The Impact of Egg Consumption on Gastrointestinal Health: A Systematic Literature Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria, Databases, Search Strategy
2.2. Study Selection and Data Extraction
2.3. Risk of Bias Assessment
2.4. Data Synthesis
3. Results
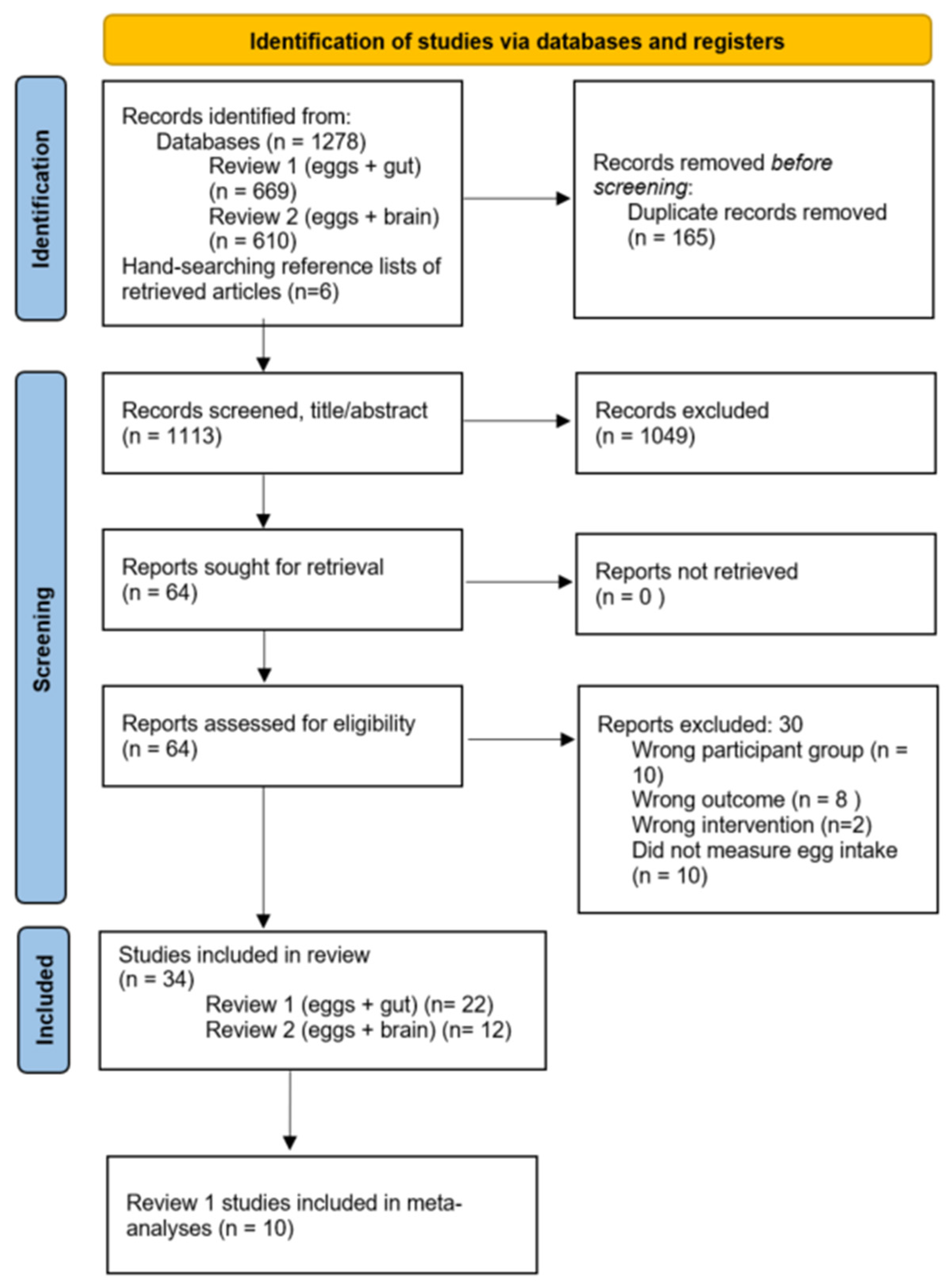
3.1. Study Selection
3.2. Study Characteristics
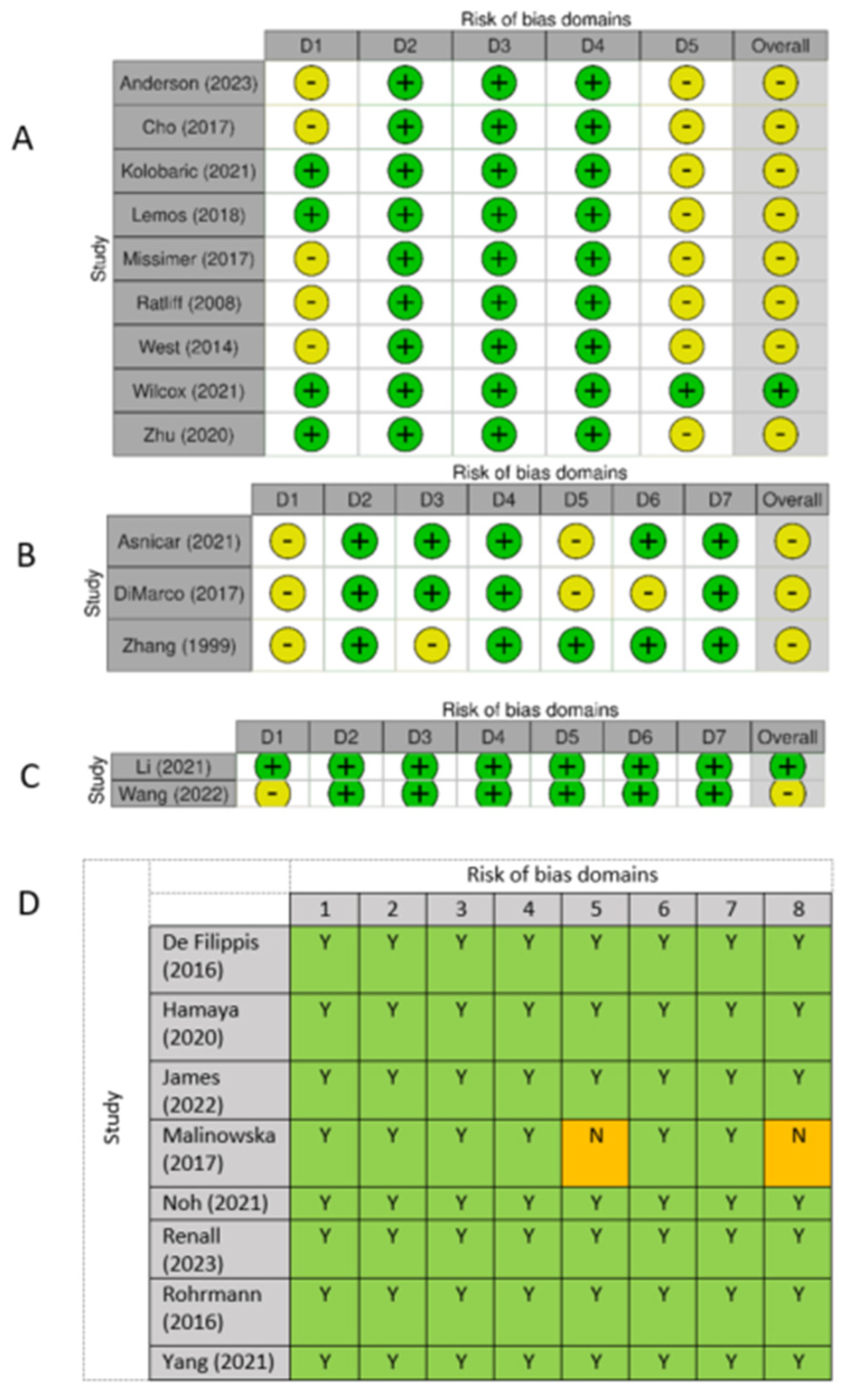
3.3. Risk of Bias Across Studies
3.4. Synthesis of Results
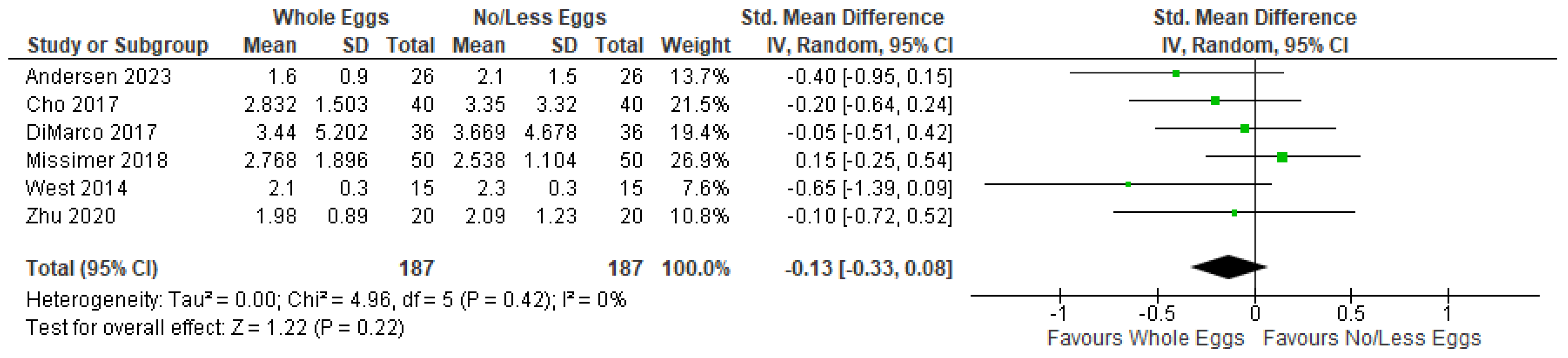
3.4.1. TMAO
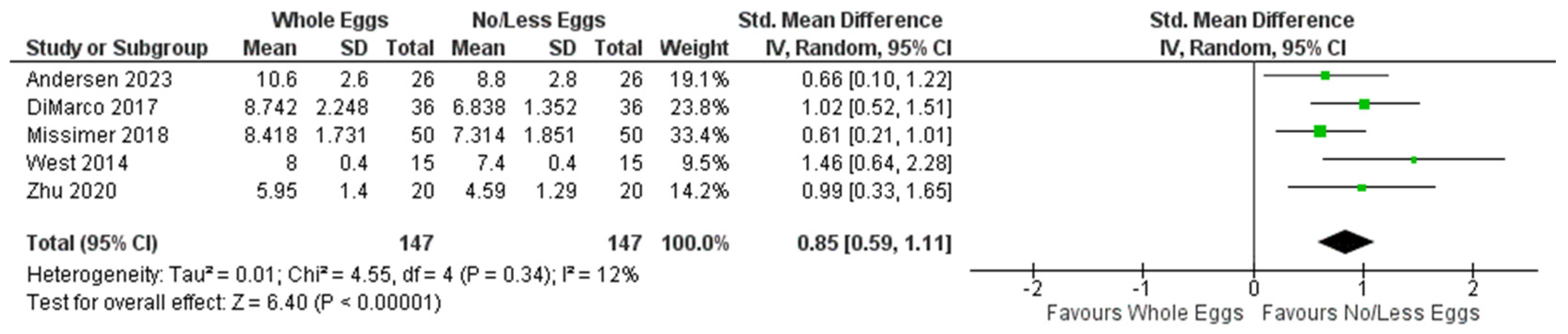
3.4.2. Plasma Choline
3.4.3. Gut Microbiome Composition and Diversity
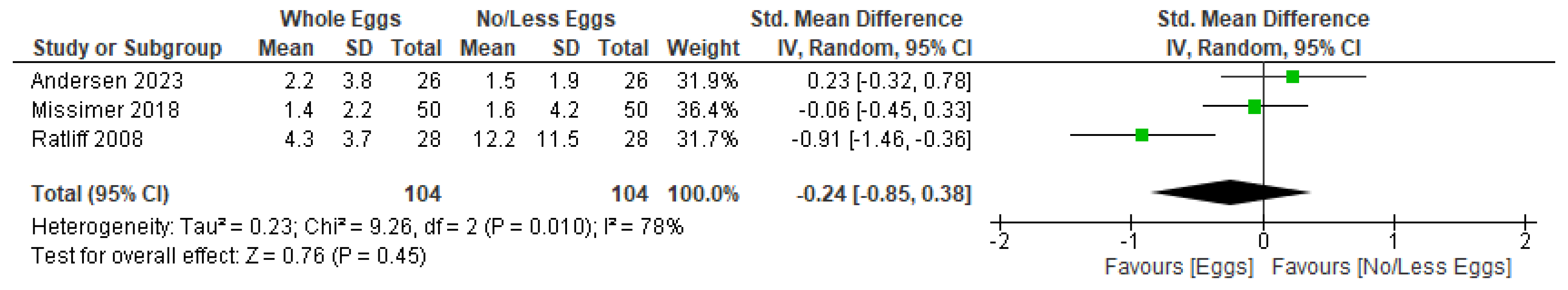
3.4.4. Inflammatory Markers
3.4.5. Faecal Short-Chain Fatty Acids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of Dietary Cholesterol or Egg Consumption with Incident Cardiovascular Disease and Mortality. JAMA 2019, 321, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Sawrey-Kubicek, L.; Zhu, C.; Bardagjy, A.S.; Rhodes, C.H.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption compared with yolk-free egg increases the cholesterol efflux capacity of high-density lipoproteins in overweight, postmenopausal women. Am. J. Clin. Nutr. 2019, 110, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, D.M.; Missimer, A.; Murillo, A.G.; Lemos, B.S.; Malysheva, O.V.; Caudill, M.A.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids 2017, 52, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.M.; Anton, X.; Redondo-Valbuena, C.; Roca-Saavedra, P.; Rodriguez, J.A.; Lamas, A.; Franco, C.M.; Cepeda, A. Egg and egg-derived foods: Effects on human health and use as functional foods. Nutrients 2015, 7, 706–729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuang, H.; Yang, F.; Zhang, Y.; Wang, T.; Chen, G. The Impact of Egg Nutrient Composition and Its Consumption on Cholesterol Homeostasis. Cholesterol 2018, 2018, 6303810. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci. Rep. 2015, 5, 15220. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, N.; Meng, Y.; Keast, R. A comparative study of the modulation of the gut microbiota in rats by dietary intervention with different sources of egg-white proteins. J. Sci. Food Agric. 2020, 100, 3622–3629. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claus, D.; Geypens, B.; Hiele, M.; Geboes, K.; Rutgeerts, P.; Ghoos, Y. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G935–G943. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Shivakumar, N.; Varkey, A.; Duraisamy, R.; Thomas, T.; Preston, T.; Devi, S.; Kurpad, A.V. Ileal digestibility of intrinsically labeled hen’s egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am. J. Clin. Nutr. 2018, 108, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, F.; Wu, J. Effects of Food Factors and Processing on Protein Digestibility and Gut Microbiota. J. Agric. Food Chem. 2023, 71, 8685–8698. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Gruppen, E.G.; Garcia, E.; Connelly, M.A.; Jeyarajah, E.J.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. TMAO is Associated with Mortality: Impact of Modestly Impaired Renal Function. Sci. Rep. 2017, 7, 13781. [Google Scholar] [CrossRef]
- Yu, D.; Shu, X.O.; Rivera, E.S.; Zhang, X.; Cai, Q.; Calcutt, M.W.; Xiang, Y.B.; Li, H.; Gao, Y.T.; Wang, T.J.; et al. Urinary Levels of Trimethylamine-N-Oxide and Incident Coronary Heart Disease: A Prospective Investigation Among Urban Chinese Adults. J. Am. Heart Assoc. 2019, 8, e010606. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Chou, R.H.; Chen, C.Y.; Chen, I.C.; Huang, H.L.; Lu, Y.W.; Kuo, C.S.; Chang, C.C.; Huang, P.H.; Chen, J.W.; Lin, S.J. Trimethylamine N-Oxide, Circulating Endothelial Progenitor Cells, and Endothelial Function in Patients with Stable Angina. Sci. Rep. 2019, 9, 4249. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Zivkovic, A.M. Are eggs good again? A precision nutrition perspective on the effects of eggs on cardiovascular risk, taking into account plasma lipid profiles and TMAO. J. Nutr. Biochem. 2022, 100, 108906. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, M.J.; Fernandez, M.L. The Health Benefits of Egg Protein. Nutrients 2022, 14, 2904. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Gomes, G.K.; Schoenfeld, B.J.; de Oliveira, E.P. The Effect of Whole Egg Intake on Muscle Mass: Are the Yolk and Its Nutrients Important? Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 514–521. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Frutos Rde, L.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Wong, M.L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- Aromaratis, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2024. [Google Scholar]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Huang, L.; Zhai, F.; Palancia Esposito, C.; Greco, J.M.; Zhang, R.; Woodruff, R.; Sloan, A.; Van Dyke, A.R. Consumption of Different Egg-Based Diets Alters Clinical Metabolic and Hematological Parameters in Young, Healthy Men and Women. Article. Nutrients 2023, 15, 3747. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef] [PubMed]
