Optimizing Body Composition During Weight Loss: The Role of Amino Acid Supplementation
Abstract
1. Introduction
2. Research Methodology
3. Mechanisms: How Amino Acids May Preserve Muscle During Calorie Deficit
3.1. Metabolic Fate and Oxidation
3.2. Anabolic Signaling via mTORC1
3.3. Anti-Catabolic Mechanisms
3.4. Energy Substrate Role
3.5. Hormonal and Appetite-Related Effects
4. Evidence from Preclinical Trials
| Model | Intervention | Main Findings | |
|---|---|---|---|
| Pedrosa et al. [28] | Adult male Wistar rats under 50% food restriction | Leucine supplementation (0.59%) | Increased liver protein content but no effect on body fat |
| Faure et al. [29] | Aged Sprague Dawley rats with dietary restriction | Leucine or citrulline during refeeding | Improved muscle mass and function; only leucine increased muscle force |
| Bozadjieva Kramer et al. [30] | Post-vertical sleeve gastrectomy in mice | BCAA levels after bariatric surgery | Lower BCAA levels post-surgery; BCAA decrease not required for improved glucose tolerance |
| Zhang et al. [32] | Obese and lean mice with/without exercise | BCAA supplementation during running exercise | In obese mice, BCAA supplementation reversed exercise-induced improvements in insulin sensitivity; ↑ lipogenesis and ↓ Akt phosphorylation in adipose tissue |
| Binder et al. [33] | HFD-fed mice ± leucine | Leucine supplementation for 17 wks | Reduced fat mass, improved insulin sensitivity, and increased UCP-3; no benefit in already obese mice |
| Huang et al. [34] | HFD-fed mice + preadipocyte culture | BCAA supplementation | Inhibited adipogenesis via the NADPH-FTO-m6A pathway; reduced CDK2 and CCNA2 |
| Li et al. [35] | HFD-fed mice | Leucine supplementation | Prevented obesity, hyperglycemia, and dyslipidemia (details truncated) |
| Zhang et al. [36] | Mice on chow or HFDs | Leucine in drinking water (↑ intake) | ↓ Weight gain (32%), ↓ adiposity (25%), ↓ LDL (53%), ↑ UCP3 expression, ↓ glucose, and ↑ insulin sensitivity in HFD mice |
5. Evidence from Clinical Trials
5.1. Lifestyle-Induced Weight Loss
5.2. Bariatric Surgery
5.3. Pharmacological Weight Loss
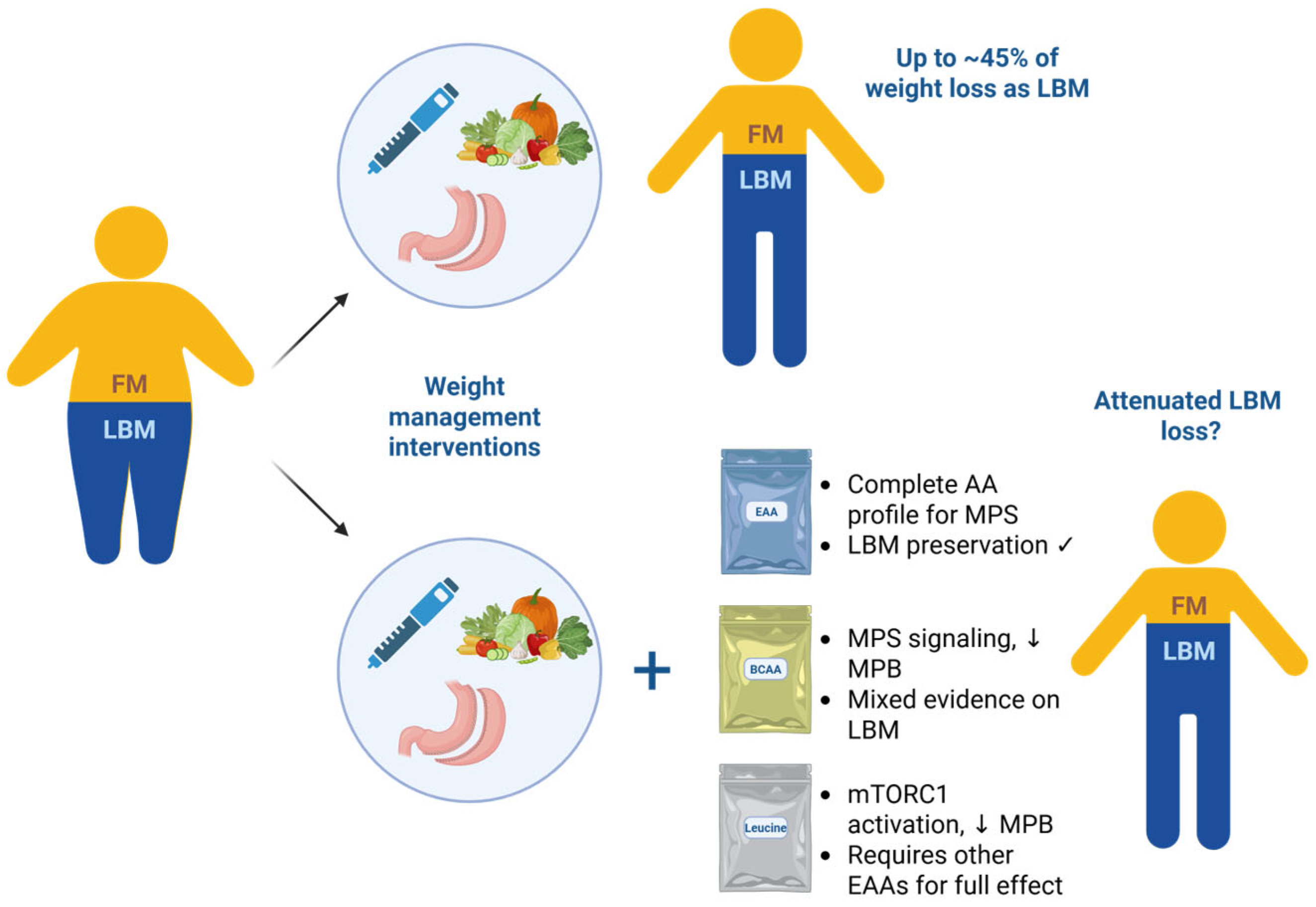
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCAA | Branched-chain amino acid |
| CP | Complex protein |
| EAA | Essential amino acid |
| GLP-1 | Glucagon-like peptide 1 |
| GLP-1 RA | Glucagon-like peptide 1 receptor agonist |
| HMB | β-Hydroxy β-methylbutyrate |
| LBM | Lean body mass |
| MAFbx | Muscle atrophy F-box (atrogin-1) |
| MPB | Muscle protein breakdown |
| MPS | Muscle protein synthesis |
| MuRF-1 | Muscle RING-finger protein-1 |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| OMM | Obesity management medication |
| VLEKD | Very-low-energy ketogenic diet |
| 1-RM | One-repetition maximum |
References
- Buckell, J.; Mei, X.W.; Clarke, P.; Aveyard, P.; Jebb, S.A. Weight loss interventions on health-related quality of life in those with moderate to severe obesity: Findings from an individual patient data meta-analysis of randomized trials. Obes. Rev. 2021, 22, e13317. [Google Scholar] [CrossRef] [PubMed]
- Tahrani, A.A.; Morton, J. Benefits of weight loss of 10% or more in patients with overweight or obesity: A review. Obesity 2022, 30, 802–840. [Google Scholar] [CrossRef] [PubMed]
- Linge, J.; Birkenfeld, A.L.; Neeland, I.J. Muscle Mass and Glucagon-Like Peptide-1 Receptor Agonists: Adaptive or Maladaptive Response to Weight Loss? Circulation 2024, 150, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Sylivris, A.; Mesinovic, J.; Scott, D.; Jansons, P. Body composition changes at 12 months following different surgical weight loss interventions in adults with obesity: A systematic review and meta-analysis of randomized control trials. Obes. Rev. 2022, 23, e13442. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Heymsfield, S.B. Fundamental Body Composition Principles Provide Context for Fat-Free and Skeletal Muscle Loss With GLP-1 RA Treatments. J. Endocr. Soc. 2024, 8, bvae164. [Google Scholar] [CrossRef]
- Beals, J.W.; Kayser, B.D.; Smith, G.I.; Schweitzer, G.G.; Kirbach, K.; Kearney, M.L.; Yoshino, J.; Rahman, G.; Knight, R.; Patterson, B.W.; et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes. Nat. Metab. 2023, 5, 1221–1235. [Google Scholar] [CrossRef]
- Stokes, T.; Hector, A.J.; Morton, R.W.; McGlory, C.; Phillips, S.M. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients 2018, 10, 180. [Google Scholar] [CrossRef]
