Ultra-Processed Foods and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): What Is the Evidence So Far?
Abstract
1. Introduction
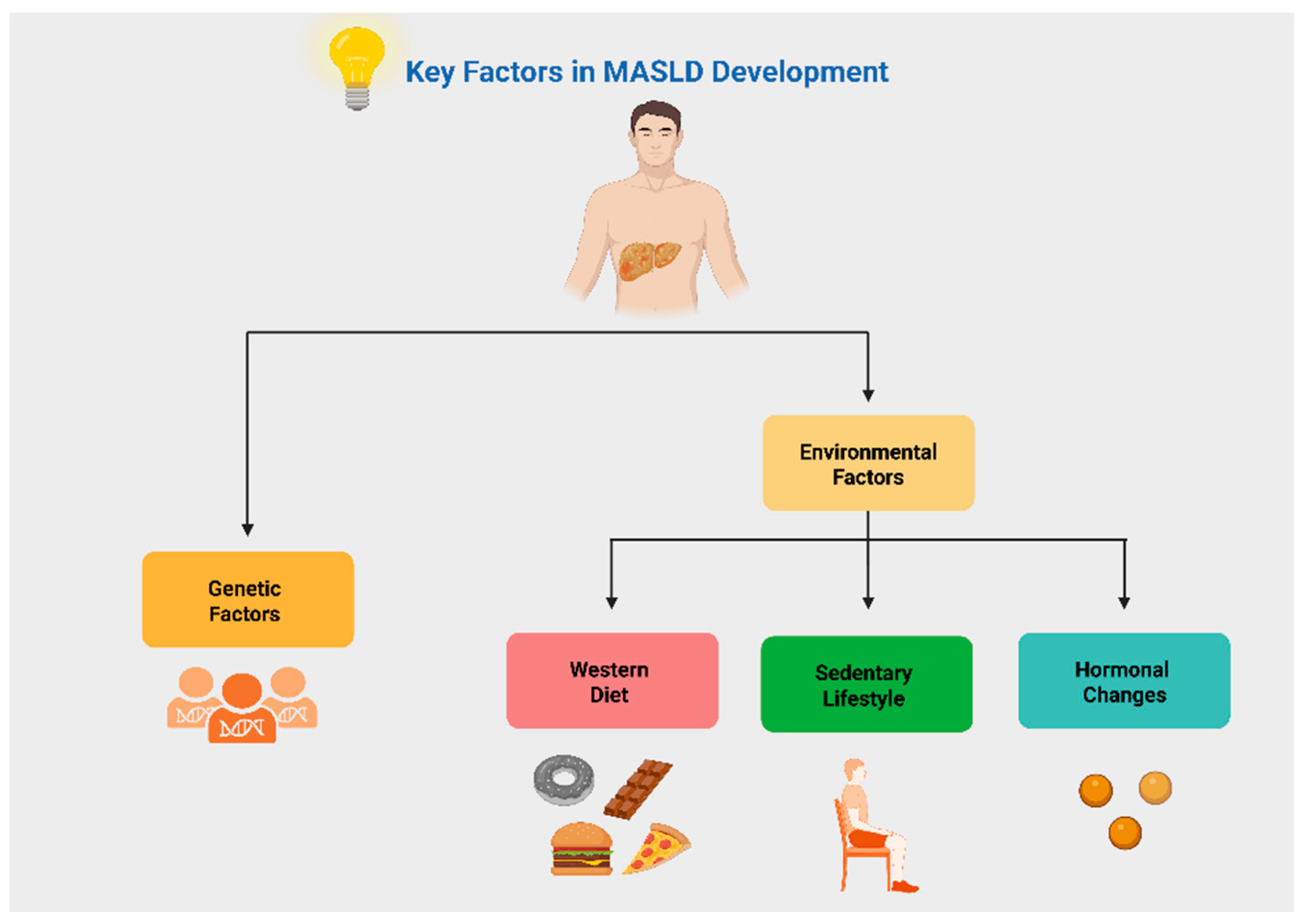
2. Key Factors in MASLD Pathogenesis
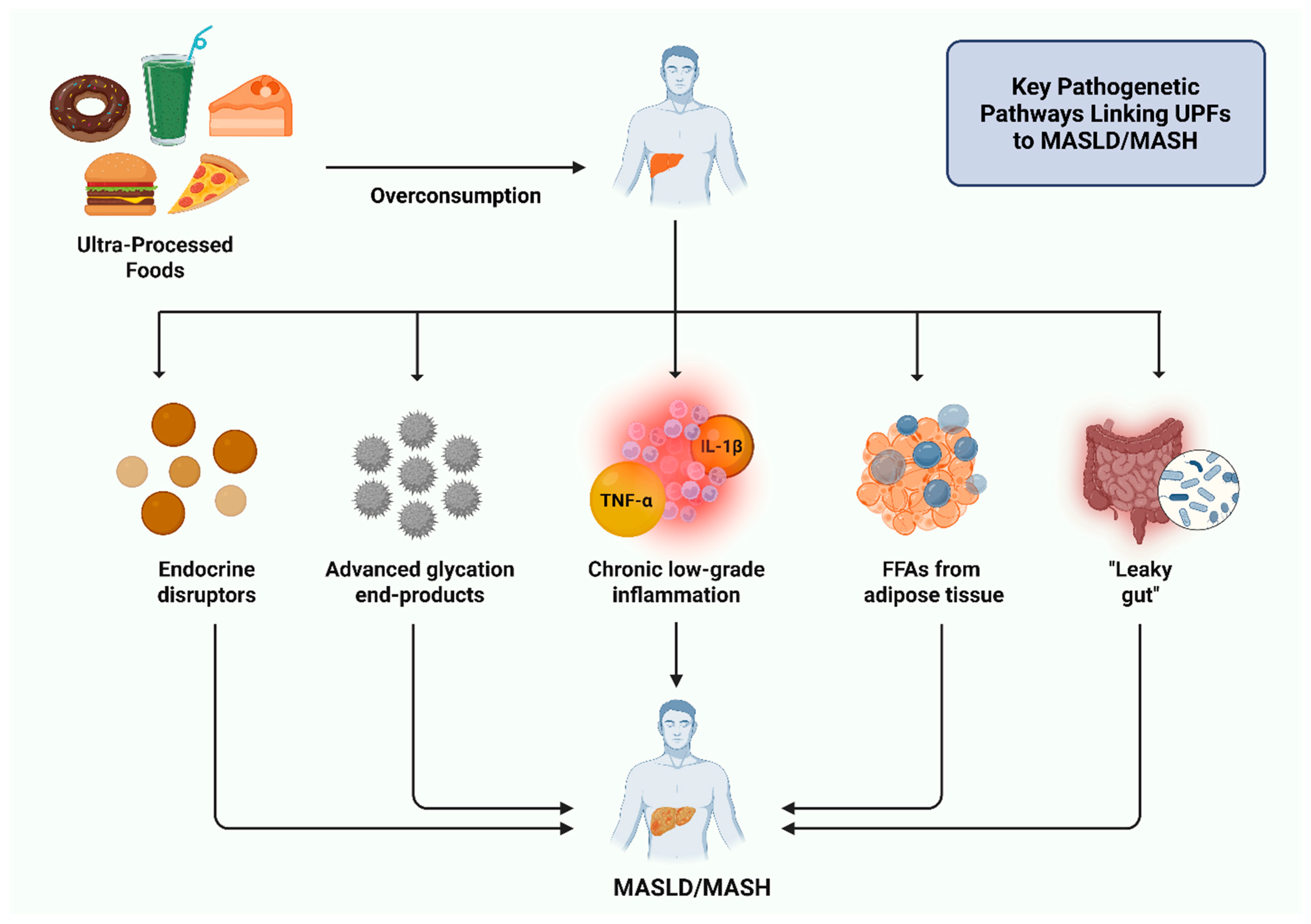
3. Pathophysiological Mechanisms Linking Ultra-Processed Foods to MASLD
4. Associating Ultra-Processed Foods with MASLD
4.1. Current Concepts
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Kébé, S.D.; Diouf, A.; Sylla, P.M.D.D.; Badiane, A.; Mama, O.M.; Idohou-Dossou, N. Ultra-Processed Foods Consumption Is Associated With Intakes of Critical Nutrients Related to Non-Communicable Diseases Among Adults in Dakar, Senegal. Int. J. Public Health 2025, 70, 1608374. [Google Scholar] [CrossRef] [PubMed]
- Gibney, M.J. Ultra-Processed Foods: Definitions and Policy Issues. Curr. Dev. Nutr. 2019, 3, nzy077. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kounatidis, D.; Tzivaki, I.; Zafeiri, G.C.M.; Rigatou, A.; Daskalopoulou, S.; Stratigou, T.; Karampela, I.; Dalamaga, M. Ultra-Processed Foods and Childhood Obesity: Current evidence and perspectives. Curr. Nutr. Rep. 2025, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Tzivaki, I.; Daskalopoulou, S.; Adamou, A.; Michalaki Zafeiri, G.C.; Karampela, I.; Dalamaga, M.; Kounatidis, D. Ultra-Processed Foods and Type 2 Diabetes Mellitus: What Is the Evidence So Far? Biomolecules 2025, 15, 307. [Google Scholar] [CrossRef]
- Abdelhameed, F.; Kite, C.; Lagojda, L.; Dallaway, A.; Chatha, K.K.; Chaggar, S.S.; Dalamaga, M.; Kassi, E.; Kyrou, I.; Randeva, H.S. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review from NAFLD to MAFLD and MASLD. Curr. Obes. Rep. 2024, 13, 510–531. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Hardy, T.; Wonders, K.; Younes, R.; Aithal, G.P.; Aller, R.; Allison, M.; Bedossa, P.; Betsou, F.; Boursier, J.; Brosnan, M.J.; et al. The European NAFLD Registry: A real-world longitudinal cohort study of nonalcoholic fatty liver disease. Contemp. Clin. Trials. 2020, 98, 106175. [Google Scholar] [CrossRef]
