Inositols and Bone Health: Potential Therapeutic Applications in Osteoporosis Prevention and Treatment
Abstract
1. Introduction
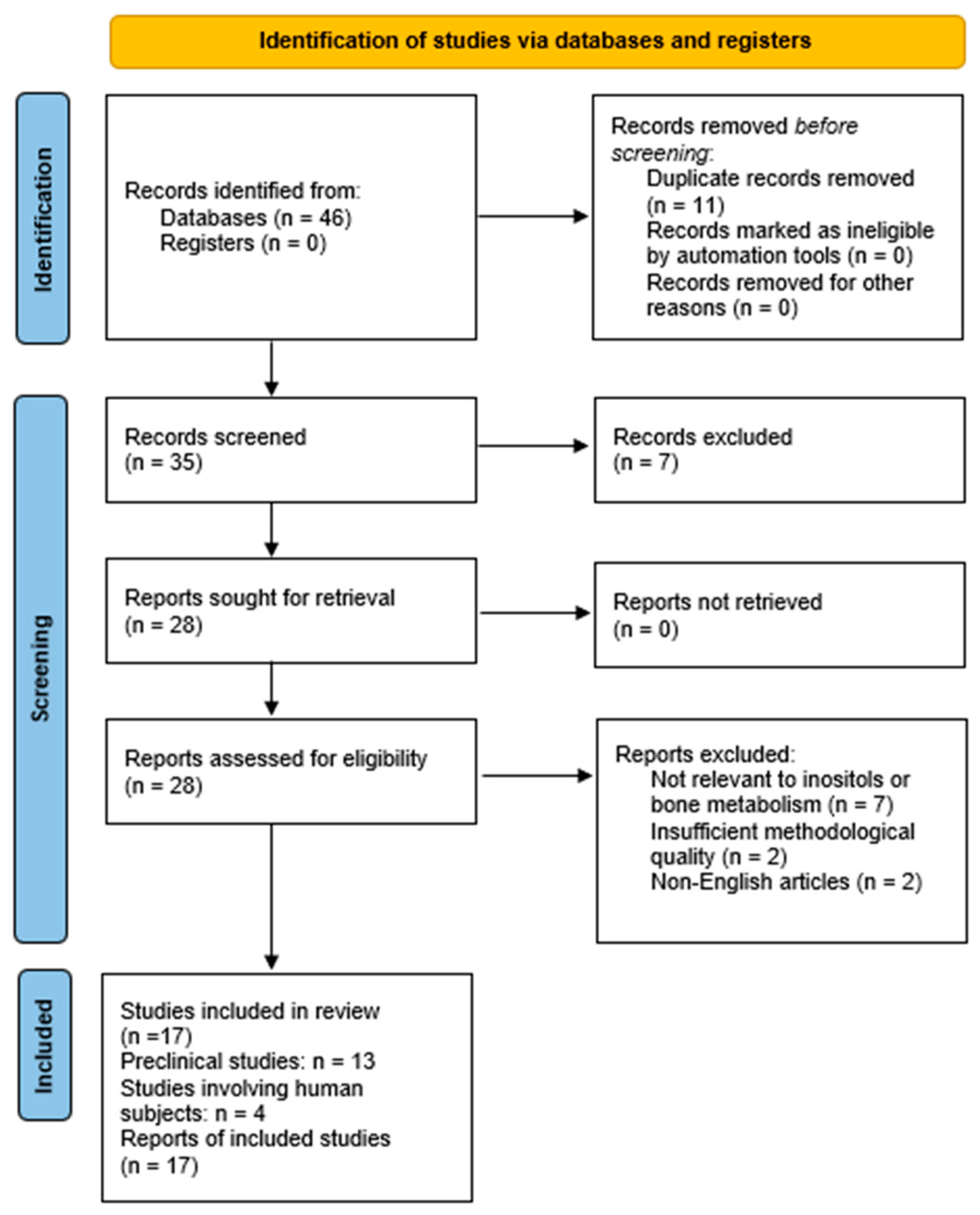
2. Materials and Methods
3. Results
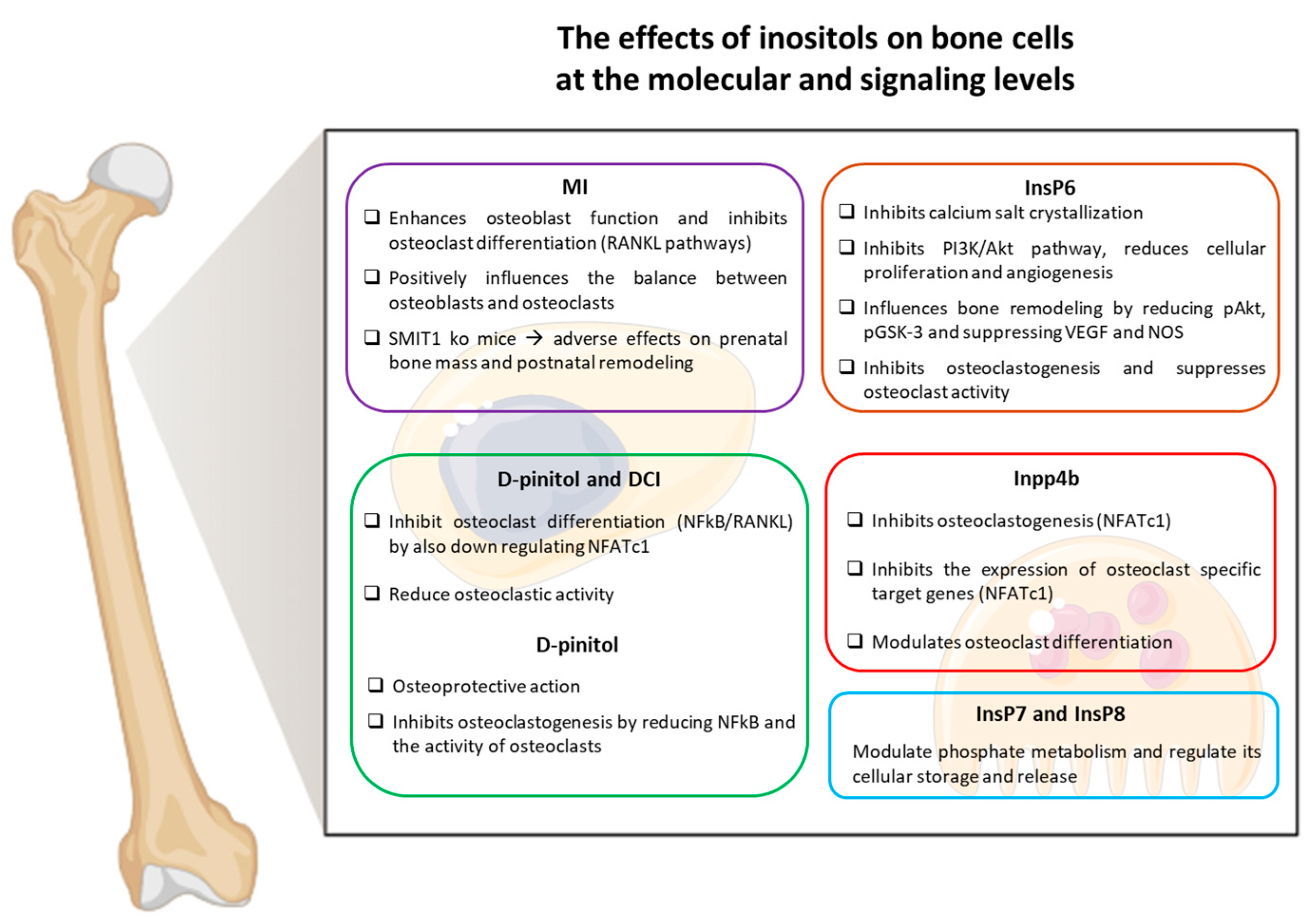
Inositols and Their Role in Bone Metabolism
| Author | Study Design | Primary Outcome | Sample Size or Study Model | Main Results |
|---|---|---|---|---|
| Sanchis et al. [35] | Human | Changes in BMD after phytate intake | n = 440 women | Higher phytate intake associated with higher BMD |
| López-González et al. [38] | Human | Changes in BMD after phytate intake | n = 157 postmenopausal women | Positive correlation between phytate intake and BMD |
| Gonzalez et al. [39] | Human | Changes in bone mass loss and urinary phytate | n = 212 women | Higher urinary phytate linked to reduced bone loss |
| Navarro et al. [53] | Human | Hormonal response to D-pinitol | n = 25 healthy volunteers | D-pinitol altered endocrine markers |
| Sanchis et al. [33] | Preclinical—in vitro | Effect of IP6 on osteoclast activity | RAW264.7, human cell cultures | IP6 inhibited osteoclastogenesis |
| Arriero et al. [36] | Preclinical—in vitro | ALP expression and osteoblast differentiation | hUC-MSCs, MC3T3-E1 cells | Differential regulation of osteoblast markers |
| Arriero et al. [37] | Preclinical—in vitro | IP6 inhibition of osteoclastogenesis | RAW264.7, human primary osteoclasts | Inhibition of osteoclast differentiation |
| Yu et al. [55] | Preclinical—in vitro | DCI/D-pinitol and NFATc1 in osteoclasts | Murine preosteoclasts | Inhibition of osteoclastogenesis via NFATc1 |
| Liu et al. [56] | Preclinical—in vitro | RANKL-induced osteoclastogenesis and pinitol | RAW264.7 cells | D-pinitol inhibited RANKL pathway |
| Gu et al. [34] | Preclinical—in vitro | IP6 effects on PI3K–Akt signaling | Prostate carcinoma cells | Inhibition of signaling and proliferation |
| Dai et al. [27] | Preclinical—in vivo | MI supplementation in SMIT1 KO mice | KO and WT mice | Partial rescue of bone phenotype |
| Boregowda et al. [49] | Preclinical—in vitro and in vivo | IP6K1 inhibitor effects on bone | MSC cultures; WT and Ip6k1−/− mice | Preserved BMD and reduced bone loss |
| Boregowda et al. [50] | Preclinical—in vitro and in vivo | IP6K1 inhibitor effects on bone | Human MSCs; C57BL/6 mice | Preserved BMD and reduced bone loss |
| Liu et al. [57] | Preclinical—in vivo | Pinitol treatment in OVX mice | OVX mice | Improved BMD with pinitol |
| Liu & Koyama [58] | Preclinical—in vivo | Pinitol in diabetic osteoporosis | Diabetic mice | Restored bone loss with metabolic improvement |
| Ferron et al. [32] | Preclinical—in vivo | INPP4B gene and bone phenotype | INPP4B KO mice | KO mice showed bone loss |
