Iodine and Selenium Status in Relation to Thyroid and Immune Functions—The Analysis of Their Dependencies in a Group of Women of Reproductive Age from the Southern Region of Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Chemicals
2.4. Determination of Iodine in Serum by ICP-MS
2.5. Determination of Selenium in Serum by AAS
2.6. Determination of Glutathione Peroxidase 3 in Serum
2.7. Determination of Thyroid Parameters in Serum
2.8. Determination of Immunological Parameters in Serum
2.9. Determination of FRAP in Serum
2.10. Statistical Approach
3. Results
4. Discussion
5. Limitations of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenzek, C.; Boelen, A.; Westendorf, A.M.; Engel, D.R.; Moeller, L.C.; Führer, D. The Interplay of Thyroid Hormones and the Immune System—Where We Stand and Why We Need to Know about It. Eur. J. Endocrinol. 2022, 186, R65–R77. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium, Iodine and Iron–Essential Trace Elements for Thyroid Hormone Synthesis and Metabolism. Int. J. Mol. Sci. 2023, 24, 3393. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.Y.; Dambaeva, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells. Front. Immunol. 2017, 8, 1573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Kryczyk-Kozioł, J.; Zagrodzki, P.; Podsiadły, R.; Galanty, A.; Paśko, P. Evaluation of the Consumption of Potentially Goitrogenic Food Products in Various Models of Plant-Based Diets in Poland. Acta Pol. Pharm. Drug Res. 2024, 80, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Ittermann, T.; Albrecht, D.; Arohonka, P.; Bilek, R.; De Castro, J.J.; Dahl, L.; Filipsson Nystrom, H.; Gaberscek, S.; Garcia-Fuentes, E.; Gheorghiu, M.L.; et al. Standardized Map of Iodine Status in Europe. Thyroid 2020, 30, 1346–1354. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Jóźków, P.; Lwow, F.; Słowińska-Lisowska, M.; Mędraś, M. Trends in the Prevalence of Autoimmune Thyroiditis in the Leading Private Health-Care Provider in Poland. Adv. Clin. Exp. Med. 2017, 26, 497–503. [Google Scholar] [CrossRef]
- Zachara, B.A.; Pilecki, A. Selenium Concentration in the Milk of Breast-Feeding Mothers and Its Geographic Distribution. Environ. Health Perspect. 2000, 108, 1043–1046. [Google Scholar] [CrossRef]