- Kolobarić, N.; Drenjančević, I.; Matić, A.; Šušnjara, P.; Mihaljević, Z.; Mihalj, M. Dietary Intake of n-3 PUFA-Enriched Hen Eggs Changes Inflammatory Markers’ Concentration and Treg/Th17 Cells Distribution in Blood of Young Healthy Adults-A Randomised Study. Nutrients 2021, 13, 1851. [Google Scholar] [CrossRef]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. Article. J. Am. Coll. Nutr. 2018, 37, 716–723. [Google Scholar] [CrossRef]
- Missimer, A.; Fernandez, M.L.; Di Marco, D.M.; Norris, G.H.; Blesso, C.N.; Murillo, A.G.; Vergara-Jimenez, M.; Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; et al. Compared to an Oatmeal Breakfast, Two Eggs/Day Increased Plasma Carotenoids and Choline without Increasing Trimethyl Amine N-Oxide Concentrations. J. Am. Coll. Nutr. 2018, 37, 140–148. [Google Scholar] [CrossRef]
- Ratliff, J.C.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr. Metab. 2008, 5, 6. [Google Scholar] [CrossRef]
- West, A.A.; Shih, Y.; Wang, W.; Oda, K.; Jaceldo-Siegl, K.; Sabaté, J.; Haddad, E.; Rajaram, S.; Caudill, M.A.; Burns-Whitmore, B. Egg n-3 fatty acid composition modulates biomarkers of choline metabolism in free-living lacto-ovo-vegetarian women of reproductive age. J. Acad. Nutr. Diet. 2014, 114, 1594–1600. [Google Scholar] [CrossRef]
- Wilcox, J.; Skye, S.M.; Graham, B.; Zabell, A.; Li, X.S.; Li, L.; Shelkay, S.; Fu, X.; Neale, S.; O’Laughlin, C.; et al. Dietary Choline Supplements, but Not Eggs, Raise Fasting TMAO Levels in Participants with Normal Renal Function: A Randomized Clinical Trial. Am. J. Med. 2021, 134, 1160–1169.e3. [Google Scholar] [CrossRef]
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole egg consumption increases plasma choline and betaine without affecting TMAO levels or gut microbiome in overweight postmenopausal women. Nutr. Res. 2020, 78, 36–41. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, R.; Ivey, K.L.; Lee, D.H.; Wang, M.; Li, J.; Franke, A.; Sun, Q.; Rimm, E.B. Association of diet with circulating trimethylamine-N-oxide concentration. Am. J. Clin. Nutr. 2020, 112, 1448–1455. [Google Scholar] [CrossRef]
- James, K.L.; Gertz, E.R.; Cervantes, E.; Bonnel, E.L.; Stephensen, C.B.; Kable, M.E.; Bennett, B.J. Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults. Article. Nutrients 2022, 14, 1376. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, A.M.; Szwengiel, A.; Chmurzynska, A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Article. Int. J. Food Sci. Nutr. 2017, 68, 488–495. [Google Scholar] [CrossRef]
- Noh, H.; Jang, H.H.; Kim, G.; Zouiouich, S.; Cho, S.Y.; Kim, H.J.; Kim, J.; Choe, J.S.; Gunter, M.J.; Ferrari, P.; et al. Taxonomic Composition and Diversity of the Gut Microbiota in Relation to Habitual Dietary Intake in Korean Adults. Nutrients 2021, 13, 366. [Google Scholar] [CrossRef]
- Renall, N.; Lawley, B.; Vatanen, T.; Merz, B.; Douwes, J.; Corbin, M.; Te Morenga, L.; Kruger, R.; Breier, B.H.; Tannock, G.W. The fecal microbiotas of women of Pacific and New Zealand European ethnicities are characterized by distinctive enterotypes that reflect dietary intakes and fecal water content. Gut Microbes 2023, 15, 2178801. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; Von Eckardstein, A.; Müller, D. Plasma concentrations of trimethylamine- n-oxide are directly associated with dairy food consumption and low-grade inflammation in a german adult population. Article. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef]