| Ito M et al. [51] | Preclinical—in vivo | The role of IP6K2/IP7 in the enteric nervous system | WT and IP6K2-KO C57BL/6 mice | IP6K2 regulates IP7 metabolism, affecting enteric neuronal activity and gut motility |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sheik Ali, A. Osteoporosis: A narrative review. Cureus 2023, 15, e43031. [Google Scholar] [CrossRef]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Majumdar, S.R.; Lier, D.A.; McAlister, F.A.; Johnson, J.A.; Rowe, B.H.; Beaupre, L.A. Cost-effectiveness of osteoporosis interventions to improve quality of care after upper extremity fracture: Results from a randomized trial (c-stop trial). J. Bone Miner. Res. 2019, 34, 1220–1228. [Google Scholar] [CrossRef]
- Singer, A.; McClung, M.R.; Tran, O.; Morrow, C.D.; Goldstein, S.; Kagan, R.; McDermott, M.; Yehoshua, A. Treatment rates and healthcare costs of patients with fragility fracture by site of care: A real-world data analysis. Arch. Osteoporos. 2023, 18, 42. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-osteoclast communication and bone homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Yao, Z.; Getting, S.J.; Locke, I.C. Regulation of tnf-induced osteoclast differentiation. Cells 2022, 11, 132. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of rank signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Huang, W.; Zhu, C.; Xu, Y.; Yang, H.; Bai, J. Targeting strategies for bone diseases: Signaling pathways and clinical studies. Signal Transduct. Target. Ther. 2023, 8, 202. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A mini review on osteoporosis: From biology to pharmacological management of bone loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef]
- Baek, Y.H.; Cho, S.W.; Jeong, H.E.; Kim, J.H.; Hwang, Y.; Lange, J.L.; Shin, J.Y. 10-year fracture risk in postmenopausal women with osteopenia and osteoporosis in south korea. Endocrinol. Metab. 2021, 36, 1178–1188. [Google Scholar] [CrossRef]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.-L.; Wang, R.; Wang, X.-Q.; Zhang, H. Exercise for osteoporosis: A literature review of pathology and mechanism. Front. Immunol. 2022, 13, 1005665. [Google Scholar] [CrossRef]
- Heaney, R.P. Functional indices of vitamin d status and ramifications of vitamin d deficiency. Am. J. Clin. Nutr. 2004, 80, 1706s–1709s. [Google Scholar] [CrossRef]
- Avenell, A.; Mak, J.C.; O’Connell, D. Vitamin d and vitamin d analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014, 2014, CD000227. [Google Scholar]
- Cummings, S.R.; Rosen, C. Vital findings—A decisive verdict on vitamin d supplementation. N. Engl. J. Med. 2022, 387, 368–370. [Google Scholar] [CrossRef]
