Does Protein Ingestion Timing Affect Exercise-Induced Adaptations? A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Selection Criteria and Data Extraction
2.3. Quality Assessment
2.4. Meta-Analysis Procedures
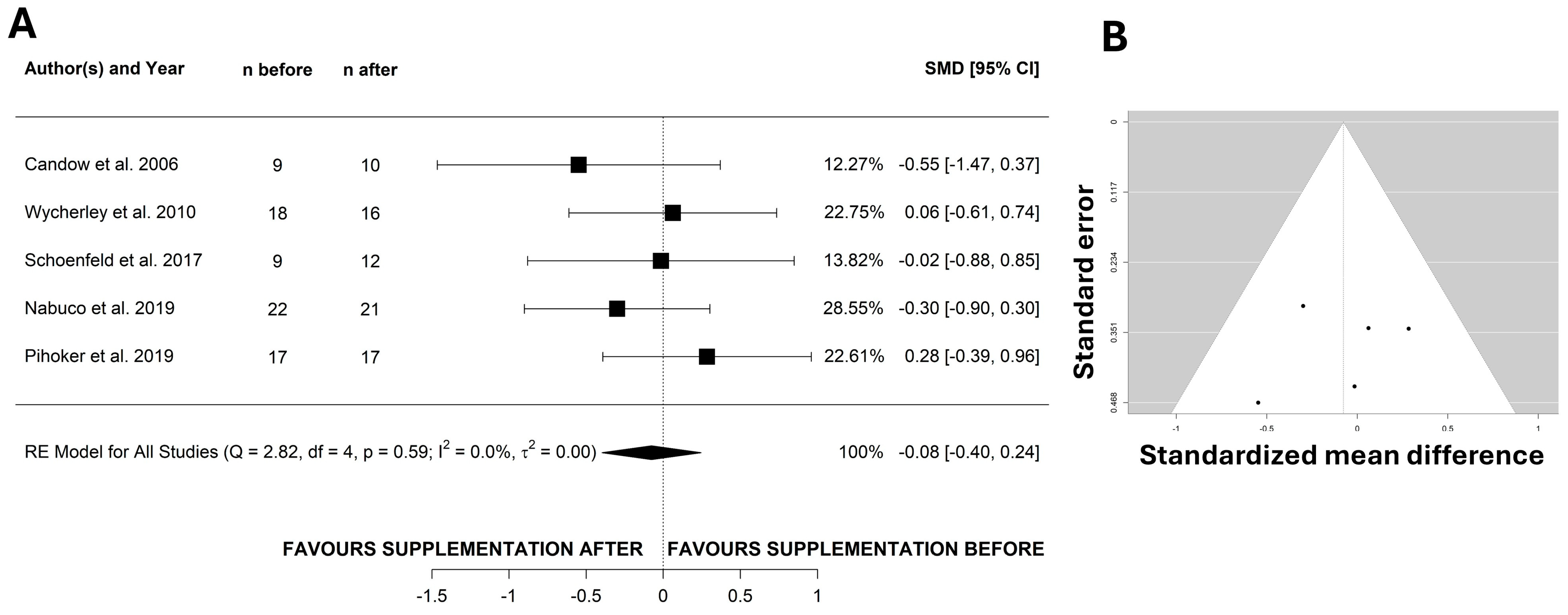
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCAA | Branched chain amino acids |
| RCT | Randomized controlled trial |
| RM | Repetition maximum |
| SMD | Standardized mean difference |
References
- Lewis, S.E.; Kelly, F.J.; Goldspink, D.F. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem. J. 1984, 217, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, D.F.; Kelly, F.J. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem. J. 1984, 217, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef]
- Phillips, S.M.; Paddon-Jones, D.; Layman, D.K. Optimizing Adult Protein Intake During Catabolic Health Conditions. Adv. Nutr. 2020, 11, S1058–S1069. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R.; Fisher, G. Protein Requirements Are Elevated in Endurance Athletes after Exercise as Determined by the Indicator Amino Acid Oxidation Method. PLoS ONE 2016, 11, e0157406. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Mazidi, M.; García, C.R.; E Lane, K.; Jafari, A.; Butler, T.; de Heredia, F.P.; Davies, I.G. Protein interventions augment the effect of resistance exercise on appendicular lean mass and handgrip strength in older adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 115, 897–913. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Galan-Lopez, P.; Casuso, R.A. Metabolic adaptations to morning versus afternoon training: A systematic review and meta-analysis. Sports Med. 2023, 53, 1951–1961. [Google Scholar] [CrossRef]
- Chang, S.-W.; Yoshihara, T.; Machida, S.; Naito, H. Circadian rhythm of intracellular protein synthesis signaling in rat cardiac and skeletal muscles. Biochem. Biophys. Rep. 2017, 9, 153–158. [Google Scholar] [CrossRef]
- Tipton, K.D.; Rasmussen, B.B.; Miller, S.L.; Wolf, S.E.; Owens-Stovall, S.K.; Petrini, B.E.; Wolfe, R.R. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E197–E206. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. The effect of protein timing on muscle strength and hypertrophy: A meta-analysis. J. Int. Soc. Sports Nutr. 2013, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J. Evidence inconclusive-comment on article by Schoenfeld et al. J. Int. Soc. Sports Nutr. 2016, 13, 37. [Google Scholar] [CrossRef]
- Wirth, J.; Hillesheim, E.; Brennan, L. The role of protein intake and its timing on body composition and muscle function in healthy adults: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2020, 150, 1443–1460. [Google Scholar] [CrossRef]
- Zhou, H.H.; Liao, Y.; Zhou, X.; Peng, Z.; Xu, S.; Shi, S.; Liu, L.; Hao, L.; Yang, W. Effects of timing and types of protein supplementation on improving muscle mass, strength, and physical performance in adults undergoing resistance training: A network meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2024, 34, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Noakes, M.; Clifton, P.M.; Cleanthous, X.; Keogh, J.B.; Brinkworth, G.D. Timing of protein ingestion relative to resistance exercise training does not influence body composition, energy expenditure, glycemic control or cardiometabolic risk factors in a hypocaloric, high protein diet in patients with type 2 diabetes. Diabetes Obes. Metab. 2010, 12, 1097–1105. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 3. [Google Scholar] [CrossRef]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: A GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Higgins, J.P.T.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence [last updated May 2025]. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5.1; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Available online: https://www.cochrane.org/handbook (accessed on 25 September 2024).
