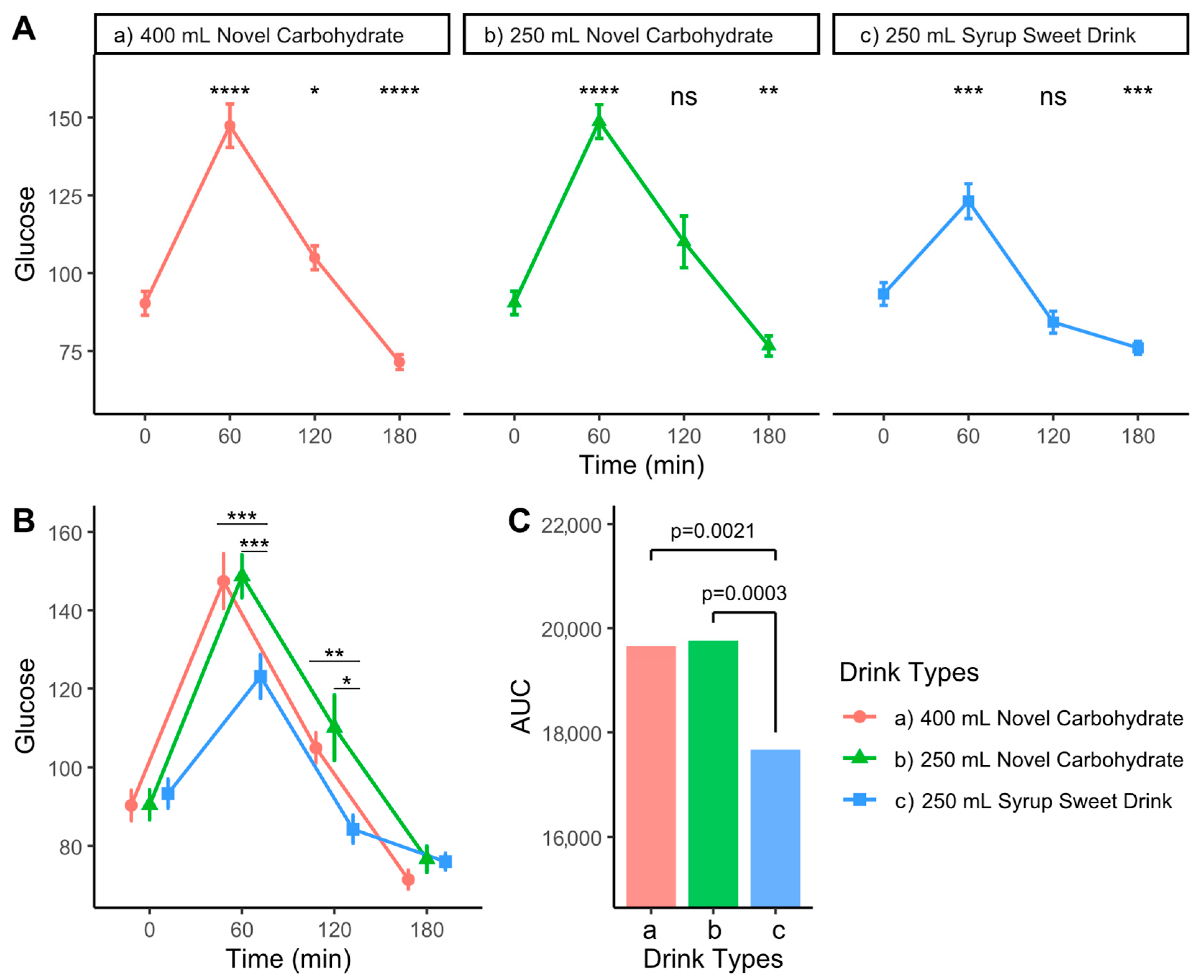
3.3. Effects on Postprandial Glycemic Response
3.4. Patient-Reported Outcomes on Fasting Discomfort
3.5. Safety and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERAS | Enhanced Recovery After Surgery |
| CSA | Cross-sectional area |
| VAS | Visual analog scales |
| SD | Standard deviation |
| IQR | Inter-quartile range |
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
Appendix A
| Variables | Time (Minutes) | Novel Carbohydrate Drink 400 mL (n = 16) | Novel Carbohydrate Drink 250 mL (n = 16) | Syrup Sweet Drink (n = 16) | p-Value |
|---|---|---|---|---|---|
| CSA: median [IQR] | 0 | 2.8 [2.6, 3.2] | 2.7 [2.3, 3.3] | 2.9 [2.5, 3.3] | 0.858 |
| 10 | 6.6 [6.1, 7.3] | 5.9 [4.6, 7.7] | 5.7 [4.4, 7.6] | 0.356 | |
| 60 | 4.5 [4.0, 5.3] | 4.2 [3.7, 5.3] | 3.8 [3.3, 4.3] | 0.166 | |
| 120 | 3.1 [2.6, 3.5] | 3.0 [2.5, 3.4] | 2.7 [2.5, 3.0] | 0.561 | |
| 180 | 2.8 [2.4, 3.3] | 2.5 [2.1, 2.9] | 2.7 [2.4, 3.3] | 0.242 | |
| Glucose (mg/dL) | 0 | 90.3 (15.3) | 90.4 (15.1) | 93.3 (14.7) | 0.816 |
| 60 | 147.4 (28.0) a | 148.7 (21.7) a | 123.1 (22.4) b | 0.006 | |
| 120 | 104.9 (15.3) a | 110.1 (33.3) a | 84.2 (14.1) b | 0.006 | |
| 180 | 71.4 (9.6) | 76.6 (13.0) | 76.0 (8.4) | 0.323 | |
| Thirst Score (cm) | 0 | 5.4 (3.2) | 5.6 (3.0) | 5.4 (3.3) | 0.962 |
| 60 | 3.7 (1.9) | 4.1 (2.1) | 4.1 (2.4) | 0.823 | |
| 120 | 4.2 (2.4) | 4.7 (2.3) | 4.7 (2.2) | 0.739 | |
| 180 | 5.0 (2.7) | 5.4 (2.9) | 5.2 (2.2) | 0.883 | |
| Hunger Score (cm) | 0 | 3.7 (2.6) | 4.0 (2.8) | 4.0 (2.9) | 0.935 |
| 60 | 4.1 (2.6) | 5.0 (2.3) | 4.4 (2.4) | 0.565 | |
| 120 | 5.0 (2.9) | 6.0 (2.5) | 5.5 (2.6) | 0.539 | |
| 180 | 6.6 (3.1) | 7.0 (2.6) | 6.5 (2.4) | 0.853 | |
| Mouth Dryness (cm) | 0 | 4.9 (2.8) | 5.5 (3.2) | 5.4 (3.1) | 0.823 |
| 60 | 3.6 (1.8) | 4.2 (2.7) | 4.4 (2.6) | 0.613 | |
| 120 | 4.4 (2.7) | 4.4 (2.5) | 4.8 (2.5) | 0.894 | |
| 180 | 5.5 (3.1) | 5.3 (3.1) | 5.3 (2.5) | 0.973 |
References
- Ricci, C.; Ingaldi, C.; Alberici, L.; Serbassi, F.; Pagano, N.; De Raffele, E.; Minni, F.; Pironi, L.; Sasdelli, A.S.; Casadei, R. Preoperative carbohydrate loading before elective abdominal surgery: A systematic review and network meta-analysis of phase II/III randomized controlled trials. Clin. Nutr. 2022, 41, 313–320. [Google Scholar] [CrossRef]
- Lin, M.-W.; Chen, C.-I.; Cheng, T.-T.; Huang, C.-C.; Tsai, J.-W.; Feng, G.-M.; Hwang, T.-Z.; Lam, C.-F. Prolonged preoperative fasting induces postoperative insulin resistance by ER-stress mediated Glut4 down-regulation in skeletal muscles. Int. J. Med. Sci. 2021, 18, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, R.S.; Tufts, C.W.; DePinto, D.G.; Chen, J.; Altshuler, J.R.; Serdiuk, A.; Cohen, J.B.; Patel, S.Y. How Sweet Is This? A Review and Evaluation of Preoperative Carbohydrate Loading in the Enhanced Recovery After Surgery Model. Nutr. Clin. Pract. 2020, 35, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Schricker, T.; Lattermann, R.; Carli, F. Physiology and Pathophysiology of ERAS. In Enhanced Recovery After Surgery; Ljungqvist, O., Francis, N., Urman, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 11–22. [Google Scholar] [CrossRef]