- Yang, J.J.; Shu, X.O.; Herrington, D.M.; Moore, S.C.; Meyer, K.A.; Ose, J.; Menni, C.; Palmer, N.D.; Eliassen, H.; Harada, S.; et al. Circulating trimethylamine N-oxide in association with diet and cardiometabolic biomarkers: An international pooled analysis. Article. Am. J. Clin. Nutr. 2021, 113, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Ivey, K.L.; Wang, D.D.; Wilkinson, J.E.; Franke, A.; Lee, K.H.; Chan, A.; Huttenhower, C.; Hu, F.B.; et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: Findings from a longitudinal cohort of US men. Article. Gut 2022, 71, 724–733. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Lee, Y.; Lai, H.T.M.; De Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.; Sotoodehnia, N.; Budoff, M.; Didonato, J.A.; et al. Dietary Meat, Trimethylamine N-Oxide-Related Metabolites, and Incident Cardiovascular Disease Among Older Adults: The Cardiovascular Health Study. Article. Arterioscler. Thromb. Vasc. Biol. 2022, 42, E273–E288. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Article. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dietary precursors of trimethylamine in man: A pilot study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; Vallim, T.Q.A.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Taesuwan, S.; Cho, C.E.; Malysheva, O.V.; Bender, E.; King, J.H.; Yan, J.; Thalacker-Mercer, A.E.; Caudill, M.A. The metabolic fate of isotopically labeled trimethylamine-N-oxide (TMAO) in humans. J Nutr. Biochem. 2017, 45, 77–82. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. Metaorganismal nutrient metabolism as a basis of cardiovascular disease. Curr. Opin. Lipidol. 2014, 25, 48–53. [Google Scholar] [CrossRef]
- Al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metab. Clin. Exp. 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Dalla Via, A.; Gargari, G.; Taverniti, V.; Rondini, G.; Velardi, I.; Gambaro, V.; Visconti, G.L.; De Vitis, V.; Gardana, C.; Ragg, E.; et al. Urinary TMAO Levels Are Associated with the Taxonomic Composition of the Gut Microbiota and with the Choline TMA-Lyase Gene (cutC) Harbored by Enterobacteriaceae. Nutrients 2020, 12, 62. [Google Scholar] [CrossRef]
- Cho, C.E.; Aardema, N.D.J.; Bunnell, M.L.; Larson, D.P.; Aguilar, S.S.; Bergeson, J.R.; Malysheva, O.V.; Caudill, M.A.; Lefevre, M. Effect of Choline Forms and Gut Microbiota Composition on Trimethylamine-N-Oxide Response in Healthy Men. Nutrients 2020, 12, 2220. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, M.; Bazeley, P.; Wang, Z.; Levison, B.S.; Li, X.S.; Jia, X.; Krauss, R.M.; Knight, R.; Lusis, A.J.; Garcia-Garcia, J.C.; et al. Fecal Microbiome Composition Does Not Predict Diet-Induced TMAO Production in Healthy Adults. J. Am. Heart Assoc. 2021, 10, e021934. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Mage, I.; Rud, I.; Aas, A. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Taylor, T.D.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef]
- Duranti, S.; Ruiz, L.; Lugli, G.A.; Tames, H.; Milani, C.; Mancabelli, L.; Mancino, W.; Longhi, G.; Carnevali, L.; Sgoifo, A.; et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 2020, 10, 14112. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.K.; Kim, D.H.; Jang, S.W.; Han, S.W.; Yoon, I.Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Sajadi Hezaveh, Z.; Sikaroudi, M.K.; Vafa, M.; Clayton, Z.S.; Soltani, S. Effect of egg consumption on inflammatory markers: A systematic review and meta-analysis of randomized controlled clinical trials. J. Sci. Food Agric. 2019, 99, 6663–6670. [Google Scholar] [CrossRef]
- Dibella, M.; Thomas, M.S.; Alyousef, H.; Millar, C.; Blesso, C.; Malysheva, O.; Caudill, M.A.; Fernandez, M.L. Choline intake as supplement or as a component of eggs increases plasma choline and reduces interleukin-6 without modifying plasma cholesterol in participants with metabolic syndrome. Article. Nutrients 2020, 12, 3120. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volk, B.; Volek, J.S.; Fernandez, M.L. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors in metabolic syndrome. Article. J. Clin. Lipidol. 2013, 7, 463–471. [Google Scholar] [CrossRef]
- Pearce, K.L.; Clifton, P.M.; Noakes, M. Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Br. J. Nutr. 2011, 105, 584–592. [Google Scholar] [CrossRef]
- Fuller, N.R.; Caterson, I.D.; Sainsbury, A.; Denyer, G.; Fong, M.; Gerofi, J.; Baqleh, K.; Williams, K.H.; Lau, N.S.; Markovic, T.P. The effect of a high-egg diet on cardiovascular risk factors in people with type 2 diabetes: The Diabetes and Egg (DIABEGG) study-a 3-mo randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.N.; Valenzuela, F.; Robles, A.E.; Artalejo, E.; Aguilar, D.; Andersen, C.J.; Valdez, H.; Fernandez, M.L. One egg per day improves inflammation when compared to an oatmeal-based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Article. Nutrients 2015, 7, 3449–3463. [Google Scholar] [CrossRef] [PubMed]
