Kidney-Gut Axis in Chronic Kidney Disease: Therapeutic Perspectives from Microbiota Modulation and Nutrition
Abstract
1. Introduction
2. Methods
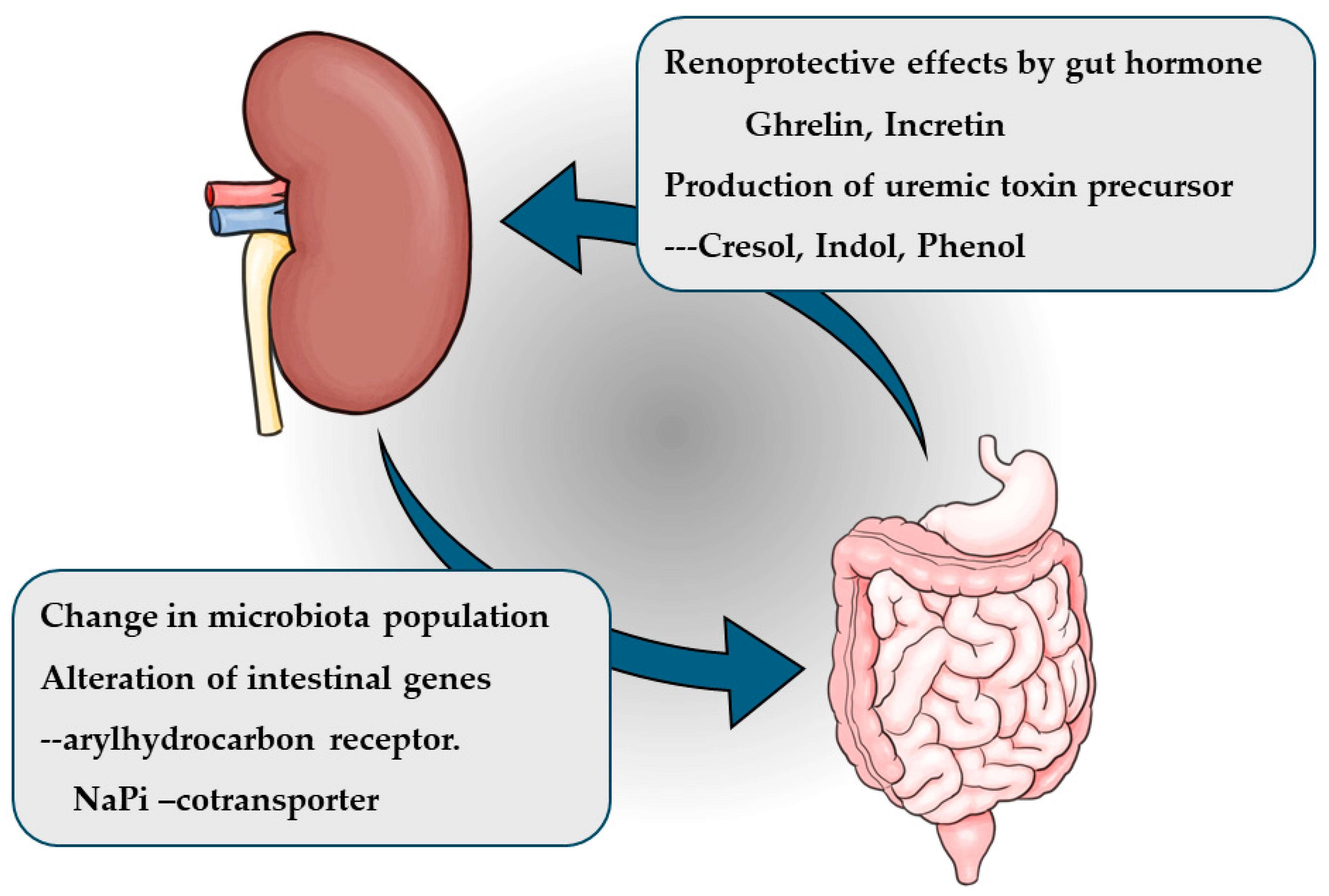
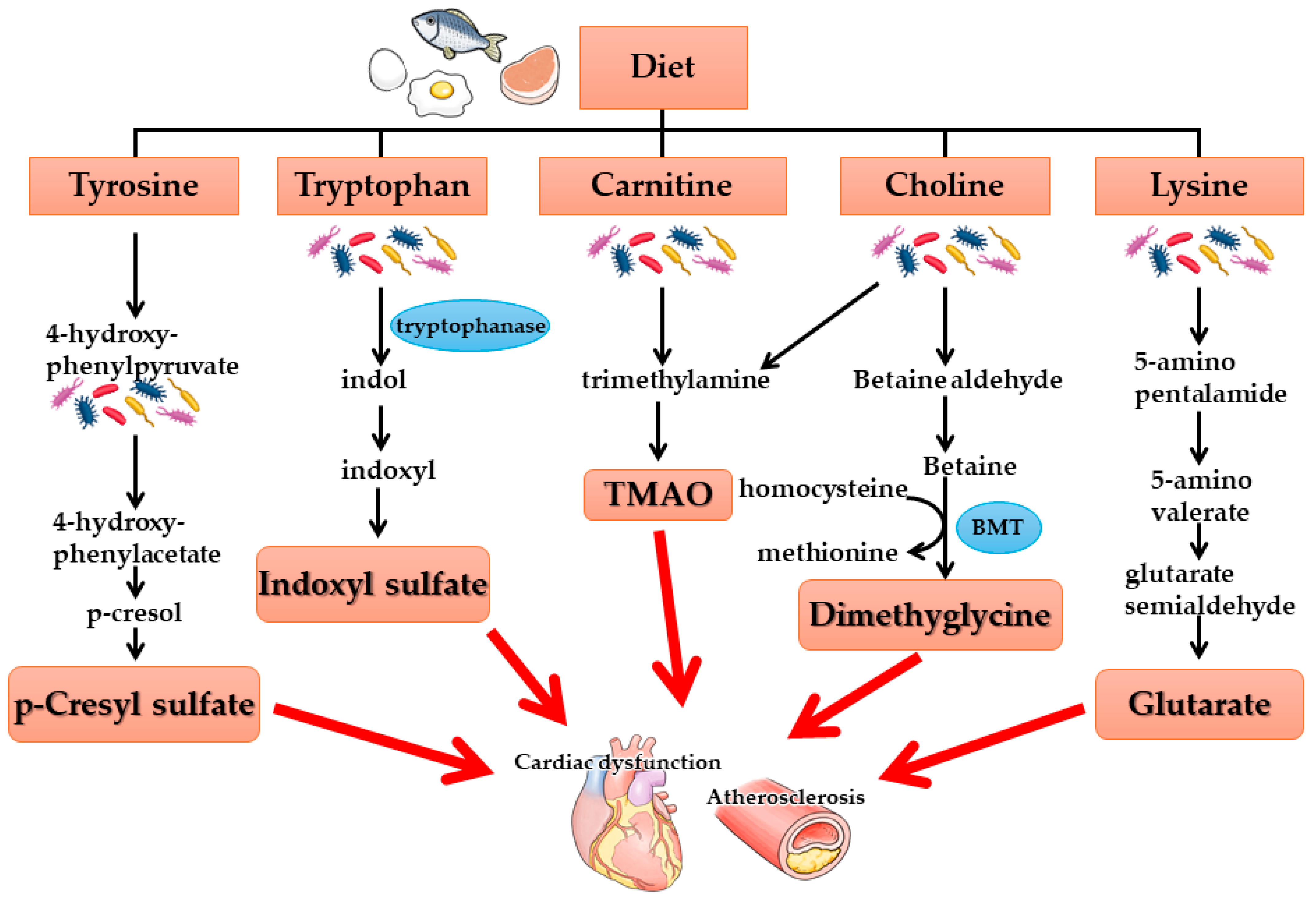
3. Kidney-Gut Axis and Intestinal Microbiota
4. Treatment Strategies Targeting Kidney-Gut Axis in CKD
4.1. Prebiotics, Probiotics, and Synbiotics
4.2. Impact of Non-Antibiotic Drugs on Human Gut Bacteria
5. Nutritional Therapy for Kidney-Gut Axis
5.1. Low-Protein Diet
5.2. Fiber Diet
5.3. Whole Grains
5.4. Mediterranean Diet and Other Healthy Dietary Patterns
5.5. Novel Strategies Targeting Kidney-Gut Axis
6. Conclusions
- A disturbed kidney-gut axis should be considered in CKD clinical practice.
- The partial correction of a disturbed kidney-gut axis by a single treatment bundle, including biotics therapy, protein-restricted diet, dietary fiber, and whole grains, is not effective in halting the progression of CKD, although it may have some favorable effects on patients’ quality of life.
