Nutrient-Wide Association Study for Dementia Risks: A Prospective Cohort Study in Middle-Aged and Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Assessment of Dietary Nutrients
2.3. Assessment of Cognition and Dementia
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Associations Between Single Nutrient and Dementia
3.3. Associations Between CNS and Dementia
3.4. Exploratory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BMI | Body mass index |
| CI | Confidence interval |
| CNS | Composite nutrient score |
| ENET | Elastic net |
| EWAS | Nutrient-wide association study |
| FDR | False discovery rate |
| FFQ | Food frequency questionnaire |
| HCNS | Health Care and Nutrition Study |
| HR | Hazard ratio |
| HRS | Health and Retirement Study |
| SCFA | Saturated short-chain fatty acid |
| TICS-m | Modified Telephone Interview for Cognitive Status |
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Bruck, C.C.; Mooldijk, S.S.; Kuiper, L.M.; Sambou, M.L.; Licher, S.; Mattace-Raso, F.; Wolters, F.J. Time to nursing home admission and death in people with dementia: Systematic review and meta-analysis. BMJ 2025, 388, e080636. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Hoost, S.S.; Honig, L.S.; Kang, M.S.; Bahl, A.; Lee, A.J.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Dage, J.L.; Brickman, A.M.; et al. Association of dietary fatty acids with longitudinal change in plasma-based biomarkers of Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2025, 12, 100117. [Google Scholar] [CrossRef]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Tublin, J.M.; Adelstein, J.M.; Del Monte, F.; Combs, C.K.; Wold, L.E. Getting to the Heart of Alzheimer Disease. Circ. Res. 2019, 124, 142–149. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Dove, A.; Dunk, M.M.; Wang, J.; Guo, J.; Whitmer, R.A.; Xu, W. Anti-Inflammatory Diet and Dementia in Older Adults with Cardiometabolic Diseases. JAMA Netw. Open 2024, 7, e2427125. [Google Scholar] [CrossRef]
- Lin, B.D.; Pries, L.K.; Sarac, H.S.; van Os, J.; Rutten, B.P.F.; Luykx, J.; Guloksuz, S. Nongenetic Factors Associated With Psychotic Experiences Among UK Biobank Participants: Exposome-Wide Analysis and Mendelian Randomization Analysis. JAMA Psychiatry 2022, 79, 857–868. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.D.; Deng, Y.T.; You, J.; He, X.Y.; Wu, X.R.; Wu, B.S.; Yang, L.; Zhang, Y.R.; Kuo, K.; et al. Identifying modifiable factors and their joint effect on dementia risk in the UK Biobank. Nat. Hum. Behav. 2023, 7, 1185–1195. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Patel, C.J.; Okamura, T.; Chan, Q.; Brown, I.J.; Miura, K.; Ueshima, H.; Zhao, L.; Van Horn, L.; Daviglus, M.L.; et al. A nutrient-wide association study on blood pressure. Circulation 2012, 126, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Muller, D.C.; van den Brandt, P.A.; Papadimitriou, N.; Critselis, E.; Gunter, M.; Vineis, P.; Weiderpass, E.; Fagherazzi, G.; Boeing, H.; et al. Nutrient-wide association study of 92 foods and nutrients and breast cancer risk. Breast Cancer Res. 2020, 22, 5. [Google Scholar] [CrossRef]
- Papadimitriou, N.; Muller, D.; van den Brandt, P.A.; Geybels, M.; Patel, C.J.; Gunter, M.J.; Lopez, D.S.; Key, T.J.; Perez-Cornago, A.; Ferrari, P.; et al. A nutrient-wide association study for risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition and the Netherlands Cohort Study. Eur. J. Nutr. 2020, 59, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Sonnega, A.; Faul, J.D.; Ofstedal, M.B.; Langa, K.M.; Phillips, J.W.; Weir, D.R. Cohort Profile: The Health and Retirement Study (HRS). Int. J. Epidemiol. 2014, 43, 576–585. [Google Scholar] [CrossRef]
- Ma, L.; Hu, Y.; Alperet, D.J.; Liu, G.; Malik, V.; Manson, J.E.; Rimm, E.B.; Hu, F.B.; Sun, Q. Beverage consumption and mortality among adults with type 2 diabetes: Prospective cohort study. BMJ 2023, 381, e073406. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.M.; Weir, D.R.; Kabeto, M.; Sonnega, A. Langa-Weir Classification of Cognitive Function (1995–2020). 2023. Available online: https://hrsdata.isr.umich.edu/data-products/langa-weir-classification-cognitive-function-1995-2020 (accessed on 30 October 2023).
