Post-Exercise Whey Protein Supplementation: Effects on IGF-1, Strength, and Body Composition in Pre-Menopausal Women, a Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Procedures
2.2.1. Screening
2.2.2. Intervention—Supplementation
2.2.3. Intervention—Resistance Training Protocol
2.2.4. Intervention—Interval Training Protocol
2.3. Measurements
Total IGF-1 Analysis (Primary Outcome)
2.4. Dietary Food Records
2.5. Body Composition
2.6. Strength Testing
2.7. Statistical Analyses
2.7.1. A Priori Sample Size Calculation
2.7.2. Analyses
3. Results
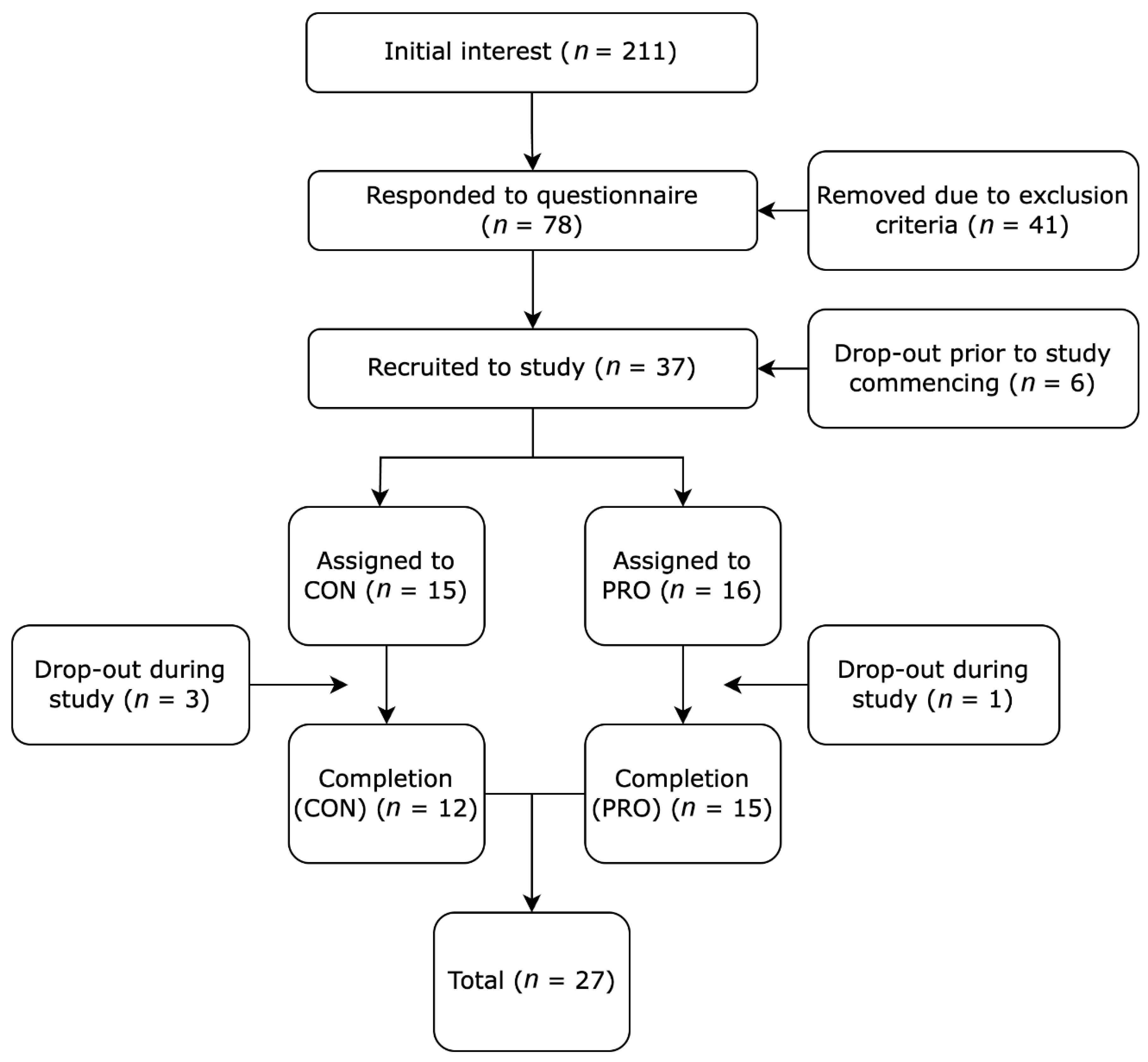
3.1. Participant Screening
3.2. Adherence
3.3. Participant Characteristics
3.4. IGF-1
3.5. Dietary Intake
3.6. Body Composition
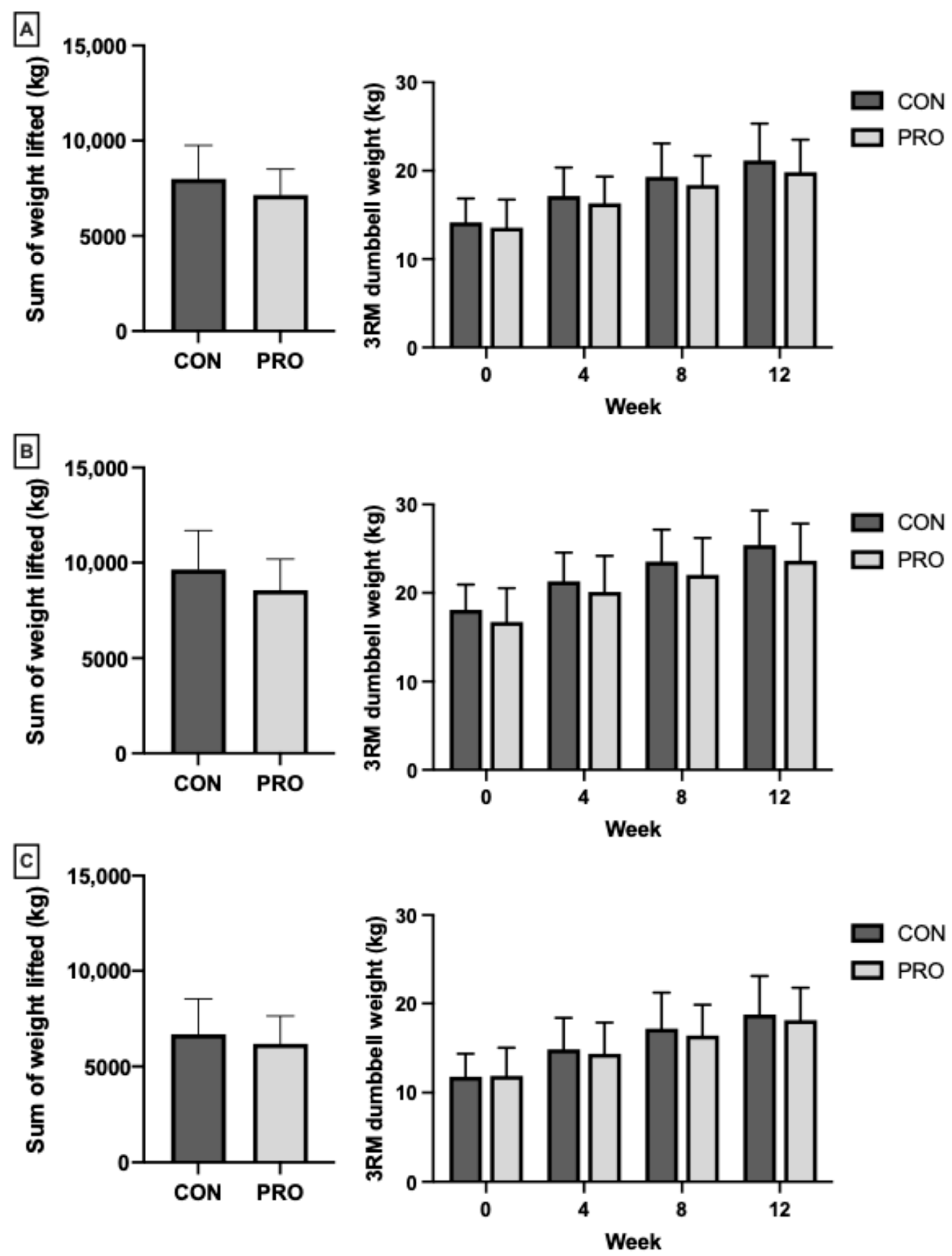
3.7. Strength and Training Volume
3.8. Sensitivity Analyses
4. Discussion
4.1. IGF-1
4.2. Diet
4.3. Body Composition
4.4. Strength
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith-Ryan, A.E.; Cabre, H.E.; Moore, S.R. Active Women Across the Lifespan: Nutritional Ingredients to Support Health and Wellness. Sports Med. 2022, 52, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.W.; Sonnier, J.H.; Johnson, E.E.; Hall, A.T.; Osman, A.; Connors, G.M.; Freedman, K.B.; Bishop, M.E. Inequalities in the Evaluation of Male Versus Female Athletes in Sports Medicine Research: A Systematic Review. Am. J. Sports Med. 2023, 51, 3335–3342. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Areta, J.; Coffey, V.G.; Stellingwerff, T.; Phillips, S.M.; Burke, L.M.; Cléroux, M.; Godin, J.-P.; Hawley, J.A. Daytime pattern of post-exercise protein intake affects whole-body protein turnover in resistance-trained males. Nutr. Metab. 2012, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.W.; Tang, J.E.; Wilkinson, S.B.; Tarnopolsky, M.A.; Lawrence, R.L.; Fullerton, A.V.; Phillips, S.M. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am. J. Clin. Nutr. 2007, 86, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol.-Endocrinol. Metab. 1997, 273, E122–E129. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am. J. Clin. Nutr. 2014, 99, 237S–242S. [Google Scholar] [CrossRef]
- Joanisse, S.; McKendry, J.; Lim, C.; Nunes, E.A.; Stokes, T.; McLeod, J.C.; Phillips, S.M. Understanding the effects of nutrition and post-exercise nutrition on skeletal muscle protein turnover: Insights from stable isotope studies. Clin. Nutr. Open Sci. 2021, 36, 56–77. [Google Scholar] [CrossRef]
- Wang, X.; Proud, C.G. The mTOR Pathway in the Control of Protein Synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Nakayama, K.; Fukasawa, T.; Koga, J.; Kanegae, M.; Kawanaka, K.; Higuchi, M. Post-exercise whey protein hydrolysate supplementation induces a greater increase in muscle protein synthesis than its constituent amino acid content. Br. J. Nutr. 2013, 110, 981–987. [Google Scholar] [CrossRef]