- The switch or shift to the healthy whole dietary pattern—involving the shift or adherence to a plant-based diet such as the MD and harboring multiple therapeutic strategies targeting the kidney-gut axis—is the most plausible measure for CKD treatment.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.Y.; Chen, D.Q.; Chen, L.; Liu, J.R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Microbiome–metabolome reveals the contribution of the gut–kidney axis in kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Tajima, T.; Hasegawa, K.; Kanda, T.; Tokuyama, H.; Hayashi, K.; Itoh, H. Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol. Dial. Transplant. 2016, 31, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.; et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Barnes, J.L.; Astroth, K.S. Gastrointestinal microbiota in patients with chronic kidney disease: A systematic review. Adv. Nutr. 2019, 10, 888–901. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Xu, X.D.; Tang, Y.Y.; Jiang, H.L.; Li, L.; Xia, W.J.; Cui, N.; Bai, J.; Dai, Z.M.; et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ. Res. 2022, 131, e120–e134. [Google Scholar] [CrossRef]
- Schepers, E.; Glorieux, G.; Vanholder, R. The gut: The forgotten organ in uremia? Blood Purif. 2010, 29, 130–136. [Google Scholar] [CrossRef]
- Simeoni, M.; Citraro, M.L.; Cerantonio, A.; Deodato, F.; Provenzano, M.; Cianfrone, P.; Capria, M.; Corrado, S.; Libri, E.; Comi, A.; et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur. J. Nutr. 2019, 58, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Zarrati-Mojarrad, M.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef] [PubMed]
- De Mauri, A.; Carrera, D.; Bagnati, M.; Rolla, R.; Vidali, M.; Chiarinotti, D.; Pane, M.; Amoruso, A.; Del Piano, M. Probiotics-Supplemented Low-Protein Diet for Microbiota Modulation in Patients with Advanced Chronic Kidney Disease (ProLowCKD): Results from a Placebo-Controlled Randomized Trial. Nutrients 2022, 14, 1637. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Kelly, J.T.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology II (SYNERGY II): A Feasibility Randomized Controlled Trial. Nutrients 2021, 13, 4481. [Google Scholar] [CrossRef]
- McFarlane, C.; Ramos, C.I.; Johnson, D.W.; Campbell, K.L. Prebiotic, Probiotic, and Synbiotic Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-analysis. J. Ren. Nutr. 2019, 29, 209–220. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Li, J.; Zhang, W.; Ou, S. Probiotics, Prebiotics, and Synbiotics for Patients on Dialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2023, 33, 126–139. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, J.; Yang, H.; Wang, D.; Zhang, Y.; Yang, Y.; Xing, G.; Kon, V. Biotic Supplements in Patients with Chronic Kidney Disease: Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2022, 32, 10–21. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Matsui, A.; Yoshifuji, A.; Irie, J.; Tajima, T.; Uchiyama, K.; Itoh, T.; Wakino, S.; Itoh, H. Canagliflozin protects the cardiovascular system through effects on the gut environment in non-diabetic nephrectomized rats. Clin. Exp. Nephrol. 2023, 27, 295–308. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Matsui, A.; Hasegawa, K.; Tokuyama, H.; Hayashi, K.; Itoh, H. Oral adsorbent AST-120 ameliorates gut environment and protects against the progression of renal impairment in CKD rats. Clin. Exp. Nephrol. 2018, 22, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Schulman, G.; Berl, T.; Beck, G.J.; Remuzzi, G.; Ritz, E.; Arita, K.; Kato, A.; Shimizu, M. Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKD. J. Am. Soc. Nephrol. 2015, 26, 1732–1746. [Google Scholar] [CrossRef] [PubMed]
- Cha, R.H.; Kang, S.W.; Park, C.W.; Cha, D.R.; Na, K.Y.; Kim, S.G.; Yoon, S.A.; Han, S.Y.; Chang, J.H.; Park, S.K.; et al. A Randomized, Controlled Trial of Oral Intestinal Sorbent AST-120 on Renal Function Deterioration in Patients with Advanced Renal Dysfunction. Clin. J. Am. Soc. Nephrol. 2016, 11, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Riccio, E.; Sabbatini, M.; Bruzzese, D.; Grumetto, L.; Marchetiello, C.; Amicone, M.; Andreucci, M.; Guida, B.; Passaretti, D.; Russo, G.; et al. Plasma p-cresol lowering effect of sevelamer in non-dialysis CKD patients: Evidence from a randomized controlled trial. Clin. Exp. Nephrol. 2018, 22, 529–538. [Google Scholar] [CrossRef]