- Langa, K.M.; Larson, E.B.; Crimmins, E.M.; Faul, J.D.; Levine, D.A.; Kabeto, M.U.; Weir, D.R. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern. Med. 2017, 177, 51–58. [Google Scholar] [CrossRef]
- Chen, H.; Dhana, K.; Huang, Y.; Huang, L.; Tao, Y.; Liu, X.; Melo van Lent, D.; Zheng, Y.; Ascherio, A.; Willett, W.; et al. Association of the Mediterranean Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet With the Risk of Dementia. JAMA Psychiatry 2023, 80, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Ryan, C.P.; Caspi, A.; Liu, Z.; Moffitt, T.E.; Sugden, K.; Zhou, J.; Belsky, D.W.; Gu, Y. Diet, Pace of Biological Aging, and Risk of Dementia in the Framingham Heart Study. Ann. Neurol. 2024, 95, 1069–1079. [Google Scholar] [CrossRef]
- Shannon, O.M.; Ranson, J.M.; Gregory, S.; Macpherson, H.; Milte, C.; Lentjes, M.; Mulligan, A.; McEvoy, C.; Griffiths, A.; Matu, J.; et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: Findings from the UK Biobank prospective cohort study. BMC Med. 2023, 21, 81. [Google Scholar] [CrossRef]
- Newsom, J.T.; Denning, E.C.; Elman, M.R.; Botoseneanu, A.; Allore, H.G.; Nagel, C.L.; Dorr, D.A.; Quinones, A.R. Physical Activity as a Mediator between Race/Ethnicity and Changes in Multimorbidity. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 77, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Ganna, A.; Ingelsson, E. 5 year mortality predictors in 498,103 UK Biobank participants: A prospective population-based study. Lancet 2015, 386, 533–540. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, Y.; Cheng, Y.; Ma, Y.; Liu, J.; Li, C.; Shang, H. Associations of sugar intake, high-sugar dietary pattern, and the risk of dementia: A prospective cohort study of 210,832 participants. BMC Med. 2024, 22, 298. [Google Scholar] [CrossRef]
- Agarwal, P.; Ford, C.N.; Leurgans, S.E.; Beck, T.; Desai, P.; Dhana, K.; Evans, D.A.; Halloway, S.; Holland, T.M.; Krueger, K.R.; et al. Dietary Sugar Intake Associated with a Higher Risk of Dementia in Community-Dwelling Older Adults. J. Alzheimers Dis. 2023, 95, 1417–1425. [Google Scholar] [CrossRef]