- Melick, C.H.; Jewell, J.L. Regulation of mTORC1 by Upstream Stimuli. Genes 2020, 11, 989. [Google Scholar] [CrossRef]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Liu, J.-L.; Fernandez, A.M.; Wu, Y.; Schally, A.V.; Frystyk, J.; Chernausek, S.D.; Mejia, W.; Le Roith, D. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes 2001, 50, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.; Szewczyk, A.; Kiszałkiewicz, J.; Migdalska-Sęk, M.; Domańska-Senderowska, D.; Brzeziański, M.; Lulińska, E.; Jegier, A.; Brzeziańska-Lasota, E. Type of training has a significant influence on the GH/IGF-1 axis but not on regulating miRNAs. Biol. Sport 2020, 37, 217–228. [Google Scholar] [CrossRef]
- Sullivan, B.P.; Weiss, J.A.; Nie, Y.; Garner, R.T.; Drohan, C.J.; Kuang, S.; Stout, J.; Gavin, T.P. Skeletal muscle IGF-1 is lower at rest and after resistance exercise in humans with obesity. Eur. J. Appl. Physiol. 2020, 120, 2835–2846. [Google Scholar] [CrossRef]
- Arazi, H.; Babaei, P.; Moghimi, M.; Asadi, A. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021, 21, 50. [Google Scholar] [CrossRef]
- Pierce, J.R.; Martin, B.J.; Rarick, K.R.; Alemany, J.A.; Staab, J.S.; Kraemer, W.J.; Hymer, W.C.; Nindl, B.C. Growth Hormone and Insulin-like Growth Factor-I Molecular Weight Isoform Responses to Resistance Exercise Are Sex-Dependent. Front. Endocrinol 2020, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lou, K.; Hou, L.; Lu, Y.; Sun, L.; Tan, S.C.; Low, T.Y.; Kord-Varkaneh, H.; Pang, S. The effect of resistance training on serum insulin-like growth factor 1(IGF-1): A systematic review and meta-analysis. Complement. Ther. Med. 2020, 50, 102360. [Google Scholar] [CrossRef] [PubMed]
- Rosendal, L.; Langberg, H.; Flyvbjerg, A.; Frystyk, J.; Ørskov, H.; Kjær, M. Physical capacity influences the response of insulin-like growth factor and its binding proteins to training. J. Appl. Physiol. 2002, 93, 1669–1675. [Google Scholar] [CrossRef]
- Arnarson, A.; Gudny Geirsdottir, O.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I. Insulin-Like Growth Factor-1 and Resistance Exercise in Community Dwelling Old Adults. J. Nutr. Health Aging 2015, 19, 856–860. [Google Scholar] [CrossRef]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef]
