Human Milk Oligosaccharide Composition at 6 Weeks Is Associated with Temperament and Eating Behaviors of Children in the STRONG Kids 2 Cohort Through 4 Years of Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and HMO Analysis
2.2. Covariates
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Summary Statistics for Demographic Information of the Participants
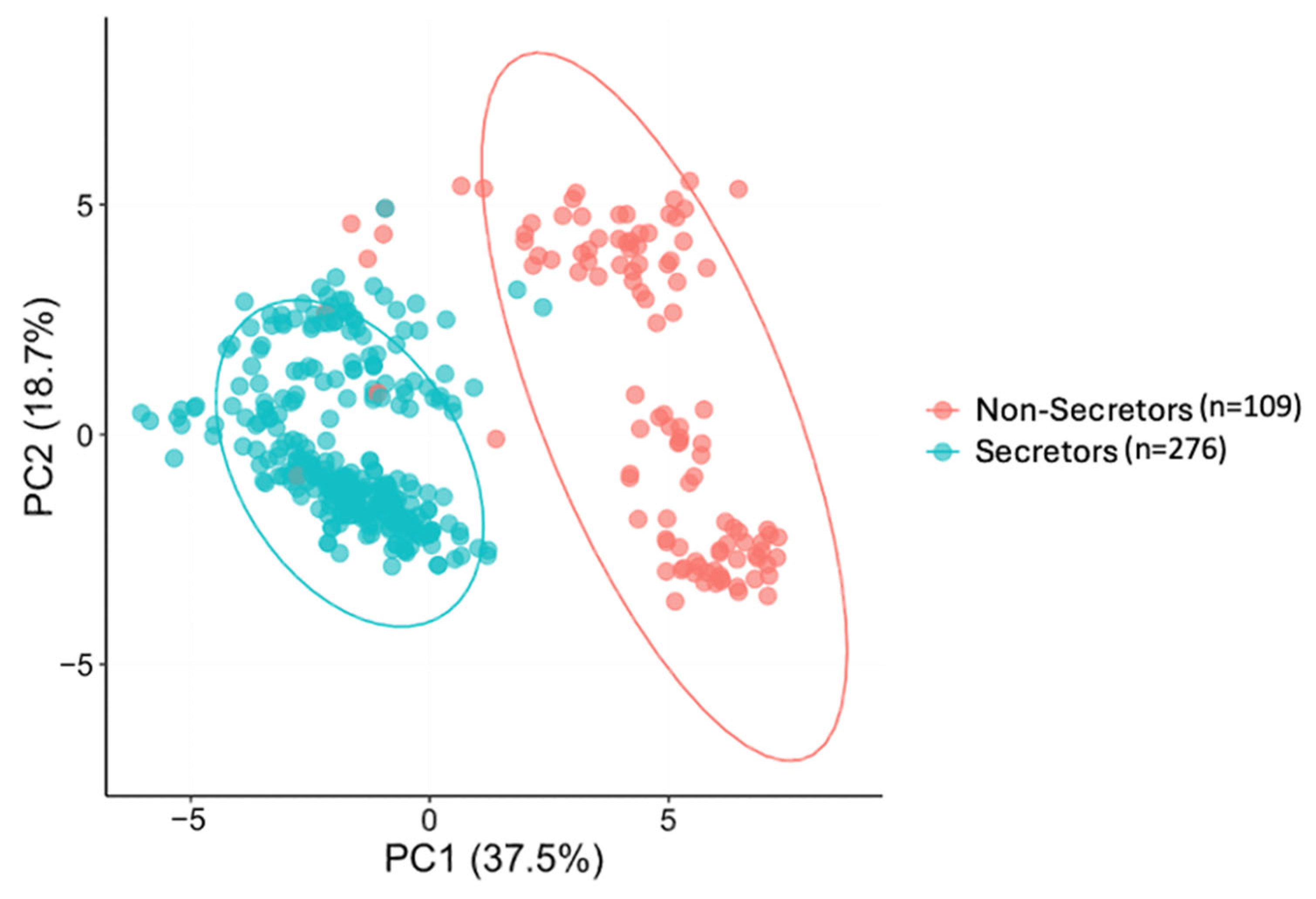
3.2. HMO Clustering
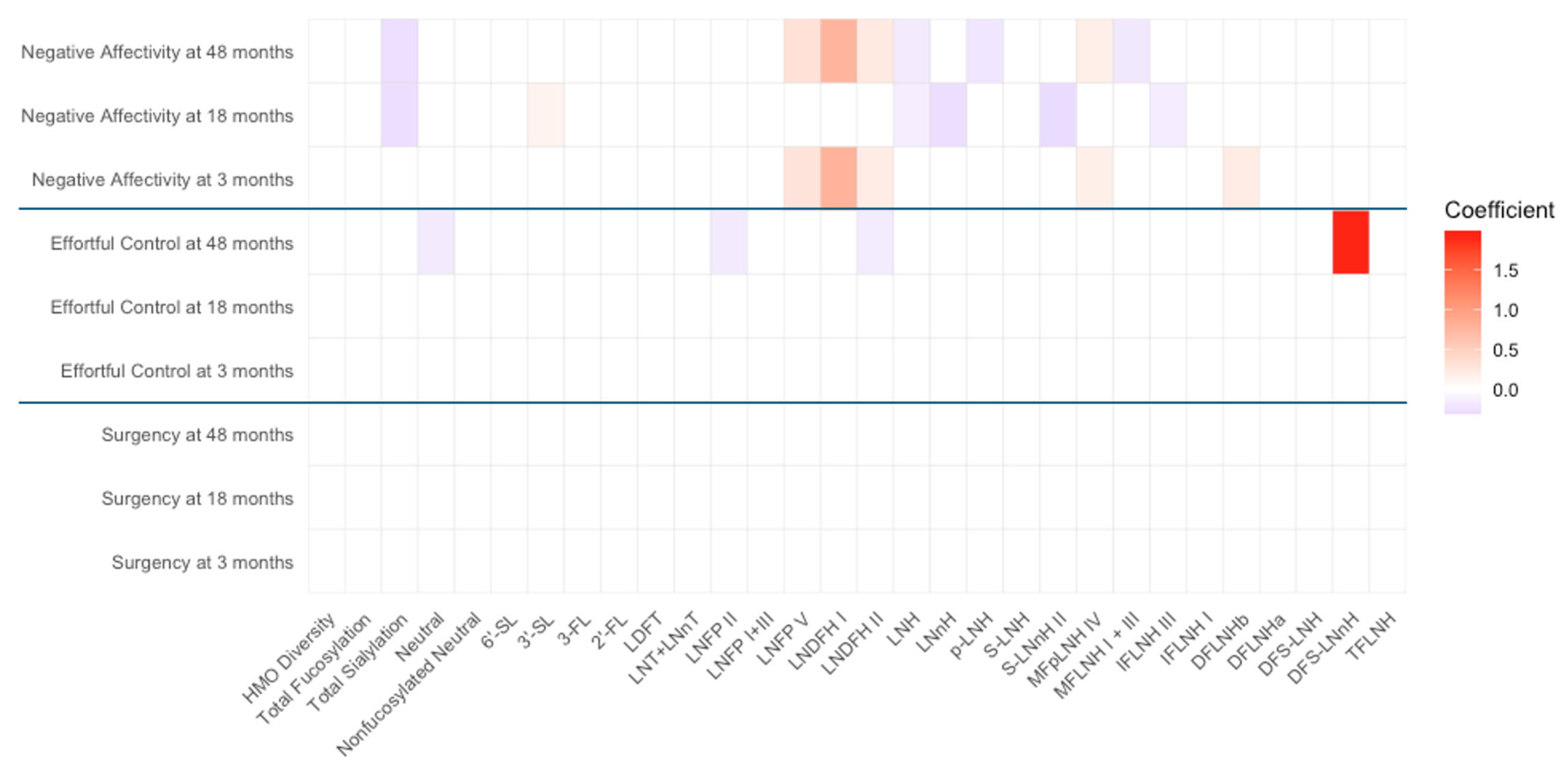
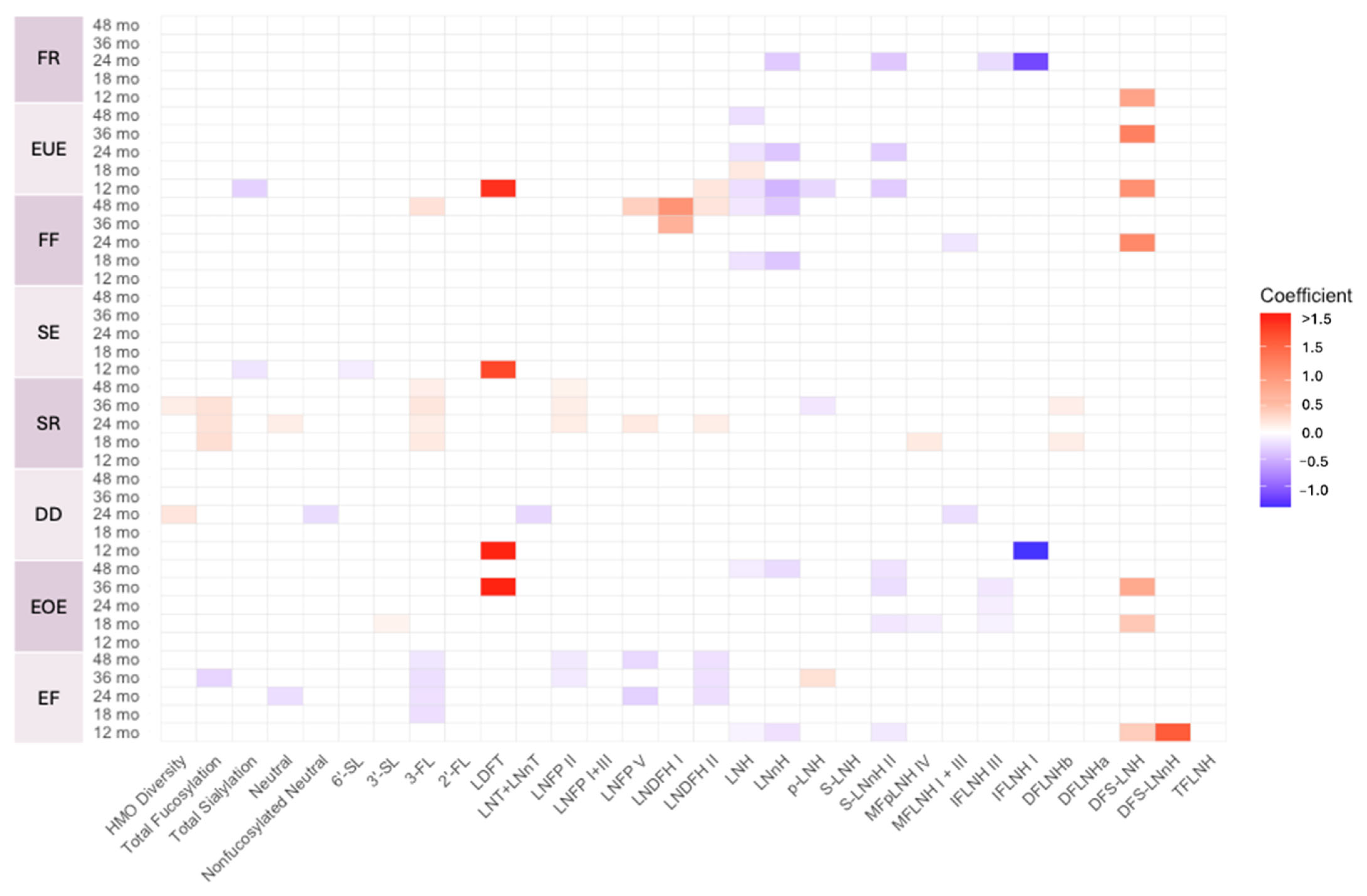
3.3. Associations Between HMO Profiles and Temperament Outcomes
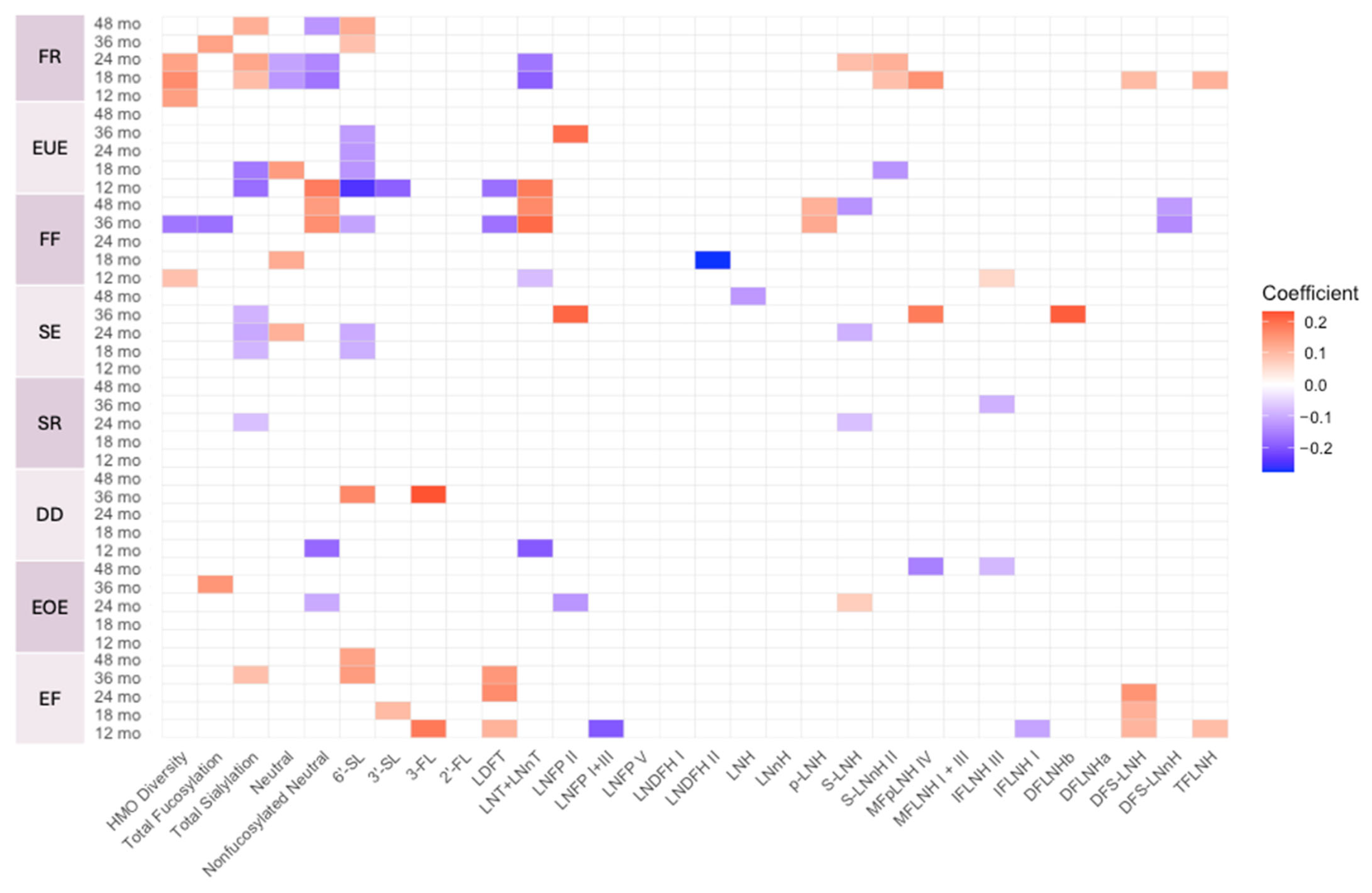
3.4. Associations Between HMO Profiles and Infant and Child Eating Behaviors
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2′-FL | 2′-Fucosyllactose |
| 3-FL | 3-Fucosyllactose |
| 3′-SL | 3′-Sialyllactose |
| 6′-SL | 6′-Sialyllactose |
| LNnT | Lacto-N-neotetraose |
| LNT | Lacto-N-tetrose |
| LDFT | Lactodifucotetraose |
| LNFP I/II/III/V | Lacto-N-fucopentaose-I/II/III/V |
| DFLNHa/b | Difucosyllacto-N-hexaose a/b |
| LNDFH I/II | Lacto-N-difucohexaose I/II |
| LNH | Lacto-N-hexaose |
| LNnH | Lacto-N-neohexaose |
| p-LNH | Para-lacto-N-hexaose |
| S-LNH | Sialyl-lacto-N-hexaose |
| S-LNnH II | Sialyllacto-N-neohexaose II |
| MFpLNH IV | Fucosyl-para-lacto-N-hexaose IV |
| MFLNH I + III | Monofucosyllacto-N-hexaose I/III |
| IFLNH I/III | Fucosyl-para-lacto-N-hexaose I/III |
| DFS-LNH | Difucosylmonosialyllacto-N-hexaose |
| DFS-LNnH | Difucosylmonosialyllacto-N-neohexaose |
| TFLNH | Trifucosyllacto-N-hexaose |
| HMO | Human Milk Oligosaccharides |
| HPLC-MS | High performance liquid chromatography-mass spectrometry |
| BMI | Body Mass Index |
| EPDS | Edinburgh Postnatal Depression Scale |
| IBQR-VS | Infant Behavior Questionnaire Revised-Very Short |
| ECBQ-VS | Early Childhood Behavior Questionnaire-Very Short |
| CEBQ | Children’s Eating Behaviour Questionnaire |
| BEBQ | Baby Eating Behaviour Questionnaire |
| yr(s) | Year/Years |
| mo(s) | Month/Months |
| FR | Food responsiveness |
| EUE | Emotional undereating |
| FF | Food fussiness |
| SE | Slowness in eating |
| SR | Satiety responsiveness; |
| DD | Desire to drink |
| EOE | Emotional overeating |
| GDM | Gestational diabetes mellitus |
| SCFAs | Short-chain fatty acids |
Appendix A



References
- Wadsworth, M.; Butterworth, S. Early life. In Social Determinants of Health, 2nd ed; Marmot, T.M., Wilkinson, R.G., Eds.; Oxford University Press: Oxford, UK, 2006; ISBN 9780198565895. [Google Scholar]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 2003, 9, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Tregellas, J.R.; Legget, K.T. Rapid early brain development highlights a critical period and possible intervention window. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 937–938. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.Y.; Noble, L.; Breastfeeding, S.O. Policy statement: Breastfeeding and the use of human milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Xanthou, M. Immune protection of human milk. Biol. Neonate 1998, 74, 121–133. [Google Scholar] [CrossRef]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of human milk bioactives on infants’ gut and immune health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]
- Anatolitou, F. Human milk benefits and breastfeeding. J. Pediatr. Neonatal Individ. Med. (JPNIM) 2012, 1, 11–18. [Google Scholar]
- Xu, G.; Davis, J.C.; Goonatilleke, E.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Absolute quantitation of human milk oligosaccharides reveals phenotypic variations during lactation. J. Nutr. 2017, 147, 117–124. [Google Scholar] [CrossRef]
- Soyyılmaz, B.; Mikš, M.H.; Röhrig, C.H.; Matwiejuk, M.; Meszaros-Matwiejuk, A.; Vigsnæs, L.K. The mean of milk: A review of human milk oligosaccharide concentrations throughout lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef]
- Vinjamuri, A.; Davis, J.C.; Totten, S.M.; Wu, L.D.; Klein, L.D.; Martin, M.; Quinn, E.; Scelza, B.; Breakey, A.; Gurven, M. Human milk oligosaccharide compositions illustrate global variations in early nutrition. J. Nutr. 2022, 152, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Bruni, S.; Morelli, L.; Soldi, S.; Gabrielli, O. The first prebiotics in humans: Human milk oligosaccharides. J. Clin. Gastroenterol. 2004, 38, S80–S83. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Fente, C.; Regal, P.; Lamas, A.; Lorenzo, M.P. Human milk oligosaccharides (HMOs) and infant microbiota: A scoping review. Foods 2021, 10, 1429. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 41–51. [Google Scholar] [CrossRef]
- Triantis, V.; Bode, L.; Van Neerven, R.J. Immunological effects of human milk oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef]