- Billing, A.M.; Kim, Y.C.; Gullaksen, S.; Schrage, B.; Raabe, J.; Hutzfeldt, A.; Demir, F.; Kovalenko, E.; Lassé, M.; Dugourd, A.; et al. Metabolic Communication by SGLT2 Inhibition. Circulation 2024, 149, 860–884. [Google Scholar] [CrossRef]
- Hsu, C.K.; Su, S.C.; Chang, L.C.; Yang, K.J.; Lee, C.C.; Hsu, H.J.; Chen, Y.T.; Sun, C.Y.; Wu, I.W. Oral Absorbent AST-120 Is Associated with Compositional and Functional Adaptations of Gut Microbiota and Modification of Serum Short and Medium-Chain Fatty Acids in Advanced CKD Patients. Biomedicines 2022, 10, 2234. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Patil, K.R.; Typas, A.; Maier, L. Towards a mechanistic understanding of reciprocal drug-microbiome interactions. Mol. Syst. Biol. 2021, 17, e10116. [Google Scholar] [CrossRef]
- Hsu, C.K.; Su, S.C.; Chang, L.C.; Shao, S.C.; Yang, K.J.; Chen, C.Y.; Chen, Y.T.; Wu, I.W. Effects of Low Protein diet on Modulating Gut Microbiota in Patients with Chronic Kidney Disease: A Systemic Review and Meta-analysis of International Studies. Int. J. Med. Sci. 2021, 18, 3839–3850. [Google Scholar] [CrossRef]
- Black, A.P.; Anjos, J.S.; Cardozo, L.; Carmo, F.L.; Dolenga, C.J.; Nakao, L.S.; de Carvalho-Ferreira, D.; Rosado, A.; Carraro-Eduardo, J.C.; Mafra, D. Does Low-Protein Diet Influence the Uremic Toxin Serum Levels from the Gut Microbiota in Nondialysis Chronic Kidney Disease Patients? J. Ren. Nutr. 2018, 28, 208–214. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, B.; Sha, T.; Li, X. Changes in the Intestinal Microbiota in Patients with Stage 5 Chronic Kidney Disease on a Low-Protein Diet and the Effects of Human to Rat Fecal Microbiota Transplantation. Med. Sci. Monit. 2020, 26, e921557. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef]
- Wu, I.W.; Lee, C.C.; Hsu, H.J.; Sun, C.Y.; Chen, Y.C.; Yang, K.J.; Yang, C.W.; Chung, W.H.; Lai, H.C.; Chang, L.C.; et al. Compositional and Functional Adaptations of Intestinal Microbiota and Related Metabolites in CKD Patients Receiving Dietary Protein Restriction. Nutrients 2020, 12, 2799. [Google Scholar] [CrossRef]
- Pavan, M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol. Nefrol. 2016, 68, 222–226. [Google Scholar] [PubMed]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of Low-Protein Diet and Inulin on Microbiota and Clinical Parameters in Patients with Chronic Kidney Disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Mazzaferro, S.; Muscaritoli, M.; Mastroluca, D.; Testorio, M.; Perrotta, A.; Esposito, Y.; Carta, M.; Campagna, L.; Di Grado, M.; et al. Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters. Toxins 2020, 12, 381. [Google Scholar] [CrossRef]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cederholm, T.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Cuerda, C.; Cupisti, A.; Sabatino, A.; Schneider, S.; Torreggiani, M.; et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease—Implications for low protein intake and nutritional care: A critical review endorsed by ERN-ERA and ESPEN. Clin. Nutr. 2023, 42, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Brunori, G.; Viola, B.F.; Parrinello, G.; De Biase, V.; Como, G.; Franco, V.; Garibotto, G.; Zubani, R.; Cancarini, G.C. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: A prospective randomized multicenter controlled study. Am. J. Kidney Dis. 2007, 49, 569–580. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef]
- Li, A.; Lee, H.Y.; Lin, Y.C. The Effect of Ketoanalogues on Chronic Kidney Disease Deterioration: A Meta-Analysis. Nutrients 2019, 11, 957. [Google Scholar] [CrossRef]