- Ćurić, Ž.B.; Masle, A.M.; Kibel, A.; Selthofer-Relatić, K.; Stupin, A.; Mihaljević, Z.; Jukić, I.; Stupin, M.; Matić, A.; Kozina, N.; et al. Effects of n-3 Polyunsaturated Fatty Acid-Enriched Hen Egg Consumption on the Inflammatory Biomarkers and Microvascular Function in Patients with Acute and Chronic Coronary Syndrome—A Randomized Study. Biology 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. Conference Paper. J. Nutr. 2004, 134, 3011–3015. [Google Scholar] [CrossRef]
- Durazzi, F.; Sala, C.; Castellani, G.; Manfreda, G.; Remondini, D.; De Cesare, A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 2021, 11, 3030. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Yao, C.K.; Chey, W.D.; Whelan, K. Optimal Design of Clinical Trials of Dietary Interventions in Disorders of Gut-Brain Interaction. Am. J. Gastroenterol. 2022, 117, 973–984. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Adults without chronic disease aged 18 years and over | Animals; adults who were breastfeeding or pregnant for the duration of the study; humans aged < 18 years; adults diagnosed with any chronic disease |
| Intervention | Whole chicken egg consumption as a dietary intervention or measured via the collection of prospective or retrospective diet intake data using food frequency questionnaires, food records, or dietary recall | Dietary interventions that did not include whole chicken eggs, including trials that included the provision of part of an egg, such as the egg whites or egg yolks only; observational studies that did not specifically measure egg intake |
| Comparison | A control group that was provided with an alternative intervention that did not include whole chicken eggs or that was not provided with an intervention; in the case of observational studies, the comparison group would include participants who reported a low egg intake | A control group that did not report levels of egg consumption or a control group that consumed a similar volume of whole eggs as the intervention group |
| Outcome | Any outcomes related to gastrointestinal factors, including symptoms, microbiome composition, inflammation, colonic fermentation, and gastrointestinal metabolites (TMAO) | Outcomes that are unrelated to gastrointestinal factors; outcomes related to appetite or satiety |
| Study Type | Randomised controlled trials; Non-randomised intervention trials; observational studies (cross-sectional, prospective, and retrospective cohort studies included) | Reviews; case studies |
| First Author (Year of Publication) | Participants (Country, No. of Participants, Mean Age (Years), Mean BMI (kg/m2), Gender) | Study Design | Outcomes | Sample Collection Methods | Analysis Methods | Summary of Findings | ||
|---|---|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||||
| Design | Intervention | Duration | ||||||
| Andersen (2023) [36] | USA, n = 26, healthy young adults, 21.7 ± 4.7 years, 22.5 ± 2.8 kg/m2, 19% males | Crossover trial. Lead-in: 4 weeks egg-free diet Washout: 4 weeks egg-free diet Blinding: not described | Group 1: 3 whole eggs/day Group 2: 3 egg whites/day | 4 weeks | a. Plasma TMAO b. Plasma choline c. High-sensitivity C-reactive protein (hsCRP) | a, b, & c. Overnight fasted blood samples pre- and post-intervention | a & b. Measured by a commercial lab (LabCorp) c. Automated multiplex assay analyser | a. ∅ b. ↑ significantly higher in 3 whole-egg group compared to egg-free washout c. ∅ |
| Cho (2017) [37] | USA, n = 40, healthy males, 27.8 ± 1.0 years, 24.2 ± 0.4 kg/m2 | Crossover trial. Lead-in: n/a Washout: 1 week Blinding: not described | Group 1: 3 whole eggs Group 2: 170 g beef steak Group 3: 170 g fish fillet Group 4: control phase, 2 packages of applesauce 180 g | 6 h (post-prandial) | a. Plasma and urine TMAO b. Gut microbiome composition c. α diversity—Faith, Chao1, observed species d. β diversity—unweighted UniFrac | a. Blood and urine samples at baseline (fasting), 15 min, 30 min, 1 h, 2 h, 4 h, and 6 h after eating b, c, & d. Stool samples at baseline | a. LCMS b, c, & d. 16S rRNA gene amplicon sequencing | a. ∅ in plasma. ↑ significantly in urine at 6 h compared to baseline b, c & d. did not assess correlation with egg consumption |