- Fan, Y.; McMath, A.L.; Donovan, S.M. Review on the impact of milk oligosaccharides on the brain and neurocognitive development in early life. Nutrients 2023, 15, 3743. [Google Scholar] [CrossRef]
- Berger, P.K.; Ong, M.L.; Bode, L.; Belfort, M.B. Human milk oligosaccharides and infant neurodevelopment: A narrative review. Nutrients 2023, 15, 719. [Google Scholar] [CrossRef]
- Sesker, A.A.; Strickhouser, J.E.; Luchetti, M.; Lee, J.H.; Aschwanden, D.; Terracciano, A.; Sutin, A.R. Cognition and the development of temperament from late childhood to early adolescence. J. Res. Pers. 2021, 95, 104163. [Google Scholar] [CrossRef]
- Rothbart, M.K.; Derryberry, D. Development of individual differences in temperament. In Advances in Developmental Psychology; Psychology Press: London, UK, 2013; pp. 37–86. [Google Scholar]
- Kristal, J. The Temperament Perspective: Working with Children’s Behavioral Styles; Paul H Brookes Publishing: Baltimore, MD, USA, 2005. [Google Scholar]
- Henderson, H.A.; Wachs, T.D. Temperament theory and the study of cognition–emotion interactions across development. Dev. Rev. 2007, 27, 396–427. [Google Scholar] [CrossRef]
- Rothbart, M.K. Measurement of temperament in infancy. Child Dev. 1981, 52, 569–578. [Google Scholar] [CrossRef]
- Burton, P.; Wells, J.; Kennedy, K.; Nicholl, R.; Khakoo, A.; Fewtrell, M. Association between infant correlates of impulsivity-surgency (extraversion)-and early infant growth. Appetite 2011, 57, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, K. The construct of surgency. In Encyclopedia of Personality and Individual Differences; Springer: Cham, Switzerland, 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A. Negative affectivity: The disposition to experience aversive emotional states. Psychol. Bull. 1984, 96, 465. [Google Scholar] [CrossRef]
- King, N.J.; Ollendick, T.H.; Gullone, E. Negative affectivity in children and adolescents: Relations between anxiety and depression. Clin. Psychol. Rev. 1991, 11, 441–459. [Google Scholar] [CrossRef]
- Rothbart, M.K.; Ellis, L.K.; Rosario Rueda, M.; Posner, M.I. Developing mechanisms of temperamental effortful control. J. Pers. 2003, 71, 1113–1144. [Google Scholar] [CrossRef]
- Rueda, M.R. Effortful control. In Handbook of Temperament; The Guilford Press: New York, NY, USA, 2012; pp. 145–167. [Google Scholar]
- Gartstein, M.A.; Skinner, M.K. Prenatal influences on temperament development: The role of environmental epigenetics. Dev. Psychopathol. 2018, 30, 1269–1303. [Google Scholar] [CrossRef]
- Suryawan, A.; Jalaludin, M.Y.; Poh, B.; Sanusi, R.; Tan, V.; Geurts, J.; Muhardi, L. Malnutrition in early life and its neurodevelopmental and cognitive consequences: A scoping review. Nutr. Res. Rev. 2022, 35, 136–149. [Google Scholar] [CrossRef]
- Umekar, S.; Joshi, A. Obesity and Preventive Intervention Among Children: A Narrative Review. Cureus 2024, 16, e54520. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Schmidt, I.; Schoelch, C.; Ziska, T.; Schneider, D.; Simon, E.; Plagemann, A. Interaction of genetic and environmental programming of the leptin system and of obesity disposition. Physiol. Genomics 2000, 3, 113–120. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Dinsa, G.D.; Goryakin, Y.; Fumagalli, E.; Suhrcke, M. Obesity and socioeconomic status in developing countries: A systematic review. Obes. Rev. 2012, 13, 1067–1079. [Google Scholar] [CrossRef]
- Bates, C.; Buscemi, J.; Nicholson, L.; Cory, M.; Jagpal, A.; Bohnert, A. Links between the organization of the family home environment and child obesity: A systematic review. Obes. Rev. 2018, 19, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Demir, D.; Bektas, M. The effect of childrens’ eating behaviors and parental feeding style on childhood obesity. Eat. Behav. 2017, 26, 137–142. [Google Scholar] [CrossRef]
- Obregón, A.M.; Pettinelli, P.P.; Santos, J.L. Childhood obesity and eating behaviour. J. Pediatr. Endocrinol. Metab. 2015, 28, 497–502. [Google Scholar] [CrossRef]
- Peuckert, M.Z.; Ayala, C.O.; Mattiello, R.; Viola, T.W.; Walker, M.S.; Feoli, A.M.P.; Drumond Costa, C.A. Validation evidence for the Children’s Eating Behaviour Questionnaire (CEBQ) in Brazil: A cross-sectional study. Nutrients 2025, 17, 851. [Google Scholar] [CrossRef]
- Fildes, A.; Mallan, K.M.; Cooke, L.; Van Jaarsveld, C.H.; Llewellyn, C.H.; Fisher, A.; Daniels, L. The relationship between appetite and food preferences in British and Australian children. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 116. [Google Scholar] [CrossRef]
- Herle, M.; De Stavola, B.; Hübel, C.; Abdulkadir, M.; Ferreira, D.S.; Loos, R.J.; Bryant-Waugh, R.; Bulik, C.M.; Micali, N. A longitudinal study of eating behaviours in childhood and later eating disorder behaviours and diagnoses. Br. J. Psychiatry 2020, 216, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Steinsbekk, S.; Bjørklund, O.; Llewellyn, C.; Wichstrøm, L. Temperament as a predictor of eating behavior in middle childhood–A fixed effects approach. Appetite 2020, 150, 104640. [Google Scholar] [CrossRef]