- Weijs, P.J.M. Protein requirement in obesity. Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 27–32. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227S–231S. [Google Scholar] [CrossRef]
- Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: An update. Nutr. Res. Rev. 2024, 37, 273–286. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, S.; Ho, M.Y.; Ng, K.K.; Cheng, K.K. Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obes. Rev. 2025, 26, e13856. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Bond, P. Regulation of mTORC1 by growth factors, energy status, amino acids and mechanical stimuli at a glance. J. Int. Soc. Sports Nutr. 2016, 13, 8. [Google Scholar] [CrossRef]
- Fujita, S.; Dreyer, H.C.; Drummond, M.J.; Glynn, E.L.; Cadenas, J.G.; Yoshizawa, F.; Volpi, E.; Rasmussen, B.B. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007, 582, 813–823. [Google Scholar] [CrossRef]
- Gwin, J.A.; Church, D.D.; Allen, J.T.; Wilson, M.A.; Carrigan, C.T.; Murphy, N.E.; Varanoske, A.N.; Margolis, L.M.; Wolfe, R.R.; Ferrando, A.A.; et al. Consuming Whey Protein with Added Essential Amino Acids, Not Carbohydrate, Maintains Postexercise Anabolism While Underfed. Med. Sci. Sports Exerc. 2025, 57, 70–80. [Google Scholar] [CrossRef]
- Knudsen, J.R.; Li, Z.; Persson, K.W.; Li, J.; Henriquez-Olguin, C.; Jensen, T.E. Contraction-regulated mTORC1 and protein synthesis: Influence of AMPK and glycogen. J. Physiol. 2020, 598, 2637–2649. [Google Scholar] [CrossRef]
- Maretty, L.; Gill, D.; Simonsen, L.; Soh, K.; Zagkos, L.; Galanakis, M.; Sibbesen, J.; Iglesias, M.T.; Secher, A.; Valkenborg, D.; et al. Proteomic changes upon treatment with semaglutide in individuals with obesity. Nat. Med. 2025, 31, 267–277. [Google Scholar] [CrossRef]
- Borgenvik, M.; Apro, W.; Blomstrand, E. Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E510–E521. [Google Scholar] [CrossRef]
- Holecek, M. The role of skeletal muscle in the pathogenesis of altered concentrations of branched-chain amino acids (valine, leucine, and isoleucine) in liver cirrhosis, diabetes, and other diseases. Physiol. Res. 2021, 70, 293–305. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019, 29, 417–429.e414. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Hermans, W.J.H.; Holwerda, A.M.; Smeets, J.S.J.; Senden, J.M.; van Kranenburg, J.; Gijsen, A.P.; Wodzig, W.; Schierbeek, H.; Verdijk, L.B.; et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: A double-blind, randomized trial. Am. J. Clin. Nutr. 2019, 110, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134, 1583S–1587S. [Google Scholar] [CrossRef]
- Church, D.D.; Schwarz, N.A.; Spillane, M.B.; McKinley-Barnard, S.K.; Andre, T.L.; Ramirez, A.J.; Willoughby, D.S. l-Leucine Increases Skeletal Muscle IGF-1 but Does Not Differentially Increase Akt/mTORC1 Signaling and Serum IGF-1 Compared to Ursolic Acid in Response to Resistance Exercise in Resistance-Trained Men. J. Am. Coll. Nutr. 2016, 35, 627–638. [Google Scholar] [CrossRef]
- Yang, J.; Chi, Y.; Burkhardt, B.R.; Guan, Y.; Wolf, B.A. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr. Rev. 2010, 68, 270–279. [Google Scholar] [CrossRef]
- Yuan, X.W.; Han, S.F.; Zhang, J.W.; Xu, J.Y.; Qin, L.Q. Leucine supplementation improves leptin sensitivity in high-fat diet fed rats. Food Nutr. Res. 2015, 59, 27373. [Google Scholar] [CrossRef]
- Pedrosa, R.G.; Donato, J.; Pires, I.S.; Tirapegui, J. Leucine supplementation favors liver protein status but does not reduce body fat in rats during 1 week of food restriction. Appl. Physiol. Nutr. Metab. 2010, 35, 180–183. [Google Scholar] [CrossRef]
- Faure, C.; Raynaud-Simon, A.; Ferry, A.; Dauge, V.; Cynober, L.; Aussel, C.; Moinard, C. Leucine and citrulline modulate muscle function in malnourished aged rats. Amino Acids 2012, 42, 1425–1433. [Google Scholar] [CrossRef]
- Bozadjieva Kramer, N.; Evers, S.S.; Shin, J.H.; Silverwood, S.; Wang, Y.; Burant, C.F.; Sandoval, D.A.; Seeley, R.J. The Role of Elevated Branched-Chain Amino Acids in the Effects of Vertical Sleeve Gastrectomy to Reduce Weight and Improve Glucose Regulation. Cell Rep. 2020, 33, 108239. [Google Scholar] [CrossRef]
