d-Allulose Ameliorates Fructose-Induced Skeletal Muscle Insulin Resistance via Regulation of Ectopic Lipid Accumulation Independent of Body Weight Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Rats and Diets
2.2. Experimental Protocols
2.2.1. Hyperinsulinemic Euglycemic (HE)-Clamp Test
2.2.2. Intraperitoneal Glucose Tolerance Test (IPGTT) and Blood, Liver, and Muscle Sampling
2.2.3. Blood Glucose and Plasma Insulin
2.2.4. Intracellular Signaling Analysis in Skeletal Muscle Using Western Blotting
2.2.5. Triglyceride (TG) Measurement
2.3. Statistical Analysis
3. Results
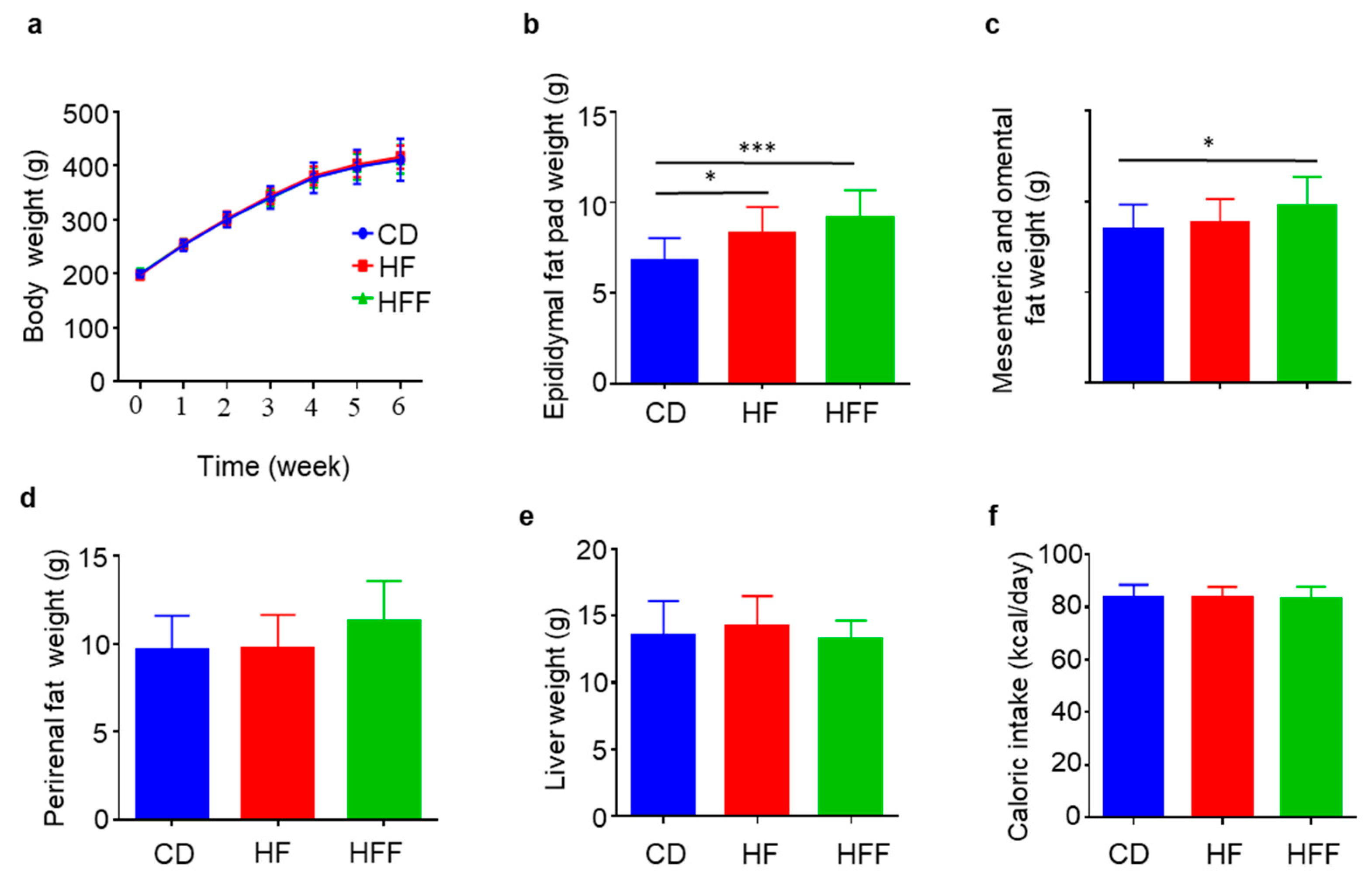
3.1. Increased Visceral Fat but Not Body Weights in the HFF Group
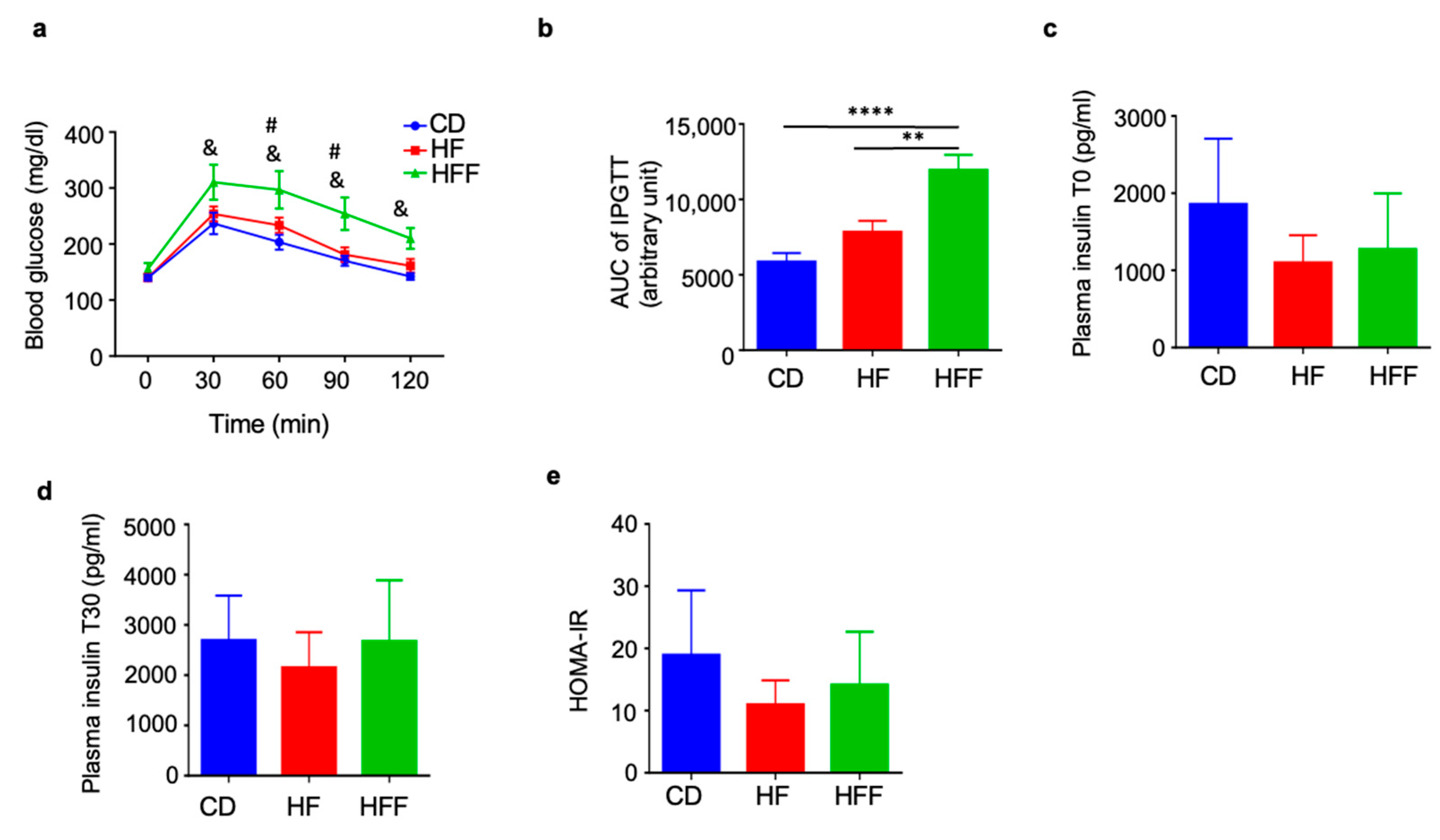
3.2. Elevated Blood Glucose Levels During IPGTT in the HFF Group
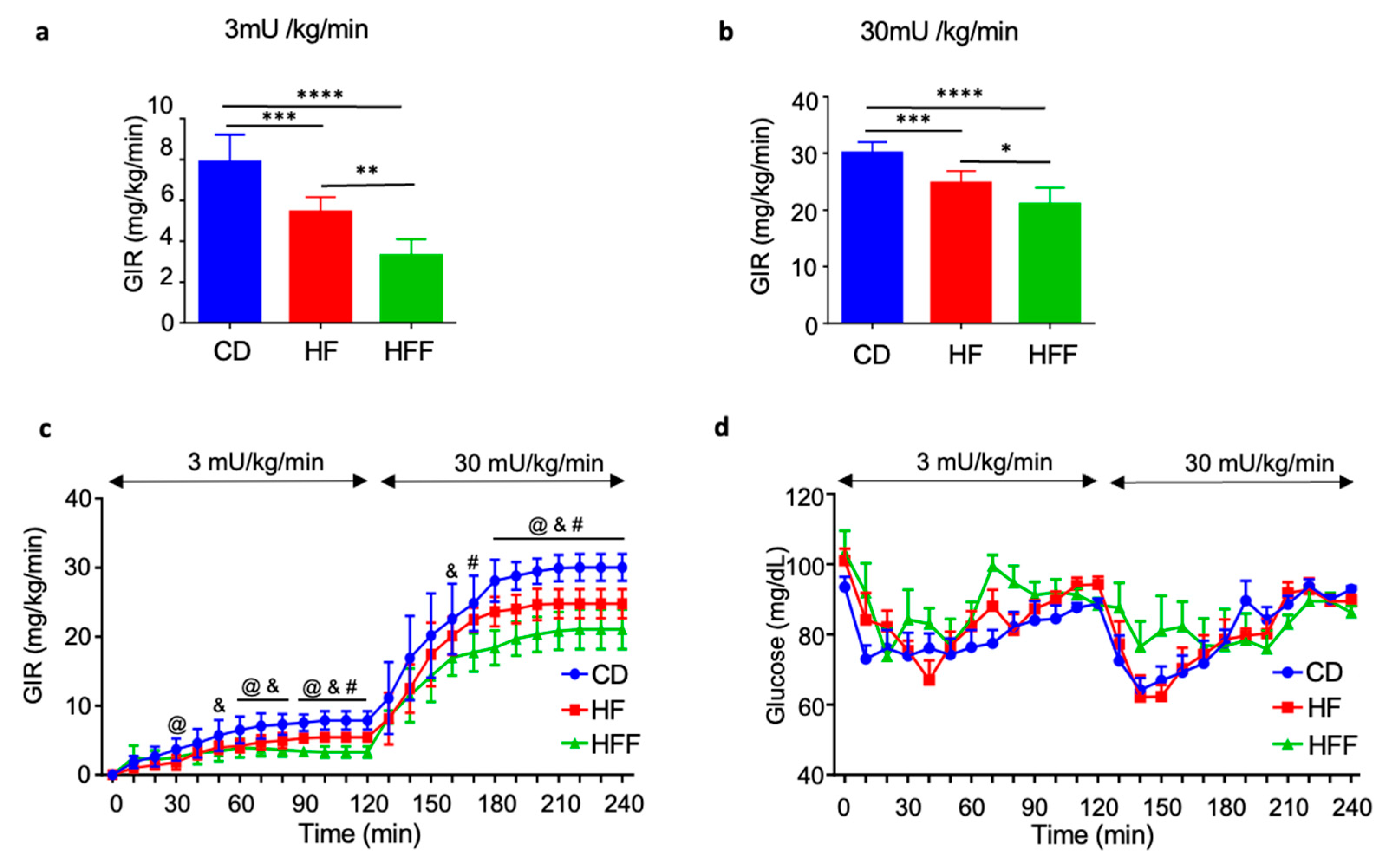
3.3. Muscle and Systemic Insulin Resistance in HE-Clamp Test
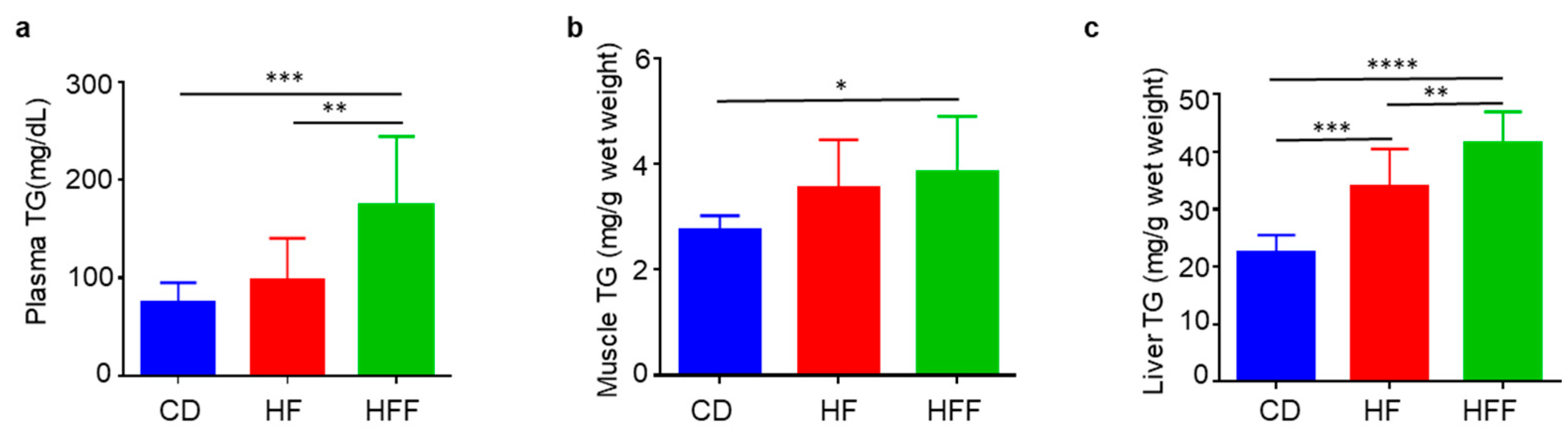
3.4. Changes in Blood, Muscle, and Liver TG
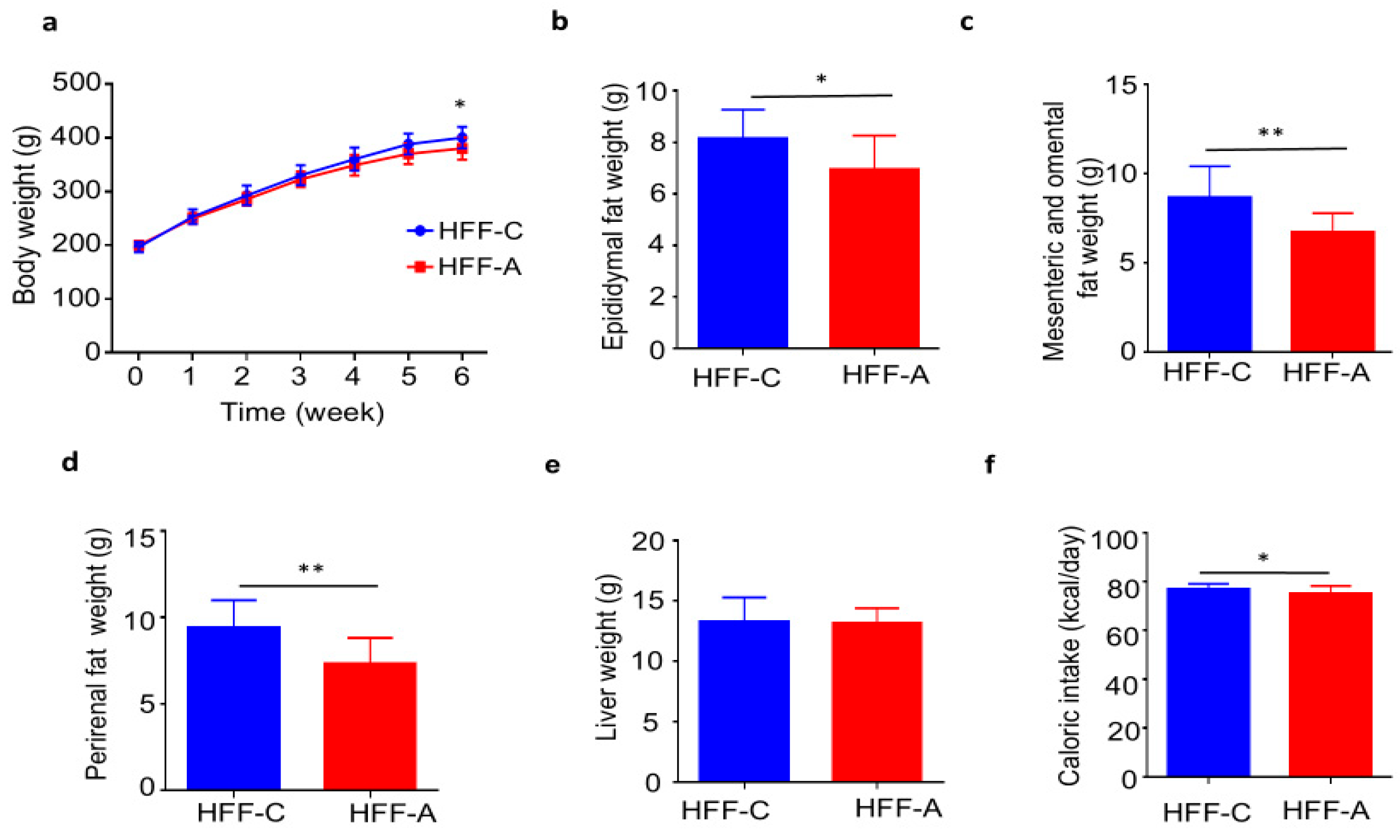
3.5. Effect of d-Allulose on Body Weights and Fat Weights in the HFF Group
3.6. Effect of d-Allulose on Blood Glucose in IPGTT in the HFF Group
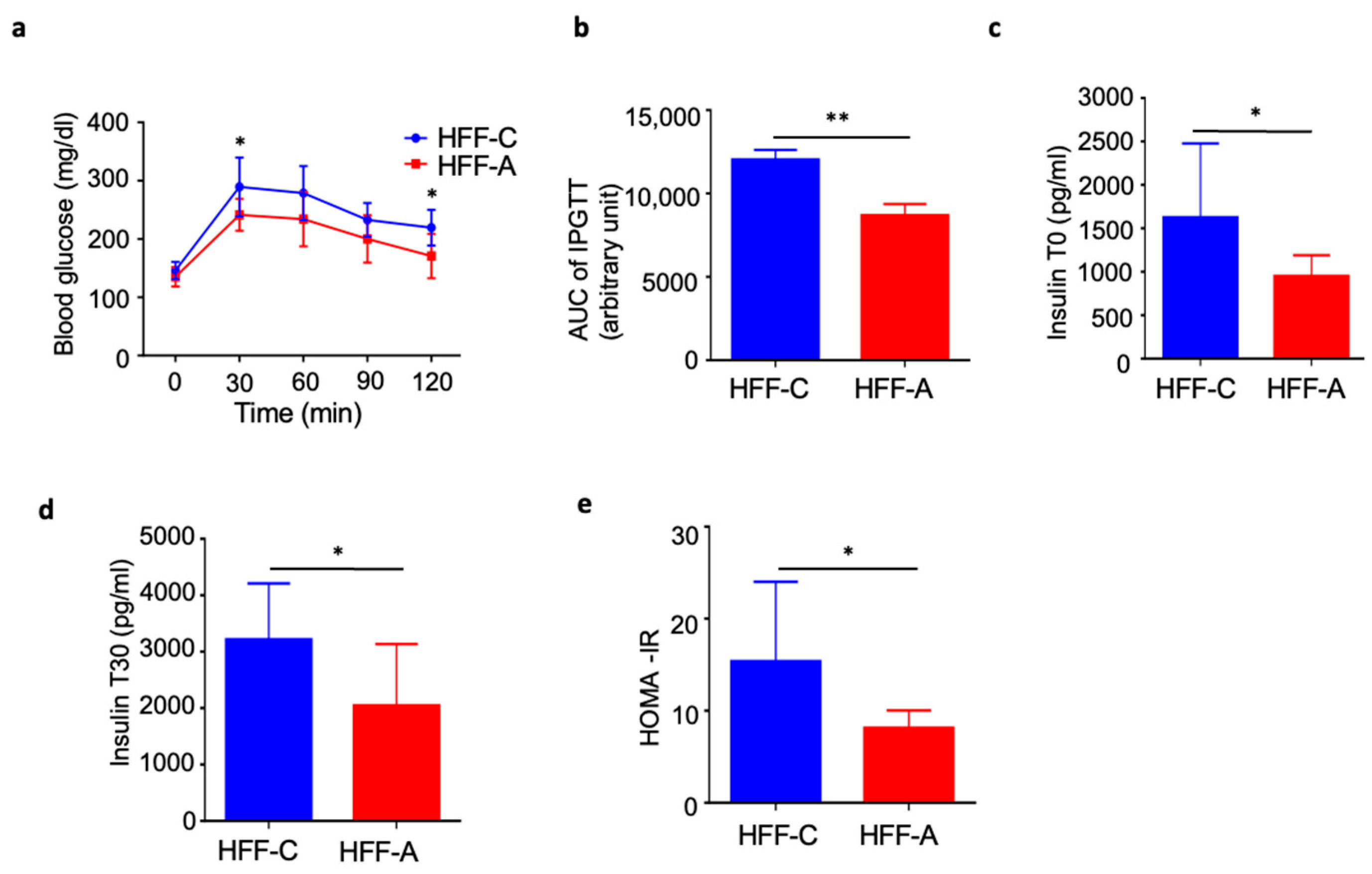
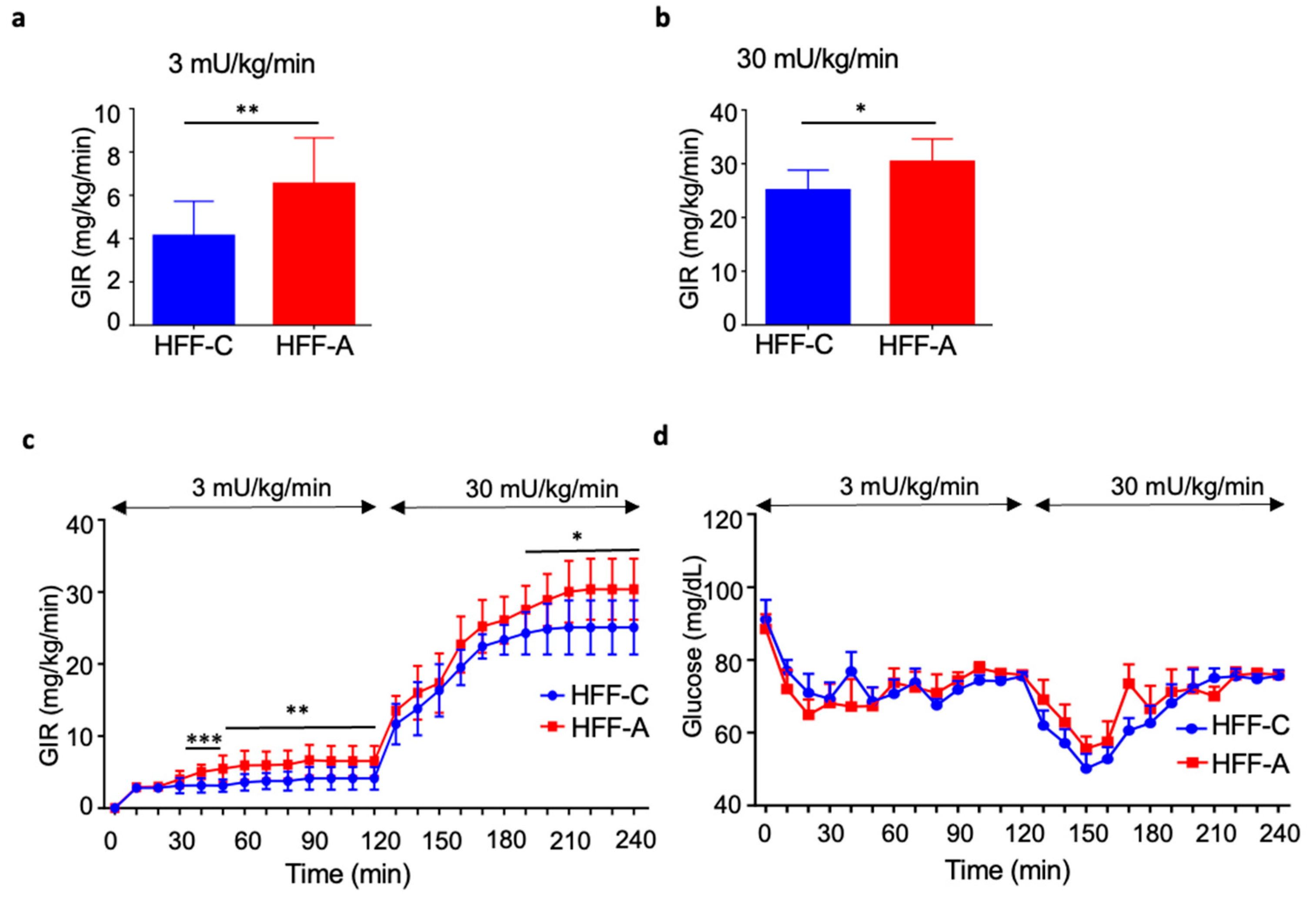
3.7. Effect of d-Allulose on Insulin Resistance in the HFF Group
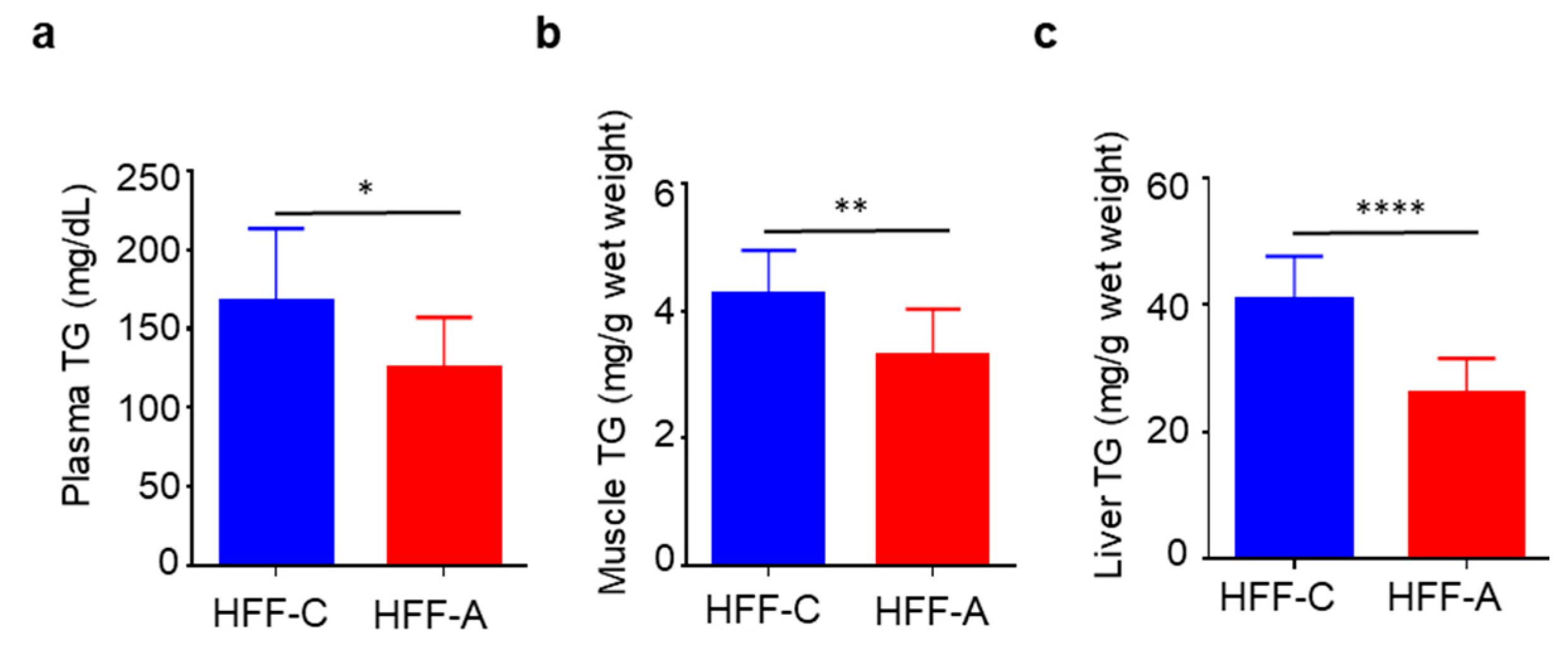