- Orsatti, F.L.; Nahas, E.A.P.; Maesta, N.; Nahas-Neto, J.; Burini, R.C. Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas 2008, 59, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Matsubara, T.; Tobina, T.; Shindo, M.; Tokuyama, K.; Tanaka, K.; Tanaka, H. Effect of low-intensity aerobic exercise on insulin-like growth factor-I and insulin-like growth factor-binding proteins in healthy men. Int. J. Endocrinol. 2010, 2010, 452820. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, Y.; Gong, F.; Shan, G.; Yang, H.; Xu, K.; Zhang, D.; Cheng, X.; Zhang, Z.; Chen, S.; et al. Reference ranges for serum insulin-like growth factor I (IGF-I) in healthy Chinese adults. PLoS ONE 2017, 12, e0185561. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.L.P.; Clapper, J.A.; Specker, B.L.; Binkley, T.L.; Vukovich, M.D. Effect of protein supplementation during a 6-mo strength and conditioning program on insulin-like growth factor I and markers of bone turnover in young adults1–3. Am. J. Clin. Nutr. 2005, 81, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.M.; Spiering, B.A.; Alemany, J.A.; Tuckow, A.P.; Rarick, K.R.; Staab, J.S.; Hatfield, D.L.; Kraemer, W.J.; Maresh, C.M.; Nindl, B.C. Exercise-induced insulin-like growth factor I system concentrations after training in women. Med. Sci. Sports Exerc. 2013, 45, 420–428. [Google Scholar] [CrossRef]
- Gunbatar, N.; Kaya, M.S.; Kahraman, T.; Bayiroglu, F. Paradoxical Advantage of Middle Aged Sedentary over Young Sedentary on Starting Exercise in Terms of GH/IGF-1 System. J. Appl. Biol. Sci. 2018, 12, 21–25. [Google Scholar]
- Eliakim, A.; Brasel, J.A.; Mohan, S.; Barstow, T.J.; Berman, N.; Cooper, D.M. Physical fitness, endurance training, and the growth hormone-insulin-like growth factor I system in adolescent females. J. Clin. Endocrinol. Metab. 1996, 81, 3986–3992. [Google Scholar] [CrossRef]
- Azadi, B.; BolBoli, L.; Khani, M.; Siyahkohyan, M.; Pourrahim, A. Comparison of the Effect of Eight Weeks of Continuous and High Intensity Interval Training on GH/IGF-1 Serum Indices and Aerobic performance of Active Young Males. J. Sport Biosci. 2022, 14, 101–118. [Google Scholar]
- Avazpour, S.; Kalkhoran, J.F.; Avazpour, K.; Mohseni, F. The effect of two types of high-intensity interval training on serum value of GH and IGF-1 in overweight nurses. Asian J. Sports Med. 2020, 11, e103135. [Google Scholar] [CrossRef]
- Hejazi, S.M. Effects of high intensity interval training on plasma levels of GH and IGF-I. Int. J. Med. Res. Health Sci. 2017, 6, 55–59. [Google Scholar]
- Nijenhuis-Noort, E.C.; Berk, K.A.; Neggers, S.J.; van der Lely, A.J. The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin. Endocrinol. Metab. 2024, 39, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Brüngger, M.; Hulter, H.N.; Krapf, R. Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: New cause of growth hormone insensitivity in humans. Kidney Int. 1997, 51, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Nindl, B.C.; Alemany, J.A.; Tuckow, A.P.; Rarick, K.R.; Staab, J.S.; Kraemer, W.J.; Maresh, C.M.; Spiering, B.A.; Hatfield, D.L.; Flyvbjerg, A. Circulating bioactive and immunoreactive IGF-I remain stable in women, despite physical fitness improvements after 8 weeks of resistance, aerobic, and combined exercise training. J. Appl. Physiol. 2010, 109, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sporer, B.C.; Wenger, H.A. Effects of aerobic exercise on strength performance following various periods of recovery. J. Strength Cond. Res. 2003, 17, 638–644. [Google Scholar] [PubMed]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Training and Conditioning; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Epley, B. Poundage Chart. Boyd Epley Workout; Body Enterprises: Lincoln, NE, USA, 1985; p. 86. [Google Scholar]
- DiStasio, T.J. Validation of the Brzycki and Epley Equations for the 1 Repetition Maximum Back Squat Test in Division I College Football Players. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2014. [Google Scholar]
- Ives, S.J.; Norton, C.; Miller, V.; Minicucci, O.; Robinson, J.; O’Brien, G.; Escudero, D.; Paul, M.; Sheridan, C.; Curran, K.; et al. Multi-modal exercise training and protein-pacing enhances physical performance adaptations independent of growth hormone and BDNF but may be dependent on IGF-1 in exercise-trained men. Growth Horm. IGF Res. 2017, 32, 60–70. [Google Scholar] [CrossRef]
- Georgiev, G. Sample Size Calculator. Available online: https://www.gigacalculator.com/calculators/power-sample-size-calculator.php (accessed on 25 November 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing; The R Core Team: Vienna, Austria, 2025. [Google Scholar]
- O’Connor, K.G.; Tobin, J.D.; Harman, S.M.; Plato, C.C.; Roy, T.A.; Sherman, S.S.; Blackman, M.R. Serum Levels of Insulin-like Growth Factor-I Are Related to Age and Not to Body Composition in Healthy Women and Men. J. Gerontol. Ser. A 1998, 53A, M176–M182. [Google Scholar] [CrossRef]
- Nasir, Y.; Hoseinipouya, M.R.; Eshaghi, H.; Rahimi, M.H. The impact of exercise on growth factors in postmenopausal women: A systematic review and meta-analysis. BMC Womens Health 2024, 24, 396. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Montesanto, A.; Giovannucci, E.; Zand, H.; Barati, M.; Kopchick, J.J.; Mirisola, M.G.; Lagani, V.; Bawadi, H.; Vardavas, R.; et al. Association between IGF-1 levels ranges and all-cause mortality: A meta-analysis. Aging Cell 2022, 21, e13540. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Johnson, W.D.; Rood, J.; Heaton, A.L.; Greenway, F.L. Increased Human Growth Hormone After Oral Consumption of an Amino Acid Supplement: Results of a Randomized, Placebo-Controlled, Double-Blind, Crossover Study in Healthy Subjects. Am. J. Ther. 2020, 27, e333–e337. [Google Scholar] [CrossRef] [PubMed]