- Li, X.; Sun, D.; Zhou, T.; Ma, H.; Heianza, Y.; Liang, Z.; Bray, G.A.; Sacks, F.M.; Qi, L. Changes of Branched-Chain Amino Acids and Ectopic Fat in Response to Weight-loss Diets: The POUNDS Lost Trial. J. Clin. Endocrinol. Metab. 2020, 105, e3747–e3756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiang, L.; Huo, M.; Wu, Y.; Yu, M.; Lau, C.W.; Tian, D.; Gou, L.; Huang, Y.; Luo, J.Y.; et al. Branched-chain amino acid supplementation impairs insulin sensitivity and promotes lipogenesis during exercise in diet-induced obese mice. Obesity 2022, 30, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermudez-Silva, F.J.; Andre, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo, I.; Duchampt, A.; Clark, S.; Aubert, A.; et al. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS ONE 2013, 8, e74705. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Luo, Y.; Zeng, B.; Chen, Y.; Liu, Y.; Chen, W.; Liao, X.; Liu, Y.; Wang, Y.; Wang, X. Branched-chain amino acids prevent obesity by inhibiting the cell cycle in an NADPH-FTO-m6A coordinated manner. J. Nutr. Biochem. 2023, 122, 109437. [Google Scholar] [CrossRef]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1234–E1244. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef]
- Ooi, D.S.Q.; Ling, J.Q.R.; Sadananthan, S.A.; Velan, S.S.; Ong, F.Y.; Khoo, C.M.; Tai, E.S.; Henry, C.J.; Leow, M.K.S.; Khoo, E.Y.H.; et al. Branched-Chain Amino Acid Supplementation Does Not Preserve Lean Mass or Affect Metabolic Profile in Adults with Overweight or Obesity in a Randomized Controlled Weight Loss Intervention. J. Nutr. 2021, 151, 911–920. [Google Scholar] [CrossRef]
- Wilson, K. Obesity: Lifestyle Modification and Behavior Interventions. FP Essent. 2020, 492, 19–24. [Google Scholar]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Gregory, J.M.; Muldowney, J.A.; Engelhardt, B.G.; Tyree, R.; Marks-Shulman, P.; Silver, H.J.; Donahue, E.P.; Edgerton, D.S.; Winnick, J.J. Aerobic exercise training improves hepatic and muscle insulin sensitivity, but reduces splanchnic glucose uptake in obese humans with type 2 diabetes. Nutr. Diabetes 2019, 9, 25. [Google Scholar] [CrossRef]
- Chen, C.; Xie, L.; Zhang, M.; Shama; Cheng, K.K.Y.; Jia, W. The interplay between the muscle and liver in the regulation of glucolipid metabolism. J. Mol. Cell Biol. 2024, 15, mjad073. [Google Scholar] [CrossRef] [PubMed]
- Koudelkova, K.; Moro, C.; Gojda, J. Commentary on “Are Individuals with Type 2 Diabetes Metabolically Inflexible? A Systematic Review and Meta-Analysis”. Endocrinol. Diabetes Metab. 2025, 8, e70068. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Angeles-Valencia, M.; Morales-Gonzalez, A.; Madrigal-Santillan, E.O.; Morales-Martinez, M.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I.; Gutierrez-Salinas, J.; Esquivel-Chirino, C.; Chamorro-Cevallos, G.; et al. Oxidative Stress, Mitochondrial Function and Adaptation to Exercise: New Perspectives in Nutrition. Life 2021, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Reisman, T. Interventions to preserve beta-cell function in the management and prevention of type 2 diabetes. Curr. Diabetes Rep. 2013, 13, 252–260. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Chen, R.; He, R.; Qian, L.; Zou, J.; Luo, Y.; Zhang, Y.; Ji, M.; Liu, Y. Differences in Insulin Sensitivity, Secretion, and the Metabolic Clearance Rate of Glucose in Newly Diagnosed Type 2 Diabetes Mellitus Patients: The Influences of Body Mass Index and Fatty Liver. Metab. Syndr. Relat. Disord. 2022, 20, 451–458. [Google Scholar] [CrossRef]
- Jachthuber Trub, C.; Balikcioglu, M.; Freemark, M.; Bain, J.; Muehlbauer, M.; Ilkayeva, O.; White, P.J.; Armstrong, S.; Ostbye, T.; Grambow, S.; et al. Impact of lifestyle Intervention on branched-chain amino acid catabolism and insulin sensitivity in adolescents with obesity. Endocrinol. Diabetes Metab. 2021, 4, e00250. [Google Scholar] [CrossRef]
- Ardestani, A.; Lupse, B.; Kido, Y.; Leibowitz, G.; Maedler, K. mTORC1 Signaling: A Double-Edged Sword in Diabetic beta Cells. Cell Metab. 2018, 27, 314–331. [Google Scholar] [CrossRef]
- Luan, C.; Wang, Y.; Li, J.; Zhou, N.; Song, G.; Ni, Z.; Xu, C.; Tang, C.; Fu, P.; Wang, X.; et al. Branched-Chain Amino Acid Supplementation Enhances Substrate Metabolism, Exercise Efficiency and Reduces Post-Exercise Fatigue in Active Young Males. Nutrients 2025, 17, 1290. [Google Scholar] [CrossRef]
- Garcia-Flores, L.A.; Green, C.L.; Mitchell, S.E.; Promislow, D.E.L.; Lusseau, D.; Douglas, A.; Speakman, J.R. The effects of graded calorie restriction XVII: Multitissue metabolomics reveals synthesis of carnitine and NAD, and tRNA charging as key pathways. Proc. Natl. Acad. Sci. USA 2021, 118, e2101977118. [Google Scholar] [CrossRef]