- Schoenfeld, B.J.; Aragon, A.; Wilborn, C.; Urbina, S.L.; Hayward, S.E.; Krieger, J. Pre- versus post-exercise protein intake has similar effects on muscular adaptations. PeerJ 2017, 5, e2825. [Google Scholar] [CrossRef] [PubMed]
- Pihoker, A.A.; Peterjohn, A.M.; Trexler, E.T.; Hirsch, K.R.; Blue, M.N.; Anderson, K.C.; Ryan, E.D.; Smith-Ryan, A.E. The effects of nutrient timing on training adaptations in resistance-trained females. J. Sci. Med. Sport 2019, 22, 472–477. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Facci, M.; Abeysekara, S.; Zello, G.A. Protein supplementation before and after resistance training in older men. Eur. J. Appl. Physiol. 2006, 97, 548–556. [Google Scholar] [CrossRef]
- Nabuco, H.C.G.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B.; et al. Effects of whey protein supplementation pre- or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older women: A randomized clinical trial. Nutrients 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.; Tomeleri, C.; Junior, P.S.; Fernandes, R.; Cavalcante, E.; Venturini, D.; Barbosa, D.; Silva, A.; Sardinha, L.; Cyrino, E. Effects of pre- or post-exercise whey protein supplementation on body fat and metabolic and inflammatory profile in pre-conditioned older women: A randomized, double-blind, placebo-controlled trial. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef]
- Dohm, G.L.; Tapscott, E.B.; Kasperek, G.J. Protein degradation during endurance exercise and recovery. Med. Sci. Sports Exerc. 1987, 19, S166–S171. [Google Scholar] [CrossRef]
- Martín, J.C.H.; Ansa, B.S.; Álvarez-Rivera, G.; Domínguez-Zorita, S.; Rodríguez-Pombo, P.; Pérez, B.; Calvo, E.; Paradela, A.; Miguez, D.G.; Cifuentes, A.; et al. An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis. Nat. Metab. 2024, 6, 209–225. [Google Scholar] [CrossRef]
- Zinner, C.; Morales-Alamo, D.; Ørtenblad, N.; Larsen, F.J.; Schiffer, T.A.; Willis, S.J.; Gelabert-Rebato, M.; Perez-Valera, M.; Boushel, R.; Calbet, J.A.; et al. The physiological mechanisms of performance enhancement with sprint interval training differ between the upper and lower extremities in humans. Front. Physiol. 2016, 7, 426. [Google Scholar] [CrossRef]
- Rodiles-Guerrero, L.; Cornejo-Daza, P.J.; Sánchez-Valdepeñas, J.; Alcazar, J.; Rodriguez-López, C.; Sánchez-Moreno, M.; Alegre, L.M.; León-Prados, J.A.; Pareja-Blanco, F. Specific adaptations to 0%, 15%, 25%, and 50% velocity-loss thresholds during bench press training. Int. J. Sports Physiol. Perform. 2022, 17, 1231–1241. [Google Scholar] [CrossRef] [PubMed]


| True Randomization | Allocation Concealed | Group Similarity at Baseline | Blinded Participants | Blinded Treatment Deliverers | Blinded Outcomes Assessors | Identically Treated Groups | Follow up Complete/Adequately Described and Analyzed | Participants Analyzed in Randomized Groups | Same Way of Outcome Measurement for Treatment Groups | Reliable Outcome Measurement | Appropriate Statistical Analysis | Appropriate Trial Design/Deviations Considered in the Conduct and Analysis | Overall Appraisal | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candow et al., 2006 [26] | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Wycherley et al., 2010 [19] | Unclear | Unclear | Yes | No | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Schoenfeld et al., 2017 [24] | Unclear | Unclear | Unclear | No | Unclear | Unclear | Yes | No | Yes | Yes | Yes | Yes | Yes | Include |
| Nabuco et al., 2018 [27] Nabuco et al., 2019 [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Pihoker et al., 2019 [25] | Unclear | Unclear | Yes | No | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Study | Subjects | Supplement | Supplementation Timing | Protein Matched with Control? | Assessment Methods | Training Protocol | Significant Results on Strength? | Significant Results on Body Composition? |
|---|---|---|---|---|---|---|---|---|
