Clinical and Gut Microbiome Characteristics of Medically Complex Patients Receiving Blenderized Tube Feeds vs. Standard Enteral Feeds †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Sample Size Estimation
2.5. Sample Collection and Processing
2.6. Bioinformatics
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Participants
3.2. Microbiome Composition
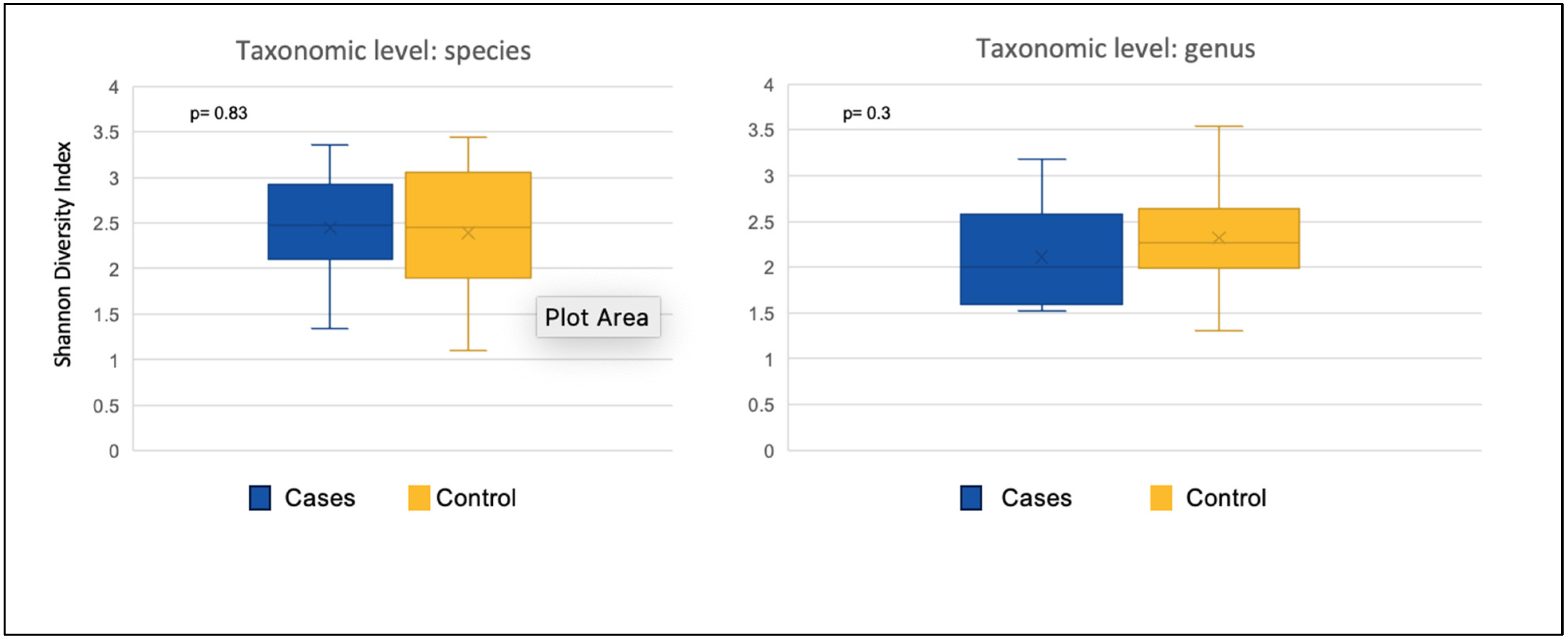
3.2.1. Alpha Diversity
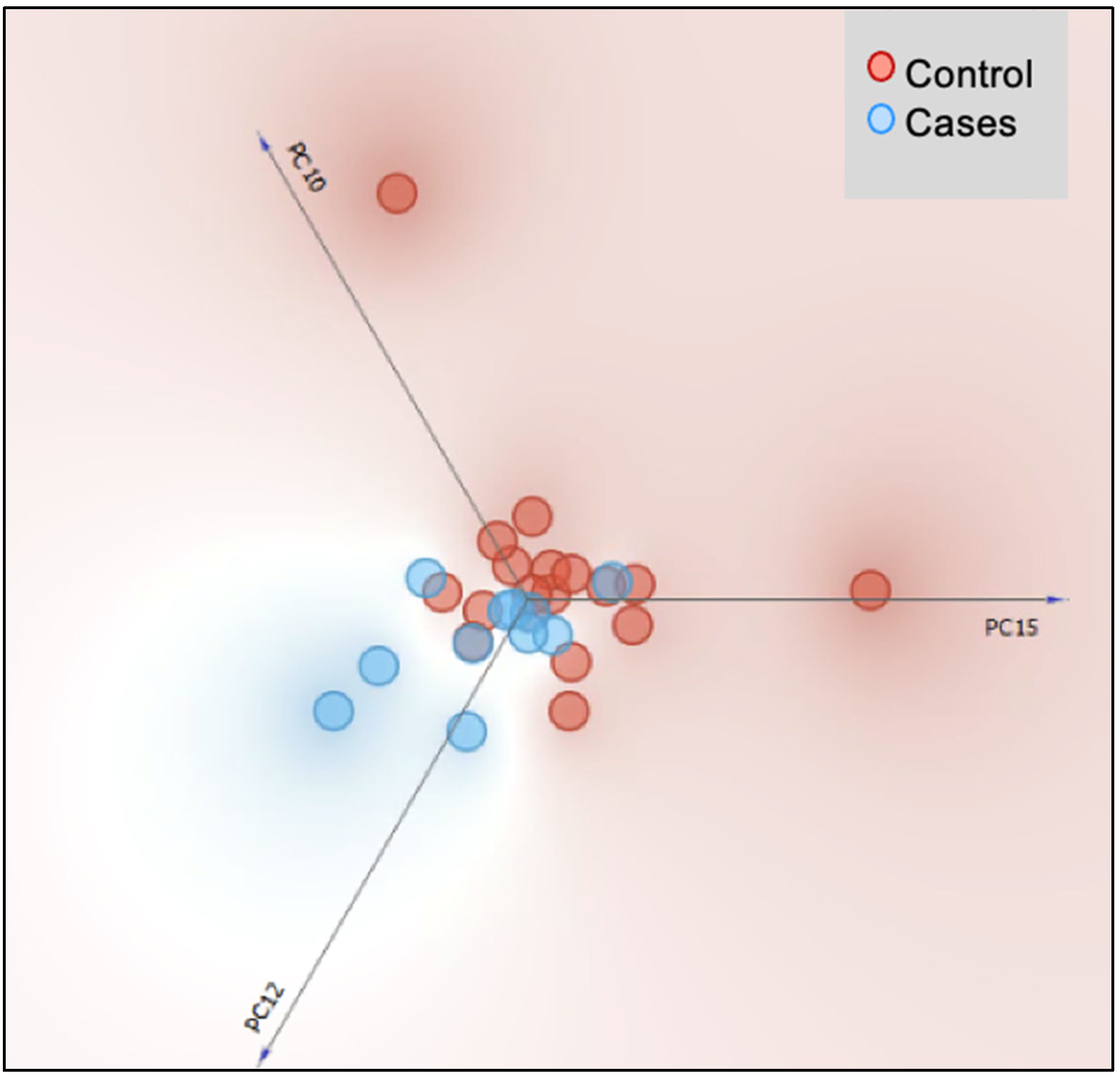
3.2.2. Beta Diversity
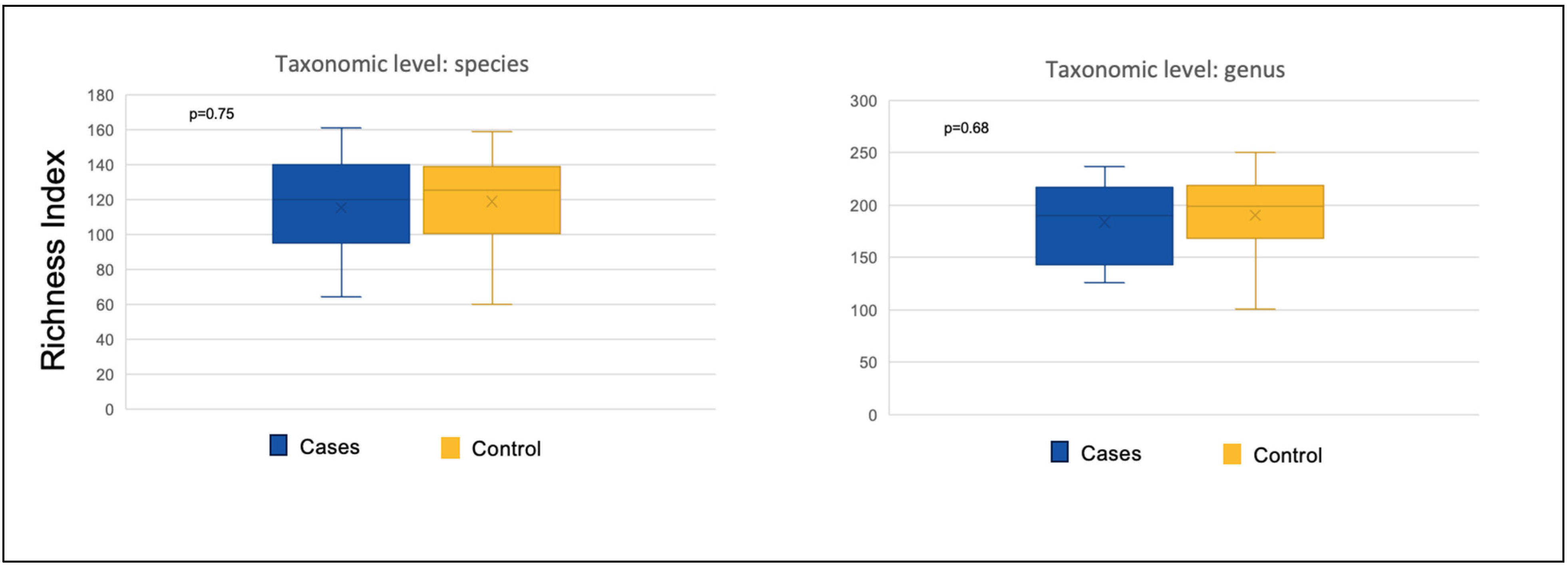
3.2.3. Richness and Evenness Indices
3.2.4. Relative Abundance
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leyenaar, J.K.; Schaefer, A.P.; Freyleue, S.D.; Austin, A.M.; Simon, T.D.; Van Cleave, J.; Moen, E.L.; O’Malley, A.J.; Goodman, D.C. Prevalence of children with medical complexity and associations with health care utilization and in-hospital mortality. JAMA Pediatr. 2022, 176, e220687. [Google Scholar] [CrossRef] [PubMed]
- Rahnemai-Azar, A.A.; Rahnemaiazar, A.A.; Naghshizadian, R.; Kurtz, A.; Farkas, D.T. Percutaneous endoscopic gastrostomy: Indications; technique; complications; management. World J. Gastroenterol. 2014, 20, 7739. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J.I.; Carrera, A.L.; Harvey, L.; Escuro, A.A.; Hudson, L.; Mays, A.; McGinnis, C.; Wessel, J.J.; Bajpai, S.; Beebe, M.L. ASPEN safe practices for enteral nutrition therapy. J. Parenter. Enter. Nutr. 2017, 41, 15–103. [Google Scholar] [CrossRef]
- Hauer, J. Feeding intolerance in children with severe impairment of the central nervous system: Strategies for treatment and prevention. Children 2017, 5, 1. [Google Scholar] [CrossRef]
- Dipasquale, V.; Aumar, M.; Ley, D.; Antoine, M.; Romano, C.; Gottrand, F. Tube feeding in neurologically disabled children: Hot topics and new directions. Nutrients 2022, 14, 3831. [Google Scholar] [CrossRef]