- Chewcharat, A.; Takkavatakarn, K.; Wongrattanagorn, S.; Panrong, K.; Kittiskulnam, P.; Eiam-Ong, S.; Susantitaphong, P. The Effects of Restricted Protein Diet Supplemented with Ketoanalogue on Renal Function, Blood Pressure, Nutritional Status, and Chronic Kidney Disease-Mineral and Bone Disorder in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2020, 30, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Garofalo, C.; Ferrara, C.; Calella, P. Ketoanalogue Supplementation in Patients with Non-Dialysis Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Fois, A.; Chatrenet, A.; Cataldo, E.; Lippi, F.; Kaniassi, A.; Vigreux, J.; Froger, L.; Mongilardi, E.; Capizzi, I.; Biolcati, M.; et al. Moderate Protein Restriction in Advanced CKD: A Feasible Option in An Elderly.; High-Comorbidity Population. A Stepwise Multiple-Choice System Approach. Nutrients 2018, 11, 36. [Google Scholar] [CrossRef]
- Baragetti, I.; De Simone, I.; Biazzi, C.; Buzzi, L.; Ferrario, F.; Luise, M.C.; Santagostino, G.; Furiani, S.; Alberghini, E.; Capitanio, C.; et al. The low-protein diet for chronic kidney disease: 8 years of clinical experience in a nephrology ward. Clin. Kidney J. 2019, 13, 253–260. [Google Scholar] [CrossRef]
- Otani, H.; Okada, T.; Saika, Y.; Sakagashira, M.; Oda, H.; Ito, Y.; Yasuda, T.; Kanno, T.; Shimazui, M.; Yamao, S.; et al. Effect of Nonsupplemented Low-Protein Diet on the Initiation of Renal Replacement Therapy in Stage 4 and 5 Chronic Kidney Disease: A Retrospective Multicenter Cohort Study in Japan. J. Ren. Nutr. 2023, 33, 649–656. [Google Scholar] [CrossRef]
- Garibotto, G.; Picciotto, D.; Saio, M.; Esposito, P.; Verzola, D. Muscle protein turnover and low-protein diets in patients with chronic kidney disease. Nephrol. Dial. Transpl. 2020, 35, 741–751. [Google Scholar] [CrossRef]
- Munro, H.N. Energy and protein intakes as determinants of nitrogen balance. Kidney Int. 1978, 14, 313–316. [Google Scholar] [CrossRef]
- Pereira, C.D.; Guimarães, C.; Ribeiro, V.S.; Vaz, D.C.; Martins, M.J. Low-Protein Diets, Malnutrition, and Bone Metabolism in Chronic Kidney Disease. Nutrients 2024, 16, 3098. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Anteyi, E. The Role of Dietary Fiber and Gut Microbiome Modulation in Progression of Chronic Kidney Disease. Toxins 2022, 14, 183. [Google Scholar] [CrossRef]
- Letourneau, P.; Bataille, S.; Chauveau, P.; Fouque, D.; Koppe, L. Source and Composition in Amino Acid of Dietary Proteins in the Primary Prevention and Treatment of CKD. Nutrients 2020, 12, 3892. [Google Scholar] [CrossRef]
- Su, G.; Qin, X.; Yang, C.; Sabatino, A.; Kelly, J.T.; Avesani, C.M.; Carrero, J.J. Fiber intake and health in people with chronic kidney disease. Clin. Kidney J. 2021, 15, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Gross, L.A.; de Azevedo, M.J.; Viana, L.V. Dietary fiber intake (supplemental or dietary pattern rich in fiber) and diabetic kidney disease: A systematic review of clinical trials. Nutrients 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Gross, L.A.; de Azevedo, M.J.; Viana, L.V. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar]
- Wu, M.; Cai, X.; Lin, J.; Zhang, X.; Scott, E.M.; Li, X. Association between fibre intake and indoxyl sulphate/P-cresyl sulphate in patients with chronic kidney disease: Meta-analysis and systematic review of experimental studies. Clin. Nutr. 2019, 38, 2. [Google Scholar] [CrossRef]
- Wu, M.; Cai, X.; Lin, J.; Zhang, X.; Scott, E.M.; Li, X. The role of dietary fiber supplementation in regulating uremic toxins in patients with chronic kidney disease: A meta-analysis of randomized controlled trials. J. Ren. Nutr. 2021, 31, 438–447. [Google Scholar]
- Saglimbene, V.M.; Wong, G.; Ruospo, M.; Palmer, S.C.; Garcia-Larsen, V.; Natale, P.; Teixeira-Pinto, A.; Campbell, K.L.; Carrero, J.J.; Stenvinkel, P.; et al. Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Dansby, L.; Shanklin, N. Glucose modulation of the disposal of an acute potassium load in patients with end-stage renal disease. Am. J. Med. 1993, 94, 475–482. [Google Scholar] [CrossRef]
- Cummings, J.H.; Hill, M.J.; Jenkins, D.J.; Pearson, J.R.; Wiggins, H.S. Changes in fecal composition and colonic function due to cereal fiber. Am. J. Clin. Nutr. 1976, 29, 1468–1473. [Google Scholar] [CrossRef]