| Candow et al., 2006 [26] | 29 untrained, older men | Supplement of 0.3 g protein/kg body mass (from whey, milk, egg) (in 0.54 g/kg of Myoplex®), and 0.09 g/kg chocolate cocoa. | (1) Protein immediately before and placebo immediately after; (2) placebo immediately before and protein immediately after; (3) placebo immediately before and placebo immediately after. | No. Dietary records for 3 days during the first and final week indicated that the ‘before’ group consumed significantly more dietary protein before training compared to the ‘after’ group. | Air-displacement plethysmography; B-Mode ultrasound; 1-RM | 12 weeks of progressively increased resistance training, 3 non-consecutive days/week, 60 min | Chest press 1-RM/leg press 1-RM | Lean tissue mass/muscle thickness |
| Wycherley et al., 2010 [19] | 34 overweight and obese, sedentary men and women with type 2 diabetes | Supplement of 25 g skim milk powder in 250 mL low fat (<1%) milk (total 860 kJ, 21 g protein, 0.7 g fat, 29.6 g carbohydrate). | (1) Protein immediately before; (2) protein at least 2 h after. | Yes. Total energy intake and macronutrient composition were similar between the treatment groups. | Dual X-ray absorptiometry; 1-RM | 16 weeks of progressively increased resistance training, 3 non-consecutive days/week, 45 min | Chest press 1-RM/lat pull-down 1-RM | Fat-free mass/ fat mass |
| Schoenfeld et al., 2017 [24] | 21 young male, experienced lifters | Supplement of 25 g protein and 1 g carbohydrate (Iso100 Hydrolyzed Whey Protein Isolate). | (1) Protein immediately before; (2) protein immediately after. | Yes. There was no significant group effect for self-reported calorie intake. | B-mode ultrasound imaging; dual x-ray absorptiometry; 1-RM | 10 weeks of progressively increased resistance training, 3 non-consecutive days/week | Chest press 1-RM/back squat 1-RM | Lean tissue mass/muscle thickness/ fat mass |
| Nabuco et al., 2018 [27]; Nabuco et al., 2019 [28] | 66 untrained, older women | Supplement of hydrolyzed whey protein (27.1 g of protein, 5.2 g of carbohydrates, 0.2 g of fat). | (1) Protein immediately before and placebo immediately after; (2) placebo immediately before and protein immediately after; (3) placebo immediately before and placebo immediately after. | Yes. The habitual daily energy and macronutrients intake were not different between groups. | Dual X-ray absorptiometry; 1-RM | 12 weeks of progressively increased resistance training, 3 non-consecutive days/week | Chest press 1-RM/preacher curl 1-RM/knee extension 1-RM | Lean body mass/ fat mass |
| Pihoker et al., 2019 [25] | 43 trained, young women | Supplement of 200 kcal (3.5 g fat, 16 g carbohydrates, 25 g lean protein). | (1) Protein within fifteen minutes before; (2) protein within 15 min after; (3) no supplementation. | Yes. Dietary logs demonstrated no differences in kilocalories. | Dual X-ray absorptiometry; 1-RM | 6 weeks of progressively increased resistance training, 2 non-consecutive days/week | Chest press 1-RM/leg press 1-RM | Lean body mass/ fat mass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casuso, R.A.; Goossens, L. Does Protein Ingestion Timing Affect Exercise-Induced Adaptations? A Systematic Review with Meta-Analysis. Nutrients 2025, 17, 2070. https://doi.org/10.3390/nu17132070
Casuso RA, Goossens L. Does Protein Ingestion Timing Affect Exercise-Induced Adaptations? A Systematic Review with Meta-Analysis. Nutrients. 2025; 17(13):2070. https://doi.org/10.3390/nu17132070
Chicago/Turabian StyleCasuso, Rafael A., and Lennert Goossens. 2025. "Does Protein Ingestion Timing Affect Exercise-Induced Adaptations? A Systematic Review with Meta-Analysis" Nutrients 17, no. 13: 2070. https://doi.org/10.3390/nu17132070
APA StyleCasuso, R. A., & Goossens, L. (2025). Does Protein Ingestion Timing Affect Exercise-Induced Adaptations? A Systematic Review with Meta-Analysis. Nutrients, 17(13), 2070. https://doi.org/10.3390/nu17132070