- Fawcett, W.J.; Ljungqvist, O. Starvation, carbohydrate loading, and outcome after major surgery. BJA Educ. 2017, 17, 312–316. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardiothorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Melloul, E.; Lassen, K.; Roulin, D.; Grass, F.; Perinel, J.; Adham, M.; Wellge, E.B.; Kunzler, F.; Besselink, M.G.; Asbun, H.; et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) Recommendations 2019. World J. Surg. 2020, 44, 2056–2084. [Google Scholar] [CrossRef]
- Brustia, R.; Monsel, A.; Skurzak, S.; Schiffer, E.; Carrier, F.M.; Patrono, D.; Kaba, A.; Detry, O.; Malbouisson, L.; Andraus, W.; et al. Guidelines for Perioperative Care for Liver Transplantation: Enhanced Recovery After Surgery (ERAS) Society Recommendations. Transplantation 2021, 106, 552–561. [Google Scholar] [CrossRef]
- Joshi, G.P.; Abdelmalak, B.B.; Weigel, W.A.; Harbell, M.W.; Kuo, C.I.; Soriano, S.G.; Stricker, P.A.; Tipton, T.; Grant, M.D.; Marbella, A.M.; et al. 2023 American Society of Anesthesiologists Practice Guidelines for Preoperative Fasting: Carbohydrate-containing Clear Liquids with or without Protein, Chewing Gum, and Pediatric Fasting Duration-A Modular Update of the 2017 American Society of Anesthesiologists Practice Guidelines for Preoperative Fasting. Anesthesiology 2023, 138, 132–151. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Zhao, T.; Zhao, B.; Yu, D.; Jiang, X.; Ye, L.; Zhao, L.; Lv, W.; Zhang, Y.; et al. Safety and efficacy of a novel neurosurgical enhanced recovery after surgery protocol for elective craniotomy: A prospective randomized controlled trial. J. Neurosurg. 2019, 130, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Van De Putte, P.; Perlas, A. The link between gastric volume and aspiration risk. In search of the Holy Grail? Anaesthesia 2018, 73, 274–279. [Google Scholar] [CrossRef]
- Nygren, J.; Soop, M.; Thorell, A.; Efendic, S.; Nair, K.S.; Ljungqvist, O. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin. Nutr. 1998, 17, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Soop, M.; Thorell, A.; Sree Nair, K.; Ljungqvist, O. Preoperative oral carbohydrates and postoperative insulin resistance. Clin. Nutr. 1999, 18, 117–120. [Google Scholar] [CrossRef]
- Tong, E.; Chen, Y.; Ren, Y.; Zhou, Y.; Di, C.; Zhou, Y.; Shao, S.; Qiu, S.; Hong, Y.; Yang, L.; et al. Effects of preoperative carbohydrate loading on recovery after elective surgery: A systematic review and Bayesian network meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 951676. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.T. Gastric emptying in ethnic populations: Possible relationship to development of diabetes and metabolic syndrome. Ethn. Dis. 2006, 16, 682–692. [Google Scholar]