- Iresjö, B.M.; Diep, L.; Lundholm, K. Initiation of muscle protein synthesis was unrelated to simultaneously upregulated local production of IGF-1 by amino acids in non-proliferating L6 muscle cells. PLoS ONE 2022, 17, e0270927. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, J.E.; Wardle, S.L.; O’Leary, T.J.; Greeves, J.P.; Cegielski, J.; Bass, J.; Brook, M.S.; Wilkinson, D.J.; Smith, K.; Atherton, P.J.; et al. Protein dose requirements to maximize skeletal muscle protein synthesis after repeated bouts of resistance exercise in young trained women. Scand. J. Med. Sci. Sports 2023, 33, 2470–2481. [Google Scholar] [CrossRef]
- Nemet, D.; Connolly, P.H.; Pontello-Pescatello, A.M.; Rose-Gottron, C.; Larson, J.K.; Galassetti, P.; Cooper, D.M. Negative energy balance plays a major role in the IGF-I response to exercise training. J. Appl. Physiol. 2004, 96, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Rarick, K.R.; Pikosky, M.A.; Grediagin, A.; Smith, T.J.; Glickman, E.L.; Alemany, J.A.; Staab, J.S.; Young, A.J.; Nindl, B.C. Energy flux, more so than energy balance, protein intake, or fitness level, influences insulin-like growth factor-I system responses during 7 days of increased physical activity. J. Appl. Physiol. 2007, 103, 1613–1621. [Google Scholar] [CrossRef]
- Hida, A.; Hasegawa, Y.; Mekata, Y.; Usuda, M.; Masuda, Y.; Kawano, H.; Kawano, Y. Effects of egg white protein supplementation on muscle strength and serum free amino acid concentrations. Nutrients 2012, 4, 1504–1517. [Google Scholar] [CrossRef]
- Mallard, A.R.; McLay-Cooke, R.T.; Rehrer, N.J. Protein supplements: Do they alter dietary intakes? Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 333–340. [Google Scholar] [CrossRef]
- Hagobian, T.A.; Sharoff, C.G.; Stephens, B.R.; Wade, G.N.; Silva, J.E.; Chipkin, S.R.; Braun, B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R233–R242. [Google Scholar] [CrossRef]
- Cornier, M.A.; Salzberg, A.K.; Endly, D.C.; Bessesen, D.H.; Tregellas, J.R. Sex-based differences in the behavioral and neuronal responses to food. Physiol. Behav. 2010, 99, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Horvath, T.L. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E817–E826. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- Martens, E.A.; Lemmens, S.G.; Westerterp-Plantenga, M.S. Protein leverage affects energy intake of high-protein diets in humans. Am. J. Clin. Nutr. 2013, 97, 86–93. [Google Scholar] [CrossRef]