| Kolobarić (2021) [38] | Croatia, n = 40, healthy young adults, 23.8 ± 2.8 years, 24.2 ± 3.0 kg/m2, 50% males | Parallel arm, 2 groups Blinding: double blinding | Group 1 (intervention, n = 19): 3 hard-boiled n-3 PUFA-enriched eggs/day, providing 1053 mg of n-3 PUFA Group 2 (control, n = 21): 3 hard-boiled regular eggs/day, providing 249 mg of n-3 PUFA | 3 weeks | a. T lymphocytes (Th17, Tregs) b. Pro-inflammatory cytokines (IL-17A, IL-23, IL-6, MCP-1, TGF-β1) c. Anti-inflammatory cytokines (IL-10) | a, b, & c. Overnight fasted blood plasma in EDTA tubes pre- and post-intervention | a. Isolated from peripheral blood mononuclear cells by flow cytometry. Cell staining with mouse anti-human antibodies b & c. Multiplex assay kits | a. nTreg oocytescytes and Th17cells ↓ significantly after regular eggs and n-3 PUFA-enriched eggs b. TGF-β1 ↑ significantly after n-3 PUFA-enriched eggs but not after regular eggs ∅ in IL-6 ∅ in IL-17A ∅ in MCP-1. Results not reported for IL-23 c. ∅ |
| Lemos (2018) [39] | USA, n = 29, healthy young adults, 25.6 ± 2.3 years, 24.3 ± 2.9 kg/m2, 47% males | Crossover trial. Lead-in: 2 weeks Washout: 3 weeks Blinding: not described | Group 1 (intervention): 3 whole eggs/day providing 400 mg choline Group 2 (control): 1.5tablets of choline bitartrate/day, providing 400 mg of choline | 4 weeks | a. Plasma TMAO b. Plasma choline | a & b. Overnight fasted blood plasma in EDTA tubes pre- and post-intervention | a & b. LCMS | a. ∅ b. ↑ significantly after whole eggs intervention but not after choline supplementation |
| Missimer (2018) [40] | USA, n = 50, healthy young adults, 23.3 ± 3.1 years, 23.2 ± 2.1 kg/m2, 50% males | Crossover trial. Lead-in: not described Washout: 3 weeks Blinding: not described | Group 1: 2 whole eggs/day Group 2: 1 packet of oatmeal/day | 4 weeks | a. Plasma TMAO b. Plasma choline c. CRP d. Cytokines (IL-6, TNF-α) | a, b, c, & d. Fasting blood samples pre- and post-intervention | a & b. LCMS c. Automated multiplex assay analyser d. Commercially available assay kits | a. ∅ b. ↑ after eggs compared to oatmeal c. ∅ d. IL6 ∅. TNF-α ↓ significantly after eggs compared to oatmeal |
| Ratliff (2008) [41] | USA, n = 28, overweight males (BMI 26–37 kg/m2), aged 40–70, mean age and mean BMI not reported | Parallel arm, 2 groups Blinding: single blinding (participants) | All participants placed on the same carbohydrate-restricted diet (% energy from CHO:fat:protein = 17:57:26) Group 1 (intervention, n = 15): liquid whole eggs, 640 mg cholesterol per day, equivalent to 3–4 eggs/day Group 2 (placebo control, n = 13): liquid fat-free eggs | 12 weeks | a. CRP b. Cytokines (TNF-α, IL-8, MCP-1) | a & b. Overnight fasted blood plasma in EDTA tubes, pre- and post-intervention | a & b. Multiplex assay kits | a. ↓ significantly in whole-egg group. Non-significant ↑ in fat-free egg group b. ∅ in TNF-α ∅ IL-8 MCP-1 significantly ↓ in fat-free egg group only, ∅ in whole-egg group |
| West (2014) [42] | USA, n = 15, lacto-ovo vegetarian females of reproductive age, 35.7 ± 12.9 years, 23.7 ± 4.7 kg/m2 | Crossover trial. Lead-in: 2 weeks Washout: 4 weeks Blinding: single blinding (participants) | Group 1: 6 n-3 fatty acids-enriched eggs/week Group 2: 6 non-enriched eggs/week Group 3: egg-free control phase, walnuts consumed in place of eggs | 4 weeks | a. Plasma TMAO b. Plasma choline | a & b. Fasted blood samples pre- and post-intervention | a & b. LCMS | a. ∅ b. ↑ significantly in groups 1 and 2 compared to control (group 3). Non-significant between groups 1 and 2. |
| Wilcox (2021) [43] | USA, n = 82, healthy adults, 28 (24.0–38.8) years, 36.9 (22.8–31.9) kg/m2, 42% males | Parallel arm, 5 groups Blinding: single blinding (researcher) | Group 1 (n = 18): 4 whole hard-boiled eggs Group 2 (n = 20): 2 500 mg choline bitartrate tablets Group 3 (n = 16): 4 whole hard-boiled eggs and 2 500 mg choline bitartrate tablets Group 4 (n = 18): 4 hard-boiled egg whites and 2 500 mg choline bitartrate tablets Group 5 (n = 10): 6 420 mg phosphatidylcholine tablets | 4 weeks | a. Plasma TMAO b. Plasma choline c. 24-h urinary TMAO and spot urine TMAO | a & b. 8 h fasted bloods taken weekly, results reported pre- and post-intervention c. Three 24-h urine samples collected. Spot urine collected weekly, results reported pre- and post-intervention | a & b. LCMS | a. ↑ in groups 2, 3, 4 ∅ in groups 1, 5 b. ↑ in all groups c. 24 h urine ↑ in groups 2, 3, 4 ∅ in groups 1, 5 Spot urine ↑ in group 2, ∅ in groups 1, 3, 4, 5 |