- Palermo, A.; Tuccinardi, D.; D’Onofrio, L.; Watanabe, M.; Maggi, D.; Maurizi, A.R.; Greto, V.; Buzzetti, R.; Napoli, N.; Pozzilli, P.; et al. Vitamin k and osteoporosis: Myth or reality? Metab. Clin. Exp. 2017, 70, 57–71. [Google Scholar] [CrossRef]
- Finnes, T.E.; Lofthus, C.M.; Meyer, H.E.; Søgaard, A.J.; Tell, G.S.; Apalset, E.M.; Gjesdal, C.; Grimnes, G.; Schei, B.; Blomhoff, R.; et al. A combination of low serum concentrations of vitamins k1 and d is associated with increased risk of hip fractures in elderly norwegians: A norepos study. Osteoporos. Int. 2016, 27, 1645–1652. [Google Scholar] [CrossRef]
- Hao, G.; Zhang, B.; Gu, M.; Chen, C.; Zhang, Q.; Zhang, G.; Cao, X. Vitamin k intake and the risk of fractures: A meta-analysis. Medicine 2017, 96, e6725. [Google Scholar] [CrossRef]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Iervasi, G.; La Manna, G.; et al. Vitamin k and osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef]
- Spector, T.D.; Calomme, M.R.; Anderson, S.H.; Clement, G.; Bevan, L.; Demeester, N.; Swaminathan, R.; Jugdaohsingh, R.; Berghe, D.A.; Powell, J.J. Choline-stabilized orthosilicic acid supplementation as an adjunct to calcium/vitamin d3 stimulates markers of bone formation in osteopenic females: A randomized, placebo-controlled trial. BMC Musculoskelet. Disord. 2008, 9, 85. [Google Scholar] [CrossRef]
- Scarpa, E.-S.; Antonelli, A.; Balercia, G.; Sabatelli, S.; Maggi, F.; Caprioli, G.; Giacchetti, G.; Micucci, M. Antioxidant, anti-inflammatory, anti-diabetic, and pro-osteogenic activities of polyphenols for the treatment of two different chronic diseases: Type 2 diabetes mellitus and osteoporosis. Biomolecules 2024, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of inositols and inositol phosphates in energy metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef]
- Chhetri, D.R. Myo-inositol and its derivatives: Their emerging role in the treatment of human diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Heart 2022, 9, e001989. [Google Scholar] [CrossRef] [PubMed]
- Su, X.B.; Ko, A.-L.A.; Saiardi, A. Regulations of myo-inositol homeostasis: Mechanisms, implications, and perspectives. Adv. Biol. Regul. 2023, 87, 100921. [Google Scholar] [CrossRef]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From established knowledge to novel approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef]
- Dai, Z.; Chung, S.K.; Miao, D.; Lau, K.S.; Chan, A.W.; Kung, A.W. Sodium/myo-inositol cotransporter 1 and myo-inositol are essential for osteogenesis and bone formation. J. Bone Miner. Res. 2011, 26, 582–590. [Google Scholar] [CrossRef]