- Messerli-Bürgy, N.; Stülb, K.; Kakebeeke, T.H.; Arhab, A.; Zysset, A.E.; Leeger-Aschmann, C.S.; Schmutz, E.A.; Meyer, A.H.; Ehlert, U.; Garcia-Burgos, D. Emotional eating is related with temperament but not with stress biomarkers in preschool children. Appetite 2018, 120, 256–264. [Google Scholar] [CrossRef]
- Fiese, B.H.; Musaad, S.; Bost, K.K.; McBride, B.A.; Lee, S.-Y.; Teran-Garcia, M.; Donovan, S.M. The Strong Kids 2 birth cohort study: A cell-to-society approach to dietary habits and weight trajectories across the first 5 years of life. Curr. Dev. Nutr. 2019, 3, nzz007. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Vinjamuri, A.; Tu, D.; Lebrilla, C.B.; Donovan, S.M. Determinants of human milk oligosaccharides profiles of participants in the STRONG kids 2 cohort. Front. Nutr. 2023, 10, 1105668. [Google Scholar] [CrossRef]
- Cox, J.; Holden, J. Perinatal Mental Health: A Guide to the Edinburgh Postnatal Depression Scale (EPDS); Royal College of Psychiatrists: London, England, 2003. [Google Scholar]
- Levis, B.; Negeri, Z.; Sun, Y.; Benedetti, A.; Thombs, B.D. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: Systematic review and meta-analysis of individual participant data. BMJ 2020, 371, m4022. [Google Scholar] [CrossRef] [PubMed]
- Shamseddeen, W.; Bou Ali, L.; El Bejjani, M.; Safieddine, B.; Abou Chaar, E.; Akoury Dirani, L. Infant and Toddler Emotion and Behavior Regulation Evaluation Tools: Infant Behavior Questionnaire–Revised (IBQR) and the Early Childhood Behavior Questionnaire-Short (ECBQ-S); Technical Working Paper; American University of Beirut: Beirut, Lebanon, 2019. [Google Scholar]
- Putnam, S.P.; Helbig, A.L.; Gartstein, M.A.; Rothbart, M.K.; Leerkes, E. Development and assessment of short and very short forms of the Infant Behavior Questionnaire–Revised. J. Pers. Assess. 2014, 96, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-S. A validation study for the Early Childhood Behavior Questionnaire. Korean J. Child Stud. 2009, 30, 191–204. [Google Scholar]
- Putnam, S.P.; Gartstein, M.A.; Rothbart, M.K. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant. Behav. Dev. 2006, 29, 386–401. [Google Scholar] [CrossRef]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the children’s eating behaviour questionnaire. J. Child Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef]
- Sleddens, E.F.; Kremers, S.P.; Thijs, C. The Children’s Eating Behaviour Questionnaire: Factorial validity and association with Body Mass Index in Dutch children aged 6–7. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Viana, V.; Sinde, S.; Saxton, J. Children’s Eating Behaviour Questionnaire: Associations with BMI in Portuguese children. Br. J. Nutr. 2008, 100, 445–450. [Google Scholar] [CrossRef]
- Domoff, S.E.; Miller, A.L.; Kaciroti, N.; Lumeng, J.C. Validation of the Children’s Eating Behaviour Questionnaire in a low-income preschool-aged sample in the United States. Appetite 2015, 95, 415–420. [Google Scholar] [CrossRef]
- Mallan, K.M.; Liu, W.-H.; Mehta, R.J.; Daniels, L.A.; Magarey, A.; Battistutta, D. Maternal report of young children’s eating styles. Validation of the Children’s Eating Behaviour Questionnaire in three ethnically diverse Australian samples. Appetite 2013, 64, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-T.; Svensson, V.; Marcus, C.; Zhang, J.; Zhang, J.-D.; Sobko, T. Eating behaviour patterns in Chinese children aged 12-18 months and association with relative weight-factorial validation of the Children’s Eating Behaviour Questionnaire. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Obregón, A.M.; Valladares, M.; Guzmán-Gutierrez, E.; Pettinelli, P.; Hunot-Alexander, C.; Smith, A.; Llewellyn, C.; Goldfield, G. Validation of the baby eating behaviour questionnaire in a Chilean population. Curr. Psychol. 2024, 43, 5377–5387. [Google Scholar] [CrossRef]
- Kurita, T. Principal component analysis (PCA). In Computer Vision: A Reference Guide; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1013–1016. [Google Scholar]
- Seger, C. An Investigation of Categorical Variable Encoding Techniques in Machine Learning: Binary Versus One-Hot and Feature Hashing. Bachelor’s Thesis, School of Electrical Engineering and Computer Science (EECS), Stockholm, Sweden, 2018. [Google Scholar]
- Cabrera-Rubio, R.; Kunz, C.; Rudloff, S.; García-Mantrana, I.; Crehuá-Gaudiza, E.; Martínez-Costa, C.; Collado, M.C. Association of maternal secretor status and human milk oligosaccharides with milk microbiota: An observational pilot study. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Carey, W.B. The importance of temperament-environment interaction for child health and development. In The Uncommon Child; Springer: Berlin/Heidelberg, Germany, 1981; pp. 31–55. [Google Scholar]