- Köglmeier, J.; Assecaira, I.; Banci, E.; De Koning, B.; Haiden, N.; Indrio, F.; Kastelijn, W.; Kennedy, D.; Luque, V.; Norsa, L. The use of blended diets in children with enteral feeding tubes: A joint position paper of the ESPGHAN committees of allied health professionals and nutrition. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Shah, N.D.; Sheikh, G.N.; Allen, K. Classifying enteral nutrition: Tailored for clinical practice. Curr. Gastroenterol. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Musial, A.; Schondelmeyer, A.; Densel, O.; Younts, A.; Kelley, J.; Herbst, L.; Statile, A.M. Decreasing time to full enteral feeds in hospitalized children with medical complexity experiencing feeding intolerance. Hosp. Pediatr. 2022, 12, 806–815. [Google Scholar] [CrossRef]
- Beauchamp-Walters, J.; Aleti, G.; Herrera, L.; Debelius, J.; Lima, N.; Dalal, P.; Hong, S.; Knight, R.; Rhee, K.E. Impact of exclusive enteral nutrition on the gut microbiome of children with medical complexity. J. Parenter. Enter. Nutr. 2023, 47, 77–86. [Google Scholar] [CrossRef]
- Gallagher, K.; Flint, A.; Mouzaki, M.; Carpenter, A.; Haliburton, B.; Bannister, L.; Norgrove, H.; Hoffman, L.; Mack, D.; Stintzi, A. Blenderized enteral nutrition diet study: Feasibility, clinical, and microbiome outcomes of providing blenderized feeds through a gastric tube in a medically complex pediatric population. J. Parenter. Enter. Nutr. 2018, 42, 1046–1060. [Google Scholar] [CrossRef]
- Pentiuk, S.; O’Flaherty, T.; Santoro, K.; Willging, P.; Kaul, A. Pureed by gastrostomy tube diet improves gagging and retching in children with fundoplication. J. Parenter. Enter. Nutr. 2011, 35, 375–379. [Google Scholar] [CrossRef]
- Batsis, I.D.; Davis, L.; Prichett, L.; Wu, L.; Shores, D.; Yeung, K.A.; Oliva-Hemker, M. Efficacy and tolerance of blended diets in children receiving gastrostomy feeds. Nutr. Clin. Pract. 2020, 35, 282–288. [Google Scholar] [CrossRef]
- O’Connor, G.; Watson, M.; Van Der Linde, M.; Bonner, R.S.; Hopkins, J.; Saduera, S. Monitor gastrointestinal tolerance in children who have switched to an “enteral formula with food-derived ingredients”: A national, multicenter retrospective chart review (RICIMIX study). Nutr. Clin. Pract. 2022, 37, 929–934. [Google Scholar] [CrossRef]
- Epp, L.; Blackmer, A.; Church, A.; Ford, I.; Grenda, B.; Larimer, C.; Lewis-Ayalloore, J.; Malone, A.; Pataki, L.; Rempel, G. Blenderized tube feedings: Practice recommendations from the American Society for Parenteral and Enteral Nutrition. Nutr. Clin. Pract. 2023, 38, 1190–1219. [Google Scholar] [CrossRef]