- Caputo, M.; Bona, E.; Leone, I.; Samà, M.T.; Nuzzo, A.; Ferrero, A.; Aimaretti, G.; Marzullo, P.; Prodam, F. Inositols and metabolic disorders: From farm to bedside. J. Tradit. Complement. Med. 2020, 10, 252–259. [Google Scholar] [CrossRef]
- Dinicola, S.; Minini, M.; Unfer, V.; Verna, R.; Cucina, A.; Bizzarri, M. Nutritional and acquired deficiencies in inositol bioavailability. Correlations with metabolic disorders. Int. J. Mol. Sci. 2017, 18, 2187. [Google Scholar]
- Lee, B.; Park, S.J.; Hong, S.; Kim, K.; Kim, S. Inositol polyphosphate multikinase signaling: Multifaceted functions in health and disease. Mol. Cells 2021, 44, 187–194. [Google Scholar] [CrossRef]
- Vacher, J. Inpp4b is a novel negative modulator of osteoclast differentiation and a prognostic locus for human osteoporosis. Ann. N. Y. Acad. Sci. 2013, 1280, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Boudiffa, M.; Arsenault, M.; Rached, M.; Pata, M.; Giroux, S.; Elfassihi, L.; Kisseleva, M.; Majerus, P.W.; Rousseau, F.; et al. Inositol polyphosphate 4-phosphatase b as a regulator of bone mass in mice and humans. Cell Metab. 2011, 14, 466–477. [Google Scholar] [CrossRef]
- Sanchis, P.; López-González, Á.A.; Costa-Bauzá, A.; Busquets-Cortés, C.; Riutord, P.; Calvo, P.; Grases, F. Understanding the Protective Effect of Phytate in Bone Decalcification Related-Diseases. Nutrients 2021, 13, 2859. [Google Scholar] [CrossRef]
- Gu, M.; Roy, S.; Raina, K.; Agarwal, C.; Agarwal, R. Inositol hexaphosphate suppresses growth and induces apoptosis in prostate carcinoma cells in culture and nude mouse xenograft: PI3K-Akt pathway as potential target. Cancer Res. 2009, 69, 9465–9472. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, P.; Prieto, R.M.; Konieczna, J.; Grases, F.; Abete, I.; Salas-Salvadó, J.; Martín, V.; Ruiz-Canela, M.; Babio, N.; García-Gavilán, J.F.; et al. Estimated phytate intake is associated with bone mineral density in mediterranean postmenopausal women. Nutrients 2023, 15, 1791. [Google Scholar] [CrossRef]
- Arriero Mdel, M.; Ramis, J.M.; Perelló, J.; Monjo, M. Differential response of MC3T3-E1 and human mesenchymal stem cells to inositol hexakisphosphate. Cell. Physiol. Biochem. 2012, 30, 974–986. [Google Scholar] [CrossRef]
- Arriero Mdel, M.; Ramis, J.M.; Perelló, J.; Monjo, M. Inositol hexakisphosphate inhibits osteoclastogenesis on raw 264.7 cells and human primary osteoclasts. PLoS ONE 2012, 7, e43187. [Google Scholar] [CrossRef] [PubMed]
- López-González, Á.; Grases, F.; Monroy, N.; Marí, B.; Vicente-Herrero, M.; Tur, F.; Perelló, J. Protective effect of myo-inositol hexaphosphate (phytate) on bone mass loss in postmenopausal women. Eur. J. Nutr. 2012, 52, 717–726. [Google Scholar] [CrossRef]