- Wasowicz, W.; Gromadzinska, J.; Rydzynski, K.; Tomczak, J. Selenium Status of Low-Selenium Area Residents: Polish Experience. Toxicol. Lett. 2003, 137, 95–101. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Płonka-Półtorak, E.; Zagrodzki, P.; Nicol, F.; Kryczyk, J.; Bartoń, H.; Westermarck, T.; Kaipainen, P.; Ounjaijean, S.; Kaski, M.; Atroshi, F. Antioxidant Agents and Physiological Responses in Adult Epileptic Patients Treated with Lamotrigine. Pharmacol. Rep. PR 2013, 65, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sainani, K.L. Introduction to Principal Components Analysis. PM&R 2014, 6, 275–278. [Google Scholar] [CrossRef]
- König, F.; Andersson, M.; Hotz, K.; Aeberli, I.; Zimmermann, M.B. Ten Repeat Collections for Urinary Iodine from Spot Samples or 24-Hour Samples Are Needed to Reliably Estimate Individual Iodine Status in Women. J. Nutr. 2011, 141, 2049–2054. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, P.; Liu, L.; Jia, Q.; Liu, P.; Meng, F.; Zhang, X.; Guan, Y.; Pang, Y.; Lu, Z.; et al. The Application of Serum Iodine in Assessing Individual Iodine Status. Clin. Endocrinol. 2017, 87, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, D.; Cheng, X.; Zhang, Q.; Wang, M.; Guo, H.; Yu, B.; Zhang, X.; Xia, L.; Sun, D.; et al. Establishing Reference Intervals for Urine and Serum Iodine Levels: A Nationwide Multicenter Study of a Euthyroid Chinese Population. Clin. Chim. Acta 2020, 502, 34–40. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. German Nutrition Society (DGE) Revised Reference Values for Selenium Intake. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Jablonska, E.; Gromadzinska, J.; Peplonska, B.; Fendler, W.; Reszka, E.; Krol, M.B.; Wieczorek, E.; Bukowska, A.; Gresner, P.; Galicki, M.; et al. Lipid Peroxidation and Glutathione Peroxidase Activity Relationship in Breast Cancer Depends on Functional Polymorphism of GPX1. BMC Cancer 2015, 15, 657. [Google Scholar] [CrossRef]
- Schneider-Matyka, D.; Cybulska, A.M.; Szkup, M.; Pilarczyk, B.; Panczyk, M.; Tomza-Marciniak, A.; Grochans, E. Selenium as a Predictor of Metabolic Syndrome in Middle Age Women. Aging 2023, 15, 1734–1747. [Google Scholar] [CrossRef]
- Bizerea-Moga, T.O.; Pitulice, L.; Bizerea-Spiridon, O.; Moga, T.V. Evaluation of Serum Selenium Status by Age and Gender: A Retrospective Observational Cohort Study in Western Romania. Nutrients 2021, 13, 1497. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Smith, A.M. Plasma Selenium and Plasma and Erythrocyte Glutathione Peroxidase Activity Increase with Estrogen during the Menstrual Cycle. J. Am. Coll. Nutr. 2003, 22, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, L.C.; Feitosa, M.M.; Freitas, T.E.C.; Severo, J.S.; Morais, J.B.S.; Henriques, G.S.; Oliveira, F.E.; Moita Neto, J.M.; Marreiro, D.d.N. Selenium Status and Its Relationship with Thyroid Hormones in Obese Women. Clin. Nutr. ESPEN 2021, 41, 398–404. [Google Scholar] [CrossRef]