- Bobo, E. Reemergence of blenderized tube feedings: Exploring the evidence. Nutr. Clin. Pract. 2016, 31, 730–735. [Google Scholar] [CrossRef]
- Nakai, Y.; Akagawa, S.; Fujishiro, S.; Akagawa, Y.; Yamagishi, M.; Yamanouchi, S.; Kimata, T.; Ohashi, A.; Hashiyada, M.; Akane, A. Dysbiosis of the gut microbiota in children with severe motor and intellectual disabilities receiving enteral nutrition: A pilot study. J. Parenter. Enter. Nutr. 2023, 47, 67–76. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q. Factors affecting gut microbiome in daily diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Isobe, K.; Martiny, J.B. Short-term dietary fiber interventions produce consistent gut microbiome responses across studies. Msystems 2024, 9, e00133-24. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary fiber intake and gut microbiota in human health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Katagiri, S.; Ohsugi, Y.; Shiba, T.; Yoshimi, K.; Nakagawa, K.; Nagasawa, Y.; Uchida, A.; Liu, A.; Lin, P.; Tsukahara, Y. Homemade blenderized tube feeding improves gut microbiome communities in children with enteral nutrition. Front. Microbiol. 2023, 14, 1215236. [Google Scholar] [CrossRef]
- McClanahan, D.; Yeh, A.; Firek, B.; Zettle, S.; Rogers, M.; Cheek, R.; Nguyen, M.V.; Gayer, C.P.; Wendell, S.G.; Mullett, S.J. Pilot study of the effect of plant-based enteral nutrition on the gut microbiota in chronically ill tube-fed children. J. Parenter. Enter. Nutr. 2019, 43, 899–911. [Google Scholar] [CrossRef]
- Andersen, S.; Banks, M.; Bauer, J. Nutrition support and the gastrointestinal microbiota: A systematic review. J. Acad. Nutr. Diet. 2020, 120, 1498–1516. [Google Scholar] [CrossRef]
- Thavamani, A.; Sankararaman, S.; Al-Shakhshir, H.; Retuerto, M.; Velayuthan, S.; Sferra, T.J.; Ghannoum, M. Impact of Erythromycin as a Prokinetic on the Gut Microbiome in Children with Feeding Intolerance—A Pilot Study. Antibiotics 2023, 12, 1606. [Google Scholar] [CrossRef]