- Gonzalez, A.A.L.; Grases, F.; Mari, B.; Tomas-Salva, M.; Rodriguez, A. Urinary phytate concentration and risk of fracture determined by the frax index in a group of postmenopausal women. Turk. J. Med. Sci. 2019, 49, 458–463. [Google Scholar] [CrossRef]
- Yoshiko, Y.; Vucenik, I. Inositol Hexaphosphate in Bone Health and Disease. Biomolecules 2024, 14, 1072. [Google Scholar] [CrossRef]
- Vucenik, I.; Druzijanic, A.; Druzijanic, N. Inositol Hexaphosphate (IP6) and Colon Cancer: From Concepts and First Experiments to Clinical Application. Molecules 2020, 25, 5931. [Google Scholar] [CrossRef] [PubMed]
- Petroski, W.; Minich, D.M. Is there such a thing as “anti-nutrients”? A narrative review of perceived problematic plant compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F. Phytic acid and whole grains for health controversy. Nutrients 2021, 14, 25. [Google Scholar] [CrossRef]
- Arsov, A.; Tsigoriyna, L.; Batovska, D.; Armenova, N.; Mu, W.; Zhang, W.; Petrov, K.; Petrova, P. Bacterial degradation of antinutrients in foods: The genomic insight. Foods 2024, 13, 2408. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Livermore, T.M.; Saiardi, A. Inositol pyrophosphates: Between signalling and metabolism. Biochem. J. 2013, 452, 369–379. [Google Scholar] [CrossRef]
- Nagpal, L.; He, S.; Rao, F.; Snyder, S.H. Inositol Pyrophosphates as Versatile Metabolic Messengers. Annu. Rev. Biochem. 2024, 93, 317–338. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Mukherjee, S.; Haubner, J.; Chakraborty, A. Targeting the inositol pyrophosphate biosynthetic enzymes in metabolic diseases. Molecules 2020, 25, 1403. [Google Scholar] [CrossRef]
- Boregowda, S.V.; Ghoshal, S.; Booker, C.N.; Krishnappa, V.; Chakraborty, A.; Phinney, D.G. Ip6k1 reduces mesenchymal stem/stromal cell fitness and potentiates high fat diet-induced skeletal involution. Stem Cells 2017, 35, 1973–1983. [Google Scholar] [CrossRef]
- Boregowda, S.V.; Nanjappa, M.K.; Booker, C.N.; Strivelli, J.; Supper, V.M.; Cooke, P.S.; Phinney, D.G. Pharmacological inhibition of inositol hexakisphosphate kinase 1 protects mice against obesity-induced bone loss. Biology 2022, 11, 1257. [Google Scholar] [CrossRef]
- Ito, M.; Fujii, N.; Kohara, S.; Hori, S.; Tanaka, M.; Wittwer, C.; Kikuchi, K.; Iijima, T.; Kakimoto, Y.; Hirabayashi, K.; et al. Inositol pyrophosphate profiling reveals regulatory roles of IP6K2-dependent enhanced IP7 metabolism in the enteric nervous system. J. Biol. Chem. 2023, 299, 102928. [Google Scholar] [CrossRef] [PubMed]