- Reddy, V.; Gouroju, S.; Suchitra, M.; Suresh, V.; Sachan, A.; Srinivasa Rao, P.; Bitla, A. Antioxidant Defense in Overt and Subclinical Hypothyroidism. Horm. Metab. Res. 2013, 45, 754–758. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global Prevalence and Epidemiological Trends of Hashimoto’s Thyroiditis in Adults: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef]
- Ng, C.T.; Fong, L.Y.; Abdullah, M.N.H. Interferon-Gamma (IFN-γ): Reviewing Its Mechanisms and Signaling Pathways on the Regulation of Endothelial Barrier Function. Cytokine 2023, 166, 156208. [Google Scholar] [CrossRef]
- Matowicka-Karna, J.; Dymicka-Piekarska, V.; Kemona, H. IFN-Gamma, IL-5, IL-6 and IgE in Patients Infected with Giardia Intestinalis. Folia Histochem. Cytobiol. 2009, 47, 93–97. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, H.; Su, Z.; Chen, J.; Xue, Y.; Wang, S.; Xue, Y.; He, Z.; Yang, H.; Zhou, C.; et al. Differentiation Imbalance of Th1/Th17 in Peripheral Blood Mononuclear Cells Might Contribute to Pathogenesis of Hashimoto’s Thyroiditis. Scand. J. Immunol. 2010, 72, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, K.; Kozłowska, M.; Kaszuba, A.; Lesiak, A.; Narbutt, J.; Zalewska-Janowska, A. Increased Serum Levels of IFN- γ, IL-1 β, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid. Med. Cell. Longev. 2020, 2020, 5693572. [Google Scholar] [CrossRef]
- Heeb, L.E.M.; Egholm, C.; Boyman, O. Evolution and Function of Interleukin-4 Receptor Signaling in Adaptive Immunity and Neutrophils. Genes Immun. 2020, 21, 143–149. [Google Scholar] [CrossRef]
- Mourtzikou, A.; Alepaki, M.; Stamouli, M.; Pouliakis, A.; Skliris, A.; Karakitsos, P. Evaluation of Serum Levels of IL-6, TNF-α, IL-10, IL-2 and IL-4 in Patients with Chronic Hepatitis. Inmunología 2014, 33, 41–50. [Google Scholar] [CrossRef]
- Zajkowska, A.; Garkowski, A.; Świerzbińska, R.; Kułakowska, A.; Król, M.E.; Ptaszyńska-Sarosiek, I.; Nowicka-Ciełuszecka, A.; Pancewicz, S.; Czupryna, P.; Moniuszko, A.; et al. Evaluation of Chosen Cytokine Levels among Patients with Herpes Zoster as Ability to Provide Immune Response. PLoS ONE 2016, 11, e0150301. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming Growth Factor-β Regulation of Immune Responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Aleksandrova, E.; Mindov, I.; Petrov, B.; Dimitrova, I.; Petrov, N.; Ananiev, J.; Vlaykova, T.; Valkanov, S. Role of Elevated Serum TGF-Β1 and the Common Promoter TGFB1-509C/T Polymorphism in the Development and Progression of Primary Glial Tumors and Brain Metastases. Medicina 2024, 60, 146. [Google Scholar] [CrossRef]