- Song, S.J.; Lai, J.C.; Wong, G.L.; Wong, V.W.; Yip, T.C. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. 2024, 80, e54–e56. [Google Scholar] [CrossRef]
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open. 2025, 8, e2454707. [Google Scholar] [CrossRef]
- Balkhed, W.; Bergram, M.; Iredahl, F.; Holmberg, M.; Edin, C.; Carlhäll, C.J.; Ebbers, T.; Henriksson, P.; Simonsson, C.; Rådholm, K.; et al. Evaluating the prevalence and severity of metabolic dysfunction-associated steatotic liver disease in patients with type 2 diabetes mellitus in primary care. J. Intern. Med. 2025. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Valério, C.M.; Júnior, W.S.S.; de Araujo-Neto, J.M.; Sposito, A.C.; Suassuna, J.H.R. CARDIAL-MS (CArdio-Renal-DIAbetes-Liver-Metabolic Syndrome): A new proposition for an integrated multisystem metabolic disease. Diabetol. Metab. Syndr. 2025, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.T.; Lee, W.C. Impact of Transgenerational Nutrition on Nonalcoholic Fatty Liver Disease Development: Interplay between Gut Microbiota, Epigenetics and Immunity. Nutrients 2024, 16, 1388. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.G.; Geladari, E.; Panoilia, M.P.; Daskou, A.; Stratigou, T.; Karampela, I.; Tsilingiris, D.; Dalamaga, M. NAFLD in the 21st Century: Current Knowledge Regarding Its Pathogenesis, Diagnosis and Therapeutics. Biomedicines 2024, 12, 826. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, X.; Zhang, Y.; Wang, T.; Lv, S.; Zhang, X.; Zhou, Y.; Peng, T.; Song, Y. Associations Between APOC3 and ANGPTL8 Gene Polymorphisms with MASLD Risk and the Mediation Effect of Triglyceride on MASLD in the Chinese Population. J. Cell. Mol. Med. 2025, 29, e70542. [Google Scholar] [CrossRef]
- Chen, V.L.; Kuppa, A.; Oliveri, A.; Chen, Y.; Ponnandy, P.; Patel, P.B.; Palmer, N.D.; Speliotes, E.K. Human genetics of metabolic dysfunction-associated steatotic liver disease: From variants to cause to precision treatment. J. Clin. Investig. 2025, 135, e186424. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, B. Unraveling the pathogenesis of non-alcoholic fatty liver diseases through genome-wide association studies. J. Gastroenterol. Hepatol. 2023, 38, 1877–1885. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Psallida, S.; Vythoulkas-Biotis, N.; Adamou, A.; Zachariadou, T.; Kargioti, S.; Karampela, I.; Dalamaga, M. NAFLD/MASLD and the Gut-Liver Axis: From Pathogenesis to Treatment Options. Metabolites 2024, 14, 366. [Google Scholar] [CrossRef]