- Fernandez Ferrer, M.; Sankararaman, S.; Thavamani, A.; Sferra, T. Intestinal Microbiome in Pediatric Patients: Blended vs. Non-blended Enteral Feeds. In Proceedings of the Seventh World Congress of Pediatric Gastroenterology Hepatology and Nutrition, Buenos Aires, Argentina, 4–7 December 2024. Abstract No.627/595. [Google Scholar] [CrossRef]
- Sforza, E.; Limongelli, D.; Giorgio, V.; Margiotta, G.; Proli, F.; Kuczynska, E.M.; Leoni, C.; Rigante, D.; Contaldo, I.; Veredice, C. The impact of blenderized tube feeding on gastrointestinal symptoms, a scoping review. Appl. Sci. 2023, 13, 2173. [Google Scholar] [CrossRef]
- Hron, B.; Fishman, E.; Lurie, M.; Clarke, T.; Chin, Z.; Hester, L.; Burch, E.; Rosen, R. Health outcomes and quality of life indices of children receiving blenderized feeds via enteral tube. J. Pediatr. 2019, 211, 139–145.e131. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Allen, S.; Scott, E.; Jenkins, H.; Sadlier, C.; Thomas, S. An observational investigation of the faecal microbiota and metabonome of gastrostomy fed children, on blended and formula diets. Gut Microbes 2022, 14, 2138661. [Google Scholar] [CrossRef]
- King, C.H.; Desai, H.; Sylvetsky, A.C.; LoTempio, J.; Ayanyan, S.; Carrie, J.; Crandall, K.A.; Fochtman, B.C.; Gasparyan, L.; Gulzar, N. Baseline human gut microbiota profile in healthy people and standard reporting template. PLoS ONE 2019, 14, e0206484. [Google Scholar] [CrossRef]
- Parsaei, M.; Sarafraz, N.; Moaddab, S.; Leylabadlo, H.E. The importance of Faecalibacterium prausnitzii in human health and diseases. New Microbes New Infect. 2021, 43, 100928. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; de Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Leylabadlo, H.E.; Ghotaslou, R.; Feizabadi, M.M.; Farajnia, S.; Moaddab, S.Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B. The critical role of Faecalibacterium prausnitzii in human health: An overview. Microb. Pathog. 2020, 149, 104344. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Gu, X.; Su, Y.; Zheng, Q.; Yuan, X.; Bao, Z.; Lu, J.; Li, L. Health and disease: Akkermansia muciniphila, the shining star of the gut flora. Research 2023, 6, 0107. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Bradley, P.H.; Pollard, K.S. Proteobacteria explain significant functional variability in the human gut microbiome. Microbiome 2017, 5, 36. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef]