- Simonson, M.; Boirie, Y.; Guillet, C. Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 2020, 21, 341–353. [Google Scholar] [CrossRef]
- Kokura, Y.; Ueshima, J.; Saino, Y.; Maeda, K. Enhanced protein intake on maintaining muscle mass, strength, and physical function in adults with overweight/obesity: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2024, 63, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Verde, L.; D’Orsi, V.; Caprio, M.; Gorini, S.; Savastano, S.; Colao, A.; Muscogiuri, G.; Barrea, L. Supplementation with essential amino acids in the early stage of carbohydrate reintroduction after a very-low energy ketogenic therapy (VLEKT) improves body cell mass, muscle strength and inflammation. J. Transl. Med. 2025, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.H.; Miller, S.; Schutzler, S.; Deutz, N.; Wolfe, R.R. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr. J. 2012, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Coker, M.S.; Barati, Z.; Murphy, C.J.; Bateman, T.; Newcomer, B.R.; Wolfe, R.R.; Coker, R.H. Essential amino acid enriched meal replacement improves body composition and physical function in obese older adults: A randomized controlled trial. Clin. Nutr. ESPEN 2022, 51, 104–111. [Google Scholar] [CrossRef]
- Dudgeon, W.D.; Kelley, E.P.; Scheett, T.P. In a single-blind, matched group design: Branched-chain amino acid supplementation and resistance training maintains lean body mass during a caloric restricted diet. J. Int. Soc. Sports Nutr. 2016, 13, 1. [Google Scholar] [CrossRef]
- Brunani, A.; Cancello, R.; Gobbi, M.; Lucchetti, E.; Di Guglielmo, G.; Maestrini, S.; Cattaldo, S.; Pitera, P.; Ruocco, C.; Milesi, A.; et al. Comparison of Protein- or Amino Acid-Based Supplements in the Rehabilitation of Men with Severe Obesity: A Randomized Controlled Pilot Study. J. Clin. Med. 2023, 12, 4257. [Google Scholar] [CrossRef]
- Almeida, C.C.; Alvares, T.S.; Costa, M.P.; Conte-Junior, C.A. Protein and Amino Acid Profiles of Different Whey Protein Supplements. J. Diet. Suppl. 2016, 13, 313–323. [Google Scholar] [CrossRef]
- Kudelka, W.; Kowalska, M.; Popis, M. Quality of Soybean Products in Terms of Essential Amino Acids Composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef]
- Ooi, D.S.Q.; Ling, J.Q.R.; Ong, F.Y.; Tai, E.S.; Henry, C.J.; Leow, M.K.S.; Khoo, E.Y.H.; Tan, C.S.; Chong, M.F.F.; Khoo, C.M.; et al. Branched Chain Amino Acid Supplementation to a Hypocaloric Diet Does Not Affect Resting Metabolic Rate but Increases Postprandial Fat Oxidation Response in Overweight and Obese Adults after Weight Loss Intervention. Nutrients 2021, 13, 4245. [Google Scholar] [CrossRef]
- Ram Sohan, P.; Mahakalkar, C.; Kshirsagar, S.; Bikkumalla, S.; Reddy, S.; Hatewar, A.; Dixit, S. Long-Term Effectiveness and Outcomes of Bariatric Surgery: A Comprehensive Review of Current Evidence and Emerging Trends. Cureus 2024, 16, e66500. [Google Scholar] [CrossRef]
- Mulla, C.M.; Middelbeek, R.J.W.; Patti, M.E. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. Acad. Sci. 2018, 1411, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Yan, K. Recent advances in the effect of adipose tissue inflammation on insulin resistance. Cell Signal 2024, 120, 111229. [Google Scholar] [CrossRef]
- Castagneto-Gissey, L.; Mingrone, G. Insulin sensitivity and secretion modifications after bariatric surgery. J. Endocrinol. Investig. 2012, 35, 692–698. [Google Scholar] [CrossRef]
- Tan, H.C.; Khoo, C.M.; Tan, M.Z.; Kovalik, J.P.; Ng, A.C.; Eng, A.K.; Lai, O.F.; Ching, J.H.; Tham, K.W.; Pasupathy, S. The Effects of Sleeve Gastrectomy and Gastric Bypass on Branched-Chain Amino Acid Metabolism 1 Year After Bariatric Surgery. Obes. Surg. 2016, 26, 1830–1835. [Google Scholar] [CrossRef]
- Barati-Boldaji, R.; Esmaeilinezhad, Z.; Babajafari, S.; Kazemi, A.; Clark, C.C.T.; Mazidi, M.; Ofori-Asenso, R.; Haghighat, N.; Shafiee, M.; Mazloomi, S.M. Bariatric surgery reduces branched-chain amino acids’ levels: A systematic review and meta-analysis. Nutr. Res. 2021, 87, 80–90. [Google Scholar] [CrossRef]
- Yoon, M.S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef]
- Nuijten, M.A.H.; Eijsvogels, T.M.H.; Monpellier, V.M.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13370. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Butsch, W.S.; Christensen, S.M.; Hamdy, O.; Li, Z.; Prado, C.M.; Heymsfield, S.B. Strategies for minimizing muscle loss during use of incretin-mimetic drugs for treatment of obesity. Obes. Rev. 2025, 26, e13841. [Google Scholar] [CrossRef]