- Petruski-Ivleva, N.; Kucharska-Newton, A.; Palta, P.; Couper, D.; Meyer, K.; Graff, M.; Haring, B.; Sharrett, R.; Heiss, G. Milk Intake at Midlife and Cognitive Decline over 20 Years. The Atherosclerosis Risk in Communities (ARIC) Study. Nutrients 2017, 9, 1134. [Google Scholar] [CrossRef]
- Lu, Y.; Sugawara, Y.; Tsuji, I. Association between dairy intake and risk of incident dementia: The Ohsaki Cohort 2006 Study. Eur. J. Nutr. 2023, 62, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tsapanou, A.; Manly, J.; Schupf, N.; Brickman, A.M.; Gu, Y. Vitamin D intake is associated with dementia risk in the Washington Heights-Inwood Columbia Aging Project (WHICAP). Alzheimer’s Dement. 2020, 16, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.P.; Shahar, S.; Haron, H.; Din, N.C. Habitual sugar intake and cognitive impairment among multi-ethnic Malaysian older adults. Clin. Interv. Aging 2019, 14, 1331–1342. [Google Scholar] [CrossRef]
- Liu, L.; Volpe, S.L.; Ross, J.A.; Grimm, J.A.; Van Bockstaele, E.J.; Eisen, H.J. Dietary sugar intake and risk of Alzheimer’s disease in older women. Nutr. Neurosci. 2022, 25, 2302–2313. [Google Scholar] [CrossRef]
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in D-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.F.; Miao, J.; Zheng, R.F.; Li, J.Y. Short-chain fatty acids: Important components of the gut-brain axis against AD. Biomed. Pharmacother. 2024, 175, 116601. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.R.; Backman, K.; Scarmeas, N.; Stern, Y.; Manly, J.J.; Mayeux, R.; Gu, Y. Dietary fatty acids and risk of Alzheimer’s disease and related dementias: Observations from the Washington Heights-Hamilton Heights-Inwood Columbia Aging Project (WHICAP). Alzheimers Dement. 2020, 16, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Meng, L.; Shen, L. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.Y.; Yan, H.Y.; Zhong, J.F.; Diao, Z.Q.; Li, J.; Li, C.P.; Chen, L.H.; Huang, W.Q.; Xu, M.; Xu, Z.T.; et al. Association of dietary choline intake with incidence of dementia, Alzheimer disease, and mild cognitive impairment: A large population-based prospective cohort study. Am. J. Clin. Nutr. 2025, 121, 5–13. [Google Scholar] [CrossRef]
- Agnew-Blais, J.C.; Wassertheil-Smoller, S.; Kang, J.H.; Hogan, P.E.; Coker, L.H.; Snetselaar, L.G.; Smoller, J.W. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the Women’s Health Initiative Memory Study. J. Acad. Nutr. Diet. 2015, 115, 231–241. [Google Scholar] [CrossRef]