- Valenti, L.; Hagström, H. Bringing genetic testing into the clinical management of people with MASLD: Are we there yet? J. Hepatol. 2025. [Google Scholar] [CrossRef]
- Bianco, C.; Jamialahmadi, O.; Pelusi, S.; Baselli, G.; Dongiovanni, P.; Zanoni, I.; Santoro, L.; Maier, S.; Liguori, A.; Meroni, M.; et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J. Hepatol. 2021, 74, 775–782. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- García, S.; Monserrat-Mesquida, M.; Ugarriza, L.; Casares, M.; Gómez, C.; Mateos, D.; Angullo-Martínez, E.; Tur, J.A.; Bouzas, C. Ultra-Processed Food Consumption and Metabolic-Dysfunction Associated Steatotic Liver Disease (MASLD): A Longitudinal and Sustainable Analysis. Nutrients 2025, 17, 472. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, J.; Fiol, M.; Colom, A.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Soria-Florido, M.T.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Does Consumption of Ultra-Processed Foods Matter for Liver Health? Prospective Analysis among Older Adults with Metabolic Syndrome. Nutrients 2022, 14, 4142. [Google Scholar] [CrossRef] [PubMed]
- Aramburu, A.; Alvarado-Gamarra, G.; Cornejo, R.; Curi-Quinto, K.; Díaz-Parra, C.d.P.; Rojas-Limache, G.; Lanata, C.F. Ultra-processed foods consumption and health-related outcomes: A systematic review of randomized controlled trials. Front. Nutr. 2024, 11, 1421728. [Google Scholar] [CrossRef]
- Dicken, S.J.; Batterham, R.L. Ultra-processed Food and Obesity: What Is the Evidence? Curr. Nutr. Rep. 2024, 13, 23–38. [Google Scholar] [CrossRef]
- Global Food Research Program. Ultra-Processed Foods: A Global Threat to Public Health. 2023. Available online: https://www.globalfoodresearchprogram.org/wp-content/uploads/2023/11/GFRP_FactSheet_UltraProcessedFoods_2023_11.pdf (accessed on 13 April 2025).