- Golzarand, M.; Toolabi, K.; Mirmiran, P. The effects of protein intake higher than the recommended value on body composition changes after bariatric surgery: A meta-analysis of randomized controlled trials. Clin. Nutr. 2024, 43, 708–718. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Lahav, Y.; Cohen, T.R.; Bacon, S.L.; Buch, A.; Moize, V.; Sherf-Dagan, S. Is There a Need to Reassess Protein Intake Recommendations Following Metabolic Bariatric Surgery? Curr. Obes. Rep. 2025, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Afsar, N.; Ozdogan, Y. Protein supplementation preserves muscle mass in persons against sleeve gastrectomy. Front. Nutr. 2024, 11, 1476258. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, L.; Valentini, R.; Zattarin, A.; Belligoli, A.; Bettini, S.; Vettor, R.; Foletto, M.; Spinella, P.; Busetto, L. Assessment of Protein Intake in the First Three Months after Sleeve Gastrectomy in Patients with Severe Obesity. Nutrients 2021, 13, 771. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Kamali, Z.; Mirmiran, P.; Barzin, M.; Khalaj, A. Association of postoperative dietary macronutrient content and quality with total weight loss and fat-free mass loss at midterm after sleeve gastrectomy. Nutrition 2024, 120, 112331. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Madura, J.A.; Roust, L.R. Essential amino acid ingestion as an efficient nutritional strategy for the preservation of muscle mass following gastric bypass surgery. Nutrition 2016, 32, 9–13. [Google Scholar] [CrossRef]
- Schiavo, L.; Santella, B.; Paolini, B.; Rahimi, F.; Giglio, E.; Martinelli, B.; Boschetti, S.; Bertolani, L.; Gennai, K.; Arolfo, S.; et al. Adding Branched-Chain Amino Acids and Vitamin D to Whey Protein Is More Effective than Protein Alone in Preserving Fat Free Mass and Muscle Strength in the First Month after Sleeve Gastrectomy. Nutrients 2024, 16, 1448. [Google Scholar] [CrossRef]
- Maimoun, L.; Lefebvre, P.; Jaussent, A.; Fouillade, C.; Mariano-Goulart, D.; Nocca, D. Body composition changes in the first month after sleeve gastrectomy based on gender and anatomic site. Surg. Obes. Relat. Dis. 2017, 13, 780–787. [Google Scholar] [CrossRef]
- Ranjbar, M.; Fallah, M.; Djafarian, K.; Mohammadi, H.; Mohammadi Farsani, G.; Shab-Bidar, S. The effects of protein supplementation on body composition after bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Obesity 2025, 33, 1027–1036. [Google Scholar] [CrossRef]
- Comas Martinez, M.; Fidilio Meli, E.; Palmas Candia, F.; Cordero, E.; Hernandez, I.; Vilallonga, R.; Burgos, R.; Vila, A.; Simo, R.; Ciudin, A. Protein Supplementation with Short Peptides Prevents Early Muscle Mass Loss after Roux-en-Y-Gastric Bypass. Nutrients 2022, 14, 5095. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2025, 392, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Kitzman, D.W.; Shah, S.J.; Ronnback, C.; Abildstrom, S.Z.; Liisberg, K.; et al. Inflammation in Obesity-Related HFpEF: The STEP-HFpEF Program. J. Am. Coll. Cardiol. 2024, 84, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K.; Fernandez Lando, L.; Bray, R.; Brouwers, B.; Rodriguez, A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022, 10, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Kakegawa, T.; Sugimoto, K.; Saito, K.; Yunaiyama, D.; Araki, Y.; Wada, T.; Takahashi, H.; Yoshimasu, Y.; Takeuchi, H.; Itoi, T. Favorable liver and skeletal muscle changes in patients with MASLD and T2DM receiving glucagon-like peptide-1 receptor agonist: A prospective cohort study. Medicine 2024, 103, e38444. [Google Scholar] [CrossRef]
- Pandey, A.; Patel, K.V.; Segar, M.W.; Ayers, C.; Linge, J.; Leinhard, O.D.; Anker, S.D.; Butler, J.; Verma, S.; Joshi, P.H.; et al. Effect of liraglutide on thigh muscle fat and muscle composition in adults with overweight or obesity: Results from a randomized clinical trial. J. Cachexia Sarcopenia Muscle 2024, 15, 1072–1083. [Google Scholar] [CrossRef]
- Sattar, N.; Neeland, I.J.; Dahlqvist Leinhard, O.; Fernandez Lando, L.; Bray, R.; Linge, J.; Rodriguez, A. Tirzepatide and muscle composition changes in people with type 2 diabetes (SURPASS-3 MRI): A post-hoc analysis of a randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 2025, 13, 482–493. [Google Scholar] [CrossRef]
- Mastrototaro, L.; Roden, M. Insulin resistance and insulin sensitizing agents. Metabolism 2021, 125, 154892. [Google Scholar] [CrossRef]