- Camerotto, C.; Cupisti, A.; D’Alessandro, C.; Muzio, F.; Gallieni, M. Dietary Fiber and Gut Microbiota in Renal Diets. Nutrients 2019, 11, 2149. [Google Scholar] [CrossRef]
- Snelson, M.; Kellow, N.J.; Coughlan, M.T. Modulation of the Gut Microbiota by Resistant Starch as a Treatment of Chronic Kidney Diseases: Evidence of Efficacy and Mechanistic Insights. Adv. Nutr. 2019, 10, 303–320. [Google Scholar] [CrossRef]
- Khosroshahi, H.T.; Abedi, B.; Ghojazadeh, M.; Samadi, A.; Jouyban, A. Effects of fermentable high fiber diet supplementation on gut de rived and conventional nitrogenous product in patients on maintenance hemodialysis: A randomized controlled trial. Nutr. Metab. 2019, 16, 18. [Google Scholar] [CrossRef]
- Esgalhado, M.; Kemp, J.A.; Paiva, B.R.; Brito, J.S.; Cardozo, L.F.M.F.; Azevedo, R.; Cunha, D.B.; Nakao, L.S.; Mafra, D. Resistant starch type-2 enriched cookies modulate uremic toxins and in f lammation in hemodialysis patients: A randomized, double-blind, crossover and placebo-controlled trial. Food Funct. 2020, 11, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.S.; Sardá, F.A.H.; Pereira, N.B.F.; Teixeira, R.R.; Rodrigues, S.D.; de Lima, J.D.; Dalboni, M.A.; Aoike, D.T.; Nakao, L.S.; Cuppari, L. Effect of unripe banana flour on gut-derived uremic toxins in in dividuals undergoing peritoneal dialysis: A randomized, double-blind, placebo-controlled, crossover trial. Nutrients 2021, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Esgalhado, M.; Kemp, J.A.; Azevedo, R.; Paiva, B.R.; Stockler-Pinto, M.B.; Dolenga, C.J.; Borges, N.A.; Nakao, L.S.; Mafra, D. Could resistant starch supplementation improve inflammatory and oxida tive stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 2018, 9, 6508–6516. [Google Scholar] [CrossRef]
- Kemp, J.A.; Regis de Paiva, B.; Fragoso Dos Santos, H.; Emiliano de Jesus, H.; Craven, H.; Ijaz, U.Z.; Alvarenga Borges, N.; Shiels, P.G.; Mafra, D. The impact of enriched resistant starch type-2 cookies on the gut microbiome in hemodialysis patients: A ran domized controlled trial. Mol. Nutr. Food Res. 2021, 65, e2100374. [Google Scholar] [CrossRef]
- Khosroshahi, H.T.; Vaziri, N.D.; Abedi, B.; Asl, B.H.; Ghojazadeh, M.; Jing, W.; Vatankhah, A.M. Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: A randomized clinical trial. Hemodial. Int. 2018, 22, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Laffin, M.R.; Khosroshahi, H.T.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose resis tant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 2019, 23, 343–347. [Google Scholar] [CrossRef]
- Meng, Y.; Bai, H.; Yu, Q.; Yan, J.; Zhao, L.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. High-resistant starch, low-protein flour intervention on patients with early type 2 diabetic nephropathy: A randomized trial. J. Ren. Nutr. 2019, 29, 386–393. [Google Scholar] [CrossRef]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.Y.; Yang, S.Y.; Hu, Y.C.; Duan, K. Effects of resistant starch supplementation on renal function and inflammatory markers in patients with chronic kidney disease: A meta-analysis of randomized controlled trials. Ren. Fail. 2024, 46, 2416609. [Google Scholar] [CrossRef]
- Zarantonello, D.; Brunori, G.J. The Role of Plant-Based Diets in Preventing and Mitigating Chronic Kidney Disease: More Light than Shadows. Clin. Med. 2023, 12, 6137. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Masedunskas, A.; Willett, W.C.; Fontana, L. Vegetarian and vegan diets: Benefits and drawbacks. Eur. Heart J. 2023, 44, 3423–3439. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Zong, G.; Gao, A.; Hu, F.B.; Sun, Q. Whole grain intake and mortality from all Causes, Cardiovascular Disease, and Cancer a meta-analysis of prospective cohort studies. Circulation 2016, 133, 2370–2380. [Google Scholar] [CrossRef]