- Bouvet, L.; Desgranges, F.P.; Aubergy, C.; Boselli, E.; Dupont, G.; Allaouchiche, B.; Chassard, D. Prevalence and factors predictive of full stomach in elective and emergency surgical patients: A prospective cohort study. Br. J. Anaesth. 2017, 118, 372–379. [Google Scholar] [CrossRef]
- Ntuk, U.E.; Gill, J.M.; Mackay, D.F.; Sattar, N.; Pell, J.P. Ethnic-specific obesity cutoffs for diabetes risk: Cross-sectional study of 490,288 UK biobank participants. Diabetes Care 2014, 37, 2500–2507. [Google Scholar] [CrossRef]
- Rattarasarn, C. Dysregulated lipid storage and its relationship with insulin resistance and cardiovascular risk factors in non-obese Asian patients with type 2 diabetes. Adipocyte 2018, 7, 71–80. [Google Scholar] [CrossRef]
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef]
- Kaewborisutsakul, A.; Kitsiripant, C.; Kaewsridam, S.; Kaewborisutsakul, W.K.; Churuangsuk, C. The influence of enhanced recovery after surgery protocol adherence in patients undergoing elective neuro-oncological craniotomies. World Neurosurg. X 2023, 19, 100196. [Google Scholar] [CrossRef] [PubMed]
- Kitsiripant, C.; Rujirapat, T.; Chatmongkolchart, S.; Tanasansuttiporn, J.; Khanungwanitkul, K. Comparison of Gastric Residual Volume After Ingestion of A Carbohydrate Drink and Water in Healthy Volunteers with Obesity: A Randomized Crossover Study. Obes. Surg. 2024, 34, 3813–3820. [Google Scholar] [CrossRef] [PubMed]
- Wongyingsinn, M.; Luangchan, S.; Tungsongsawat, S.; Trakarnsanga, A.; Lohsiriwat, V. A randomized controlled trial of preoperative carbohydrate drinks on postoperative walking capacity in elective colorectal surgery. Asia Pac. J. Clin. Nutr. 2019, 28, 727–733. [Google Scholar] [CrossRef]
- Leiper, J.B. Fate of ingested fluids: Factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr. Rev. 2015, 73 (Suppl. S2), 57–72. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Uchida, K.; Akahane, M.; Watanabe, Y.; Ohtomo, K.; Yamada, Y. The effects on gastric emptying and carbohydrate loading of an oral nutritional supplement and an oral rehydration solution: A crossover study with magnetic resonance imaging. Anesth. Analg. 2014, 118, 1268–1273. [Google Scholar] [CrossRef]
- Perlas, A.; Arzola, C.; Van De Putte, P. Point-of-care gastric ultrasound and aspiration risk assessment: A narrative review. Can. J. Anaesth. 2018, 65, 437–448. [Google Scholar] [CrossRef]
- Jian, W.-L.; Zhang, Y.-L.; Xu, J.-M.; Xia, S.-Y.; Zeng, H.; Dai, R.-P.; Li, H. Effects of a carbohydrate loading on gastric emptying and fasting discomfort: An ultrasonography study. Int. J. Clin. Exp. Med. 2017, 10, 788–794. [Google Scholar]
- Crowe, P.J.; Dennison, A.; Royle, G.T. The effect of pre-operative glucose loading on postoperative nitrogen metabolism. Br. J. Surg. 2005, 71, 635–637. [Google Scholar] [CrossRef]