- Thomas, M.K.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D.A. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 388–396. [Google Scholar] [CrossRef]
- Miguens-Gomez, A.; Casanova-Marti, A.; Blay, M.T.; Terra, X.; Beltran-Debon, R.; Rodriguez-Gallego, E.; Ardevol, A.; Pinent, M. Glucagon-like peptide-1 regulation by food proteins and protein hydrolysates. Nutr. Res. Rev. 2021, 34, 259–275. [Google Scholar] [CrossRef]
- Kido, K.; Sase, K.; Yokokawa, T.; Fujita, S. Enhanced skeletal muscle insulin sensitivity after acute resistance-type exercise is upregulated by rapamycin-sensitive mTOR complex 1 inhibition. Sci. Rep. 2020, 10, 8509. [Google Scholar] [CrossRef]
- Dubin, R.L.; Heymsfield, S.B.; Ravussin, E.; Greenway, F.L. Glucagon-like peptide-1 receptor agonist-based agents and weight loss composition: Filling the gaps. Diabetes Obes. Metab. 2024, 26, 5503–5518. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Lin, C.; Cai, X.; Wang, J.; Wang, Y.; Lv, F.; Yang, W.; Ji, L. Characterizing body composition modifying effects of a glucagon-like peptide 1 receptor-based agonist: A meta-analysis. Diabetes Obes. Metab. 2025, 27, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Mantzoros, C.S. Effect of glucagon-like peptide-1 receptor agonists and co-agonists on body composition: Systematic review and network meta-analysis. Metabolism 2025, 164, 156113. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Hall, K.D.; Klein, S. Is Weight Loss-Induced Muscle Mass Loss Clinically Relevant? JAMA 2024, 332, 9–10. [Google Scholar] [CrossRef]
- Caturano, A.; Amaro, A.; Berra, C.C.; Conte, C. Sarcopenic obesity and weight loss-induced muscle mass loss. Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 339–350. [Google Scholar] [CrossRef]
- Look, M.; Dunn, J.P.; Kushner, R.F.; Cao, D.; Harris, C.; Gibble, T.H.; Stefanski, A.; Griffin, R. Body composition changes during weight reduction with tirzepatide in the SURMOUNT-1 study of adults with obesity or overweight. Diabetes Obes. Metab. 2025, 27, 2720–2729. [Google Scholar] [CrossRef]
- Gudzune, K.A.; Stefanski, A.; Cao, D.; Mojdami, D.; Wang, F.; Ahmad, N.; Ling Poon, J. Association between weight reduction achieved with tirzepatide and quality of life in adults with obesity: Results from the SURMOUNT-1 study. Diabetes Obes. Metab. 2025, 27, 539–550. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Verma, S.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Jon Jensen, T.; Rasmussen, S.; Erlang Marstrand, P.; Petrie, M.C.; Shah, S.J.; et al. Effects of Semaglutide on Symptoms, Function, and Quality of Life in Patients With Heart Failure With Preserved Ejection Fraction and Obesity: A Prespecified Analysis of the STEP-HFpEF Trial. Circulation 2024, 149, 204–216. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Le Grazie, G.; Caruso, I.; Marrano, N.; Biondi, G.; D’Oria, R.; Sorice, G.P.; Natalicchio, A.; Perrini, S.; et al. Mini Review: Effect of GLP-1 Receptor Agonists and SGLT-2 Inhibitors on the Growth Hormone/IGF Axis. Front Endocrinol 2022, 13, 846903. [Google Scholar] [CrossRef]
- Takegaki, J.; Sase, K.; Yasuda, J.; Shindo, D.; Kato, H.; Toyoda, S.; Yamada, T.; Shinohara, Y.; Fujita, S. The Effect of Leucine-Enriched Essential Amino Acid Supplementation on Anabolic and Catabolic Signaling in Human Skeletal Muscle after Acute Resistance Exercise: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients 2020, 12, 2421. [Google Scholar] [CrossRef]
- Christensen, S.; Robinson, K.; Thomas, S.; Williams, D.R. Dietary intake by patients taking GLP-1 and dual GIP/GLP-1 receptor agonists: A narrative review and discussion of research needs. Obes. Pillars 2024, 11, 100121. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, J.C.; Costa, J.G.; Haynes, A.; Naylor, L.H.; Fegan, P.G.; Yeap, B.B.; Green, D.J. Incretin-Based Weight Loss Pharmacotherapy: Can Resistance Exercise Optimize Changes in Body Composition? Diabetes Care 2024, 47, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Linge, J.; Birkenfeld, A.L. Changes in lean body mass with glucagon-like peptide-1-based therapies and mitigation strategies. Diabetes Obes. Metab. 2024, 26 (Suppl. S4), 16–27. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; Koscien, C.P.; Monteyne, A.J.; Wall, B.T.; Stephens, F.B. Association of postprandial postexercise muscle protein synthesis rates with dietary leucine: A systematic review. Physiol. Rep. 2023, 11, e15775. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.B.; O’Brien, P.E. Changes in fat-free mass during significant weight loss: A systematic review. Int. J. Obes. 2007, 31, 743–750. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Wolfe, R.R.; Hirsch, K.R.; Church, D.D.; Kviatkovsky, S.A.; Roberts, M.D.; Stout, J.R.; Gonzalez, D.E.; Sowinski, R.J.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Effects of essential amino acid supplementation on exercise and performance. J. Int. Soc. Sports Nutr. 2023, 20, 2263409. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, C.; Yan, Q.; Tan, Z.; Kang, J.; Tang, S. Elucidating the underlying mechanism of amino acids to regulate muscle protein synthesis: Effect on human health. Nutrition 2022, 103–104, 111797. [Google Scholar] [CrossRef]
- Beals, J.W.; Sukiennik, R.A.; Nallabelli, J.; Emmons, R.S.; van Vliet, S.; Young, J.R.; Ulanov, A.V.; Li, Z.; Paluska, S.A.; De Lisio, M.; et al. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am. J. Clin. Nutr. 2016, 104, 1014–1022. [Google Scholar] [CrossRef]
- Guillet, C.; Delcourt, I.; Rance, M.; Giraudet, C.; Walrand, S.; Bedu, M.; Duche, P.; Boirie, Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J. Clin. Endocrinol. Metab. 2009, 94, 3044–3050. [Google Scholar] [CrossRef]
- Beals, J.W.; Burd, N.A.; Moore, D.R.; van Vliet, S. Obesity Alters the Muscle Protein Synthetic Response to Nutrition and Exercise. Front. Nutr. 2019, 6, 87. [Google Scholar] [CrossRef]
- Kouw, I.W.K.; van Dijk, J.W.; Horstman, A.M.H.; Kramer, I.F.; Goessens, J.P.B.; van Dielen, F.M.H.; Verdijk, L.B.; van Loon, L.J.C. Basal and Postprandial Myofibrillar Protein Synthesis Rates Do Not Differ between Lean and Obese Middle-Aged Men. J. Nutr. 2019, 149, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Beals, J.W.; Skinner, S.K.; McKenna, C.F.; Poozhikunnel, E.G.; Farooqi, S.A.; van Vliet, S.; Martinez, I.G.; Ulanov, A.V.; Li, Z.; Paluska, S.A.; et al. Altered anabolic signalling and reduced stimulation of myofibrillar protein synthesis after feeding and resistance exercise in people with obesity. J. Physiol. 2018, 596, 5119–5133. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.I.; Xhuti, D.; de Maat, N.M.; Hettinga, B.P.; Tarnopolsky, M.A. Obesity and Metabolic Disease Impair the Anabolic Response to Protein Supplementation and Resistance Exercise: A Retrospective Analysis of a Randomized Clinical Trial with Implications for Aging, Sarcopenic Obesity, and Weight Management. Nutrients 2024, 16, 4407. [Google Scholar] [CrossRef] [PubMed]
- Smeuninx, B.; McKendry, J.; Wilson, D.; Martin, U.; Breen, L. Age-Related Anabolic Resistance of Myofibrillar Protein Synthesis Is Exacerbated in Obese Inactive Individuals. J. Clin. Endocrinol. Metab. 2017, 102, 3535–3545. [Google Scholar] [CrossRef]
- Pinel, A.; Guillet, C.; Capel, F.; Pouget, M.; De Antonio, M.; Pereira, B.; Topinkova, E.; Eglseer, D.; Barazzoni, R.; Cruz-Jentoft, A.J.; et al. Identification of factors associated with sarcopenic obesity development: Literature review and expert panel voting. Clin. Nutr. 2024, 43, 1414–1424. [Google Scholar] [CrossRef]
- Hooper, L.; Liu, S.; Pai, M.P. GLP-1RA-induced delays in gastrointestinal motility: Predicted effects on coadministered drug absorption by PBPK analysis. Pharmacotherapy 2025, 45, 211–219. [Google Scholar] [CrossRef]
- Jalleh, R.J.; Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Umapathysivam, M.M.; Wu, T.; Quast, D.R.; Plummer, M.P.; Nauck, M.A.; Horowitz, M. Physiology and Pharmacology of Effects of GLP-1-based Therapies on Gastric, Biliary and Intestinal Motility. Endocrinology 2024, 166, bqae155. [Google Scholar] [CrossRef]
- Trommelen, J.; Tomé, D.; van Loon, L.J.C. Gut amino acid absorption in humans: Concepts and relevance for postprandial metabolism. Clin. Nutr. Open Sci. 2021, 36, 43–55. [Google Scholar] [CrossRef]
- Calvarysky, B.; Dotan, I.; Shepshelovich, D.; Leader, A.; Cohen, T.D. Drug-Drug Interactions Between Glucagon-Like Peptide 1 Receptor Agonists and Oral Medications: A Systematic Review. Drug Saf. 2024, 47, 439–451. [Google Scholar] [CrossRef]
- Li, W.; Kirchner, T.; Ho, G.; Bonilla, F.; D’Aquino, K.; Littrell, J.; Zhang, R.; Jian, W.; Qiu, X.; Zheng, S.; et al. Amino acids are sensitive glucagon receptor-specific biomarkers for glucagon-like peptide-1 receptor/glucagon receptor dual agonists. Diabetes Obes. Metab. 2020, 22, 2437–2450. [Google Scholar] [CrossRef]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Grady, J.J.; Volpi, E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E745–E754. [Google Scholar] [CrossRef]