3.8. Effect of d-Allulose on Blood, Muscle, and Liver TG in the HFF Group
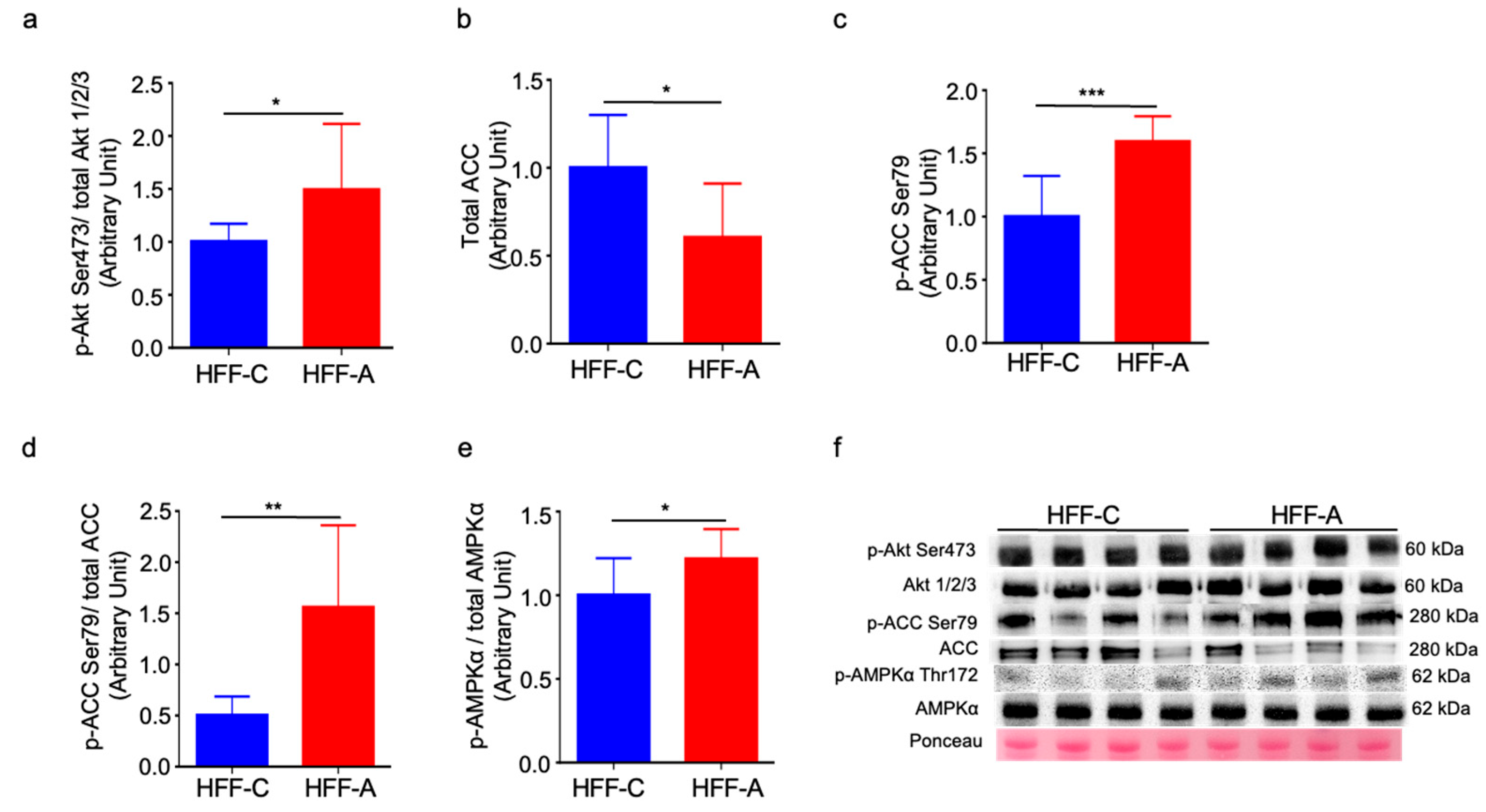
3.9. Effect of d-Allulose on Signaling Molecules in Skeletal Muscle
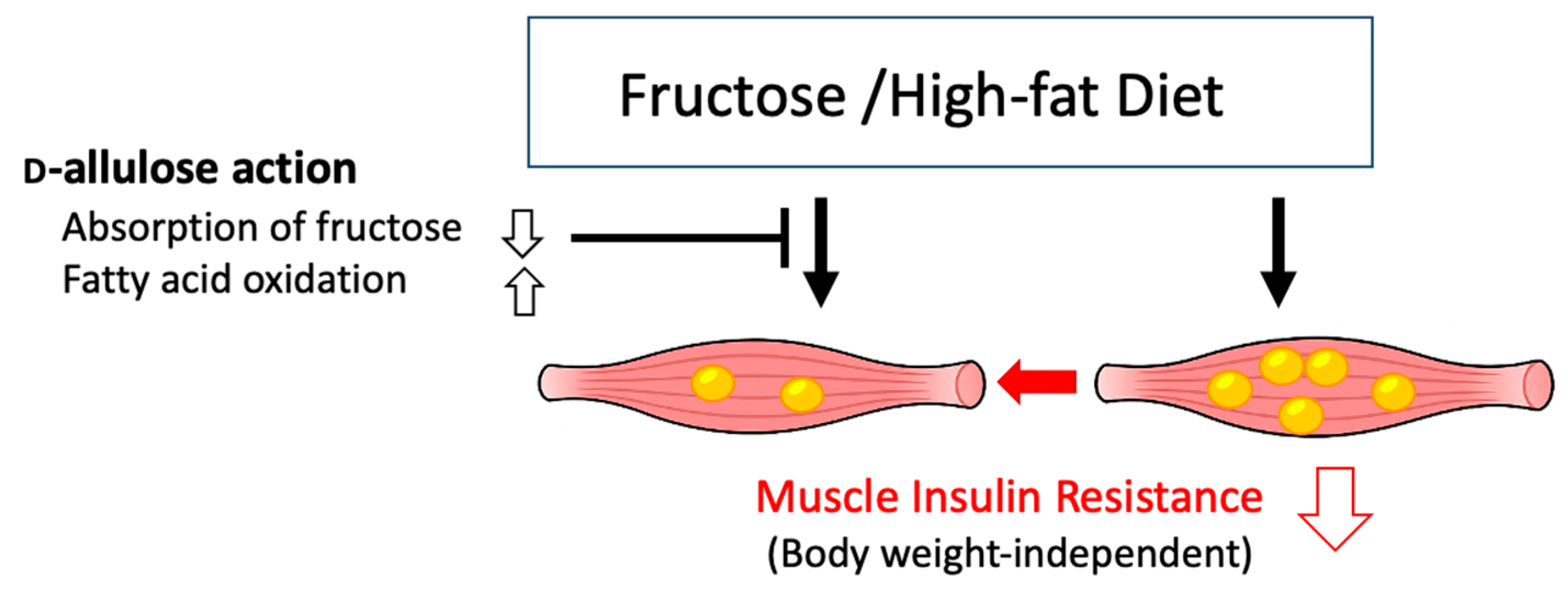
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Control diet |
| GIR | Glucose infusion rate |
| HF | High-fat |
| HFF | High-fat/fructose |
| HFF-A | High-fat/fructose-d-allulose |
| HFF-C | High-fat/fructose-cellulose |
| IPGTT | Intraperitoneal glucose tolerance test |
| TG | Triglyceride |
References
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Morio, B.; Fardet, A.; Legrand, P.; Lecerf, J.M. Involvement of dietary saturated fats, from all sources or of dairy origin only, in insulin resistance and type 2 diabetes. Nutr. Rev. 2016, 74, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Holm, J.B.; Sjøberg, K.A.; Bojsen-Møller, K.N.; Myrmel, L.S.; Fjære, E.; Jensen, B.A.H.; Nicolaisen, T.S.; Hingst, J.R.; Hansen, S.L.; et al. Mechanisms preserving insulin action during high dietary fat intake. Cell Metab. 2019, 29, 50–63.e4. [Google Scholar] [CrossRef]
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987, 10, 512–516. [Google Scholar] [CrossRef]
- Reaven, G.M.; Risser, T.R.; Chen, Y.D.; Reaven, E.P. Characterization of a model of dietary-induced hypertriglyceridemia in young, nonobese rats. J. Lipid Res. 1979, 20, 371–378. [Google Scholar] [CrossRef]