- Alving-Jessep, E.; Botchway, E.; Wood, A.G.; Hilton, A.C.; Blissett, J.M. The development of the gut microbiome and temperament during infancy and early childhood: A systematic review. Dev. Psychobiol. 2022, 64, e22306. [Google Scholar] [CrossRef]
- Nigg, J.T. Temperament and developmental psychopathology. J. Child Psychol. Psychiatry 2006, 47, 395–422. [Google Scholar] [CrossRef]
- ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 3′ Sialyllactose and 6′ Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut–brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef]
- Tilson, H.; Harry, G.; Nanry, K.; Hudson, P.; Hong, J. Ganglioside interactions with the dopaminergic system of rats. J. Neurosci. Res. 1988, 19, 88–93. [Google Scholar] [CrossRef]
- Nyman, E.S.; Loukola, A.; Varilo, T.; Ekelund, J.; Veijola, J.; Joukamaa, M.; Taanila, A.; Pouta, A.; Miettunen, J.; Freimer, N. Impact of the dopamine receptor gene family on temperament traits in a population-based birth cohort. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150, 854–865. [Google Scholar] [CrossRef]
- Sheese, B.E.; Voelker, P.M.; Rothbart, M.K.; Posner, M.I. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Dev. Psychopathol. 2007, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shepard, D.N.; Chandler-Laney, P.C. Prospective associations of eating behaviors with weight gain in infants. Obesity 2015, 23, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.J.; Paul, I.M.; Anzman-Frasca, S.; Savage, J.S.; Hohman, E.E. Protective Eating Behaviors Among Children at Higher Risk for Obesity in the INSIGHT Study. Child. Obes. 2024, 21, 76–83. [Google Scholar] [CrossRef]
- Cummings, J.R.; Lipsky, L.M.; Faith, M.S.; Nansel, T.R. Developmental trajectory of appetitive traits and their bidirectional relations with body mass index from infancy to early childhood. Clin. Obes. 2024, 14, e12620. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Yonemitsu, C.; Ryoo, J.H.; Alderete, T.L.; Bode, L.; Goran, M.I. Associations between human milk oligosaccharides (HMOs) and eating behaviour in Hispanic infants at 1 and 6 months of age. Pediatr. Obes. 2020, 15, e12686. [Google Scholar] [CrossRef] [PubMed]
- Mannino, A.; Sarapis, K.; Moschonis, G. The effect of maternal overweight and obesity pre-pregnancy and during childhood in the development of obesity in children and adolescents: A systematic literature review. Nutrients 2022, 14, 5125. [Google Scholar] [CrossRef]
- Schneider-Worthington, C.R.; Berger, P.K.; Goran, M.I.; Salvy, S.J. Learning to overeat in infancy: Concurrent and prospective relationships between maternal BMI, feeding practices and child eating response among Hispanic mothers and children. Pediatr. Obes. 2021, 16, e12756. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sharma, A.J.; Callaghan, W.M. Gestational diabetes and childhood obesity: What is the link? Curr. Opin. Obstet. Gynecol. 2012, 24, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Kohlhoff, R.; Rohde, W.; Dörner, G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 451–456. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, J.; Choi, J. Maternal pre-pregnancy obesity and gestational diabetes mellitus increase the risk of childhood obesity. Children 2022, 9, 928. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, M.; Papandreou, D.; Pavlidou, E.; Papadopoulou, S.K.; Tolia, M.; Mentzelou, M.; Poutsidi, A.; Antasouras, G.; Vasios, G.K.; Giaginis, C. Maternal gestational diabetes is associated with high risk of childhood overweight and obesity: A cross-sectional study in pre-school children aged 2–5 years. Medicina 2023, 59, 455. [Google Scholar] [CrossRef]
- Thum, C.; Wall, C.R.; Weiss, G.A.; Wang, W.; Szeto, I.M.-Y.; Day, L. Changes in HMO concentrations throughout lactation: Influencing factors, health effects and opportunities. Nutrients 2021, 13, 2272. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Next-generation functions and questions. In Human Milk: Composition, Clinical Benefits and Future Opportunities; Karger Publishers: Basel, Switzerland, 2019; Volume 90, pp. 191–201. [Google Scholar]
- Mulinge, M.M.; Abisi, H.K.; Kabahweza, H.M.; Okutoyi, L.; Wamalwa, D.C.; Nduati, R.W. The role of maternal secretor status and human milk oligosaccharides on early childhood development: A systematic review and meta-analysis. Breastfeed. Med. 2024, 19, 409–424. [Google Scholar] [CrossRef]