- Lefevre-Arbogast, S.; Feart, C.; Dartigues, J.F.; Helmer, C.; Letenneur, L.; Samieri, C. Dietary B Vitamins and a 10-Year Risk of Dementia in Older Persons. Nutrients 2016, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mi, N.; Liao, K.; Li, Y.; Sun, Y.; Xie, P.; Hu, L.; Wu, S.; Liang, Z.; He, Q.; et al. Associations among dietary 1-carbon metabolism nutrients, genetic risk, and Alzheimer disease: A prospective cohort study. Am. J. Clin. Nutr. 2024, 120, 1009–1018. [Google Scholar] [CrossRef]
- Beaudry-Richard, A.; Abdelhak, A.; Saloner, R.; Sacco, S.; Montes, S.C.; Oertel, F.C.; Cordano, C.; Jabassini, N.; Ananth, K.; Gomez, A.; et al. Vitamin B12 Levels Association with Functional and Structural Biomarkers of Central Nervous System Injury in Older Adults. Ann. Neurol. 2025, 97, 1190–1204. [Google Scholar] [CrossRef]
- Creekmore, B.C.; Watanabe, R.; Lee, E.B. Neurodegenerative Disease Tauopathies. Annu. Rev. Pathol. 2024, 19, 345–370. [Google Scholar] [CrossRef]
- Gu, Y.; Nieves, J.W.; Stern, Y.; Luchsinger, J.A.; Scarmeas, N. Food combination and Alzheimer disease risk: A protective diet. Arch. Neurol. 2010, 67, 699–706. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary fats and the risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 194–200. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.C.; Lee-Chen, G.J.; Chen, C.M.; Chen, Y.; Lo, Y.S.; Chang, K.H. Isorhamnetin Attenuated the Release of Interleukin-6 from beta-Amyloid-Activated Microglia and Mitigated Interleukin-6-Mediated Neurotoxicity. Oxid. Med. Cell Longev. 2022, 2022, 3652402. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S.; Aggarwal, N.T.; Scherr, P.A. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am. J. Clin. Nutr. 2005, 81, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Solomon, A.; Kareholt, I.; Hooshmand, B.; Cecchetti, R.; Fratiglioni, L.; Soininen, H.; Laatikainen, T.; Mecocci, P.; Kivipelto, M. Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp. Gerontol. 2013, 48, 1428–1435. [Google Scholar] [CrossRef]