- Gosby, A.K.; Conigrave, A.D.; Raubenheimer, D.; Simpson, S.J. Protein leverage and energy intake. Obes. Rev. 2014, 15, 183–191. [Google Scholar] [CrossRef]
- Cermak, N.M.; de Groot, L.C.; Saris, W.H.; Van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. The effect of protein timing on muscle strength and hypertrophy: A meta-analysis. J. Int. Soc. Sports Nutr. 2013, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Willingham, B.D.; Marchant, T.; Binkley, T.L.; Specker, B.L.; Vukovich, M.D. Protein supplementation during a 6-month concurrent training program: Effect on body composition and muscular strength in sedentary individuals. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Josse, A.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Body composition and strength changes in women with milk and resistance exercise. Med. Sci. Sports Exerc. 2010, 42, 1122–1130. [Google Scholar] [CrossRef]
- Arciero, P.J.; Ives, S.J.; Norton, C.; Escudero, D.; Minicucci, O.; O’Brien, G.; Paul, M.; Ormsbee, M.J.; Miller, V.; Sheridan, C. Protein-pacing and multi-component exercise training improves physical performance outcomes in exercise-trained women: The PRISE 3 study. Nutrients 2016, 8, 332. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.L.; Staron, R.S.; Phillips, S.M. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J. Physiol. 2005, 568, 283–290. [Google Scholar] [CrossRef]
- Timmons, J.A. Variability in training-induced skeletal muscle adaptation. J. Appl. Physiol. 2011, 110, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.D.; Marshall, P.W.; Halaki, M.; Hackett, D.A. The Effect of Resistance Training in Women on Dynamic Strength and Muscular Hypertrophy: A Systematic Review with Meta-analysis. Sports Med. 2020, 50, 1075–1093. [Google Scholar] [CrossRef]
- Campbell, B.I.; Aguilar, D.; Conlin, L.; Vargas, A.; Schoenfeld, B.J.; Corson, A.; Gai, C.; Best, S.; Galvan, E.; Couvillion, K. Effects of high versus low protein intake on body composition and maximal strength in aspiring female physique athletes engaging in an 8-week resistance training program. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 580–585. [Google Scholar] [CrossRef]
- Lamont, L.S.; Lemon, P.; Bruot, B.C. Menstrual cycle and exercise effects on protein catabolism. Med. Sci. Sports Exerc. 1987, 19, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Kriengsinyos, W.; Wykes, L.J.; Goonewardene, L.A.; Ball, R.O.; Pencharz, P.B. Phase of menstrual cycle affects lysine requirement in healthy women. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E489–E496. [Google Scholar] [CrossRef]
- Roberts, B.M.; Nuckols, G.; Krieger, J.W. Sex Differences in Resistance Training: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2020, 34, 1448–1460. [Google Scholar] [CrossRef]