| Parameters | Blenderized Tube Feeds (n = 11) | Standard Enteral Feeds (n = 17) | p-Value |
|---|---|---|---|
| Median age (in years) | 4.4 (1.5 to 16.5) | 2.2 (1.65 to 5.9) | 0.14 |
| Sex | 0.46 | ||
| Male | 7 (64%) | 8 (47%) | |
| Female | 4 (36%) | 9 (53%) | |
| Malnutrition | 3 (27.3%) | 1 (5.9%) | 0.12 |
| Diarrhea | 0 (0%) | 1 (5.9%) | 1.00 |
| Constipation | 6 (54.5%) | 8 (47.1%) | 1.00 |
| Vomiting | 0 (0%) | 7 (41.1%) | 0.02 |
| Oral feeds | 5 (45.4%) | 4 (25%) | 0.58 |
| Acid suppression | 0.38 | ||
| None | 5 (45.5%) | 4 (23.5%) | |
| H2RAs | 0 (0%) | 1 (5.9%) | |
| PPI | 6 (54.5%) | 12 (70.6%) | |
| Use of laxatives | 6 (54.5%) | 9 (52.9%) | 1.00 |
| Use of erythromycin | 3 (27.3%) | 4 (23.5%) | 1.00 |
| Use of probiotics | 3 (27.3%) | 1 (5.9%) | 0.27 |
| Parameters | Blenderized Tube Feeds (n = 11) | Standard Enteral Feeds (n = 17) | p-Value |
|---|---|---|---|
| Cerebral palsy | 4 (36.4%) | 3 (17.6%) | 0.38 |
| Autism | 2 (18.2%) | 1 (5.9%) | 0.54 |
| Developmental delay | 5 (45.5%) | 10 (58.8%) | 0.70 |
| Genetic disorders | 3 (27.3%) | 3 (17.6%) | 0.65 |
| Prematurity | 3 (27.3%) | 5 (29.4%) | 1 |
| Oral aversion | 6 (54.5%) | 7 (41.2%) | 0.70 |
| Aspiration | 2 (18.2%) | 2 (11.8%) | 1 |
| GERD | 8 (72.7%) | 10 (58.8%) | 0.68 |
| Food allergies | 3 (27.3%) | 1 (5.9%) | 0.27 |
| CMPI | 1 (9.1%) | 1 (5.9%) | 1 |
| Gastroparesis | 2 (18.2%) | 2 (11.8%) | 1 |
| Epilepsy | 4 (36.4%) | 4 (23.5%) | 0.67 |
| Failure to thrive | 5 (45.5%) | 3 (17.6%) | 0.20 |
| Cardiac disorders | 2 (18.2%) | 3 (17.6%) | 1 |
| Chronic lung disease | 4 (36.4%) | 7 (41.2%) | 1 |
| At Species Level | |||
|---|---|---|---|
| Mean Value | Shannon’s Diversity Index | Richness (No. of OTUs) | Pielou’s Evenness Index |
| BTF (11) | 2.44 | 115 | 0.52 |
| SEF (17) | 2.40 | 114 | 0.51 |
| p-value BTF vs. SEF | 0.83 | 0.75 | 0.97 |
| At Genus Level | |||
| BTF (11) | 2.11 | 184 | 0.40 |
| SEF (17) | 2.35 | 183 | 0.46 |
| BTF vs. SEF p-value | 0.30 | 0.68 | 0.20 |
| Taxonomic Level | p-Value | Elevated in | RA in Cases (%) | RA in Controls (%) | Fold Change (Case/Control) |