- Taylor, R.; Barnes, A.C.; Hollingsworth, K.G.; Irvine, K.M.; Solovyova, A.S.; Clark, L.; Kelly, T.; Martin-Ruiz, C.; Romeres, D.; Koulman, A.; et al. Aetiology of Type 2 diabetes in people with a ‘normal’ body mass index: Testing the personal fat threshold hypothesis. Clin. Sci. 2023, 137, 1333–1346. [Google Scholar] [CrossRef]
- Millar, S.R.; Harrington, J.M.; Perry, I.J.; Phillips, C.M. Associations between ultra-processed food and drink consumption and biomarkers of chronic low-grade inflammation: Exploring the mediating role of adiposity. Eur. J. Nutr. 2025, 64, 150. [Google Scholar] [CrossRef]
- Bastos, A.A.; Félix, P.V.; Carnaúba, R.A.; Valentini Neto, J.; Vicente, B.M.; Ferreira, L.M.; Batista, L.D.; de Melo, C.M.; Fisberg, R.M.; Yannakoulia, M.; et al. Evaluating the influence of ultra-processed food intake on associations between dietary indices with systemic inflammation in adulthood and old ages. Clin. Nutr. ESPEN 2024, 61, 8–14. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Zhu, J.H.; Liu, B.P.; Jia, C.X. The joint associations of physical activity and ultra-processed food consumption with depression: A cohort study in the UK Biobank. J. Affect. Disord. 2024, 367, 184–192. [Google Scholar] [CrossRef]
- Alabdul Razzak, I.; Fares, A.; Stine, J.G.; Trivedi, H.D. The Role of Exercise in Steatotic Liver Diseases: An Updated Perspective. Liver Int. 2025, 45, e16220. [Google Scholar] [CrossRef]
- Mosca, A.; Manco, M.; Braghini, M.R.; Cianfarani, S.; Maggiore, G.; Alisi, A.; Vania, A. Environment, Endocrine Disruptors, and Fatty Liver Disease Associated with Metabolic Dysfunction (MASLD). Metabolites 2024, 14, 71. [Google Scholar] [CrossRef]
- Cano, R.; Perez, J.L.; Davila, L.A.; Ortega, Á.; Gómez, Y.; Valero-Cedeño, N.J.; Parra, H.; Manzano, A.; Véliz Castro, T.I.; Albornoz, M.P.D.; et al. Role of endocrine-disrupting chemicals in the pathogenesis of non-alcoholic fatty liver disease: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 4807. [Google Scholar] [CrossRef]
- Ravichandran, G.; Lakshmanan, D.K.; Arunachalam, A.; Thilagar, S. Food obesogens as emerging metabolic disruptors; A toxicological insight. J. Steroid Biochem. Mol. Biol. 2022, 217, 106042. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L. Ultra-processed foods and type 2 diabetes: More fundamental research is needed. Lancet Reg. Health Eur. 2024, 46, 101084. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. Int. J. Mol. Sci. 2024, 25, 675. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wan, S.; Yu, L.; Liu, W.; Song, J.; Shi, D.; Zhang, Y.; Chen, W.; Qiu, W.; Wang, B. Phthalates exposure, biological aging, and increased risks of insulin resistance, prediabetes, and diabetes in adults with metabolic dysfunction-associated steatotic liver disease. Diabetes Metab. 2025, 51, 101602. [Google Scholar] [CrossRef]
- Lolescu, B.M.; Furdui-Lința, A.V.; Ilie, C.A.; Sturza, A.; Zară, F.; Muntean, D.M.; Blidișel, A.; Crețu, O.M. Adipose tissue as target of environmental toxicants: Focus on mitochondrial dysfunction and oxidative inflammation in metabolic dysfunction-associated steatotic liver disease. Mol. Cell. Biochem. 2025, 480, 2863–2879. [Google Scholar] [CrossRef]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Harrison, S.A.; Allen, A.M.; Dubourg, J.; Noureddin, M.; Alkhouri, N. Challenges and opportunities in NASH drug development. Nat. Med. 2023, 29, 562–573. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signaling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.; Evangelopoulos, A.; Vlahodimitris, I.; Grivakou, E.; Kotsi, E.; Dimitriou, K.; Skourtis, A.; Mourouzis, I. SGLT-2 Inhibitors and the Inflammasome: What’s Next in the 21st Century? Nutrients 2023, 15, 2294. [Google Scholar] [CrossRef]
- Saghir, S.A.; Shams, N.; Veliz, L.; Alfuraih, S.; Omidi, Y.; Barar, J.; Sharma, A.; Ansari, R. Non-alcoholic fatty liver disease: Genetic susceptibility. Arch. Clin. Toxicol. 2024, 6, 33–47. [Google Scholar] [CrossRef]
- Afrisham, R.; Alasvand, G.; Jadidi, Y.; Farrokhi, V.; Moradi, N.; Alizadeh, S.; Fadaei, R. CCN3/NOV Serum Levels in Non-alcoholic Fatty Liver Disease (NAFLD) Patients in Comparison with the Healthy Group and its Correlation with TNF-α and IL-6. Curr. Mol. Med. 2025, 25, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H. Multiple organs involved in the pathogenesis of non-alcoholic fatty liver disease. Cell. Biosci. 2020, 10, 140. [Google Scholar] [CrossRef]
- Litwinowicz, K.; Waszczuk, E.; Gamian, A. Advanced glycation end-products in common non-infectious liver diseases: Systematic review and meta analysis. Nutrients 2021, 13, 3370. [Google Scholar] [CrossRef]
- Yu, G.; Wen, W.; Li, Q.; Chen, H.; Zhang, S.; Huang, H.; Zhang, Q.; Fu, L. Heat-Processed Diet Rich in Advanced Glycation End Products Induced the Onset and Progression of NAFLD via Disrupting Gut Homeostasis and Hepatic Lipid Metabolism. J. Agric. Food Chem. 2025, 73, 2510–2526. [Google Scholar] [CrossRef]