| Zhu (2020) [44] | USA, n = 20, overweight postmenopausal females with hypercholesterolaemia, 57.7 ± 5.6 years, 28.3 ± 3.0 kg/m2 | Crossover trial. Lead-in: 2 weeks Washout: 4 weeks Blinding: not described | Group 1 (intervention): 100 g liquid whole egg Group 2 (control): 100 g liquid egg whites | 4 weeks | a. Plasma TMAO b. Plasma choline c. Gut microbiome composition d. α diversity—Shannon | a & b. Overnight fasted blood plasma in EDTA tubes pre- and post-intervention c & d. Stool samples collected with uBiome kits pre- and post-intervention | a & b. LCMS c & d. 16S rRNA gene amplicon sequencing | a. ∅ b. ↑ after whole-egg intervention c & d. ∅ |
| Cross-sectional trials | ||||||||
| Participant data sources/recruitment | Measurement of egg intake | |||||||
| De Filippis (2016) [45] | Italy, n = 153, healthy adults (n = 51 vegetarians, n = 51 vegans, n = 51 omnivores), 39 ± 9 years, 21.9 ± 2.5 kg/m2, 42% males | Participants recruited from 4 geographically distanced cities in Italy (Bari, Bologna, Parma, Torino). Vegan and vegetarians recruited with collation of the Italian Society of Vegetarian Nutrition. | 7-day weighted food diaries Egg intake categorised as: High Mediterranean diet adherence: 0 g eggs/day Low Mediterranean diet adherence: 9.7 g eggs/day | a. Gut microbiome composition b. α diversity—weighted and unweighted UniFrac distance c. Faecal short-chain fatty acids (SCFAs) | a, b, & c. Three stool samples collected on the same day of 3 consecutive weeks and were homogenised | a & b. 16S rRNA gene amplicon sequencing c. GC-MS/SPME | a. Non-significant but stronger positive association between eggs and Adlercreutzia and Coriobacteriaceae and negative association with Eubacterium and Lachnospiraceae c. Non-significant but stronger negative association between eggs and butanoate and propyl acetate | |
| Hamaya (2020) [46] | USA, n = 620, healthy middle-aged adult males, 67.7 ± 7.7 years, 25.5 (23.6–28.0) kg/m2 | 2011–2012 Men’s Lifestyle Validation Study | 1. Two 152-item FFQs completed at baseline and 6 months. Median (IQR) of egg intake according to FFQs: 0.43 (0.1–0.9) eggs/day 2. Two 7-day food diaries, completed at baseline and 6 months. Median (IQR) of egg intake according to 7-day food diaries: 0.39 (0.17–0.68) eggs/day | a. Plasma TMAO | a. Two fasted blood plasma samples 6 months apart | a. LCMS | a. ↑ significant positive association with egg intake when using FFQ, but not when using 7-day food diary | |
| James (2022) [47] | USA, n = 361, healthy adults, 39.9 (13.3) years, 27.0 (4.7) kg/m2, 48% males (age and BMI reported for n = 120 participants of TMAO tertile 2 as average age and BMI of total cohort not reported) | The Nutritional Phenotyping Study 2015–2019 | Three 24-h dietary recalls. Two on weekdays, one on a weekend. Categories of egg intake not defined. The evening prior to the study visit, they consumed a standardised meal, providing 280.7 mg of choline (included 80 g of eggs). | a. Plasma TMAO b. Plasma choline c. CRP d. Cytokines (TNF-α, IL-6) e. Gut microbiome composition f. α diversity—Shannon, Pielou’s evenness, observed species | a, b, c, & d. fasted blood plasma samples e & f. stool samples collected using sanitary collection supplies | a & b. LCMS c & d. Multiplex assay kits e & f. 16S rRNA gene amplicon sequencing | a. ∅ based on egg consumption b, c, d, e, & f. did not assess correlation with egg consumption | |
| Malinowska (2017) [48] | Poland, n = 122, healthy elderly females, 68.5 ± 7.4 years, 26.7 ± 4.1 kg/m2 | Participants recruited from the University of the Third Age and a public nursing home | 90 item-FFQ Categories of egg intake not defined. | a. Plasma TMA and TMAO b. Plasma-free choline | a & b. fasted blood plasma samples | a & b. LCMS | a. ∅ based on egg consumption b. ↑ significant positive association with egg intake | |
| Noh (2021) [49] | South Korea, n= 222, healthy adults, 29.6 (20–51) years, 22.9 (19.1–28.5) kg/m2, 49% males | National Institute of Agricultural Sciences of Korea and the International Agency for Research on Cancer (NAS-IARC) study, 2018 | 106-item semi-quantitative FFQ Categories of egg intake not defined. | a. Gut microbiome composition b. α diversity—Chao1, Shannon, Faith c. β diversity—Bray–Curtis, weighted and unweighted UniFrac | a, b, & c. stool samples collected on-site during the study visit. Stored in nucleic acid collection tubes. | a, b, & c. 16S rRNA gene amplicon sequencing. | a, b, & c. ∅ significant patterns noted based on egg consumption | |