|---|---|---|---|---|---|
| Species | |||||
| Bacteroides caccae | 0.0222 | Cases | 1.059 | 0.213 | 5.0 |
| Enterobacter nickellidurans | 0.0486 | Cases | 0.171 | 0.028 | 6.1 |
| Pseudoalteromonas piscicida | 0.0468 | Cases | 0.047 | 0.022 | 2.2 |
| Genus | |||||
| Aggregatibacter | 0.0032 | Cases | 0.010 | 0.002 | 4.7 |
| Deinococcus | 0.0041 | Cases | 0.002 | 0.000 | 11.5 |
| Exiguobacterium | 0.0070 | Cases | 0.004 | 0.001 | 6.2 |
| Geobacter | 0.0079 | Controls | 0.000 | 0.003 | Unique to control group |
| Thermomonas | 0.0253 | Controls | 0.000 | 0.003 | −52.4 |
| Clostridium | 0.0263 | Controls | 0.001 | 0.009 | −9.9 |
| Ramlibacter | 0.0294 | Controls | 0.001 | 0.006 | −4.6 |
| Streptococcus | 0.0408 | Controls | 0.084 | 0.427 | −5.1 |
| Phylum | |||||
| Spirochaetes | 0.0321 | Controls | 0.007 | 0.015 | −2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, M.F.; Retuerto, M.; Thavamani, A.; San Valentin, E.M.; Sferra, T.J.; Ghannoum, M.; Sankararaman, S. Clinical and Gut Microbiome Characteristics of Medically Complex Patients Receiving Blenderized Tube Feeds vs. Standard Enteral Feeds. Nutrients 2025, 17, 2018. https://doi.org/10.3390/nu17122018
Ferrer MF, Retuerto M, Thavamani A, San Valentin EM, Sferra TJ, Ghannoum M, Sankararaman S. Clinical and Gut Microbiome Characteristics of Medically Complex Patients Receiving Blenderized Tube Feeds vs. Standard Enteral Feeds. Nutrients. 2025; 17(12):2018. https://doi.org/10.3390/nu17122018
Chicago/Turabian StyleFerrer, Marianelly Fernandez, Mauricio Retuerto, Aravind Thavamani, Erin Marie San Valentin, Thomas J. Sferra, Mahmoud Ghannoum, and Senthilkumar Sankararaman. 2025. "Clinical and Gut Microbiome Characteristics of Medically Complex Patients Receiving Blenderized Tube Feeds vs. Standard Enteral Feeds" Nutrients 17, no. 12: 2018. https://doi.org/10.3390/nu17122018
APA StyleFerrer, M. F., Retuerto, M., Thavamani, A., San Valentin, E. M., Sferra, T. J., Ghannoum, M., & Sankararaman, S. (2025). Clinical and Gut Microbiome Characteristics of Medically Complex Patients Receiving Blenderized Tube Feeds vs. Standard Enteral Feeds. Nutrients, 17(12), 2018. https://doi.org/10.3390/nu17122018