- Ravelli, M.N.; Schoeller, D.A. Traditional Self-Reported Dietary Instruments Are Prone to Inaccuracies and New Approaches Are Needed. Front. Nutr. 2020, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Delafontaine, P.; Song, Y.-H.; Li, Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
| CON | PRO | |
|---|---|---|
| Serving size (g) | 31.1 | 32.0 |
| Energy (kJ) | 510.4 | 510.4 |
| Protein (g) | 2.7 | 24 |
| Carbohydrate (g) | 21.7 | 1.7 |
| Fat (g) | 3.4 | 1.8 |
| Block 1 | Testing | Block 2 | Testing | Block 3 | |
|---|---|---|---|---|---|
| Sets | 3 | X | 3 | X | 4 |
| Repetitions | 10 | X | 10 | X | 8 |
| Load (%1RM) | 75 | X | 75 | X | 80 |
| Rest (s) | 150 | X | 150 | X | 180 |
| Block | Intervals * | Warm-Up | Work: Rest | Warm-Down | RPE Work: Rest |
|---|---|---|---|---|---|
| Week 1 & 2 | 6 | 5 min | 60:90 s | 5 min | 10:2 |
| Week 3 | 6 | 5 min | 60:60 s | 5 min | 10:2 |
| T0 | T12 | Mean Difference | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| CON (n = 12) | PRO (n = 15) | CON (n = 12) | PRO (n = 15) | T12 | Group | Time | Interaction | |
| Age (y) | 32.8 (9.7) | 34.2 (9.1) | ||||||
| Height (cm) | 167.3 (2.2) | 163.3 (8.0) | ||||||
| Body mass (kg) | 75.3 (7.5) | 64.2 (14.5) | 75.7 (6.9) | 64.4 (13.7) | −0.28 (−1.52, 0.97) | 0.021 | 0.189 | 0.975 |
| BMI (kg/m2) | 27.1 (3.1) | 24.0 (3.9) | 27.2 (2.9) | 24.1 (3.8) | −0.07 (−0.48, 0.33) | 0.033 | 0.760 | 0.961 |
| Total IGF-1 (µg/L) | 203 (67.9) | 178 (61.1) | 197 (67.4) | 176 (43.0) | 1.38 (−26.6, 29.4) | 0.280 | 0.923 | 0.920 |
| T0 | T4–T12 | Mean Difference | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| LP (n = 11) | HP (n = 13) | LP (n = 11) | HP (n = 13) | Group | Time | Interaction | ||
| Energy intake (kJ) | 7742 (1361) | 6868 (1743) | 7867 (1092) | 7205 (1133) | 211 (−1001, 1425) | 0.122 | 0.439 | 0.721 |
| Protein intake (g) | 79 (12) | 70 (20) | 84 (12) | 91 (15) | 16 (2, 29) | 0.845 | <0.001 | 0.025 * |
| Carbohydrate intake (g) | 176 (65) | 157 (55) | 190 (43) | 155 (56) | −15 (−55, 24) | 0.200 | 0.526 | 0.432 |
| Fat intake (g) | 83 (19) | 75 (30) | 83 (23) | 70 (16) | −4 (−24, 16) | 0.209 | 0.570 | 0.670 |
| Relative protein intake (g/kg BW) | 1.1 (0.2) | 1.2 (0.4) | 1.1 (0.2) | 1.5 (0.3) | 0.27 (0.07, 0.47) | 0.040 | <0.001 | 0.011 * |
| Relative protein intake (g/kg lean) | 1.8 (0.2) | 1.9 (0.6) | 1.9 (0.3) | 2.3 (0.5) | 0.43 (0.09, 0.77) | 0.150 | 0.003 | 0.016 * |
| T0 | T12 | Mean Difference | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| CON (n = 12) | PRO (n = 15) | CON (n = 11) | PRO (n = 15) | Group | Time | Interaction | ||
| Lean mass (kg): | ||||||||