- Leerach, N.; Harashima, A.; Munesue, S.; Kimura, K.; Oshima, Y.; Goto, H.; Yamamoto, H.; Higashida, H.; Yamamoto, Y. Glycation reaction and the role of the receptor for advanced glycation end-products in immunity and social behavior. Glycoconj. J. 2021, 38, 303–310. [Google Scholar] [CrossRef]
- Sakasai-Sakai, A.; Takeda, K.; Takeuchi, M. Involvement of Intracellular TAGE and the TAGE-RAGE-ROS Axis in the Onset and Progression of NAFLD/NASH. Antioxidants 2023, 12, 748. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zheng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Sig. Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Nier, A.; Ulrich, C.; Volk, C.; Wolffgang, M.C.; Brandsch, C.; Wensch-Dorendorf, M.; Girndt, M.; Stangl, G.I. Effects of a single phosphate-enriched test meal on inflammasome activity and postprandial inflammatory markers in healthy subjects. Eur. J. Nutr. 2024, 63, 797–807. [Google Scholar] [CrossRef]
- Xiang, M.; Tian, X.; Wang, H.; Gan, P.; Zhang, Q. Inappropriate Diet Exacerbates Metabolic Dysfunction-Associated Steatotic Liver Disease via Abdominal Obesity. Nutrients 2024, 16, 4208. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, S.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Gu, Y.; Wang, Y.; Zhang, T.; et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin chronic low grade systemic inflammation and health cohort study. Int. J. Epidemiol. 2022, 51, 237–249. [Google Scholar] [CrossRef]
- Jahromi, M.K.; Tehrani, A.N.; Teymoori, F.; Asghari, G.; Kalantari, M.; Mirmiran, P.; Azizi, F. Dietary advanced glycation end products are associated with an increased risk of non-alcoholic fatty liver disease in Iranian adults. BMC Endocr. Disord. 2023, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, Z.; Ma, X.; Wang, J.; Wu, Y.; Zhu, Y.; Wang, Y.; Yang, Y.; Luo, M.; Li, J.; et al. Effects of ultra-processed foods on the liver: Insights from gut microbiome and metabolomics studies in rats. Front. Nutr. 2025, 11, 1503879. [Google Scholar] [CrossRef] [PubMed]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The Detrimental Impact of Ultra-Processed Foods on the Human Gut Microbiome and Gut Barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef] [PubMed]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Ma, R.; Shi, G.; Li, Y.; Shi, H. Trimethylamine N-oxide, choline and its metabolites are associated with the risk of non-alcoholic fatty liver disease. Br. J. Nutr. 2024, 6, 1915–1923. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qiao, W.; Zhuang, J.; Feng, H.; Zhang, Z.; Zhang, Y. Association of ultra-processed food intake with severe non-alcoholic fatty liver disease: A prospective study of 143073 UK Biobank participants. J. Nutr. Health Aging. 2024, 28, 100352. [Google Scholar] [CrossRef]
- Zhao, L.; Clay-Gilmour, A.; Zhang, J.; Zhang, X.; Steck, S.E. Higher ultra-processed food intake is associated with adverse liver outcomes: A prospective cohort study of UK Biobank participants. Am. J. Clin. Nutr. 2024, 119, 49–57. [Google Scholar] [CrossRef]
- Rahimi-Sakak, F.; Maroofi, M.; Emamat, H.; Hekmatdoost, A. Red and Processed Meat Intake in Relation to Non-Alcoholic Fatty Liver Disease Risk: Results from a Case-Control Study. Clin. Nutr. Res. 2022, 11, 42–49. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Martinez Steele, E.; Lo, C.H.; Zhang, F.F.; Zhang, X. Higher ultra-processed food intake was positively associated with odds of NAFLD in both US adolescents and adults: A national survey. Hepatol. Commun. 2023, 7, e0240. [Google Scholar] [CrossRef]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Cuthbertson, D.J. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2266. [Google Scholar] [CrossRef] [PubMed]
- Grinshpan, L.S.; Eilat-Adar, S.; Ivancovsky-Wajcman, D.; Kariv, R.; Gillon-Keren, M.; Zelber-Sagi, S. Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: A systematic review. JHEP. Rep. 2023, 6, 100964. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef]
- Noureddin, M.; Zelber-Sagi, S.; Wilkens, L.R.; Porcel, J.; Boushey, C.J.; Le Marchand, L.; Rosen, H.R.; Setiawan, V.W. Diet Associations with Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology 2020, 71, 1940–1952. [Google Scholar] [CrossRef]
- Yari, Z.; Cheraghpour, M.; Aghamohammadi, V.; Alipour, M.; Ghanei, N.; Hekmatdoost, A. Energy-dense nutrient-poor snacks and risk of non-alcoholic fatty liver disease: A case-control study in Iran. BMC Res. Notes 2020, 13, 221. [Google Scholar] [CrossRef]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Bentov, I.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021, 41, 2635–2645. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R.; Van Wagner, L.B.; Pereira, M.A. Levels of abdominal adipose tissue and metabolic-associated fatty liver disease (MAFLD) in middle age according to average fast-food intake over the preceding 25 years: The CARDIA Study. Am. J. Clin. Nutr. 2022, 116, 255–262. [Google Scholar] [CrossRef]