- Boccardi, V.; Baroni, M.; Mangialasche, F.; Mecocci, P. Vitamin E family: Role in the pathogenesis and treatment of Alzheimer’s disease. Alzheimers Dement (NY) 2016, 2, 182–191. [Google Scholar] [CrossRef]
- Lorenzo-Mora, A.M.; Lopez-Sobaler, A.M.; Bermejo, L.M.; Gonzalez-Rodriguez, L.G.; Cuadrado-Soto, E.; Peral-Suarez, A.; Salas-Gonzalez, M.D.; Delgado-Losada, M.L.; Rodriguez-Rojo, I.C.; Barabash, A.; et al. Association between Mineral Intake and Cognition Evaluated by Montreal Cognitive Assessment (MoCA): A Cross-Sectional Study. Nutrients 2023, 15, 4505. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Zhang, Y.; Zhou, R.; Lin, X.; Du, Y.; Xu, J.; Xu, Q.; Zang, J. The associations of dietary manganese, iron, copper, zinc, selenium and magnesium with cognitive outcomes in Chinese adults: A cross sectional study in Shanghai. Front. Nutr. 2024, 11, 1424614. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Vercambre, M.N.; Boutron-Ruault, M.C.; Ritchie, K.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef]
- Yamagishi, K.; Maruyama, K.; Ikeda, A.; Nagao, M.; Noda, H.; Umesawa, M.; Hayama-Terada, M.; Muraki, I.; Okada, C.; Tanaka, M.; et al. Dietary fiber intake and risk of incident disabling dementia: The Circulatory Risk in Communities Study. Nutr. Neurosci. 2023, 26, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, X.; Wu, X.; Liu, Y.; Chen, Y.; Li, L.; Chen, J.; Wang, Z. Sex differences in association of healthy eating pattern with all-cause mortality and cardiovascular mortality. BMC Public Health 2024, 24, 2363. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef]
- Gilsanz, P.; Mayeda, E.R.; Glymour, M.M.; Quesenberry, C.P.; Mungas, D.M.; DeCarli, C.; Dean, A.; Whitmer, R.A. Female sex, early-onset hypertension, and risk of dementia. Neurology 2017, 89, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Gannon, O.J.; Robison, L.S.; Salinero, A.E.; Abi-Ghanem, C.; Mansour, F.M.; Kelly, R.D.; Tyagi, A.; Brawley, R.R.; Ogg, J.D.; Zuloaga, K.L. High-fat diet exacerbates cognitive decline in mouse models of Alzheimer’s disease and mixed dementia in a sex-dependent manner. J. Neuroinflamm. 2022, 19, 110. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, M.; Paye, S.; Benayoun, B.A. Sex as a Biological Variable in Nutrition Research: From Human Studies to Animal Models. Annu. Rev. Nutr. 2022, 42, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.K.; Narasimhan, B.; Hastie, T. Elastic Net Regularization Paths for All Generalized Linear Models. J. Stat. Softw. 2023, 106, 1–31. [Google Scholar] [CrossRef]