- Onalan, E.; Andsoy, I.I.; Ersoy, O.F. The Effect of Preoperative Oral Carbohydrate Administration on Insulin Resistance and Comfort Level in Patients Undergoing Surgery. J. Perianesth Nurs. 2019, 34, 539–550. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Thorell, A.; Gutniak, M.; Häggmark, T.; Efendic, S. Glucose infusion instead of preoperative fasting reduces postoperative insulin resistance. J. Am. Coll. Surg. 1994, 178, 329–336. [Google Scholar] [CrossRef]
- Pillinger, N.L.; Robson, J.L.; Kam, P.C.A. Nutritional Prehabilitation: Physiological Basis and Clinical Evidence. Anaesth. Intensive Care 2018, 46, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Rizvanovic, N.; Nesek Adam, V.; Causevic, S.; Dervisevic, S.; Delibegovic, S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int. J. Color. Dis. 2019, 34, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Taché, Y.; Martinez, V.; Million, M.; Wang, L., III. Stress-related alterations of gut motor function: Role of brain corticotropin-releasing factor receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G173–G177. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Values (n = 16) |
|---|---|
| Age, years | 33.7 (8.1) |
| Female sex, n (%) | 15 (93.8) |
| Weight, kg | 61.2 (14.2) |
| Height, cm | 1.6 (0.0) |
| Body mass index, kg/m2 | 24.0 (5.1) |
| Baseline blood glucose, mg/dL | 91.4 (14.8) |
| Baseline gastric CSA, cm2 (Median, [IQR]) | 2.8 [2.6, 3.3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Churuangsuk, C.; Khanungwanitkul, K.; Kaewborisutsakul, A.; Kitsiripant, C.; Rattanaburi, A.; Suntornlohanakul, O.; Charupanit, K.; Ingviya, T.; Intusoma, U.; Puttarak, P. Novel Preoperative Carbohydrate Drinks Versus Commercial Syrup-Based Drinks on Gastric Emptying, Glycemic Responses, and Fasting Discomfort: A Pilot Randomized Crossover Trial. Nutrients 2025, 17, 2131. https://doi.org/10.3390/nu17132131
Churuangsuk C, Khanungwanitkul K, Kaewborisutsakul A, Kitsiripant C, Rattanaburi A, Suntornlohanakul O, Charupanit K, Ingviya T, Intusoma U, Puttarak P. Novel Preoperative Carbohydrate Drinks Versus Commercial Syrup-Based Drinks on Gastric Emptying, Glycemic Responses, and Fasting Discomfort: A Pilot Randomized Crossover Trial. Nutrients. 2025; 17(13):2131. https://doi.org/10.3390/nu17132131
Chicago/Turabian StyleChuruangsuk, Chaitong, Khanin Khanungwanitkul, Anukoon Kaewborisutsakul, Chanatthee Kitsiripant, Athithan Rattanaburi, Onnicha Suntornlohanakul, Krit Charupanit, Thammasin Ingviya, Utcharee Intusoma, and Panupong Puttarak. 2025. "Novel Preoperative Carbohydrate Drinks Versus Commercial Syrup-Based Drinks on Gastric Emptying, Glycemic Responses, and Fasting Discomfort: A Pilot Randomized Crossover Trial" Nutrients 17, no. 13: 2131. https://doi.org/10.3390/nu17132131
APA StyleChuruangsuk, C., Khanungwanitkul, K., Kaewborisutsakul, A., Kitsiripant, C., Rattanaburi, A., Suntornlohanakul, O., Charupanit, K., Ingviya, T., Intusoma, U., & Puttarak, P. (2025). Novel Preoperative Carbohydrate Drinks Versus Commercial Syrup-Based Drinks on Gastric Emptying, Glycemic Responses, and Fasting Discomfort: A Pilot Randomized Crossover Trial. Nutrients, 17(13), 2131. https://doi.org/10.3390/nu17132131