- Fridén, M.; Kullberg, J.; Ahlström, H.; Lind, L.; Rosqvist, F. Intake of Ultra-Processed Food and Ectopic-, Visceral- and Other Fat Depots: A Cross-Sectional Study. Front. Nutr. 2022, 9, 774718. [Google Scholar] [CrossRef]
- Kwanten, W.J. Diet and non-alcoholic fatty liver disease, a short narrative review. Acta Gastroenterol. Belg. 2023, 86, 306–310. [Google Scholar] [CrossRef]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40 (Suppl. S1), 102–108. [Google Scholar] [CrossRef]
- Kardashian, A.; Dodge, J.L.; Terrault, N.A. Quantifying the Negative Impact of Fast-food Consumption on Liver Steatosis Among United States Adults with Diabetes and Obesity. Clin. Gastroenterol. Hepatol. 2023, 21, 3176–3178.e3. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef] [PubMed]
- Angelidi, A.M.; Papadaki, A.; Nolen-Doerr, E.; Boutari, C.; Mantzoros, C.S. The effect of dietary patterns on non-alcoholic fatty liver disease diagnosed by biopsy or magnetic resonance in adults: A systematic review of randomised controlled trials. Metabolism 2022, 129, 155136. [Google Scholar] [CrossRef] [PubMed]
- Jeznach-Steinhagen, A.; Ostrowska, J.; Czerwonogrodzka-Senczyna, A.; Boniecka, I.; Shahnazaryan, U.; Kuryłowicz, A. Dietary and Pharmacological Treatment of Nonalcoholic Fatty Liver Disease. Medicina 2019, 55, 166. [Google Scholar] [CrossRef] [PubMed]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Hassani Zadeh, S.; Mozaffari-Khosravi, H.; Hosseinzadeh, M. Effect of Mediterranean diet on liver enzymes: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2022, 128, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, G.; Barabási, A.L.; Loscalzo, J. Chemical Complexity of Food and Implications for Therapeutics. N. Engl. J. Med. 2025, 392, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- George, E.S.; Reddy, A.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Abbott, G.; Johnson, N.A.; Sood, S.; Roberts, S.K.; Tierney, A.C. Impact of a Mediterranean diet on hepatic and metabolic outcomes in non-alcoholic fatty liver disease: The MEDINA randomised controlled trial. Liver. Int. 2022, 42, 1308–1322. [Google Scholar] [CrossRef]
- Zhou, Q.; Hu, H.; Hu, L.; Liu, S.; Chen, J.; Tong, S. Association between processed and unprocessed red meat consumption and risk of nonalcoholic fatty liver disease: A systematic review and dose-response meta-analysis. J. Glob. Health 2024, 14, 04060. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Feng, L.; Cao, C.; Fei, X. A study of correlation of the dietary index for gut microbiota with non-alcoholic fatty liver disease based on 2007-2018 National Health and Nutrition Examination Survey. Front. Nutr. 2025, 12, 1573249. [Google Scholar] [CrossRef]
- Donghia, R.; Tatoli, R.; Campanella, A.; Cuccaro, F.; Bonfiglio, C.; Giannelli, G. Adding a Leafy Vegetable Fraction to Diets Decreases the Risk of Red Meat Mortality in MASLD Subjects: Results from the MICOL Cohort. Nutrients 2024, 16, 1207. [Google Scholar] [CrossRef] [PubMed]
- Aerts, M.; Rosseel, Z.; De Waele, E. The Evolution in Non Alcoholic Fatty Liver Disease Patients’ Profile and the Associated Sustainable Challenges: A Multidisciplinary Perspective. Nutrients 2024, 16, 1584. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Ramaiah, P.; Jamel Baljon, K.; Alsulami, S.A.; Lindsay, G.M.; Chinnasamy, L. Diet quality indices and odds of metabolic dysfunction-associated fatty liver disease: A case-control study. Front. Nutr. 2024, 10, 1251861. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Zelber-Sagi, S.; Henry, L.; Gerber, L.H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 708–722. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Gómez, C.; Mateos, D.; Ripoll-Vera, T.; Tur, J.A.; Sureda, A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants 2022, 11, 901. [Google Scholar] [CrossRef]
- Jurek, J.M.; Zablocka-Sowinska, K.; Clavero Mestres, H.; Reyes Gutiérrez, L.; Camaron, J.; Auguet, T. The Impact of Dietary Interventions on Metabolic Outcomes in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Comorbid Conditions, Including Obesity and Type 2 Diabetes. Nutrients 2025, 17, 1257. [Google Scholar] [CrossRef]
- Mercurio, G.; Giacco, A.; Scopigno, N.; Vigliotti, M.; Goglia, F.; Cioffi, F.; Silvestri, E. Mitochondria at the Crossroads: Linking the Mediterranean Diet to Metabolic Health and Non-Pharmacological Approaches to NAFLD. Nutrients 2025, 17, 1214. [Google Scholar] [CrossRef]
- Tsitsou, S.; Bali, T.; Adamantou, M.; Saridaki, A.; Poulia, K.A.; Karagiannakis, D.S.; Papakonstantinou, E.; Cholongitas, E. Effects of a 12-Week Mediterranean-Type Time-Restricted Feeding Protocol in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomised Controlled Trial-The ‘CHRONO-NAFLD Project’. Aliment. Pharmacol. Ther. 2025, 61, 1290–1309. [Google Scholar] [CrossRef]