- Thorburn, A.W.; Storlien, L.H.; Jenkins, A.B.; Khouri, S.; Kraegen, E.W. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am. J. Clin. Nutr. 1989, 49, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, Q.; Mu, W.; Hu, X.; Sun, Z.; Qiu, Y.; Liu, X.; Wang, Z. Research advances of d-allulose: An overview of physiological functions, enzymatic biotransformation technologies, and production processes. Foods 2021, 10, 2186. [Google Scholar] [CrossRef]
- Ahmed, A.; Khan, T.A.; Dan Ramdath, D.; Kendall, C.W.C.; Sievenpiper, J.L. Rare sugars and their health effects in humans: A systematic review and narrative synthesis of the evidence from human trials. Nutr. Rev. 2022, 80, 255–270. [Google Scholar] [CrossRef]
- Fukunaga, K.; Yoshimura, T.; Imachi, H.; Kobayashi, T.; Saheki, T.; Sato, S.; Saheki, N.; Jiang, W.; Murao, K. A pilot study on the efficacy of a diabetic diet containing the rare sugar d-allulose in patients with type 2 diabetes mellitus: A prospective, randomized, single-blind, crossover study. Nutrients 2023, 15, 2802. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Tokuda, M.; Nishimoto, N.; Yokoi, H.; Izumori, K. Allulose for the attenuation of postprandial blood glucose levels in healthy humans: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0281150. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kwon, E.Y.; Yu, M.K.; Lee, S.J.; Kim, H.J.; Kim, S.B.; Kim, Y.H.; Choi, M.S. A preliminary study for evaluating the dose-dependent effect of d-allulose for fat mass reduction in adult humans: A randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Gou, Y.; Liu, B.; Cheng, M.; Yamada, T.; Iida, T.; Wang, S.; Banno, R.; Koike, T. d-Allulose ameliorates skeletal muscle insulin resistance in high-fat diet-fed rats. Molecules 2021, 26, 6310. [Google Scholar] [CrossRef]
- Natsume, Y.; Yamada, T.; Iida, T.; Ozaki, N.; Gou, Y.; Oshida, Y.; Koike, T. Investigation of d-allulose effects on high-sucrose diet-induced insulin resistance via hyperinsulinemic-euglycemic clamps in rats. Heliyon 2021, 7, e08013. [Google Scholar] [CrossRef]
- Liu, B.; Gou, Y.; Tsuzuki, T.; Yamada, T.; Iida, T.; Wang, S.; Banno, R.; Toyoda, Y.; Koike, T. d-Allulose improves endurance and recovery from exhaustion in male C57BL/6J mice. Nutrients 2022, 14, 404. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Suzuki, R.; Kajun, R.; Yamada, T.; Iida, T.; Liu, B.; Koike, T.; Toyoda, Y.; Negishi, T.; Yukawa, K. Combined effects of exercise training and d-allulose intake on endurance capacity in mice. Physiol. Rep. 2022, 10, e15297. [Google Scholar] [CrossRef]
- Oshida, Y.; Tachi, Y.; Morishita, Y.; Kitakoshi, K.; Fuku, N.; Han, Y.Q.; Ohsawa, I.; Sato, Y. Nitric oxide decreases insulin resistance induced by high-fructose feeding. Horm. Metab. Res. 2000, 32, 339–342. [Google Scholar] [CrossRef]