- Seal, C.J.; Brownlee, I.A. Whole-grain foods and chronic disease: Evidence from epidemiological and intervention studies. Proc. Nutr. Soc. 2015, 74, 313–319. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Steffen, L.M.; Jacobs, D.R., Jr.; Murtaugh, M.A.; Moran, A.; Steinberger, J.; Hong, C.-P.; Sinaiko, A.R. Whole grain intake is Associated with Lower Body Mass and Greater insulin sensitivity among adolescents. Am. J. Epidemiol. 2003, 158, 243–250. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Matsushita, K.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and Incident CKD. J. Am. Soc. Nephrol. 2017, 28, 1248–1258. [Google Scholar] [CrossRef]
- Cho, D.H.; Lim, S.T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown Rice Versus White Rice: Nutritional Quality, Potential Health Benefits, Development of Food Products, and Preservation Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ohtsubo, K. Low-Protein Diet: History and Use of Processed Low-Protein Rice for the Treatment of Chronic Kidney Disease. Foods 2021, 10, 2255. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Ruszkowski, J.; Styburski, D.; Dębska-Ślizień, A.; Małgorzewicz, S. The associations between nutrition and circulating gut microbiota-derived uremic toxins in patients undergoing kidney replacement therapy: An observational, cross-sectional study. Clin. Nutr. ESPEN 2025, 65, 105–114. [Google Scholar] [CrossRef]
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G.; De Angelis, M.; Gobbetti, M.; Gesualdo, L. What would you like to eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney Blood Press. Res. 2014, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Podadera-Herreros, A.; Arenas-de Larriva, A.P.; Gutierrez-Mariscal, F.M.; Alcala-Diaz, J.F.; Ojeda-Rodriguez, A.; Rodriguez-Cantalejo, F.; Cardelo, M.P.; Rodriguez-Cano, D.; Torres-Peña, J.D.; Luque, R.M.; et al. Mediterranean diet as a strategy for preserving kidney function in patients with coronary heart disease with type 2 diabetes and obesity: A secondary analysis of CORDIOPREV randomized controlled trial. Nutr. Diabetes. 2024, 14, 27. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Joo, Y.S.; Yun, H.R.; Lim, L.R.; Yang, J.; Lee, H.S.; Kim, H.M.; Lee, H.; Lee, J.E.; Lee, J.W. Safety and impact of the Mediterranean diet in patients with chronic kidney disease: A pilot randomized crossover trial. Front. Nutr. 2024, 11, 1463502. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Giannese, D.; Panichi, V.; Cupisti, A. Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet. Nutrients 2023, 15, 1256. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Wiese, G.N.; Biruete, A.; Moorthi, R.N.; Moe, S.M.; Lindemann, S.R.; Hill Gallant, K.M. Plant-Based Diets, the Gut Microbiota, and Trimethylamine N-Oxide Production in Chronic Kidney Disease: Therapeutic Potential and Methodological Considerations. J. Ren. Nutr. 2021, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Soulage, C.O. The impact of dietary nutrient intake on gut microbiota in the progression and complications of chronic kidney disease. Kidney Int. 2022, 102, 728–739. [Google Scholar] [CrossRef]
- MacLaughlin, H.L.; Friedman, A.N.; Ikizler, T.A. Nutrition in Kidney Disease: Core Curriculum 2022. Am. J. Kidney Dis. 2022, 79, 437–449. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Kim, K.M. Association of vegetables and fruits consumption with sarcopenia in older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2015, 44, 96–102. [Google Scholar] [CrossRef]
- Inoshita, H.; Asaoka, D.; Matsuno, K.; Yanagisawa, N.; Suzuki, Y.; Miyauchi, K. Cross-Sectional Study on the Association between Dietary Patterns and Sarcopenia in Elderly Patients with Chronic Kidney Disease Receiving Conservative Treatment. Nutrients 2023, 15, 4994. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-Based Diets and Incident CKD and Kidney Function. Clin. J. Am. Soc. Nephrol. 2019, 14, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Lew, Q.-L.J.; Jafar, T.H.; Koh, H.W.L.; Jin, A.; Chow, K.Y.; Yuan, J.-M.; Koh, W.P. Red meat intake and risk of ESRD. J. Am. Soc. Nephrol. 2017, 28, 304–312. [Google Scholar] [CrossRef]