- Davami, M.; Baharlou, R.; Ahmadi Vasmehjani, A.; Ghanizadeh, A.; Keshtkar, M.; Dezhkam, I.; Atashzar, M. Elevated IL-17 and TGF-β Serum Levels: A Positive Correlation between T-Helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin. Neurosci. J. 2016, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Manolova, I.; Gerenova, J.; Ivanova, M. Serum Levels of Transforming Growth Factor-Β1 (TGF-Β1) in Patients with Systemic Lupus Erythematosus and Hashimoto’s Thyroiditis. Eur. Cytokine Netw. 2013, 24, 69–74. [Google Scholar] [CrossRef]
- Eskalli, Z.; Achouri, Y.; Hahn, S.; Many, M.-C.; Craps, J.; Refetoff, S.; Liao, X.-H.; Dumont, J.E.; Van Sande, J.; Corvilain, B.; et al. Overexpression of Interleukin-4 in the Thyroid of Transgenic Mice Upregulates the Expression of Duox1 and the Anion Transporter Pendrin. Thyroid 2016, 26, 1499–1512. [Google Scholar] [CrossRef]
- Merakchi, K.; Djerbib, S.; Dumont, J.-E.; Miot, F.; De Deken, X. Severe Autoimmune Thyroiditis in Transgenic NOD.H2 h4 Mice Expressing Interleukin-4 in the Thyroid. Thyroid 2023, 33, 351–364. [Google Scholar] [CrossRef]
- Wang, B.; He, W.; Li, Q.; Jia, X.; Yao, Q.; Song, R.; Qin, Q.; Zhang, J. U-Shaped Relationship between Iodine Status and Thyroid Autoimmunity Risk in Adults. Eur. J. Endocrinol. 2019, 181, 255–266. [Google Scholar] [CrossRef]
- Cuenca-Micó, O.; Delgado-González, E.; Anguiano, B.; Vaca-Paniagua, F.; Medina-Rivera, A.; Rodríguez-Dorantes, M.; Aceves, C. Effects of Molecular Iodine/Chemotherapy in the Immune Component of Breast Cancer Tumoral Microenvironment. Biomolecules 2021, 11, 1501. [Google Scholar] [CrossRef]
- Filippini, T.; Fairweather-Tait, S.; Vinceti, M. Selenium and Immune Function: A Systematic Review and Meta-Analysis of Experimental Human Studies. Am. J. Clin. Nutr. 2023, 117, 93–110. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mean ± SD | Median (Min.–Max.) |
|---|---|---|
| Iodine (μg/L) | 92.3 ± 14.6 | 91.0 (69.5–159.6) |
| Selenium (μg/L) | 95.4 ± 17.2 | 91.2 (65.3–148.1) |
| GPX3 (U/L) | 161.2 ± 23.4 | 160.7 (107.4–210.2) |
| FRAP (μmolFe2+/L) | 750.5 ± 89.6 | 746.7 (545.7–979.1) |
| Parameter | Mean ± SD 1/Mean (CI) 2 | Median (Min.–Max.) |
|---|---|---|
| TSH (mIU/mL) | 2.5 ± 1.1 | 2.4 (0.8–6.0) |
| fT4 (pmol/L) | 16.7 ± 2.6 | 16.7 (10.3–20.6) |
| anti-TPO (IU/mL) | 9.2 (4.4–19.2) | 6.4 (6.4–307.0) |
| Parameter | Mean (CI) 1 | Median (Min.–Max.) |
|---|---|---|
| INF-γ (pg/mL) | 72.8 (35.3–149.9) | 53.6 (20.6–300.7) |
| IL-4 (pg/mL) | 183.9 (84.9–398.3) | 135.3 (54.7–867.2) |