- Simon, N.; Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J. Stat. Softw. 2011, 39, 1–13. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection Via the Elastic Net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
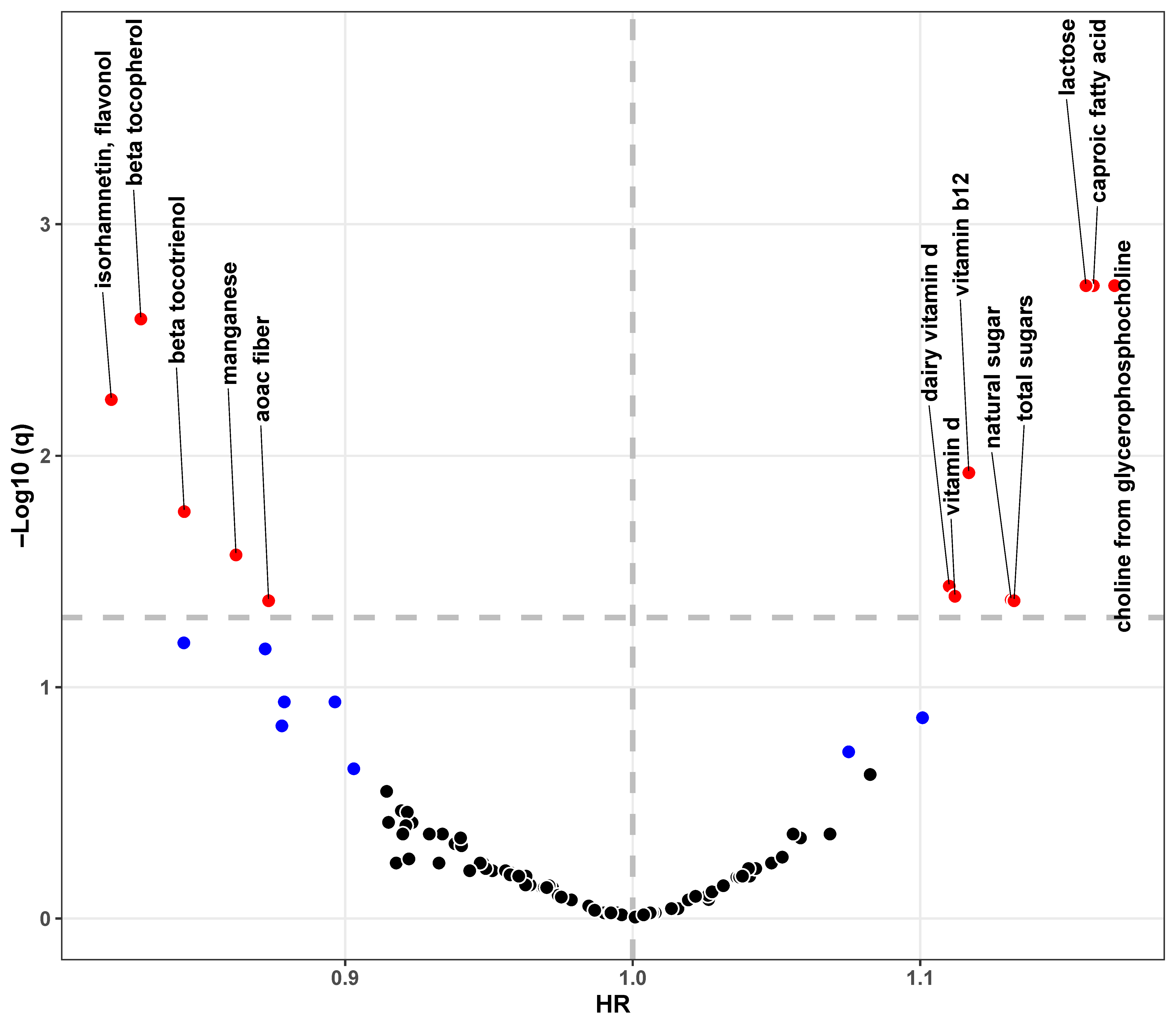
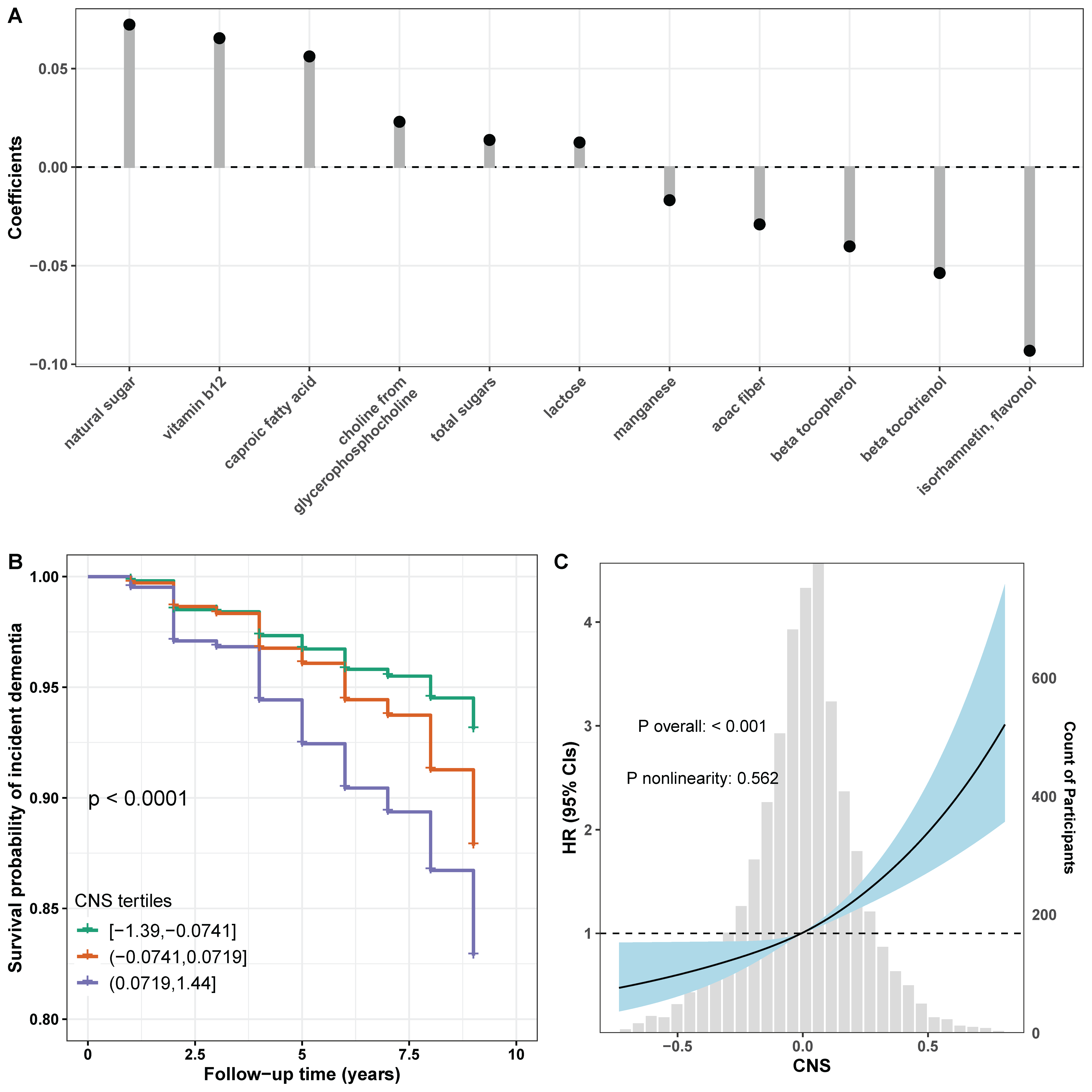
| Characteristics | Total (n = 6280) | Non-Demented (n = 5785) | Incident (n = 495) | p Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 66.88 (10.13) | 66.28 (9.87) | 73.80 (10.49) | <0.001 |
| Sex, n (%) | 0.810 | |||
| Male | 2544 (40.51) | 2346 (40.55) | 198 (40.00) | |
| Female | 3736 (59.49) | 3439 (59.45) | 297 (60.00) | |
| Race/ethnicity, n (%) | <0.001 | |||
| White/Caucasian | 4921 (78.36) | 4564 (78.89) | 357 (72.12) | |
| Black/African American | 929 (14.79) | 822 (14.21) | 107 (21.62) | |
| Other | 430 (6.85) | 399 (6.90) | 31 (6.26) | |
| Years of education, mean (SD) | 13.21 (2.79) | 13.32 (2.71) | 11.93 (3.34) | <0.001 |
| Marital status, n (%) | <0.001 | |||
| Married/partnered | 4232 (67.39) | 3955 (68.37) | 277 (55.96) | |
| Separated/divorced/widowed | 1791 (28.52) | 1594 (27.55) | 197 (39.80) | |
| Never married | 257 (4.09) | 236 (4.08) | 21 (4.24) | |
| Smoking, n (%) | 0.682 | |||
| Never | 2875 (45.78) | 2654 (45.88) | 221 (44.65) | |
| Past | 2678 (42.64) | 2458 (42.49) | 220 (44.44) | |
| Current | 727 (11.58) | 673 (11.63) | 54 (10.91) | |
| BMI categories, n (%) | <0.001 | |||
| Underweight | 67 (1.07) | 54 (0.93) | 13 (2.63) | |
| Normal weight | 1658 (26.40) | 1499 (25.91) | 159 (32.12) | |
| Overweight | 2330 (37.10) | 2130 (36.82) | 200 (40.40) | |
| Obese | 2225 (35.43) | 2102 (36.34) | 123 (24.85) | |
| Number of comorbidities, mean (SD) | 2.01 (1.43) | 1.97 (1.42) | 2.44 (1.47) | <0.001 |
| Score of physical activity, mean (SD) | 8.19 (6.66) | 8.39 (6.68) | 5.91 (6.00) | <0.001 |
| Disability, n (%) | <0.001 | |||
| No | 5462 (86.97) | 5096 (88.09) | 366 (73.94) | |
| Yes | 818 (13.03) | 689 (11.91) | 129 (26.06) | |