- Meloni, A.R.; DeYoung, M.B.; Lowe, C.; Parkes, D.G. GLP-1 receptor activated insulin secretion from pancreatic beta-cells: Mechanism and glucose dependence. Diabetes Obes. Metab. 2013, 15, 15–27. [Google Scholar] [CrossRef]
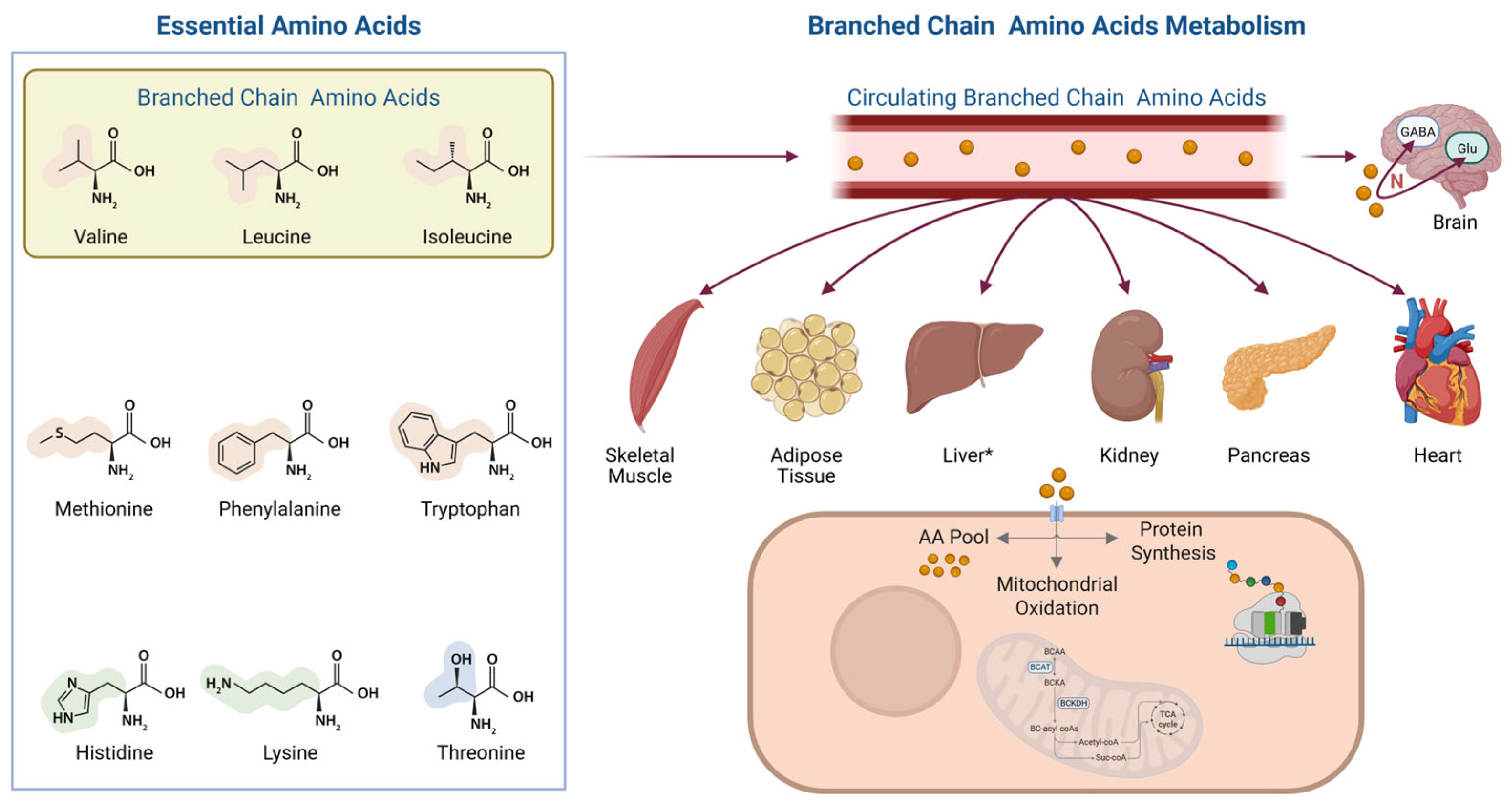
| Model | Intervention | Main Findings | |
|---|---|---|---|
| Ooi et al. [59] | RCT; 111 Chinese adults with overweight/obesity | BCAA + standard-protein diets vs. the placebo or high-protein diets | BCAA ↑ postprandial fat oxidation and ↓ carb oxidation; no RMR benefit |
| Annunziata et al. [52] | Women with grade I obesity after the ketogenic diet | 8 g/day of EAAs during the reintroduction phase | EAAs improved body composition (↓ fat mass and ↑ muscle mass), ↑ muscle strength, and ↓ hs-CRP vs. the controls |
| Coker et al. [53] | Older individuals on caloric restriction | EAAMR vs. competitive meal replacement | Both groups lost ~7% body weight; EAAMR showed greater fat loss and ↑skeletal muscle protein FSR |
| Coker et al. [54] | Older adults with obesity | EMR vs. Optifast® (once/day for 4 weeks) | EMR reduced fat and intrahepatic lipid, ↑ thigh muscle CSA, and ↑ 6 min walk distance |
| Dudgeon et al. [55] | Resistance-trained males on hypocaloric diet | BCAA vs. CHO with resistance training (8 weeks) | The BCAA group preserved lean mass and improved strength vs. CHO (which lost lean mass) |
| Brunani et al. [56] | Men with obesity (<65 years, inpatient) | PD-E07 (EAA + TCA), BCAA, and protein vs. the control | Only PD-E07 led to ↑ muscle mass; other groups lost or did not improve muscle mass |
| Model | Intervention | Main Findings | |
|---|---|---|---|
| Afsar et al. [72] | RCT-like design with two parallel groups (BSD vs. BSD + PS) | 15 g/day protein supplement post-sleeve gastrectomy plus dietitian support | Protein supplementation with dietitian-guided nutrition led to better maintenance of muscle mass compared to the diet alone. Nutritional intake remained below recommended levels in both groups. |
| Bertoni et al. [73] | Prospective observational | Assessment of protein intake and the use of supplements post-sleeve gastrectomy at 1 and 3 months | Protein intake from food was inadequate. Supplements improved intake, but it was still below recommended levels. Compliance dropped over time. |
| Moslehi et al. [74] | Cross-sectional | Analysis of dietary macronutrient quality and quantity 2–4 years after SG | Higher protein and fiber intake associated with greater total weight loss and less fat-free mass loss. Poor quality carbs and high fat linked to worse outcomes. |
| Schiavo et al. [76] | Prospective cohort | Supplementation with protein + BCAA + vitamin D vs. protein alone after SG | Combined supplementation resulted in greater fat mass loss and lower declines in FFM and muscle strength than protein alone. |
| Maïmoun et al. [77] | Longitudinal prospective | Gender-stratified body composition analysis pre-and 1-month post-SG | Both fat and lean mass decreased post-SG; patterns varied by gender and anatomical site. Men lost more trunk FM; women had lower limb muscle decline. |
| Comas Martínez et al. [79] | Prospective interventional | Comparison of short peptide-based, HMB-enriched, and complex protein formulas post-RYGB | SPB group had significantly lower %FFM loss despite higher total weight loss, compared to the CP and HMB groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannavaro, D.; Leva, F.; Caturano, A.; Berra, C.C.; Bonfrate, L.; Conte, C. Optimizing Body Composition During Weight Loss: The Role of Amino Acid Supplementation. Nutrients 2025, 17, 2000. https://doi.org/10.3390/nu17122000
Cannavaro D, Leva F, Caturano A, Berra CC, Bonfrate L, Conte C. Optimizing Body Composition During Weight Loss: The Role of Amino Acid Supplementation. Nutrients. 2025; 17(12):2000. https://doi.org/10.3390/nu17122000
Chicago/Turabian StyleCannavaro, Daniele, Francesco Leva, Alfredo Caturano, Cesare Celeste Berra, Leonilde Bonfrate, and Caterina Conte. 2025. "Optimizing Body Composition During Weight Loss: The Role of Amino Acid Supplementation" Nutrients 17, no. 12: 2000. https://doi.org/10.3390/nu17122000
APA StyleCannavaro, D., Leva, F., Caturano, A., Berra, C. C., Bonfrate, L., & Conte, C. (2025). Optimizing Body Composition During Weight Loss: The Role of Amino Acid Supplementation. Nutrients, 17(12), 2000. https://doi.org/10.3390/nu17122000