- Shirota, M.; Watanabe, N.; Suzuki, M.; Kobori, M. Japanese-Style Diet and Cardiovascular Disease Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients 2022, 14, 2008. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Osman, A.; El-Gazzar, N.; Almanaa, T.N.; El-Hadary, A.; Sitohy, M. Lipolytic Postbiotic from Lactobacillus Paracasei Manages Metabolic Syndrome in Albino Wistar Rats. Molecules 2021, 26, 472. [Google Scholar] [CrossRef]
- Lee, H.; Ji, S.Y.; Hwangbo, H.; Kim, M.Y.; Kim, D.H.; Park, B.S.; Park, J.-H.; Lee, B.-J.; Kim, G.-Y.; Jeon, Y.-J.; et al. Protective Effect of Gamma Aminobutyric Acid against Aggravation of Renal Injury Caused by High Salt Intake in Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2022, 23, 502. [Google Scholar] [CrossRef]
- Marzocco, S.; Fazeli, G.; Di Micco, L.; Autore, G.; Adesso, S.; Dal Piaz, F.; Heidland, A.; Di Iorio, B. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef]
- Li, J.; Shen, Y.; Yan, K.; Wang, S.; Jiao, J.; Chi, H.; Zhong, J.C.; Dong, Y.; Wang, P.J. The compositional and functional imbalance of the gut microbiota in CKD linked to disease patterns. Transl. Med. 2024, 22, 773. [Google Scholar] [CrossRef]
- Krukowski, H.; Valkenburg, S.; Madella, A.M.; Garssen, J.; van Bergenhenegouwen, J.; Overbeek, S.A.; Huys, G.R.B.; Raes, J.; Glorieux, G. Gut microbiome studies in CKD: Opportunities, pitfalls and therapeutic potential. Nat. Rev. Nephrol. 2023, 19, 87–101. [Google Scholar] [CrossRef]
- Lauriero, G.; Abbad, L.; Vacca, M.; Celano, G.; Chemouny, J.M.; Calasso, M.; Berthelot, L.; Gesualdo, L.; De Angelis, M.; Monteiro, R.C. Fecal Microbiota Transplantation Modulates Renal Phenotype in the Humanized Mouse Model of IgA Nephropathy. Front. Immunol. 2021, 12, 694787. [Google Scholar] [CrossRef]
- Arteaga-Muller, G.Y.; Flores-Treviño, S.; Bocanegra-Ibarias, P.; Robles-Espino, D.; Garza-González, E.; Fabela-Valdez, G.C.; Camacho-Ortiz, A. Changes in the Progression of Chronic Kidney Disease in Patients Undergoing Fecal Microbiota Transplantation. Nutrients 2024, 16, 1109. [Google Scholar] [CrossRef]

| Classification | Fibers |
|---|---|
| Non-starch polysaccharide | Cellulose Hemicellulose Mannan Heteromannan Pectin Inulin Fractan |
| Resistant oligosaccharide | α-galactosides β-fructooligosaccharides (FOS) α-galactooligosaccharides (GOS) β-galactooligosaccharides (TOS) Xylo-oligosaccharides (XOS) Arabinoxylooligosaccharides (AXOS) Polydextrose |
| Resistant starch | Type 1—starch resistant to amylase Type 2—ungelatinized starch, powdered starch Type 3—recrystallized starch Type 4—chemically modified starch Type 5—complex of amyloses and lipids |
| Other dietary fibers | Lignin Chitins |
| RS Type | Description | Examples |
|---|---|---|
| RS1 | Physically inaccessible | Coarsely milled grains or seeds, legumes |
| RS2 | Ungelatinized starch | Raw potato, unripe banana, high-amylose maize starch |
| RS3 | Retrograded starch | Cooked, cooled foods (potatoes, pasta, rice), corn flakes |
| RS4 | Chemically modified starch | Cross-linked starch and octenyl succinate starch |
| RS5 | Amylose–lipid complex | Stearic acid–complexed high-amylose starch |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakino, S.; Hasegawa, K.; Tamaki, M.; Minato, M.; Inagaki, T. Kidney-Gut Axis in Chronic Kidney Disease: Therapeutic Perspectives from Microbiota Modulation and Nutrition. Nutrients 2025, 17, 1961. https://doi.org/10.3390/nu17121961
Wakino S, Hasegawa K, Tamaki M, Minato M, Inagaki T. Kidney-Gut Axis in Chronic Kidney Disease: Therapeutic Perspectives from Microbiota Modulation and Nutrition. Nutrients. 2025; 17(12):1961. https://doi.org/10.3390/nu17121961
Chicago/Turabian StyleWakino, Shu, Kazuhiro Hasegawa, Masanori Tamaki, Masanori Minato, and Taizo Inagaki. 2025. "Kidney-Gut Axis in Chronic Kidney Disease: Therapeutic Perspectives from Microbiota Modulation and Nutrition" Nutrients 17, no. 12: 1961. https://doi.org/10.3390/nu17121961
APA StyleWakino, S., Hasegawa, K., Tamaki, M., Minato, M., & Inagaki, T. (2025). Kidney-Gut Axis in Chronic Kidney Disease: Therapeutic Perspectives from Microbiota Modulation and Nutrition. Nutrients, 17(12), 1961. https://doi.org/10.3390/nu17121961