| Dietary calories (kcal/day), mean (SD) | 1770.67 (660.22) | 1764.00 (655.44) | 1848.58 (709.85) | 0.011 |
| Model 1 a | Model 2 b | ||||
|---|---|---|---|---|---|
| Subpopulation | No. of Events/Person-Years | HR (95% CI) | p Value | HR (95% CI) | p Value |
| All (n = 6280) | |||||
| CNS categories c | |||||
| T1 (n = 2094) | 103/14,583 | Reference | Reference | ||
| T2 (n = 2093) | 157/14,248 | 1.45 (1.13, 1.86) | 0.004 | 1.43 (1.11, 1.84) | 0.005 |
| T3 (n = 2093) | 235/13,609 | 1.89 (1.50, 2.39) | <0.001 | 1.80 (1.42, 2.27) | <0.001 |
| Continuous CNS | 495/42,440 | 4.18 (2.94, 5.92) | <0.001 | 3.55 (2.49, 5.05) | <0.001 |
| Males (n = 2544) | |||||
| CNS categories c | |||||
| T1 (n = 830) | 43/5651 | Reference | Reference | ||
| T2 (n = 842) | 66/5726 | 1.38 (0.94, 2.05) | 0.104 | 1.32 (0.89, 1.96) | 0.162 |
| T3 (n = 872) | 89/5627 | 1.65 (1.14, 2.39) | 0.007 | 1.53 (1.05, 2.21) | 0.026 |
| Continuous CNS | 198/17,004 | 2.65 (1.49, 4.71) | 0.001 | 2.23 (1.24, 4.01) | 0.007 |
| Females (n = 3736) | |||||
| CNS categories c | |||||
| T1 (n = 1264) | 60/8932 | Reference | Reference | ||
| T2 (n = 1251) | 91/8522 | 1.49 (1.07, 2.07) | 0.018 | 1.55 (1.11, 2.16) | 0.010 |
| T3 (n = 1221) | 146/7982 | 2.06 (1.52, 2.78) | <0.001 | 2.01 (1.48, 2.72) | <0.001 |
| Continuous CNS | 297/25,436 | 5.47 (3.52, 8.49) | <0.001 | 4.58 (2.94, 7.13) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Gu, Y. Nutrient-Wide Association Study for Dementia Risks: A Prospective Cohort Study in Middle-Aged and Older Adults. Nutrients 2025, 17, 1960. https://doi.org/10.3390/nu17121960
Guo J, Gu Y. Nutrient-Wide Association Study for Dementia Risks: A Prospective Cohort Study in Middle-Aged and Older Adults. Nutrients. 2025; 17(12):1960. https://doi.org/10.3390/nu17121960
Chicago/Turabian StyleGuo, Jing, and Yian Gu. 2025. "Nutrient-Wide Association Study for Dementia Risks: A Prospective Cohort Study in Middle-Aged and Older Adults" Nutrients 17, no. 12: 1960. https://doi.org/10.3390/nu17121960
APA StyleGuo, J., & Gu, Y. (2025). Nutrient-Wide Association Study for Dementia Risks: A Prospective Cohort Study in Middle-Aged and Older Adults. Nutrients, 17(12), 1960. https://doi.org/10.3390/nu17121960