| IL-17 (pg/mL) | 105.7 (53.9–207.0) | 77.0 (44.0–399.8) |
| TGF-β (ng/mL) | 0.67 (0.30–1.50) | 0.47 (0.21–3.37) |
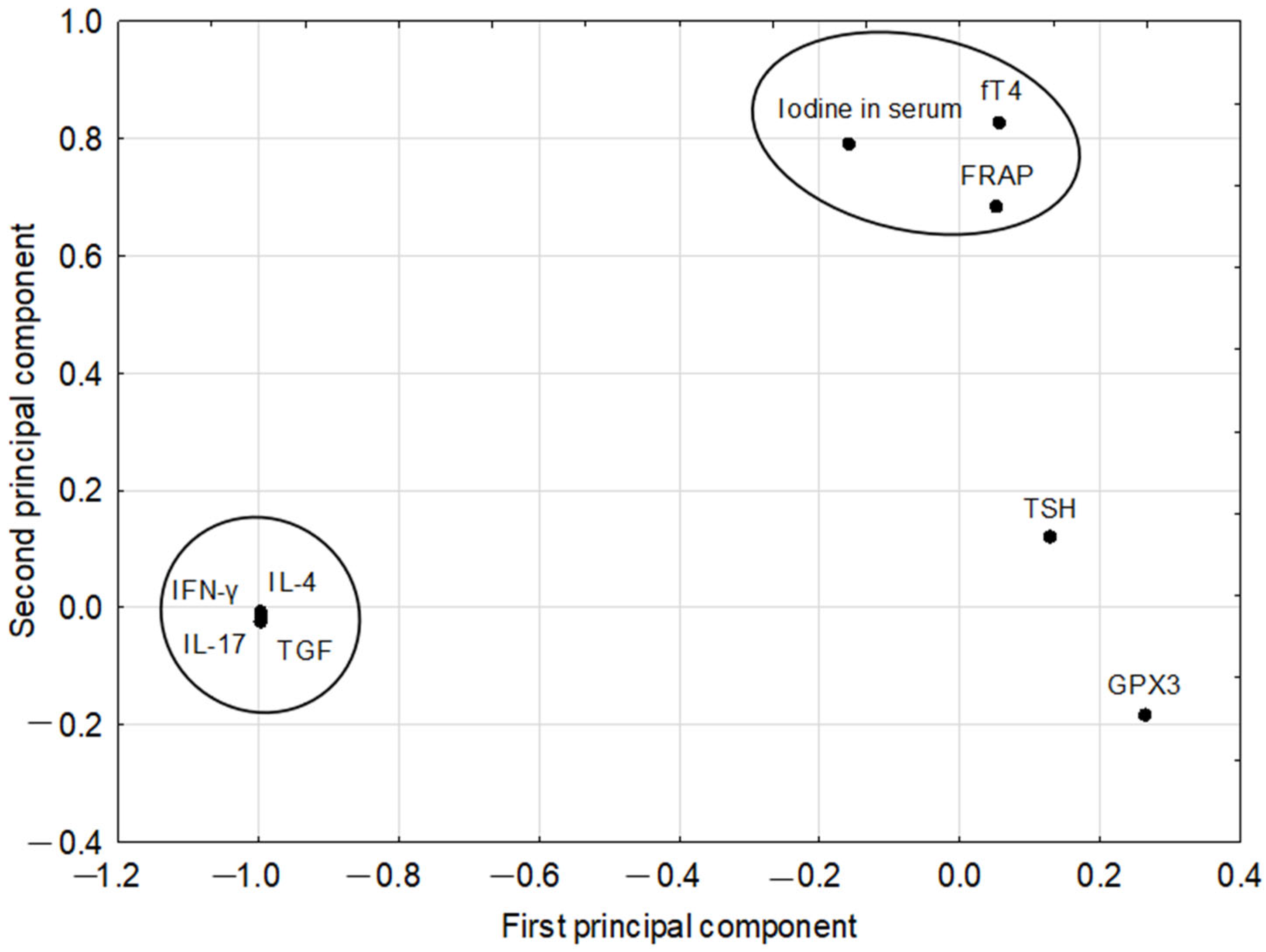
| Pairs of Correlated Parameters | Correlation Coefficients Based on PCA Model | Correlation Coefficients of Raw Parameters | |
|---|---|---|---|
| fT4 | FRAP | 1.0000 | 0.367; p = 0.004 |
| IL-17 | TGF-β | 1.0000 | 0.995; p = 0.000 |
| IL-4 | INF-γ | 1.0000 | 0.996; p = 0.000 |
| TGF-β | INF-γ | 1.0000 | 0.995; p = 0.000 |
| IL-17 | INF-γ | 1.0000 | 0.997; p = 0.000 |
| IL-4 | TGF-β | 1.0000 | 0.997; p = 0.000 |
| IL-4 | IL-17 | 1.0000 | 0.996; p = 0.000 |
| fT4 | Iodine in serum | 0.9649 | 0.544; p = 0.000 |
| FRAP | Iodine in serum | 0.9632 | 0.284; p = 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryczyk-Kozioł, J.; Prochownik, E.; Dobrowolska-Iwanek, J.; Paśko, P.; Kleszcz, K.; Francik, R.; Potok, H.; Nieckula, M.; Cisoń-Apanasewicz, U.; Jabłońska, P.; et al. Iodine and Selenium Status in Relation to Thyroid and Immune Functions—The Analysis of Their Dependencies in a Group of Women of Reproductive Age from the Southern Region of Poland. Nutrients 2025, 17, 1952. https://doi.org/10.3390/nu17121952
Kryczyk-Kozioł J, Prochownik E, Dobrowolska-Iwanek J, Paśko P, Kleszcz K, Francik R, Potok H, Nieckula M, Cisoń-Apanasewicz U, Jabłońska P, et al. Iodine and Selenium Status in Relation to Thyroid and Immune Functions—The Analysis of Their Dependencies in a Group of Women of Reproductive Age from the Southern Region of Poland. Nutrients. 2025; 17(12):1952. https://doi.org/10.3390/nu17121952
Chicago/Turabian StyleKryczyk-Kozioł, Jadwiga, Ewelina Prochownik, Justyna Dobrowolska-Iwanek, Paweł Paśko, Krzysztof Kleszcz, Renata Francik, Halina Potok, Magdalena Nieckula, Urszula Cisoń-Apanasewicz, Paulina Jabłońska, and et al. 2025. "Iodine and Selenium Status in Relation to Thyroid and Immune Functions—The Analysis of Their Dependencies in a Group of Women of Reproductive Age from the Southern Region of Poland" Nutrients 17, no. 12: 1952. https://doi.org/10.3390/nu17121952
APA StyleKryczyk-Kozioł, J., Prochownik, E., Dobrowolska-Iwanek, J., Paśko, P., Kleszcz, K., Francik, R., Potok, H., Nieckula, M., Cisoń-Apanasewicz, U., Jabłońska, P., Ogonowska, D., Kuzera, G., Krośniak, M., Klobučar, S., & Zagrodzki, P. (2025). Iodine and Selenium Status in Relation to Thyroid and Immune Functions—The Analysis of Their Dependencies in a Group of Women of Reproductive Age from the Southern Region of Poland. Nutrients, 17(12), 1952. https://doi.org/10.3390/nu17121952