- Berger, P.; Plows, J.; Jones, R.; Alderete, T.; Yonemitsu, C.; Ryoo, J.H.; Bode, L.; Goran, M. Human milk oligosaccharides and Hispanic infant weight gain in the first 6 months. Obesity 2020, 28, 1519–1525. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The gut–brain axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Kiely, L.J.; Busca, K.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Molecular strategies for the utilisation of human milk oligosaccharides by infant gut-associated bacteria. FEMS Microbiol. Rev. 2023, 47, fuad056. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Barone, M.; Garelli, S.; Rampelli, S.; Agostini, A.; Matysik, S.; D’Amico, F.; Krautbauer, S.; Mazza, R.; Salituro, N.; Fanelli, F. Multi-omics gut microbiome signatures in obese women: Role of diet and uncontrolled eating behavior. BMC Med. 2022, 20, 500. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The role of the gut microbiome, immunity, and neuroinflammation in the pathophysiology of eating disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 2014, 36, 940–949. [Google Scholar] [CrossRef]
- Berding, K.; Holscher, H.D.; Arthur, A.E.; Donovan, S.M. Fecal microbiome composition and stability in 4-to 8-year old children is associated with dietary patterns and nutrient intake. J. Nutr. Biochem. 2018, 56, 165–174. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.-Q.; Liu, F.; Wu, J.-Y. Human milk oligosaccharides and infant gut microbiota: Molecular structures, utilization strategies and immune function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, H.; Skouteris, H.; Horwood, S.; Hooley, M.; Richardson, B. Child temperament and maternal predictors of preschool children’s eating and body mass index. A prospective study. Appetite 2014, 74, 125–132. [Google Scholar] [CrossRef]
- Faith, M.S.; Hittner, J.B.; Hurston, S.R.; Yin, J.; Greenspan, L.C.; Quesenberry, C.P.; Gunderson, E.P.; Investigators, S.O.S. Association of infant temperament with subsequent obesity in young children of mothers with gestational diabetes mellitus. JAMA Pediatr. 2019, 173, 424–433. [Google Scholar] [CrossRef]
- Kidwell, K.M.; James, T.D.; Brock, R.L.; Lazarus Yaroch, A.; Hill, J.L.; Mize Nelson, J.; Alex Mason, W.; Andrews Espy, K.; Nelson, T.D. Preschool executive control, temperament, and adolescent dietary behaviors. Ann. Behav. Med. 2023, 57, 260–268. [Google Scholar] [CrossRef]
| Characteristic | n (%) or Mean ± SD |
|---|---|
| Maternal age (yrs) | 31.2 ± 4.2 |
| Pre-pregnancy BMI | 26.3 ± 6.2 |
| Maternal depression EPDS score (on a scale of 0–30) | 4.3 ± 4.0 |
| Infant birthweight (g) | 3492.7 ± 428.4 |
| Infant sex assigned at birth | |
| Female | 179 (46.5%) |
| Male | 206 (53.5%) |
| Secretor status | |
| Secretor | 276 (71.7%) |
| Non-secretor | 109 (28.3%) |
| Delivery mode | |
| Cesarean section | 88 (22.9%) |
| Vaginal | 294 (76.4%) |
| Missing | 3 (0.8%) |
| Maternal education level at enrollment | |
| Grade school/some high school/high school graduate | 13 (3.4%) |
| Some college or technical school | 56 (14.5%) |
| College graduate/post-graduate work | 303 (78.7%) |
| Missing | 13 (3.4%) |
| Socioeconomic status (monthly income) | |
| USD 3000 and under | 97 (25.2%) |
| USD 3001–5000 | 104 (27.0%) |
| USD 5001 and above | 136 (35.3%) |
| Missing | 48 (12.5%) |
| Maternal Ethnicity | |
| Hispanic/Latino | 15 (3.9%) |
| Non-Hispanic/Latino Unknown or Mixed | 61 (15.8%) |
| Non-Hispanic/Latino White | 285 (74.0%) |
| Missing | 24 (6.2%) |
| Child Ethnicity | |
| Hispanic/Latino | 19 (4.9%) |
| Non-Hispanic/Latino Unknown or Mixed | 74 (19.2%) |
| Non-Hispanic/Latino White | 270 (70.1%) |
| Missing | 22 (5.7%) |
| Exclusive Breastfeeding duration | |
| 6 weeks | 24 (6.2%) |
| 3 mos | 107 (27.8%) |
| 12 mos | 197 (51.2%) |
| Mixed feeding (human milk and formula) | 49 (12.7%) |
| Missing | 8 (2.1%) |
| Sample | Temperament Characteristic | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full cohort | Surgency | HMO Diversity | 0.128 | 0.023 |
| Total non-fucosylated neutral | −0.127 | 0.022 | ||
| 2′-FL | 0.204 | 0.028 | ||
| LNT+LNnT | −0.145 | 0.015 | ||
| LNFP I+LNFP III | −0.222 | 0.021 | ||
| LNnH | 0.127 | 0.025 | ||
| IFLNH III | 0.164 | <0.001 | ||
| Orienting/ Regulation | LNnH | 0.101 | 0.014 | |
| IFLNH III | 0.073 | 0.039 | ||
| Secretors | Surgency | 2′-FL | 0.229 | 0.015 |
| LNT+LNnT | −0.175 | 0.036 | ||
| LNnH | 0.123 | 0.046 | ||
| MFpLNH IV | 0.234 | 0.024 | ||
| IFLNH III | 0.224 | <0.001 | ||
| Orienting/ Regulation | LNnH | 0.108 | 0.014 | |
| Non-Secretors | Negative affectivity | LNFP V | 0.288 | 0.016 |
| LNDFH I | 0.785 | 0.010 | ||
| LNDFH II | 0.199 | 0.029 | ||
| MFpLNH IV | 0.168 | 0.046 | ||
| DFLNHb | 0.209 | 0.028 |
| Sample | Temperament Characteristic | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full Cohort | Surgency | p-LNH | 0.104 | 0.012 |
| Effortful control | Neutral | −0.084 | 0.040 | |
| Negative affectivity | LNH | −0.123 | <0.001 | |
| S-LNnH II | −0.094 | 0.009 | ||