- Chakkour, M.; Greenberg, M.L. Insights into the roles of inositol hexakisphosphate kinase 1 (IP6K1) in mammalian cellular processes. J. Biol. Chem. 2024, 300, 107116. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.A.; Díaz, C.; Decara, J.; Medina-Vera, D.; Lopez-Gambero, A.J.; Suarez, J.; Pavón, F.J.; Serrano, A.; Vargas, A.; Gavito, A.L.; et al. Pharmacokinetics and endocrine effects of an oral dose of d-pinitol in human fasting healthy volunteers. Nutrients 2022, 14, 4094. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Unfer, V.; Soulage, C.O.; Yap-Garcia, M.I.M.; Bevilacqua, A.; Benvenga, S.; Barbaro, D.; Wdowiak, A.; Nordio, M.; Dewailly, D.; et al. D-chiro-inositol in clinical practice: A perspective from the experts group on inositol in basic and clinical research (egoi). Gynecol. Obstet. Investig. 2024, 89, 284–294. [Google Scholar] [CrossRef]
- Yu, J.; Choi, S.; Park, E.-S.; Shin, B.; Yu, J.; Lee, S.H.; Takami, M.; Kang, J.S.; Meong, H.; Rho, J. D-chiro-inositol negatively regulates the formation of multinucleated osteoclasts by down-regulating nfatc1. J. Clin. Immunol. 2012, 32, 1360–1371. [Google Scholar] [CrossRef]
- Liu, S.C.; Chuang, S.M.; Tang, C.H. D-pinitol inhibits rankl-induced osteoclastogenesis. Int. Immunopharmacol. 2012, 12, 494–500. [Google Scholar] [CrossRef]
- Liu, X.; He, C.; Koyama, T. D-pinitol ameliorated osteoporosis via elevating d-chiro-inositol level in ovariectomized mice. J. Nutr. Sci. Vitaminol. 2023, 69, 220–228. [Google Scholar] [CrossRef]
- Liu, X.; Koyama, T. D-pinitol improved glucose metabolism and inhibited bone loss in mice with diabetic osteoporosis. Molecules 2023, 28, 3870. [Google Scholar] [CrossRef]
- Castañeda, S.; Casas, A.; González-Del-Alba, A.; Martínez-Díaz-Guerra, G.; Nogués, X.; Ojeda Thies, C.; Torregrosa Suau, Ó.; Rodríguez-Lescure, Á. Bone loss induced by cancer treatments in breast and prostate cancer patients. Clin. Transl. Oncol. 2022, 24, 2090–2106. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipriani, F.; Gnessi, L.; Watanabe, M.; Baldelli, R. Inositols and Bone Health: Potential Therapeutic Applications in Osteoporosis Prevention and Treatment. Nutrients 2025, 17, 1999. https://doi.org/10.3390/nu17121999
Cipriani F, Gnessi L, Watanabe M, Baldelli R. Inositols and Bone Health: Potential Therapeutic Applications in Osteoporosis Prevention and Treatment. Nutrients. 2025; 17(12):1999. https://doi.org/10.3390/nu17121999
Chicago/Turabian StyleCipriani, Fiammetta, Lucio Gnessi, Mikiko Watanabe, and Roberto Baldelli. 2025. "Inositols and Bone Health: Potential Therapeutic Applications in Osteoporosis Prevention and Treatment" Nutrients 17, no. 12: 1999. https://doi.org/10.3390/nu17121999
APA StyleCipriani, F., Gnessi, L., Watanabe, M., & Baldelli, R. (2025). Inositols and Bone Health: Potential Therapeutic Applications in Osteoporosis Prevention and Treatment. Nutrients, 17(12), 1999. https://doi.org/10.3390/nu17121999