- Simancas-Racines, D.; Annunziata, G.; Verde, L.; Fascì-Spurio, F.; Reytor-González, C.; Muscogiuri, G.; Frias-Toral, E.; Barrea, L. Nutritional Strategies for Battling Obesity-Linked Liver Disease: The Role of Medical Nutritional Therapy in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Management. Curr. Obes. Rep. 2025, 14, 7. [Google Scholar] [CrossRef]
- Martinez-Vazquez, S.E.; Kammar-García, A.; Moctezuma-Velázquez, C.; Mancilla-Galindo, J.; García-Juárez, I.; Uscanga-Domínguez, L.F. The Impact of Dietary Sugars and Saturated Fats on Body and Liver Fat in a Healthcare Worker Population. Nutrients 2025, 17, 1328. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wu, T.; Du, X.; Li, Q.; Hao, Y.; Zhou, T.; Yi, Y. Association of dietary inflammatory index on all-cause and cardiovascular mortality in U.S. adults with metabolic dysfunction-associated steatotic liver disease. Front. Nutr. 2025, 12, 1478165. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Zelber-Sagi, S.; Lazarus, J.V.; Wai-Sun Wong, V.; Yilmaz, Y.; Duseja, A.; Eguchi, Y.; Castera, L.; Pessoa, M.G.; Oliveira, C.P.; et al. Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Gastroenterology 2025, in press. [CrossRef] [PubMed]
- Paredes-Marin, A.; Napoli, J.; Sivakumar, V.; Near, C.; Adams, H.; Harty, A.; Agarwal, R.; Dieterich, D.; Bucuvalas, J.; Kushner, T.; et al. Identifying Environmental Factors Associated with Significant Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease/Steatohepatitis. Dig. Dis. Sci. 2025. [Google Scholar] [CrossRef]


| Author/Year | Population | Study | Findings | Remarks |
|---|---|---|---|---|
| Zelber-Sagi et al., 2018 [64] | 789 individuals, aged 40 to 70 y.o. with a valid FFQ, and 357 with a valid meat questionnaire, in Israel between 2013 and 2015 | Cross-sectional Study | Higher intake of processed red meat was associated with increased odds for NAFLD as well as IR For red meat OR:1.47 95% CI: 1.04–2.09, p = 0.031. For processed red meat OR: 1.55, 95% CI: 1.07–2.23, p = 0.020. | Increased consumption of red processed meat was related to higher odds for NAFLD. The authors conclude that avoiding red processed meat and unhealthy cooking methods may alleviate NAFLD. |
| Noureddin et al., 2020 [65] | 2974 patients with NAFLD, with and without cirrhosis, with a valid FFQ, aged 45 to 75 y.o., in Hawai and California, U.S.A., between 1993 and 1996 | Nested case-control analysis within the MEC (Multiethnic cohort) prospective study with >215,000 participants | Higher intake of red meat and processed red meat was associated with NAFLD (p = 0.010 and p = 0.004, respectively). | The association between higher red meat and processed red meat intake and increased NAFLD was more prominent, especially among patients with NAFLD and cirrhosis. The authors concluded that by reducing the intake of red meat and processed red meat, patients could decrease the risk of NAFLD, and in particular NAFLD and advanced liver disease. |
| Yari et al., 2020 [66] | 143 adult patients with newly diagnosed NAFLD and 471 controls with a valid FFQ in Iran | Case-control study | Sweet energy-dense nutrient-poor snacks were moderately associated with a higher risk of NAFLD | The authors conclude that there might be a moderate association between energy-dense snacks and risk of NAFLD. |
| Ivancovsky-Wajcman et al., 2021 [67] | 789 participants, among whom 305 individuals were diagnosed with NAFLD, with a valid FFQ in Israel | Cross-sectional study | Higher consumption of UPFs was associated with increased odds for METS (OR: 1.88 95% CI: 1.31–2.71, p = 0.001). | Increased consumption of UPFs is related to a higher risk of METS, and among patients with NAFLD, it is associated with an increased risk of NASH. |
| Odegaard et al., 2022 [68] | 5115 participants, aged 18 to 30 y.o., between 1985 and 1986 were enrolled from 4 cities in the USA | Prospective multicenter cohort study (the CARDIA Study) | Participants were followed for 25 years, and there was a significant association between MAFLD in middle age among individuals with more frequent consumption of fast food. | Although no FFQ had been used, participants answered a question regarding frequent or infrequent fast food consumption, six times during the 25 years of the follow-up. Increased VAT, liver fat, and odds of MAFLD were related to a more frequent consumption of fast food during the 25 years. |
| Rahimi-Sakak et al., 2022 [60] | 196 patients with NAFLD and 803 controls, aged >18 y.o., with a valid FFQ, in Iran | Case-control study | Patients with the highest quartile of processed red meat consumption had a 3.28 times higher risk of NAFLD, when compared to the lowest quartile of processed red meat consumption (OR: 3.28, 95% CI: 1.97–5.46, p < 0.001). | The authors conclude that there seems to be a relationship between higher consumption of processed red meat and the risk of NAFLD, which requires further evaluation. |