- Hughey, C.C.; Hittel, D.S.; Johnsen, V.L.; Shearer, J. Hyperinsulinemic-euglycemic clamp in the conscious rat. J. Vis. Exp. 2011, 48, 2432. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Western blotting. Methods 2006, 38, 283–293. [Google Scholar] [CrossRef]
- Takahashi, K.; Kitaoka, Y.; Hatta, H. Effects of endurance training on metabolic enzyme activity and transporter protein levels in the skeletal muscles of orchiectomized mice. J. Physiol. Sci. 2022, 72, 14. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Kumari, P.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of d-allulose. Nat. Commun. 2018, 9, 113. [Google Scholar] [CrossRef]
- Matsuo, T.; Izumori, K. Effects of dietary d-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 2006, 70, 2081–2085. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kitagaki, S.; Nakano, D.; Nishiyama, A.; Funamoto, Y.; Matsunaga, T.; Tsukamoto, I.; Yamaguchi, F.; Kamitori, K.; Dong, Y.; et al. Rare sugar d-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem. Biophys. Res. Commun. 2011, 405, 7–12. [Google Scholar] [CrossRef]
- Cayabyab, K.B.; Shin, M.J.; Heimuli, M.S.; Kim, I.J.; D’Agostino, D.P.; Johnson, R.J.; Koutnik, A.P.; Bellissimo, N.; Diamond, D.M.; Norwitz, N.G.; et al. The Metabolic and endocrine effects of a 12-week allulose-rich diet. Nutrients 2024, 16, 1821. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Ramsamooj, S.; Liang, R.J.; Katti, A.; Pozovskiy, R.; Vasan, N.; Hwang, S.-K.; Nahiyaan, N.; Francoeur, N.J.; Schatoff, E.M.; et al. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature 2021, 597, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Softic, S.; Gupta, M.K.; Wang, G.X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Investig. 2017, 127, 4059–4074, Erratum in: J. Clin. Investig. 2018, 128, 1199. [Google Scholar] [CrossRef]
- Chong, M.F.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [CrossRef]
- Kishida, K.; Martinez, G.; Iida, T.; Yamada, T.; Ferraris, R.P.; Toyoda, Y. d-Allulose is a substrate of glucose transporter type 5 (GLUT5) in the small intestine. Food Chem. 2019, 277, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, H.J.; Kim, A.H.; Choi, J.Y.; Cho, S.J.; Park, Y.B.; Jung, U.J.; Choi, M.S. d-Allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol. Nutr. Food Res. 2016, 60, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kanasaki, A.; Hayashi, N.; Yamada, T.; Iida, T.; Nagata, Y.; Okuma, K. d-Allulose enhances postprandial fat oxidation in healthy humans. Nutrition 2017, 43–44, 16–20. [Google Scholar] [CrossRef]
- Ochiai, M.; Onishi, K.; Yamada, T.; Iida, T.; Matsuo, T. d-psicose increases energy expenditure and decreases body fat accumulation in rats fed a high-sucrose diet. Int. J. Food Sci. Nutr. 2014, 65, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Kanasaki, A.; Tamaru, S.; Tanaka, K. d-psicose, an epimer of d-fructose, favorably alters lipid metabolism in Sprague-Dawley rats. J. Agric. Food Chem. 2015, 63, 3168–3176. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Lally, J.S.; Galic, S.; Thomas, M.; Azizi, P.D.; Fullerton, M.D.; Smith, B.K.; Pulinilkunnil, T.; Chen, Z.; Samaan, M.C.; et al. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia 2014, 57, 1693–1702. [Google Scholar] [CrossRef]
- Ingram, D.K.; Roth, G.S. Glycolytic inhibition: An effective strategy for developing calorie restriction mimetics. GeroScience 2021, 43, 1159–1169. [Google Scholar] [CrossRef]
- Shintani, H.; Shintani, T.; Ashida, H.; Sato, M. Calorie restriction mimetics: Upstream-type compounds for modulating glucose metabolism. Nutrients 2018, 10, 1821. [Google Scholar] [CrossRef]
- Gilbert, M. Role of skeletal muscle lipids in the pathogenesis of insulin resistance of obesity and type 2 diabetes. J. Diabetes Investig. 2021, 12, 1934–1941. [Google Scholar] [CrossRef]
- Stannard, S.R.; Johnson, N.A. Insulin resistance and elevated triglyceride in muscle: More important for survival than “thrifty” genes? J. Physiol. 2004, 554, 595–607. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Chen, N.; Shi, X.; Tsang, B.; Yu, Y.H. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Investig. 2007, 117, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, S.; Gou, Y.; Tsuzuki, T.; Fu, L.; Yamada, T.; Banno, R.; Koike, T. d-Allulose Ameliorates Fructose-Induced Skeletal Muscle Insulin Resistance via Regulation of Ectopic Lipid Accumulation Independent of Body Weight Changes. Nutrients 2025, 17, 2050. https://doi.org/10.3390/nu17122050
Kamal S, Gou Y, Tsuzuki T, Fu L, Yamada T, Banno R, Koike T. d-Allulose Ameliorates Fructose-Induced Skeletal Muscle Insulin Resistance via Regulation of Ectopic Lipid Accumulation Independent of Body Weight Changes. Nutrients. 2025; 17(12):2050. https://doi.org/10.3390/nu17122050
Chicago/Turabian StyleKamal, Shahriar, Yang Gou, Takamasa Tsuzuki, Linlin Fu, Takako Yamada, Ryoichi Banno, and Teruhiko Koike. 2025. "d-Allulose Ameliorates Fructose-Induced Skeletal Muscle Insulin Resistance via Regulation of Ectopic Lipid Accumulation Independent of Body Weight Changes" Nutrients 17, no. 12: 2050. https://doi.org/10.3390/nu17122050
APA StyleKamal, S., Gou, Y., Tsuzuki, T., Fu, L., Yamada, T., Banno, R., & Koike, T. (2025). d-Allulose Ameliorates Fructose-Induced Skeletal Muscle Insulin Resistance via Regulation of Ectopic Lipid Accumulation Independent of Body Weight Changes. Nutrients, 17(12), 2050. https://doi.org/10.3390/nu17122050