| Total | 43.3 (3.2) | 40.2 (7.1) | 44.3 (3.1) | 40.7 (6.2) | 0.46 (−0.54, 1.46) | 0.028 | 0.009 | 0.75 |
| Upper body | 25.1 (1.9) | 23.2 (4.0) | 25.6 (2.0) | 23.5 (3.6) | 0.39 (−0.17), 0.96) | 0.025 | 0.041 | 0.82 |
| Trunk | 20.4 (1.3) | 19.1 (3.4) | 20.7 (1.4) | 19.1 (3.0) | 0.15 (−0.71, 1.02) | 0.041 | 0.339 | 0.834 |
| Arm | 4.7 (0.8) | 4.1 (0.7) | 4.9 (0.7) | 4.4 (0.7) | −0.03 (−0.21, 0.14) | 0.017 | <0.001 | 0.913 |
| Leg | 15.1 (1.3) | 14.0 (3.0) | 15.6 (1.3) | 14.3 (2.7) | 0.07 (−0.44, 0.57) | 0.048 | 0.001 | 0.659 |
| Fat mass (kg): | ||||||||
| Total | 28.8 (5.8) | 21.5 (7.8) | 28.4 (5.4) | 21.0 (8.1) | −0.72 (−1.82, 0.39) | 0.007 | 0.269 | 0.609 |
| Upper body | 17.2 (4.7) | 16.9 (4.6) | 12.2 (5.1) | 11.9 (5.2) | −0.35 (−1.09, 0.40) | 0.008 | 0.374 | 0.825 |
| Trunk | 13.9 (4.1) | 9.8 (4.3) | 13.6 (3.9) | 9.5 (4.2) | −0.17 (−0.92, 0.57) | 0.008 | 0.325 | 0.778 |
| Arm | 3.3 (0.7) | 2.4 (0.9) | 3.3 (0.7) | 2.5 (1.0) | −0.03 (−0.28, 0.23) | 0.019 | 0.688 | 0.98 |
| Leg | 10.8 (1.6) | 8.4 (2.8) | 10.7 (1.4) | 8.3 (3.0) | −0.36 (−0.87, 0.14) | 0.016 | 0.24 | 0.384 |
| Body fat (%) | 38.2 (4.5) | 32.7 (5.8) | 37.4 (4.2) | 31.9 (6.4) | −0.09 (−1.36, 1.18) | 0.012 | 0.016 | 0.885 |
| VAT (g) | 471 (317) | 328 (297) | 471 (307) | 316 (308) | −5.3 (−83.8, 73.3) | 0.22 | 0.629 | 0.891 |
| Total BMD (g/cm2) | 1.28 (0.01) | 1.19 (0.07) | 1.27 (0.10) | 1.20 (0.07) | 0.01 (−0.01, 0.03) | 0.016 | 0.809 | 0.176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, M.; Vlietstra, L.; Best, A.M.D.; Sims, S.T.; Loehr, J.A.; Rehrer, N.J. Post-Exercise Whey Protein Supplementation: Effects on IGF-1, Strength, and Body Composition in Pre-Menopausal Women, a Randomised Controlled Trial. Nutrients 2025, 17, 2033. https://doi.org/10.3390/nu17122033
Murray M, Vlietstra L, Best AMD, Sims ST, Loehr JA, Rehrer NJ. Post-Exercise Whey Protein Supplementation: Effects on IGF-1, Strength, and Body Composition in Pre-Menopausal Women, a Randomised Controlled Trial. Nutrients. 2025; 17(12):2033. https://doi.org/10.3390/nu17122033
Chicago/Turabian StyleMurray, Marc, Lara Vlietstra, Alyssa M. D. Best, Stacy T. Sims, James A. Loehr, and Nancy J. Rehrer. 2025. "Post-Exercise Whey Protein Supplementation: Effects on IGF-1, Strength, and Body Composition in Pre-Menopausal Women, a Randomised Controlled Trial" Nutrients 17, no. 12: 2033. https://doi.org/10.3390/nu17122033
APA StyleMurray, M., Vlietstra, L., Best, A. M. D., Sims, S. T., Loehr, J. A., & Rehrer, N. J. (2025). Post-Exercise Whey Protein Supplementation: Effects on IGF-1, Strength, and Body Composition in Pre-Menopausal Women, a Randomised Controlled Trial. Nutrients, 17(12), 2033. https://doi.org/10.3390/nu17122033