| Secretors | Effortful control | Neutral | −0.098 | 0.045 |
| 6′-SL | −0.092 | 0.038 | ||
| LDFT | −0.105 | 0.040 | ||
| Negative affectivity | 3-FL | 0.142 | 0.018 | |
| LNH | −0.105 | 0.014 | ||
| Non-Secretors | Negative affectivity | Total Sialylation | −0.267 | 0.002 |
| 3′-SL | 0.129 | 0.034 | ||
| LNH | −0.150 | 0.006 | ||
| LNnH | −0.284 | 0.019 | ||
| S-LNnH II | −0.307 | 0.002 | ||
| IFLNH III | −0.159 | 0.003 |
| Sample | Temperament Characteristic | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full cohort | Surgency | DFS-LNH | −0.123 | 0.026 |
| Negative affectivity | LNFP V | 0.220 | 0.008 | |
| LNDFH I | 0.157 | 0.007 | ||
| LNDFH II | 0.208 | 0.002 | ||
| LNH | −0.118 | 0.008 | ||
| MFpLNH I+III | −0.198 | <0.001 | ||
| DFLNHa | −0.168 | 0.004 | ||
| Secretors | Negative affectivity | LNFP II | 0.197 | 0.025 |
| LNDFH I | 0.136 | 0.022 | ||
| LNDFH II | 0.275 | 0.030 | ||
| MFpLNH I+III | −0.206 | 0.004 | ||
| DFLNHa | −0.173 | 0.003 | ||
| Non-Secretors | Effortful control | Neutral | −0.179 | 0.033 |
| LNFP II | −0.180 | 0.012 | ||
| LNDFH II | −0.172 | 0.047 | ||
| DFS-LNnH | 1.988 | 0.050 | ||
| Negative affectivity | Total Sialylation | −0.280 | 0.020 | |
| LNFP V | 0.309 | 0.012 | ||
| LNDFH I | 0.767 | 0.011 | ||
| LNDFH II | 0.231 | 0.012 | ||
| LNH | −0.176 | 0.019 | ||
| p-LNH | −0.212 | 0.044 | ||
| MFpLNH IV | 0.174 | 0.048 | ||
| MFpLNH I+III | −0.190 | 0.018 |
| Sample | Eating Behavior | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full Cohort | Enjoyment of food | 6′-SL | 0.086 | 0.028 |
| LNFP I+III | −0.183 | 0.018 | ||
| DFS-LNH | 0.114 | 0.019 | ||
| Food fussiness | HMO Diversity | 0.048 | 0.038 | |
| Emotional undereating | Total Sialylation | −0.158 | 0.004 | |
| 6′-SL | −0.178 | <0.001 | ||
| LDFT | −0.156 | 0.032 | ||
| Food responsiveness | Neutral | −0.094 | 0.025 | |
| LNT+LNnT | −0.096 | 0.043 | ||
| DFS-LNnH | 0.096 | 0.033 | ||
| Secretors | Enjoyment of food | 3-FL | 0.186 | 0.008 |
| LDFT | 0.110 | 0.047 | ||
| LNFP I+III | −0.201 | 0.006 | ||
| IFLNH I | −0.109 | 0.022 | ||
| DFS-LNH | 0.108 | 0.021 | ||
| TFLNH | 0.095 | 0.047 | ||
| Desire to drink | Total nonfucosylated neutral | −0.180 | 0.021 | |
| LNT+LNnT | −0.198 | 0.025 | ||
| Food fussiness | HMO Diversity | 0.088 | 0.002 | |
| LNT+LNnT | −0.080 | 0.011 | ||
| IFLNH III | 0.058 | 0.016 | ||
| Emotional undereating | Total Sialylation | −0.176 | 0.005 | |
| Total nonfucosylated neutral | 0.182 | 0.020 | ||
| 6′-SL | −0.250 | <0.001 | ||
| 3′-SL | −0.190 | 0.003 | ||
| LDFT | −0.172 | 0.023 | ||
| LNT+LNnT | 0.183 | 0.039 | ||
| Food responsiveness | HMO Diversity | 0.138 | 0.021 | |
| Non-Secretors | Emotional overeating | LNH | −0.079 | 0.016 |
| LNnH | −0.183 | 0.016 | ||
| S-LNnH II | −0.142 | 0.015 | ||
| DFS-LNH | 0.396 | 0.026 | ||
| DFS-LNnH | 1.482 | <0.001 | ||
| Slowness in eating | Total Sialylation | −0.172 | 0.029 | |
| 6′-SL | −0.119 | 0.032 | ||
| LDFT | 4.36 | 0.031 | ||
| Desire to drink | LDFT | 7.47 | 0.022 | |
| IFLNH I | −1.383 | 0.014 | ||
| Emotional undereating | Total Sialylation | −0.291 | 0.017 | |
| LDFT | 6.24 | 0.046 | ||
| LNDFH II | 0.191 | 0.028 | ||
| LNH | −0.202 | 0.006 | ||
| LNnH | −0.473 | 0.006 | ||
| p-LNH | −0.248 | 0.016 | ||
| S-LNnH II | −0.335 | 0.011 | ||
| DFS-LNH | 0.851 | 0.033 | ||
| Food responsiveness | DFS-LNH | 0.711 | 0.036 |
| Sample | Eating Behavior | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full Cohort | Enjoyment of food | DFS-LNH | 0.089 | 0.050 |
| Emotional overeating | Total Nonfucosylated neutral | −0.066 | 0.031 | |
| Satiety responsiveness | MFpLNH IV | 0.099 | 0.022 | |
| DFLNHb | 0.094 | 0.045 | ||
| Slowness in eating | Total Sialylation | −0.080 | 0.040 | |
| 6′-SL | −0.084 | 0.017 | ||
| LNFP II | 0.094 | 0.035 | ||
| Food fussiness | LNDFH II | −0.141 | 0.022 | |
| LNH | −0.090 | 0.030 | ||
| Emotional undereating | Total Sialylation | −0.120 | 0.029 | |
| LNDFH I | 0.142 | 0.039 | ||
| LNH | −0.112 | 0.029 | ||
| S-LNnH II | −0.134 | 0.017 | ||
| Food responsiveness | LNT+LNnT | −0.091 | 0.050 | |
| DFS-LNH | 0.093 | 0.042 | ||
| TFLNH | 0.097 | 0.041 | ||
| Secretors | Enjoyment of food | 3′-SL | 0.100 | 0.028 |
| DFS-LNH | 0.114 | 0.011 | ||
| Slowness in eating | Total Sialylation | −0.088 | 0.046 | |
| 6′-SL | −0.097 | 0.027 | ||
| Emotional undereating | Total Sialylation | −0.161 | 0.008 | |
| Neutral | 0.142 | 0.041 | ||
| 6′-SL | −0.128 | 0.034 | ||
| S-LNnH II | −0.130 | 0.035 | ||
| Food fussiness | Neutral | 0.120 | 0.029 | |
| LNDFH II | −0.278 | 0.016 | ||
| Food responsiveness | HMO Diversity | 0.163 | 0.005 | |
| Total Sialylation | 0.095 | 0.030 | ||
| Neutral | −0.125 | 0.012 | ||
| Total nonfucosylated neutral | −0.166 | 0.002 | ||
| LNT+LNnT | −0.191 | 0.002 | ||
| S-LNnH II | 0.091 | 0.040 | ||