| Renall (2023) [50] | New Zealand, n = 286, females (n = 125 Pacific Islander origin, n = 161 European origin), 28 (22, 35) years, 28.1 (23.0, 33.4) kg/m2 | PRedictors linking Obesity and gut MIcrobiomE (PROMISE) 2016–2017 | 1.5-day non-consecutive estimated food record (5DFR) 2. 220-item semi-quantitative FFQ (NZWFFQ) Egg intake of 60 g/day considered a higher intake | a. Gut microbiome composition b. α diversity—Pielou’s c. β diversity—Bray–Curtis, Jaccard | a & b. stool samples stored in participant home freezers for 11–14 days prior to storage at −80 °C | a, b, & c. Shotgun metagenomic sequencing | a, b, & c. ↑ habitual egg intake linked to microbiota profiles, including butyrate-producing species ↓ habitual egg intake linked to microbiota profile with more lactic acid producing species α diversity (Pielou’s) ↑ in individuals with higher habitual egg intake Associations between microbial composition/diversity and habitual egg intake displayed in Table 3 | |
| Rohrmann (2016) [51] | Germany, n = 271, healthy adults, 50 (37, 63) years, 26.1 (24.0–29.4) kg/m2 for males and 44 (36, 59) years, 25.2 (22.4, 28.9) kg/m2 for females, 38% males | 2002–2003 Bavarian Food Consumption Survey | Three 24-h dietary recalls Egg intake categorised as: 0 g/day 0.1< 17 g/day ≥17 g/day | a. Plasma TMAO b. Plasma choline c. CRP d. Cytokines (TNF-α, IL-6) | a & b. non-fasted blood plasma samples | a & b. LCMS | a & b. ∅ based on egg consumption c & d. did not assess correlation with egg consumption | |
| Yang (2021) [52] | USA, Europe, Asia, n = 32,166 healthy adults aged 19–84 (average age of total cohort not reported), BMI not reported, 39% males | TMAO Pooling Project. Pooled data from 16 international studies. | FFQs used in 14 studies (2 studies excluded from diet analysis) Average intake of eggs across studies: 0.2–0.8 eggs/day | a. Plasma TMAO | a. blood samples | a. Targeted and untargeted assays used across studies | a. ↑ significant positive association with egg intake (associated with 1 serving eggs/day) | |
| Prospective cohort trials | ||||||||
| Participant data sources/recruitment | Measurement of egg intake | Duration (sample collection timepoints) | ||||||
| Li (2022) [53] | USA, n = 307, healthy adult males (all health professionals), 71.9 years, 26.3 kg/m2 (only mean age and BMI reported for participants of TMAO quartile 3 as average age and BMI of total cohort not reported) | 2011–2012 Men’s Lifestyle Validation Study (MLVS) | 1. Two 152-item FFQs. First FFQ completed at baseline and second FFQ completed 12–13 months later. Median (IQR) of egg intake according to FFQs: 0.43 (0.14–0.93) eggs/day 2. Two 7-day food diaries. First conducted at baseline. Second at 6 months. Median (IQR) of egg intake according to 7-day food diaries: 0.4 (0.2–0.7) eggs/day | 13 months (baseline, 6 months) | a. Plasma TMAO b. Plasma choline c. Gut microbiome composition d. β-diversity—Bray-Curtis | a & b. two fasted blood plasma samples taken at each timepoint, 24–72 h apart (four in total) c & d. two stool samples taken at each timepoint, 24–72 h apart (four in total) | a & b. LCMS c & d. Shotgun metagenomic sequencing | a. ↑ significant positive association with egg intake when using FFQ, but not when using 7-day food diary b. ∅ c. Associations between microbial composition and habitual egg intake displayed in Table 3 d. did not assess correlation with egg consumption |
| Wang (2022) [54] | USA, n = 3931, older adults, 72.9 ± 5.6 years, BMI not reported, 37% males | 1989–2015 Cardiovascular Health Study (CHS). Participants recruited between 1989 and 1993. Followed up every 6 months until 2015. | 1. Baseline 99-item picture-sort FFQ 2. Semiquantitative FFQ at follow up in 1995–1996 Average intake of eggs at baseline: 0.2 ± 0.3 eggs/day | Median study period of 12.5 years (baseline, follow up in 1996–1997) | a. Plasma TMAO b. Plasma choline | a & b. fasted blood samples taken at baseline (1989–1993) and at follow up (1996–1997) | a & b. LCMS | a. ∅ at baseline b. ↑significant positive association at baseline Follow-up data used in mediation analyses for atherosclerotic cardiovascular disease risk |
| Non-randomised intervention trials | ||||||||
| Participant data sources/recruitment | Intervention | Duration | ||||||