| Friden et al., 2022 [69] | 285 individuals, aged 50 y.o., with a valid FFQ in 2010, in Uppsala, Sweden | Cross-sectional study based on data taken from a prospective population-based cohort | There was an association between consumption of UPFs that were rich in energy and carbohydrates with VAT. This association was more significant among women than men. | Although energy intake from UPFs was not associated with liver fat nor SAT, there was an association between UPFs and VAT, especially among women rather than men. |
| Zhang et al., 2022 [52] | 16,168 participants, with a valid FFQ, aged 18 to 90 y.o., in China | Prospective study | Among the studied population, i.e., 16,168 individuals, there were 3752 cases of NAFLD during 56,935 person-years of follow-up. | The study documented a relationship between higher UPF consumption and increased risk of NAFLD. The authors conclude that UPFs may be a modifiable factor regarding risk of NAFLD. |
| Konieczna et al., 2022 [22] | 5867 patients with METS, with a valid FFQ, aged 55 to 75 y.o. were followed for 1-year enrollment during 2013 and 2016 in Spain | Prospective analysis from the PREDIMED Plus trial | A 10-fold increment in the daily consumption of UPFs was associated with a significantly higher FLI as well as HSI. | The authors conclude that a higher consumption of UPFs was associated with increased levels of NAFLD biomarkers among patients with overweight/obesity and METS. |
| Zhao et al., 2023 [61] | 806 adolescents and 2734 adults were included between 2017 and 2018 from the NHANES study, with 2 days of 24 h recall data, in the U.S.A. | Cohort study | Higher consumption of UPFs was associated with increased odds of NAFLD (In adolescents OR: 2.34 95% CI: 1.01–5.41, p = 0.15, in adults OR: 1.72 95% CI: 1.01–2.93, p = 0.002) | A higher consumption of UPFs was associated with increased risk of NAFLD, which was 68% and 71% mediated in adolescents and adults by increased body fat. |
| Zhang et al., 2024 [58] | 143,073 participants from the U.K. Biobank, with 24 h recall data in the U.K. | Prospective cohort study | After a median of 10.5 years of follow-up, 1445 participants developed NAFLD in its severe form, as hospitalization or death due to NAFLD or NASH. | The authors concluded that by decreasing the consumption of UPFs there may be a reduction in NAFLD. |
| Zhao et al., 2024 [59] | 173,889 participants from the U.K. Biobank, aged 40 to 69 y.o., with 24 h dietary recall data in the U.K. | Prospective cohort study | After a median of 8.9 years of follow-up, the authors documented an association between higher consumption of UPFs and an increased risk of NAFLD, as well as liver fibrosis/cirrhosis. | The authors commented that by reducing consumption of UPFs, there may be an amelioration in NAFLD and liver fibrosis/cirrhosis and an improvement in liver health status. |
| Encourage drinking fresh and safe water instead of beverage consumption |
| Substituting red meat and red meat products with fish or poultry |
| Encouraging learning programs about what UPFs are and how to distinguish them in order for parents, children/adolescents, and the elderly to avoid them |
| Encouraging the consumption of more fruits, vegetables, legumes, nuts, and whole grains |
| Encouraging fresh cooking and cooking skills at home |
| Educational programs regarding the potential harms of UPFs for parents, at school, and possibly at work |
| Legislations for detailed labelling of ingredients/calories of UPFs |
| Protecting parents and especially children/adolescents against uncontrolled advertising of UPFs |
| Legislations for extra taxes on UPFs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geladari, E.V.; Kounatidis, D.; Christodoulatos, G.S.; Psallida, S.; Pavlou, A.; Geladari, C.V.; Sevastianos, V.; Dalamaga, M.; Vallianou, N.G. Ultra-Processed Foods and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): What Is the Evidence So Far? Nutrients 2025, 17, 2098. https://doi.org/10.3390/nu17132098
Geladari EV, Kounatidis D, Christodoulatos GS, Psallida S, Pavlou A, Geladari CV, Sevastianos V, Dalamaga M, Vallianou NG. Ultra-Processed Foods and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): What Is the Evidence So Far? Nutrients. 2025; 17(13):2098. https://doi.org/10.3390/nu17132098
Chicago/Turabian StyleGeladari, Eleni V., Dimitris Kounatidis, Gerasimos Socrates Christodoulatos, Sotiria Psallida, Argyro Pavlou, Charalampia V. Geladari, Vassilios Sevastianos, Maria Dalamaga, and Natalia G. Vallianou. 2025. "Ultra-Processed Foods and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): What Is the Evidence So Far?" Nutrients 17, no. 13: 2098. https://doi.org/10.3390/nu17132098
APA StyleGeladari, E. V., Kounatidis, D., Christodoulatos, G. S., Psallida, S., Pavlou, A., Geladari, C. V., Sevastianos, V., Dalamaga, M., & Vallianou, N. G. (2025). Ultra-Processed Foods and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): What Is the Evidence So Far? Nutrients, 17(13), 2098. https://doi.org/10.3390/nu17132098