| MFpLNH IV | 0.157 | 0.049 | ||
| DFS-LNH | 0.100 | 0.025 | ||
| TFLNH | 0.113 | 0.014 | ||
| Non-Secretors | Enjoyment of food | 3-FL | −0.203 | 0.014 |
| Emotional overeating | 3′-SL | 0.092 | 0.035 | |
| S-LNnH II | −0.151 | 0.038 | ||
| MFpLNH IV | −0.117 | 0.012 | ||
| IFLNH III | −0.091 | 0.028 | ||
| DFS-LNH | 0.434 | 0.044 | ||
| Satiety responsiveness | Total Fucosylation | 0.261 | 0.005 | |
| 3-FL | 0.168 | 0.013 | ||
| MFpLNH IV | 0.165 | 0.007 | ||
| DFLNHb | 0.142 | 0.032 | ||
| Emotional undereating | LNH | −0.171 | 0.045 | |
| Food fussiness | LNH | −0.186 | 0.005 | |
| LNnH | −0.364 | 0.014 |
| Sample | Eating Behavior | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full cohort | Enjoyment of food | Total Sialylation | 0.086 | 0.045 |
| LDFT | 0.183 | 0.001 | ||
| DFS-LNH | 0.140 | 0.003 | ||
| Emotional overeating | Total Nonfucosylated neutral | −0.084 | 0.015 | |
| LNDFH I | −0.088 | 0.035 | ||
| S-LNH | 0.078 | 0.018 | ||
| DFLNHa | 0.085 | 0.044 | ||
| Desire to drink | HMO Diversity | 0.134 | 0.031 | |
| Total Nonfucosylated neutral | −0.121 | 0.050 | ||
| LNT+LNnT | −0.150 | 0.023 | ||
| Satiety responsiveness | Total Sialylation | −0.071 | 0.038 | |
| Neutral | 0.079 | 0.017 | ||
| LNFP II | 0.120 | 0.002 | ||
| S-LNH | −0.093 | 0.007 | ||
| DFS-LNnH | −0.089 | 0.012 | ||
| Slowness in eating | Total Sialylation | −0.083 | 0.037 | |
| 6′-SL | −0.077 | 0.032 | ||
| S-LNH | −0.099 | 0.014 | ||
| DFS-LNnH | −0.094 | 0.025 | ||
| Emotional undereating | 6′-SL | −0.103 | 0.030 | |
| Food responsiveness | S-LNH | 0.096 | 0.024 | |
| Secretors | Enjoyment of food | LDFT | 0.163 | 0.004 |
| DFS-LNH | 0.153 | 0.001 | ||
| Emotional overeating | Total nonfucosylated neutral | −0.101 | 0.018 | |
| LNFP II | −0.126 | 0.036 | ||
| S-LNH | 0.072 | 0.026 | ||
| Satiety responsiveness | Total Sialylation | −0.076 | 0.043 | |
| S-LNH | −0.076 | 0.028 | ||
| Slowness in eating | Total Sialylation | −0.104 | 0.020 | |
| Neutral | 0.113 | 0.023 | ||
| 6′-SL | −0.100 | 0.022 | ||
| S-LNH | −0.094 | 0.022 | ||
| Emotional undereating | 6′-SL | −0.125 | 0.030 | |
| Food responsiveness | HMO Diversity | 0.132 | 0.024 | |
| Total Sialylation | 0.127 | 0.005 | ||
| Neutral | −0.110 | 0.027 | ||
| Total nonfucosylated neutral | −0.144 | 0.009 | ||
| LNT+LNnT | −0.164 | 0.008 | ||
| S-LNH | 0.094 | 0.022 | ||
| S-LNnH II | 0.114 | 0.012 | ||
| Non-Secretors | Enjoyment of food | Neutral | −0.214 | 0.011 |
| 3-FL | −0.190 | 0.026 | ||
| LNFP V | −0.303 | 0.009 | ||
| LNDFH II | −0.195 | 0.021 | ||
| Emotional overeating | IFLNH III | −0.116 | 0.034 | |
| Desire to drink | HMO Diversity | 0.211 | 0.035 | |
| Total nonfucosylated neutral | −0.229 | 0.026 | ||
| LNT+LNnT | −0.254 | 0.017 | ||
| MFLNH I+III | −0.216 | 0.019 | ||
| Satiety responsiveness | Total Fucosylation | 0.231 | 0.010 | |
| Neutral | 0.133 | 0.033 | ||
| 3-FL | 0.144 | 0.022 | ||
| LNFP II | 0.149 | 0.003 | ||
| LNFP V | 0.169 | 0.048 | ||
| LNDFH II | 0.135 | 0.031 | ||
| Emotional undereating | LNH | −0.185 | 0.021 | |
| LNnH | −0.368 | 0.040 | ||
| S-LNnH II | −0.321 | 0.031 | ||
| Food fussiness | MFLNH I+III | −0.167 | 0.027 | |
| DFS-LNH | 0.905 | 0.021 | ||
| Food responsiveness | LNnH | −0.340 | 0.041 | |
| S-LNnH II | −0.349 | 0.011 | ||
| IFLNH III | −0.231 | 0.002 | ||
| IFLNH I | −1.186 | 0.024 |
| Sample | Eating Behavior | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full Cohort | Enjoyment of Food | Total Sialylation | 0.089 | 0.037 |
| 6′-SL | 0.109 | 0.004 | ||
| LDFT | 0.145 | 0.009 | ||
| LNFP II | −0.101 | 0.036 | ||
| Emotional overeating | IFLNH III | −0.077 | 0.028 | |
| Desire to drink | 6′-SL | 0.170 | 0.002 | |
| 3-FL | 0.190 | 0.012 | ||
| Satiety responsiveness | Total Sialylation | −0.069 | 0.046 | |
| LNFP II | 0.116 | 0.003 | ||
| Slowness in eating | LNFP II | 0.116 | 0.014 | |
| LNDFH I | 0.104 | 0.047 | ||
| IFLNH I | −0.094 | 0.049 | ||
| DFLNHb | 0.139 | 0.015 | ||
| TFLNH | 0.104 | 0.033 | ||
| Food fussiness | LDFT | −0.164 | 0.006 | |
| LNT+LNnT | 0.113 | 0.032 | ||
| DFS-LNnH | −0.137 | 0.004 | ||
| Emotional undereating | Total Sialylation | −0.108 | 0.045 | |
| LNFP II | 0.149 | 0.014 | ||
| LNFP V | 0.189 | 0.049 | ||
| Food responsiveness | 6′-SL | 0.078 | 0.048 | |
| Secretors | Enjoyment of food | Total Sialylation | 0.091 | 0.049 |
| 6′-SL | 0.142 | 0.002 | ||
| LDFT | 0.147 | 0.007 | ||
| Emotional overeating | Total fucosylation | 0.150 | 0.015 | |
| Desire to drink | 6′-SL | 0.168 | 0.011 | |
| 3-FL | 0.231 | 0.034 | ||
| Satiety responsiveness | IFLNH III | −0.093 | 0.022 | |
| Slowness in eating | Total Sialylation | −0.090 | 0.046 | |
| LNFP II | 0.210 | 0.006 | ||
| MFpLNH IV | 0.183 | 0.024 | ||
| DFLNHb | 0.219 | 0.014 | ||
| Food fussiness | HMO Diversity | −0.163 | 0.015 | |