| Asnicar (2021) [55] | UK and USA, n = 1098, healthy adults, 45.6 ± 11.9 years, 25.6 ± 5.0 kg/m2, 38% males in UK cohort and 41.3 years, BMI not reported, 32% males in USA cohort | Personalised Responses to Dietary Composition Trial (PREDICT) 2018–2019 | Baseline study visit (day 1): participants given ‘metabolic challenge meal’ (890 kcal, 86 g CHO, 53 g fat, 16 g protein, 2 g fibre) and ‘medium fat and carb lunch meal’ (500 kcal, 71 g CHO, 22 g fat, 10 g protein, 2 g fibre) Home-phase (days 2–14): participants given standardised test meals to consume for breakfast on all days and lunch on days 2 and 3, differing in macronutrient distribution Dietary intake during trial measured via mobile phone app. Habitual egg intake measured via FFQs (different tools used in UK vs. US). | 14 days | a. Gut microbiome composition b. α diversity—Shannon, observed species c. β diversity—Bray–Curtis | a. Stool samples collected at day 0 and day 14 using EasySampler stool collection kit in UK or FECOTAINER stool collection kit in USA | a. Shotgun metagenomic sequencing | a. associations between microbial composition and habitual egg intake displayed in Table 3 b & c. did not assess correlation with egg consumption |
| Di Marco (2017) [4] | USA, n = 36, healthy young adults, 24.1 ± 2.2 years, 24.3 ± 2.5 kg/m2, 50% males | n/a | Lead-in: 2 weeks Washout: 2 weeks Blinding: not described Group 1: 1 whole egg/day Group 2: 2 whole eggs/day Group 3: 3 whole eggs/day | 4 weeks | a. Plasma TMAO b. Plasma choline | a & b. blood plasma samples, unclear if fasting samples | a & b. LCMS | a. ∅ b. ↑ significantly with increasing egg intake in a dose-dependent manner |
| Zhang (1998) [56] | UK, n = 6, healthy males, 32 ± 5 years, BMI not reported | n/a | Participants fed a specific breakfast plus 227 g of each ‘test’ food group. Forty-six foods were tested. Each test day separated by 1 week washout period. Control phase: the specific breakfast without the addition of the ‘test’ food. | 8 h (post-prandial) | a. Urine TMAO | a. Urine samples collected over 0–8 h. Unclear how many urine samples were collected. | a. LCMS | a. ∅ |
| First Author (Year of Publication) | Study Design | Measurement of Egg Intake | Microbiota Quantification Technique | Diversity-Related Outcome (Diversity Metric) | Positively-Associated Bacteria (Genus/Species) | Negatively-Associated Bacteria (Genus/Species) |
|---|---|---|---|---|---|---|
| Asnicar (2021) [55] | Non-randomised intervention trial | FFQ | Shotgun metagenomic sequencing | α diversity (Shannon, observed species)—did not assess correlation with egg consumption β diversity (Bray–Curtis)—did not assess correlation with egg consumption | Eubacterium eligens Firmicutes bacterium CAG:95 Firmicutes bacterium CAG:170 | Bifidobacterium adolescentis Bifidobacterium catenulatum Bifidobacterium longum Cenarchaeum symbiosum Clostridium bolteae CAG:59 |
| Li (2022) [53] | Prospective cohort trial | FFQ 7-day food diary | Shotgun metagenomic sequencing | β diversity (Bray–Curtis)—did not assess correlation with egg consumption | Alistipes indistinctus Bacteroides intestinalis Bifidobacterium bifidum Streptococcus vestibularis | Alistipes putredinis Clostridium bolteae |
| Renall (2023) [50] | Cross-sectional | 5-day non-consecutive estimated food record 220-item semi-quantitative FFQ (NZWFFQ) | Shotgun metagenomic sequencing | α diversity (Pielou’s)—↑ in individuals with higher habitual egg intake β diversity (Bray–Curtis, Jaccard)—did not assess correlation with egg consumption | Akkermansia muciniphila Alistipes putredinis Collinsella aerofaciens Coprococcus sp. ART55 1 Eubacterium rectale Faecalibacterium prausnitzii Lactobacillus ruminis Ruminococcus bromii Subdoligranulum unclassified | Bifidobacterium adolescentis Bifidobacterium bifidum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, N.; Tuck, C.J.; Cheng, E.; Kellow, N.J.; Biesiekierski, J.R. The Impact of Egg Consumption on Gastrointestinal Health: A Systematic Literature Review and Meta-Analysis. Nutrients 2025, 17, 2059. https://doi.org/10.3390/nu17132059
Sultan N, Tuck CJ, Cheng E, Kellow NJ, Biesiekierski JR. The Impact of Egg Consumption on Gastrointestinal Health: A Systematic Literature Review and Meta-Analysis. Nutrients. 2025; 17(13):2059. https://doi.org/10.3390/nu17132059
Chicago/Turabian StyleSultan, Nessmah, Caroline J. Tuck, Edellyne Cheng, Nicole J. Kellow, and Jessica R. Biesiekierski. 2025. "The Impact of Egg Consumption on Gastrointestinal Health: A Systematic Literature Review and Meta-Analysis" Nutrients 17, no. 13: 2059. https://doi.org/10.3390/nu17132059
APA StyleSultan, N., Tuck, C. J., Cheng, E., Kellow, N. J., & Biesiekierski, J. R. (2025). The Impact of Egg Consumption on Gastrointestinal Health: A Systematic Literature Review and Meta-Analysis. Nutrients, 17(13), 2059. https://doi.org/10.3390/nu17132059