| Total Fucosylation | −0.173 | 0.027 | ||
| Total nonfucosylated neutral | 0.159 | 0.012 | ||
| 6′-SL | −0.110 | 0.031 | ||
| LDFT | −0.170 | 0.005 | ||
| LNT+LNnT | 0.205 | 0.004 | ||
| p-LNH | 0.122 | 0.020 | ||
| DFS-LNnH | −0.139 | 0.003 | ||
| Emotional undereating | 6′-SL | −0.119 | 0.038 | |
| LNFP II | 0.198 | 0.046 | ||
| Food responsiveness | Total Fucosylation | 0.134 | 0.049 | |
| 6′-SL | 0.089 | 0.043 | ||
| Non-Secretors | Enjoyment of food | Total Fucosylation | −0.270 | 0.022 |
| 3-FL | −0.212 | 0.007 | ||
| LNFP II | −0.136 | 0.039 | ||
| LNDFH II | −0.193 | 0.016 | ||
| p-LNH | 0.237 | 0.005 | ||
| Emotional overeating | LDFT | 6.897 | 0.019 | |
| S-LNnH II | −0.219 | 0.024 | ||
| IFLNH III | −0.155 | 0.014 | ||
| DFS-LNH | 0.664 | 0.045 | ||
| Satiety responsiveness | HMO Diversity | 0.140 | 0.017 | |
| Total Fucosylation | 0.293 | 0.001 | ||
| 3-FL | 0.203 | <0.001 | ||
| LNFP II | 0.135 | 0.007 | ||
| p-LNH | −0.150 | 0.024 | ||
| DFLNHb | 0.129 | 0.050 | ||
| Emotional undereating | DFS-LNH | 0.960 | 0.046 | |
| Food fussiness | LNDFH I | 0.606 | 0.028 |
| Sample | Eating Behavior | HMO | Estimate | p-Values |
|---|---|---|---|---|
| Full Cohort | Enjoyment of Food | LNFP II | −0.119 | 0.014 |
| LNDFH II | −0.125 | 0.035 | ||
| Emotional overeating | LNH | −0.071 | 0.046 | |
| LNnH | −0.088 | 0.024 | ||
| IFLNH III | −0.070 | 0.037 | ||
| Satiety responsiveness | LNFP II | 0.010 | 0.017 | |
| DFS-LNnH | −0.085 | 0.023 | ||
| Slowness in eating | S-LNH | −0.092 | 0.041 | |
| Food fussiness | LNFP V | 0.200 | 0.019 | |
| LNnH | −0.106 | 0.038 | ||
| S-LNH | 0.139 | 0.004 | ||
| DFS-LNnH | −0.141 | 0.005 | ||
| Food responsiveness | Total Sialylation | 0.084 | 0.040 | |
| 6′-SL | 0.114 | 0.003 | ||
| IFLNH I | 0.094 | 0.047 | ||
| Secretors | Enjoyment of food | 6′-SL | 0.132 | 0.008 |
| Emotional overeating | MFpLNH IV | −0.153 | 0.042 | |
| IFLNH III | −0.085 | 0.047 | ||
| Slowness in eating | LNH | −0.123 | 0.037 | |
| Food fussiness | Total nonfucosylated neutral | 0.143 | 0.029 | |
| LNT+LNnT | 0.166 | 0.023 | ||
| p-LNH | 0.112 | 0.041 | ||
| S-LNH | −0.131 | 0.006 | ||
| DFS-LNnH | −0.121 | 0.012 | ||
| Food responsiveness | Total Sialylation | 0.116 | 0.010 | |
| Total nonfucosylated neutral | −0.126 | 0.026 | ||
| 6′-SL | 0.120 | 0.010 | ||
| Non-Secretors | Enjoyment of food | 3-FL | −0.165 | 0.032 |
| LNFP II | −0.143 | 0.026 | ||
| LNFP V | −0.246 | 0.016 | ||
| LNDFH II | −0.192 | 0.012 | ||
| Emotional overeating | LNH | −0.119 | 0.024 | |
| LNnH | −0.236 | 0.020 | ||
| S-LNnH II | −0.176 | 0.036 | ||
| Satiety response | 3-FL | 0.125 | 0.039 | |
| LNFP II | 0.104 | 0.041 | ||
| Emotional undereating | LNH | −0.210 | 0.022 | |
| Food fussiness | 3-FL | 0.234 | 0.014 | |
| LNFP V | 0.367 | 0.003 | ||
| LNDFH I | 0.828 | 0.009 | ||
| LNDFH II | 0.222 | 0.021 | ||
| LNH | −0.155 | 0.050 | ||
| LNnH | −0.344 | 0.023 |
| Sample | Temperament Characteristics and Eating Behaviors | HMO | Estimate | FDR Adjusted p-Values |
|---|---|---|---|---|
| Full Cohort | Surgency (3 mo) | IFLNH III | 0.164 | 0.08 |
| Negative affectivity (18 mo) | LNH | −0.123 | 0.02 | |
| Negative affectivity (48 mo) | MFLNH I+III | −0.206 | 0.009 | |
| Emotional undereating (12 mo) | 6′-SL | −0.178 | 0.09 | |
| Secretors | Surgency (3 mo) | IFLNH III | 0.224 | 0.024 |
| Emotional undereating (12 mo) | 6′-SL | −0.25 | 0.014 | |
| Non-Secretors | Negative affectivity (18 mo) | Total Sialylation | −0.267 | 0.10 |
| Negative affectivity (18 mo) | S-LNnH II | −0.307 | 0.10 | |
| Negative affectivity (18 mo) | IFLNH III | −0.159 | 0.10 | |
| Emotional overeating (12 mo) | DFS-LNnH | 1.482 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Bost, K.F.; Donovan, S.M. Human Milk Oligosaccharide Composition at 6 Weeks Is Associated with Temperament and Eating Behaviors of Children in the STRONG Kids 2 Cohort Through 4 Years of Age. Nutrients 2025, 17, 2080. https://doi.org/10.3390/nu17132080
Fan Y, Bost KF, Donovan SM. Human Milk Oligosaccharide Composition at 6 Weeks Is Associated with Temperament and Eating Behaviors of Children in the STRONG Kids 2 Cohort Through 4 Years of Age. Nutrients. 2025; 17(13):2080. https://doi.org/10.3390/nu17132080
Chicago/Turabian StyleFan, Yuting, Kelly F. Bost, and Sharon M. Donovan. 2025. "Human Milk Oligosaccharide Composition at 6 Weeks Is Associated with Temperament and Eating Behaviors of Children in the STRONG Kids 2 Cohort Through 4 Years of Age" Nutrients 17, no. 13: 2080. https://doi.org/10.3390/nu17132080
APA StyleFan, Y., Bost, K. F., & Donovan, S. M. (2025). Human Milk Oligosaccharide Composition at 6 Weeks Is Associated with Temperament and Eating Behaviors of Children in the STRONG Kids 2 Cohort Through 4 Years of Age. Nutrients, 17(13), 2080. https://doi.org/10.3390/nu17132080








